Abstract
The simultaneous consumption of glucose and glycerol led to remarkably higher productivity of both biomass and ε-poly-l-lysine (ε-PL), which was of great significance in industrial microbial fermentation. To further understand the superior fermentation performances, transcriptional analysis and exogenous substrates addition were carried out to study the simultaneous utilization of glucose and glycerol by Streptomyces albulus M-Z18. Transcriptome analysis revealed that there was no mutual transcriptional suppression between the utilization of glucose and glycerol, which was quite different from typical “glucose effect”. In addition, microorganisms cultivated with single glycerol showed significant demand for ribose-5-phosphate, which resulted in potential demand for glucose and xylitol. The above demand could be relieved by glucose (in the mixed carbon source) or xylitol addition, leading to improvement of biomass production. It indicated that glucose in the mixed carbon source was more important for biomass production. Besides, transcriptional analysis and exogenous citrate addition proved that single carbon sources could not afford enough carbon skeletons for Embden Meyerhof pathway (EMP) while a glucose–glycerol combination could provided sufficient carbon skeletons to saturate the metabolic capability of EMP, which contributed to the replenishment of precursors and energy consumed in ε-PL production. This study offered insight into the simultaneous consumption of glucose and glycerol in the ε-PL batch fermentation, which deepened our comprehension on the high ε-PL productivity in the mixed carbon source.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
ε-Poly-l-lysine (ε-PL) is a special cationic homo-polymer composed of 25–35 residues of l-lysine with linkages between α-carboxyl group and ε-amino group. Differing from proteins in nature, the ε-PL is synthesized by a nonribosomal peptide synthetase rather than usual ribosome. Due to its biodegradability, edibility and innocuousness, it was approved by the US Food and Drug Administration as a natural food preservative in the food industry [1]. It has been successfully used in Japan, the United States and South Korea for several years, and was also recently approved by the Chinese government. Besides, it can also serve other purposes such as drug carrier, nanoparticles, gene carrier, liposomes, interferon inducer, lipase inhibitor, coating material, etc. [2, 3]. Therefore, it is of great significance to promote the commercial production of ε-PL.
Microbial fermentation by actinomycetes is the main approach to commercially produce ε-PL. Abundant efforts have been made to improve ε-PL production, including strain improvement, optimization of culture medium and fermentation process [4,5,6,7,8]. Since carbon source provides carbon skeletons and energy for the natural metabolism of cells, it plays an important role in medium composition. In most ε-PL fermentation, glucose is usually selected as the carbon source, while glycerol is regarded as a more efficient carbon source for ε-PL production [7, 8]. Recently, we have found that glucose and glycerol can be simultaneously used by Streptomyces sp. M-Z18. Moreover, batch fermentation time can be significantly reduced (above 20%) when 30 g L−1 glucose and 30 g L−1 glycerol are used as the mixed carbon source. The mixed carbon source leads to 1.84- and 1.35-fold higher ε-PL productivity than does glucose or glycerol, respectively, as well as 1.21- and 1.30-fold higher cell growth rate than does glucose or glycerol, individually [9]. These virtues are of great significance in microbial fermentation industry, which are attributed to the simultaneous consumption of multiple substrates.
In general, microorganisms prefer and first consume glucose in a multiple substrate environment, which is the so-called “glucose effect”. The underlying mechanism has been identified as “carbon catabolite repression (CCR)” that the metabolites of glucose utilization can repress the decomposition, transacylation, transmembrane transport and phosphorylation of other carbon sources [10]. However, exceptions always exist. Some microorganisms can simultaneously use multiple substrates and show superior features, most of which significantly promote the productivity and cell growth. For example, in lycopene fermentation, substrates mixture of glucose, arabinose and glycerol can be simultaneously consumed by E. coli, leading to higher productivity of both lycopene and biomass [11]. Besides, simultaneous consumption of glucose and glycerol by Propionibacterium acidipropionici greatly improves the propionic acid production, which are 1.9-fold of that in single glucose and 1.2-fold of that in single glycerol, respectively. In addition, the mixed carbon source also results in a 1.5-fold higher biomass production than single glycerol [12]. Moreover, a glucose–triacylglycerol triolein mixed carbon source achieves increased cell growth and actinorhodine productivity. The following study reveals that the glucose and triacylglycerol triolein can be used in a complementary manner that the triacylglycerol triolein mainly provides carbon skeletons for the biosynthesis of cellular lipids while the glucose is mainly used for actinorhodine production [13]. These exceptions proved that “glucose effect” was not universally applicable; however, it is still unclear that why multiple carbon sources are preferred in microorganisms and how could they be simultaneously used.
The present study is designed to disclose above questions by means of Illumina RNA deep sequencing (RNA-seq) technology, quantitative real-time PCR (qRT-PCR) and exogenous addition of auxiliary substrates. Firstly, the feasibility of glucose–glycerol simultaneous utilization by Streptomyces albulus M-Z18 was assessed. In addition, transcriptome analysis was employed to explore the potential demand of cells for carbon sources in ε-PL fermentation. Further, this potential demand was further confirmed by qRT-PCR and assay of exogenous addition of auxiliary substrates.
Materials and methods
Microorganism, culture media and conditions
Streptomyces albulus Z-18 was previously isolated from the soil as described by Nishikawa and Ogawa and registered in Chinese General Microbiological Culture Collection Center (CGMCC 10479) [14]. S. albulus M-Z18, a mutagenesis from S. albulus Z-18, was used throughout this study. It can produce and secrete ε-PL in acid pH conditions. Culture media were used as follows. For spores generation, the agar slant media were prepared containing 10 g L−1 glucose, 5 g L−1 yeast extract, 5 g L−1 peptone and 20 g L−1 agar, and the pH were adjusted to 7.5 by 2 M NaOH. For seeds pre-culture, the M3G media were used as the fermentation media for batch cultures. They were composed of 50 g L−1 glucose, 10 g L−1 yeast extract, 10 g L−1 (NH4)2SO4, 1.36 g L−1 KH2PO4, 0.8 g L−1 K2HPO4, 0.5 g L−1 MgSO4·7H2O, 0.04 g L−1 ZnSO4·7H2O, 0.03 g L−1 FeSO4·7H2O, and the pH were adjusted to 6.8 by 6 M NaOH. The fermentation media consisted of 10 g L−1 beef extract, 10 g L−1 (NH4)2SO4, 4 g L−1 KH2PO4, 0.8 g L−1 MgSO4·7H2O and 0.05 g L−1 FeSO4·7H2O, and the pH were adjusted to 6.8 by sterilized 12.5% (v v−1) NH3·H2O. The carbon sources of fermentation media were selected as follows: (a) 60 g L−1 glucose; (b) 60 g L−1 glycerol; (c) 30 g L−1 glucose + 30 g L−1 glycerol (glucose:glycerol 1:1, w w−1). The S. albulus M-Z18 was firstly inoculated on agar slant media and cultivated at 30 °C until the spores generated. As for the pre-culture, two loops of S. albulus M-Z18 spores (about 2 × 105 spores L−1) were incubated in 80 mL of M3G media in 500 mL flasks for 24 h in rotary shaker at 200 rpm and 30 °C. After that, the media were transferred into a 5-L jar-fermenter (Baoxing Corp., Shanghai, China) with a working volume of 3.5 L for batch fermentation with an inoculum size of 8%. The control parameters were set as the same in fermentations mentioned previously [9]. The dissolved oxygen (DO) was maintained above 30% and the aeration was set as 1.0 vvm. After the natural pH decline from initial 6.8 to 4.0, the pH values were maintained constant at 4.0 with 12.5% (v v−1) NH3·H2O solution. The agitation speed was controlled from 200 to 800 rpm to maintain the air saturation above 30%.
Analytical methods
Samples were withdrawn from the fermentor at specific time points. The broth was centrifuged at 4500g for 10 min, and the precipitate was collected for biomass determination, while the supernatant was used to determine the concentrations of ε-PL, glucose and glycerol. For the biomass (dry cell weight, DCW) measurement, the precipitate of mycelia pellets was washed twice, filtered by pre-weighted filter paper and dried at 105 °C to a constant weight. The measurement of ε-PL concentration was carried out as described by Kahar et al. [5]. The concentrations of glucose, glycerol, xylitol and citrate were determined using an HPLC system (DIONEX, U-3000, US) with a refractive index detector (Shodex RI-101, Japan) and an ion exchange column (Aminex HPX-87H, 300 × 7.8 mm; Hercules, CA, USA). The flow rate of mobile phase (5 mM H2SO4) was set at 0.6 mL min−1, and the column temperature was maintained at 60 °C. Assays were performed at least in triplicate.
RNA extraction and RNA-seq analysis
In the mixed carbon source, significantly higher specific ε-PL formation rate, cell growth rate and carbon source consumption rate were all observed at the same period during 21–31 h in ε-PL batch culture at pH 4.0 [15]. Considering the process of gene transcription and subsequent translation, mycelia pellets at 24 h were selected as the samples for the RNA extraction. The RNA was isolated from three biological replicates of S. albulus M-Z18 batch cultures. The broths were withdrawn from three independent batch cultures at 24 h and then merged together. After the centrifugation at 7000g for 1 min, the mycelial pellets were washed once before frozen treatment in liquid nitrogen and were stored at −80 °C overnight for subsequent RNA isolation. Total RNA was isolated using RiboPure™-Yeast Kit (Life technologies, USA) according to the manufacturer’s protocols. The DNA in the total RNA was digested with DNase I (NEB, USA), and the rRNA was removed with Ribo-Zero™ Magnetic Kit to enrich the mRNA samples. After the purification of mRNA with RNA Clean XP Beads (Agencourt, USA), cDNA libraries were constructed and sequenced by Illumina Hiseq 2000. Raw sequencing fragments were filtered to remove adaptors and low-quality reads. Transcriptome de novo assembly was carried out with short reads assembling program (Trinity). Analyses of differences in genes transcription were performed among cultures using single glucose, glycerol and glucose–glycerol mixed carbon source, and relevant genes were mapped to the genome of S. albulus ZPM (CP006871). The transcriptions of genes related to the carbon source utilization and metabolism were studied to obtain a deeper insight into the simultaneous utilization of glucose and glycerol in the mixed carbon source.
Validation of the understanding from RNA-seq analysis
The qRT-PCR was employed for the validation of RNA-seq analysis results. The samples were also withdrawn from the fermentors at 24 h in batch cultures with glucose, glycerol and the mixed carbon source. The total RNA from S. albulus M-Z18 was extracted using Trizol kit (SK2445, Sangon Biotech, Shanghai, China) according to the manufacturer’s procedure. Reverse transcription was achieved using AMV First Strand cDNA Synthesis Kit (SK2445, Sangon Biotech, Shanghai, China) according to the manufacturer’s protocol. The transcriptional levels of genes (MAGL006991, MAGL003823, MAGL008921, MAGL002671 and MAGL003375) were determined by a real-time fluorescent quantitative PCR (ABI Stepone plus, Applied Biosystems, USA) with SG Fast qPCR Master Mix (High Rox) (Bio Basic Inc., Toronto, Canada). Control reactions were set as pre-denature at 95 °C for 3 min and amplification occurred in two steps: 7 s at 95 °C for denature, 10 s at 57 °C for annealing and 15 s at 72 °C for extension for 40 cycles. The primer pair sequences are listed in Table 1.
Fermentation with exogenous addition of auxiliary carbon sources
Xylitol and citrate were selected as two types of exogenous auxiliary carbon sources in this study. The fermentation control parameters were set as above mentioned. 6 g L−1 of xylitol or citrate was added in the batch fermentations using glucose, glycerol and glucose–glycerol combination, respectively, at 15 h, when the pH of all cultures naturally dropped to 4.0 (data not shown). Samples were withdrawn from the fermentor for the determination of ε-PL, glucose, glycerol, xylitol, citrate and DCW as above mentioned.
Calculation
The average values of specific cell growth rate (h−1), ε-PL specific formation rate (h−1), and specific auxiliary carbon source consumption rate (h−1) are calculated as follows:
The above parameters are the averaged values performed in certain time range (between two consecutive samples drawn from the fermentor), where t is the ferment time (h), and c(X), c(P), c(S) represents the cell concentration (g L−1), ε-PL concentration (g L−1) and auxiliary carbon source concentration (g L−1) of the t h, respectively.
Results
Effects of the mixed carbon source on the ε-PL batch fermentation
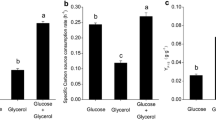
To re-evaluate the effects of the glucose–glycerol mixed carbon source on the production of ε-PL, the fermentation profiles in glucose, glycerol and glucose–glycerol are shown in Fig. 1. In single carbon sources, glucose leads to higher biomass production (Fig. 1a) while glycerol leads to higher ε-PL production (Fig. 1b). Interestingly, these two substrates could be simultaneously used by S. albulus M-Z18 (Fig. 1c), which was not common in microbial fermentation. More importantly, both the ε-PL biosynthesis and biomass production could be significantly enhanced with the consumption of multiple substrates during 21–31 h. Therefore, understanding of the carbon source utilization was of great importance to dig into the enhancements of ε-PL fermentation in the mixed carbon source.
Feasibility assessment of the glucose–glycerol simultaneous utilization
Table 2 shows the transcription levels of genes coding for the transportation and phosphorylation of glucose and glycerol in cultures using different carbon sources. The glucose–glycerol mixed carbon source could be regarded as glycerol addition in single glucose, and the same as extra glucose addition in single glycerol. Single glucose showed 2.9-fold higher RPKM of glucokinase gene than did single glycerol, while single glycerol exhibited 20.7-fold higher transcription levels of glycerol kinase than did single glucose. The glycerol kinase gene only showed basal transcription level (RPKM = 40.02) when glycerol was absent (in single glucose), while the gene transcription significantly increased when extra glycerol was added (in the mixed carbon source). Compared to single glycerol, the extra glucose addition (in the mixed carbon source) exerted no significant influence on the transcription of genes coding for glycerol uptake facilitator protein (MAGL002354) and glycerol kinase (MAGL002355). Similarly, compared with single glucose, the extra glycerol addition (in the mixed carbon source) exerted no negative influence on the transcription of glucose-specific PTS system gene (MAGL001888) either.
Transcriptome analysis of the genes related to the utilization of glucose and glycerol in ε-PL batch cultures with different carbon sources
To obtain more information about the carbon source metabolism in culture using glucose–glycerol mixed carbon source, genes transcription related to the metabolism of carbon sources was compared by RNA-seq technology among the cultures using three different carbon sources. The results are shown in Online Resource 1 and performed as Fig. 2 for a better comprehension. The significance of gene transcription differences was classified into six levels: (a) over twofold up-regulation; (b) 1.5- to 2-fold up-regulation; (c) 1.2- to 1.5-fold up-regulation; (d) no significant difference; (e) 1.5- to 2-fold down-regulation; (f) over twofold down-regulation. In the pairwise comparison between different carbon sources, the relative up-regulated transcription of genes could be read by following these principles: (1) if there are enough carbon skeletons metabolized through the specific pathway, the up-regulated genes transcription could be deemed as metabolic enhancement; (2) if there is a lack of carbon skeletons, the up-regulated genes transcription could be considered as a potential need of cells for more carbon skeletons metabolized through corresponding pathway.
Comparison of genes transcriptions related to the metabolism of glucose and glycerol among ε-PL batch cultures using single glucose, glycerol and glucose–glycerol combination as the carbon sources. a Glucose (control) vs. glycerol (treated); b glycerol (control) vs. glucose–glycerol combination (treated); c Glucose (control) vs. glucose–glycerol combination (treated). Bold red arrows indicate over twofold up-regulation, red arrows indicate 1.5- to 2-fold up-regulation, pink arrows indicate 1.2- to 1.5-fold up-regulation, black arrows indicate no significant difference in transcription levels, blue arrows indicate 1.5- to 2-fold down-regulation, bold blue arrows indicate over twofold down-regulation. The numbered biochemical pathways are according to the relevant genes in Online resource 1 (color figure online)
Figure 2a shows the transcriptional comparison between single glycerol (treated) and glucose (control). Higher transcription of glycerol-3-phosphate dehydrogenase gene (reaction 11) was observed in single glycerol, similar to the performances of the genes coding for glycerol uptake facilitator protein and glycerol kinase. Interestingly, the cells in single glycerol (without glucose) showed higher transcription of genes for glucoamylase and glucose-specific IIA component (reaction 1 and 2). In addition, without glucose addition (in single glycerol), the gene coding for 6-phosphofructokinase (reaction 5, glycerol metabolism independent) was still up-regulated. Moreover, in single glycerol, the cells showed higher transcriptions of xylulokinase (reaction 19). Figure 2b shows the effects of glucose addition (in the mixed carbon source) on the genes transcription in culture using single glycerol. The glucose addition (in the mixed carbon source) significantly decreased the transcription of genes coding for glucoamylase (reaction 1) and 6-phosphofructokinase (reaction 5). It was noteworthy to mention that similar transcriptional down-regulation could be observed in the xylulokinase gene (reaction 19) by glucose addition (in the mixed carbon source). Besides, the glucose addition (in the mixed carbon source) enhanced the genes transcription in PPP. Further, the transcriptions of genes coding for pyruvate kinase (reaction 7) and phosphoenolpyruvate carboxylase (reaction 8) were also up-regulated. Compared with single glucose, similar enhancements were observed in the mixed carbon source (extra glycerol addition) in Fig. 2c. An extra glycerol addition (in the mixed carbon source) improved the transcription of pyruvate kinase gene (reaction 7) and phosphoenolpyruvate carboxylase gene (reaction 8).
Validation of the important genes transcription involved in carbon source utilization
As shown in Fig. 3a, b, compared with that in single glucose, cells in single glycerol showed higher transcription level of genes coding for glucose-6-phosphate dehydrogenase and ribose-5-phosphate isomerase. Similar to the RNA-seq results, the mixed carbon source enhanced this two genes transcription. The cells in the mixed carbon source (with extra glucose addition) showed lower transcription level of 6-phosphofructokinase gene than those in single glycerol (Fig. 3c). Besides, the highest transcription levels of genes coding for phosphoenolpyruvate carboxylase and pyruvate kinase were observed in the mixed carbon source (Fig. 3d, e).
mRNA relative transcription levels of important genes for carbon source utilization at 24 h in ε-PL batch cultures with different carbon sources. a MAGL003375 (ribose-5-phosphate isomerase); b MAGL002671 (glucose 6-phosphate dehydrogenase); c MAGL006991 (6-phosphofructokinase); d MAGL003823 (phosphoenolpyruvate carboxylase); e MAGL008921 (pyruvate kinase)
Effects of the auxiliary carbon sources on the ε-PL batch fermentation with different carbon sources
To view the above results from another perspective, batch fermentations using glucose, glycerol and the mixed carbon source were performed with exogenous addition of auxiliary carbon sources (xylitol and citrate). The metabolism of the auxiliary carbon sources is shown in Fig. 4.
The fermentation parameters in different carbon sources with auxiliary carbon sources addition are performed in Online Resource 2, from which the kinetic parameters were calculated and shown in Fig. 5. Exogenous xylitol addition greatly improved the specific cell growth rates in single carbon sources (Fig. 5b), while it exerted no positive influence on the specific ε-PL formation rates (Fig. 5c). The cells in glycerol showed higher specific xylitol consumption rate than that in glucose (Fig. 5a), resulting in a 1.9-fold increase in cell growth rate compared to the control (in single glycerol without xylitol addition) (Fig. 5b). However, rapid xylitol consumption and growth enhancement could not be observed in the mixed carbon source with xylitol addition (Fig. 5b). Similarly, exogenous citrate addition significantly improved the specific ε-PL formation rates in single carbon sources (Fig. 5f). The cells in single carbon sources consumed more citrate (Fig. 5d), which exerted less influence on biomass production (Fig. 5e) while it effectively improved ε-PL biosynthesis (Fig. 5f). However, the cells in the mixed carbon source could hardly use citrate and showed no increase in specific ε-PL formation rate by citrate addition. Therefore, the glucose–glycerol mixed carbon source could provide sufficient carbon skeletons for the metabolism through PPP and TCA cycle, which was beneficial for both cell growth and ε-PL biosynthesis.
Discussion
In general, glucose will be firstly used by microorganisms when the media contain multiple carbon sources (including glucose), which is the so-called “glucose effect” [16]. However, our previous study found that glucose and glycerol could be simultaneously used by Streptomyces sp. M-Z18, which leads to remarkable improvement of both the ε-PL productivity and the cell growth rate. Similar improvements have been also found in other biochemicals’ fermentations using mixed carbon source [11,12,13], which are of great significance in industrial fermentation. These superior performances are mainly ascribed to the simultaneous utilization of multiple substrates. However, why multiple carbon sources are preferred by these special microorganisms and how could them be simultaneously used are still unclear. The answers contribute to the understanding of the improvements of ε-PL production in the glucose–glycerol mixed carbon source.
Different carbon sources could lead to various adaptations in genes transcription. Hence, in this study, transcriptome analyses concerning the carbon source utilization and central carbon metabolism were employed. Membrane transport of glucose and glycerol depends on glucose-specific IIA component (PTS system) [17] and glycerol uptake facilitator protein (nonPTS permease) in Streptomyces species [18], respectively. The cause of the “glucose effect” is believed as carbon catabolite repression [19] that the catabolites of glucose could repress the transcription of genes related to the decomposition, transacylation, transportation and phosphorylation of the substrates which are more difficult to be used [10]. Glycerol is one of them. When glucose is present in culture by E. coli, the uptake and metabolism of alternative carbon sources can be prevented, as the genes transcription containing glycerol kinase and other nonPTS permeases are inhibited by unphosphorylated glucose-specific IIA component [17, 20]. However, it seems as if this effect was inapplicable in the ε-PL batch fermentation by S. albulus M-Z18 with mixed carbon sources (Fig. 1c). The pairwise comparisons of genes transcription on substrates utilization were carried out among cultures using single glucose, glycerol and glucose–glycerol combination. The results revealed that the genes transcription in glycerol utilization was more inducible than that in glucose utilization, implying that the glucose was more essential than glycerol for the survival of S. albulus. Interestingly, no mutual repression of gene transcription concerning the transmembrane transport and phosphorylation of glucose and glycerol was observed in cells cultivated with the mixed carbon source. The glucose utilization exerted no negative influence on the glycerol uptake and metabolism in S. albulus M-Z18. Although the glucose kinase gene showed a transcriptional decrease in the mixed carbon source compared to single glucose (Table 2), the decrease was so little that it was more like a substrate induction in single glucose rather than a transcriptional repression by glycerol. The above assessment confirmed the feasibility of the simultaneous utilization of glucose and glycerol, which was the foundation of the fermentation enhancement in the mixed carbon source.
In Streptomyces, EMP is a common metabolic pathway for both glucose and glycerol; however, there are differences between these two substrates. On the one hand, the single glycerol has to be first metabolized through gluconeogenesis pathway for ribose-5-phosphate production through PPP, while the gluconeogenesis is not necessary in single glucose for ribose-5-phosphate biosynthesis. On the other hand, phosphofructokinase, the rate-limiting enzyme of EMP in single glucose, is not involved in the glycerol metabolism. The transcriptional differences between the cultures using single glucose and glycerol intensively appeared in PPP and the first half of EMP (Fig. 2a). Interestingly, without glucose addition, the cells in single glycerol showed higher transcription of genes for obtaining transportation and exclusive metabolism of glucose (reaction 1, 2 and 5, respectively). Moreover, ribose-5-phosphate biosynthesis was enhanced in single glycerol by up-regulated transcription of genes in PPP (reaction 14, 15 and 17) and xylulose utilization (reaction 19). It was indicated that both glucose and xylulose were in great demand by the cells in single glycerol. Xylulose is an effective substitute of glucose for ribose-5-phosphate biosynthesis, because the ribose-5-phosphate could be quickly obtained from xylulose through only two steps of biochemical reactions (reaction 19 and 18). Once extra glucose was added (in the mixed carbon source), the transcription of genes in PPP (reaction 14, 16 and 17) was increased, while genes transcription for glucose obtaining (reaction 1) and xylulose utilization (reaction 19) was greatly down-regulated. These results highlighted a fact that ribose-5-phosphate was urgently needed by the cells in single glycerol; therefore, the cells presented higher transcription of xylulokinase gene to promote potential xylulose uptake for ribose-5-phosphate production. This potential demand could be relieved by glucose addition (in the mixed carbon source), which was due to the improved ribose-5-phosphate biosynthesis through PPP from glucose (Fig. 2b). Therefore, in single glycerol, the potential glucose requirement could be mainly attributed to the deficiency of ribose-5-phosphate. In addition, extra glucose addition (in the mixed carbon source) relieved the demand of cells in single glycerol for carbon skeletons metabolized through EMP (reaction 5), and provided more carbon skeletons for TCA cycle (reaction 7 and 8). It was revealed that glucose in the mixed carbon source could also be used for compensating the carbon skeletons used in the following TCA cycle. Compared to single glucose, extra glycerol addition (in the mixed carbon source) also enhanced the provision of carbon skeletons for TCA cycle (reaction 7 and 8) (Fig. 2c), which implied that glycerol in the mixed carbon source could be metabolized through EMP and following TCA cycle. The above transcriptome results were well confirmed by qRT-PCR in Fig. 3. In single glycerol, the cells were suffering shortages of both ribose-5-phosphate and EMP intermediates, resulting in feedback transcription enhancements on glucose-6-phosphate dehydrogenase (Fig. 3a), ribose-5-phosphate isomerase (Fig. 3b) and 6-phosphofructokinase (Fig. 3c). The glucose–glycerol mixed carbon source effectively enhanced the transcription of genes in PPP (glucose-6-phosphate dehydrogenase, Fig. 3a) and EMP (phosphoenolpyruvate carboxylase, Fig. 3d; pyruvate kinase, Fig. 3e), which guaranteed the provision of carbon skeletons for production of biomass, ATP and l-lysine. The above transcription comparisons revealed that the glucose and glycerol could be used in a co-ordinate and complementary manner. In the mixed carbon source, glycerol was mainly metabolized through EMP, while glucose was used to produce ribose-5-phosphate and partly provide carbon skeletons through EMP.
The complementary manner of glucose and glycerol metabolism in S. albulus M-Z18 was due to the potential demands of cells in each single carbon source. As shown in Fig. 4, xylitol can be metabolized to produce xylulose in fungi and bacteria by xylitol dehydrogenase [21], quickly compensating the carbon skeletons used for ribose-5-phosphate biosynthesis. Besides, citrate served as a useful carbon source, which could be directly metabolized through TCA cycle (Fig. 4). It helped to produce more NADH and oxaloacetate for the synthesis of ATP and l-lysine, respectively, and finally increased the ε-PL production [22]. With different metabolic behavior, xylitol and citrate were employed to study the potential demand for carbon skeletons in cultures using different carbon sources. Single glycerol showed the lowest cell growth rate and exhibited the highest rate of xylitol consumption under exogenous xylitol addition (Fig. 5a). In addition, the cells in single glycerol showed up-regulated transcription of genes coding for xylulokinase (reaction 19 in Fig. 2a) and ribose 5-phosphate isomerase (reaction 17 in Fig. 2a) in comparison to those in single glucose. The exogenous-added xylitol did not influence the ε-PL biosynthesis in single glycerol (Fig. 5c), while it greatly enhanced the specific cell growth rate, resulting in a 1.9-fold increase compared to the control (in single glycerol without xylitol addition) (Fig. 5b). In single glucose with xylitol addition, the cells showed lower xylitol consumption rate (Fig. 5a) and smaller increase of specific cell growth rate (Fig. 5b). In fact, glycerol was more difficult to be used through PPP than glucose for multiple reactions of gluconeogenesis, which was unfavorable for ribose-5-phosphate synthesis. This shortage in single glycerol limited the cell growth (Fig. 1a) and, at the same time, led to up-regulated transcription of genes in PPP as a feedback enhancement. Interestingly, the rapid xylitol consumption and growth enhancement could not be observed in the mixed carbon source with xylitol addition (Fig. 5b). The demand for xylitol in single glycerol could be relieved by glucose addition (in the mixed carbon source). Similarly, as shown in Fig. 5d, the citrate was more acceptable in single carbon sources while it was less used in the mixed carbon source. As a result, the ε-PL biosynthesis was greatly enhanced by citrate addition in single carbon sources, while no enhancement was observed in the mixed carbon source. These results indicated that the carbon flux in EMP pathway was relatively unsaturated in single glucose or glycerol, and the glucose–glycerol combination could fully satisfy the demand for carbon fluxes through EMP and following metabolism through TCA cycle, anaplerotic and DAP pathway for ε-PL production.
In this study, the results of genes transcription and the effects of auxiliary carbon sources addition were well confirmed with each other, which offered more information about the carbon source metabolism in the mixed carbon source. There was no mutual suppression between the utilization of glucose and glycerol in S. albulus M-Z18. Glucose could be easily metabolized through PPP, while such metabolism was more tough for glycerol. Equally, glycerol could be easily metabolized through EMP without the rate-limiting enzyme (6-phosphofructokinase); however, this rate-limiting effect could not be avoided in glucose metabolism. Actually, the cells needed abundant carbon sources for different physiological purposes, but single glucose or glycerol could not afford all of them, so the cells tended to enhance the deficient metabolisms. A collaboration of carbon sources utilization occurred in the glucose–glycerol mixed carbon source. Glucose was metabolized through PPP, quickly compensating the ribose-5-phosphate consumed for biomass production. The other part of glucose was used through EMP. Besides, glycerol was mainly used through EMP. The combined carbon fluxes from glucose and glycerol in EMP improved the total carbon skeletons provision, which efficiently replenished the precursor l-lysine and ATP consumed in ε-PL production. The above information contributed to the understanding of how the S. albulus M-Z18 quickly and simultaneously used glucose and glycerol in ε-PL fermentation, which laid the foundation for the ε-PL productivity enhancements in culture using glucose–glycerol mixed carbon source. In spite of this, we were attempting to improve the comprehension. Metabolic flux analysis proved to be an effective way to study the flux distribution in culture using mixed carbon source [13, 23]. Further investigations are to be carried out on the comparison of metabolic fluxes using 13C isotopic trace among cultures using different carbon sources. It would help to depict a more accurate patten of carbon metabolism in the glucose–glycerol mixed carbon source.
References
Hiraki J, Ichikawa T, Ninomiya S, Seki H, Uohama K, Seki H, Kimura S, Yanagimoto Y, Barnett JJ (2003) Use of ADME studies to confirm the safety of ε-polylysine as a preservative in food. Regul Toxicol Pharmacol 37:328–340
Bankar SB, Singhal RS (2013) Panorama of poly-ε-lysine. RSC Adv 3(23):8586–8603
Shih IL, Shen MH (2006) Application of response surface methodology to optimize production of poly-ɛ-lysine by Streptomyces albulus IFO 14147. Enzyme Microb Technol 39(1):15–21
Li S, Tang L, Chen X, Liao L, Li F, Mao Z (2011) Isolation and characterization of a novel epsilon-poly-l-lysine producing strain: Streptomyces griseofuscus. J Ind Microbiol Biotechnol 38(4):557–563
Kahar P, Iwata T, Hiraki J, Park EY, Okabe M (2001) Enhancement of ε-polylysine production by Streptomyces albulus strain 410 using pH control. J Biosci Bioeng 91(2):190–194
Jia S, Wang G, Sun Y, Tan Z (2009) Improvement of ε-poly-l-lysine production by Streptomyces albulus TUST2 employing a feeding strategy. Paper presented at the Tianjin University of Science and Technology, Tianjin
Bankar SB, Singhal RS (2010) Optimization of poly-epsilon-lysine production by Streptomyces noursei NRRL 5126. Bioresour Technol 101(21):8370–8375
Chen XS, Tang L, Li S, Liao LJ, Zhang JH, Mao ZG (2011) Optimization of medium for enhancement of epsilon-poly-l-lysine production by Streptomyces sp. M-Z18 with glycerol as carbon source. Bioresour Technol 102(2):1727–1732
Chen XS, Ren XD, Dong N, Li S, Li F, Zhao FL, Tang L, Zhang JH, Mao ZG (2012) Culture medium containing glucose and glycerol as a mixed carbon source improves ε-poly-l-lysine production by Streptomyces sp. M-Z18. Bioproc Biosyst Eng 35(3):469–475
Magasanik B (1963) The genetic and molecular basis of catabolite repression. In: Vogel HJ, Bryson V, Lampen J (eds) Informational macromolecules. Academic Press, New York, pp 271–286
Kim YS, Lee JH, Kim NH, Yeom SJ, Kim SW, Oh DK (2011) Increase of lycopene production by supplementing auxiliary carbon sources in metabolically engineered Escherichia coli. Appl Microbiol Biotechnol 90(2):489–497
Liu Y, Zhang YG, Zhang RB, Zhang F, Zhu J (2011) Glycerol/glucose co-fermentation: one more proficient process to produce propionic acid by Propionibacterium acidipropionici. Curr Microbiol 62(1):152–158
Peacock L, Ward J, Ratledge C, Dickinson F, Ison A (2003) How Streptomyces lividans uses oils and sugars as mixed substrates? Enzyme Microb Technol 1:157–166
Nishikawa M, Ogawa K (2002) Distribution of microbes producing antimicrobial ε-poly-l-lysine polymers in soil microflora determined by a novel method. Appl Environ Microbiol 68(7):3575–3581
Zeng X, Xs Chen, Ren XD, Liu QR, Wang L, Sun QX, Tang L, Mao ZG (2014) Insights into the role of glucose and glycerol as a mixed carbon source in the improvement of ε-poly-l-lysine productivity. Appl Biochem Biotechnol 173(8):2211–2224
Monod J (1942) Recherches sur la croissance des cultures bacteriennes. Hermann, Paris
Deutscher J, Francke C, Postma PW (2006) How phosphotransferase system-related protein phosphorylation regulates carbohydrate metabolism in bacteria. Microbiol Mol Biol Rev 70(4):939–1031
Minambres B, Reglero A, Luengo JM (1992) Characterization of an inducible transport system for glycerol in Streptomyces clavuligerus. J Antibiot 45(2):269–277
Magasanik B (1961) Catabolite repression Cold Spring Harbor symposia on quantitative biology. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, pp 249–256
Kremling A, Geiselmann J, Ropers D, de Jong H (2015) Understanding carbon catabolite repression in Escherichia coli using quantitative models. Trends Microbiol 23(2):99–109
Fossitt D, Mortlock R, Anderson R, Wood W (1964) Pathways of l-arabitol and xylitol metabolism in Aerobacter aerogenes. J Biol Chem 239(7):2110–2115
Bankar SB, Singhal RS (2011) Metabolic precursors enhance the production of poly-ε-lysine by Streptomyces noursei NRRL 5126. Eng Life Sci 11(3):253–258
Borodina I, Scholler C, Eliasson A, Nielsen J (2005) Metabolic network analysis of Streptomyces tenebrarius, a Streptomyces species with an active Entner–Doudoroff pathway. Appl Environ Microb 71(5):2294–2302
Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (31301556), the Fundamental Research Funds for the Central Universities (JUSRP51504), the Open Project Program of the Key Laboratory of Industrial Biotechnology, Ministry of Education, China (KLIBKF201302), and the Jiangsu Province Collaborative Innovation Center for Advanced Industrial Fermentation Industry Development Program.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Zeng, X., Zhao, J., Chen, X. et al. Insights into the simultaneous utilization of glucose and glycerol by Streptomyces albulus M-Z18 for high ε-poly-l-lysine productivity. Bioprocess Biosyst Eng 40, 1775–1785 (2017). https://doi.org/10.1007/s00449-017-1832-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00449-017-1832-9