Abstract
Deforestation and habitat loss resulting from land use changes are some of the utmost anthropogenic impacts that threaten tropical birds in human-modified landscapes (HMLs). The degree of these impacts on birds’ diet, habitat use, and ecological niche can be measured by isotopic analysis. We investigated whether the isotopic niche width, food resources, and habitat use of bird trophic guilds differed between HMLs and natural landscapes (NLs) using stable carbon (δ13C) and nitrogen isotopes (δ15N). We analyzed feathers of 851 bird individuals from 28 landscapes in the Brazilian Atlantic Forest. We classified landscapes into two groups according to the percentage of forest cover (HMLs ≤ 30%; NLs ≥ 47%), and compared the isotopic niche width and mean values of δ13C and δ15N for each guild between landscape types. The niches of frugivores, insectivores, nectarivores, and omnivores were narrower in HMLs, whereas granivores showed the opposite pattern. In HMLs, nectarivores showed a reduction of 44% in niche width, while granivores presented an expansion of 26%. Individuals in HMLs consumed more resources from agricultural areas (C4 plants), but almost all guilds showed a preference for forest resources (C3 plants) in both landscape types, except granivores. Degraded and fragmented landscapes typically present a lower availability of habitat and food resources for many species, which was reflected by the reduction in niche width of birds in HMLs. Therefore, to protect the diversity of guilds in HMLs, landscape management strategies that offer birds more diverse habitats must be implemented in tropical regions.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Land use changes are some of the greatest threats to biodiversity, especially considering the high rates of deforestation and expansion of agricultural areas upon tropical forests worldwide (Steffen et al. 2005). Significant compositional shifts in many communities of animals and plants, and diversity shrinkage are consequences of habitat loss and fragmentation, which are mainly driven by human activities (Haddad et al. 2015; Vellend et al. 2017). As many biological organisms serve as direct food resources for other organisms, these multi-taxon losses negatively affect food webs (Estes et al. 2011; Bogoni et al. 2019). Considering that anthropogenic changes directly affect the availability of habitat and food to the fauna, human-modified landscapes (HMLs) differ from natural landscapes (NLs) in the provision of both types of resources (Magioli et al. 2019; da Silva et al. 2020).
Birds are one of the many taxa influenced by anthropogenic impacts and their communities have changed significantly in HMLs (Pardini et al. 2009; Martensen et al. 2012). In the Atlantic Forest of South America, landscapes composed of up to 30% of forest have a lower bird richness and abundance compared to landscapes with over 50% of forest (Martensen et al. 2012; Morante-Filho et al. 2015). In HMLs, bird assemblages are dominated by species that are generalists regarding the use of habitat and diet (Carrara et al. 2015; Alexandrino et al. 2017). Although generalist birds are typically less sensitive to changes in habitats than specialists (Devictor et al. 2008), they also depend on forest remnants to persist in HMLs (Pardini et al. 2009). Moreover, in degraded environments, generalist species are responsible for much of the ecological processes required to maintain ecosystem functioning (Pizo 2007; Barros et al. 2019).
Conventional methods used to assess the habitat use and diet of birds (e.g., collecting gut contents and tracking with GPS) are time and resource demanding. As an alternative, the use of stable carbon and nitrogen isotopes in bird studies across spatiotemporal scales are increasing, especially due to the efficiency of this method to obtain information on diet and habitat use (Inger and Bearhop 2008; Hobson 2011). Stable carbon isotopes (12C and 13C; δ13C) permits the estimation of food resources and habitats used by individuals (Inger and Bearhop 2008). The main processes that lead to different carbon isotopic values are related to plant photosynthetic pathways (C3 and C4), although some other factors have the potential to influence these values to some extent in terrestrial landscapes (e.g., soil moisture and characteristics, temperature, and light) (Vitória et al. 2018; Sena-Souza et al. 2019). The Atlantic Forest is mostly composed of C3 plants, whereas agricultural areas in this domain are mainly composed of C4 plants (e.g., grasses for cattle ranching and sugarcane), which are dominant components of many contemporary tropical landscapes. Stable nitrogen isotopes (14N and 15N; δ15N), on the other hand, are often used to provide insights on trophic processes and to estimate the position of the species in the trophic chain (Post 2002). These isotopes can also reflect the influence of anthropogenic nitrogen input into the environment upon organisms, mainly via nitrogen fertilizer used in agriculture (Hebert and Wassenaar 2001).
The concept of ecological niche has been widely used to understand the relationship between species and their environment (Chase and Leibold 2003). The niche comprises a multidimensional space of biotic and abiotic variables, such as the food and habitat used so that the study of niche breadth allows the understanding of relevant information about the ecology of a species (Sexton et al. 2017). Niche breadth reduction caused by environmental impacts might result in the local extinction of species (Scheele et al. 2017). Conversely, niche expansion may reveal that some species can use areas highly modified by anthropogenic activities (Pagani-Núñez et al. 2019; Magioli et al. 2019). Isotopic niches represent ecological niches as the combination of δ13C and δ15N values provides information on the use of habitat and diet of a given species (Newsome et al. 2007). Using this approach, some studies have found relevant information regarding niche width changes in anthropogenic habitats and the possible consequences of these changes for species conservation (e.g., Galetti et al. 2016; Pagani-Núñez et al. 2019). In the real world in which constant modifications occur at the landscape scale, it is essential to understand and compare how species differ in their use of habitats and food resources in NLs and HMLs.
We compared the patterns of resource and habitat use by five trophic guilds of birds (frugivores, granivores, insectivores, nectarivores, and omnivores) between HMLs and NLs in the Atlantic Forest, Brazil. To achieve this, we determined the isotopic values of carbon (δ13C) and nitrogen (δ15N) and the niche width for each guild, comparing them between NLs (≥ 47% of forest cover) and HMLs (≤ 30% of forest cover). We hypothesized that, given the negative anthropogenic impacts on tropical forests, especially regarding land use changes in response to agriculture expansion and deforestation, trophic guilds would show a significant decrease in isotopic niche width between HMLs and NLs. We also hypothesized that trophic guilds in HMLs are prone to a greater (direct or indirect) consumption of food items from both forest remnants (mostly C3 plants) and agricultural areas (mostly C4 plants), showing higher δ13C values for birds in this landscape type. Regarding δ15N values, we expected differences between landscape types as anthropogenic changes negatively affect resource availability and trophic chain structure (Morante-Filho et al. 2018; Magioli et al. 2019).
Materials and methods
Study sites and landscape type definition
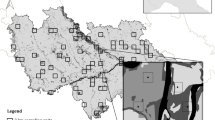
Study sites were set on evergreen and semideciduous forests of the Atlantic Forest biome, a tropical biodiversity hotspot (Mittermeier et al. 2011), in the state of São Paulo, south-eastern Brazil (Fig. 1). Before the nineteenth century, this state was covered by ~ 82% of native forests (Victor et al. 2005). Nowadays, only 28% of the forest cover remains, mostly in steep terrains of coastal regions named Serra do Mar corridor and Paranapiacaba ecological continuum—typically evergreen forests (Rezende et al. 2018; Souza et al. 2020). The high fragmentation and forest loss in the central and western portion of the state—mainly composed of semideciduous forests—results from the history of agriculture expansion in São Paulo, which was mostly established away from the coastal region (Aguiar et al. 2003). Despite the difference in vegetation type and abiotic variables (e.g., soil and climate) between inland (semideciduous forest) and coastal regions (evergreen forest), both express similar δ13C and δ15N values (Powell et al. 2012; Vitória et al. 2018; Sena-Souza et al. 2019), which enables the following comparisons.
Map of the 28 landscapes where bird species were sampled in south-eastern Atlantic Forest, Brazil. The exact location of each sampling area is represented on the grey map, which were classified into two landscape types: human-modified landscapes (HMLs; N = 12) and natural landscapes (NLs; N = 16). The white star near study site number 8 represents the city of São Paulo (capital and the biggest city of São Paulo state). The circular buffers in the right are a sample of the diversity of landscapes with different percentages of forest cover (see all landscapes in Supplementary Figs. S1 and S2, according to the referred numbers)
For the selection of landscape sampling sites, we first searched for biological samples (wing feather) available from recent field sampling collections (2011–2019) and also from the ornithological collection of the Museum of Zoology of the University of São Paulo (MZUSP), Brazil. We collected wing feather samples from different landscapes of interest (see Feather and food resource sampling) using bird capture techniques (i.e., mist nets) in 12 sites, and from birds found dead in one site. To increase the number of samples, we also included feathers of other 15 sites available in MZUSP collections, which ranged from 2001 to 2015.
We sampled a total of 28 sites (13 from primary data and 15 from museum collections) and calculated the land use composition for all sites regardless of the number of individuals obtained in each one (Supplementary Figs. S1 and S2). We identified each sample site using 5-m resolution satellite images obtained from the Brazilian Foundation for Sustainable Development (FBDS 2019), and categorized land use types considering a 2-km radial buffer around each bird sampling point (area = ~ 13 km2; following Morante-Filho et al. 2015). Land use types were categorized as follows: forest (native tree vegetation with continuous canopy), non-forest vegetation (native shrub or herbaceous vegetation), watercourse, urban area, agricultural areas, and planted forest (i.e., silviculture). Then, we identified two types of landscapes based on forest cover: landscapes with ≤ 30% of forest cover (HMLs), and with ≥ 47% of forest cover (NLs). The distinction we made between HMLs and NLs is based on the importance of forest cover at the landscape level as one of the main factors causing local extinction of bird species in the Atlantic Forest, where fragmented landscapes with less than 30% of forest cover are less biodiverse than those with more than 50% of forest cover (Uezu and Metzger 2011; Martensen et al. 2012; Estavillo et al. 2013; Morante-Filho et al. 2015). We had 12 HMLs sites with 6–26% of forest cover, mostly located inland (Supplementary Fig. S1) and 16 NLs with 47–99% of forest cover, mostly located near the coastal region (Supplementary Fig. S2). This dichotomy is clearly observed in the comparison between the western/central side of the state (more agricultural lands) and the region near the coast (more forested lands). To delimit buffers on sites and calculate the percentages of forest cover we used the QGIS version 3.2.2 software (QGIS 2018). The geographic coordinates of each study site are expressed as a UTM projection—Datum SIRGAS 2000.
Feather and food resource sampling
Feather samples came from individuals collected from 2001 to 2019, a large time span that does not affect the values of isotopic signatures (except from the Suess effect, see Statistical analysis) as no significant landscape changes, considering all land uses, occurred in the state of São Paulo in this period (see Bias evaluation; Souza et al. 2020). We classified bird species into five trophic guilds (frugivores, granivores, insectivores, nectarivores, and omnivores) based on data from the literature (see Willis 1979; Motta Junior 1990; Wilman et al. 2014). We selected 5 species as frugivores (123 individuals sampled), 5 species as granivores (88 individuals), 20 species as insectivores (359 individuals), 9 species as nectarivores (105 individuals), and 10 species as omnivores (176 individuals) (Supplementary Table S3). Then, we divided the 851 individuals into the 2 landscape categories: HMLs (652 individuals) and NLs (199 samples).
In addition, we sampled food resources from eight HMLs (invertebrates and seeds) to use as a baseline for the isotopic analysis of feathers. In total, we had 56 samples of seeds, belonging to 6 genera and 1 morphospecies, that were removed from the feces of birds captured in mist nets, washed, and sorted for isotopic analysis (Supplementary Table S4). We collected 70 samples of invertebrates opportunistically from the ground and vegetation next to mist nets during field expeditions (Supplementary Table S4). To use as baseline for NLs, we extracted isotopic values of food resources (invertebrates, fruits and seeds) from Galetti et al. (2016), which conducted a study in the same Atlantic Forest region near the coast.
Isotopic analysis
We cleaned the feathers with 70% alcohol to remove possible contaminants, and then cut and packed approximately 0.5 cm2 of the vane (without the rachis) into small tin capsules (8 × 5 mm or 5 × 2.5 mm) (Wiley et al. 2010). We used mortar and pestle to ground separately the food resource samples from HMLs (invertebrates and seeds) until obtaining a homogeneous mixture. Approximately 2 and 1 mg of seed and invertebrate samples, respectively, were separately packed in tin capsules (5 × 2.5 mm).
All samples were combusted in a CNH-1110 elemental analyser (Carlo Erba; Milan, Italy) generating gases that were subsequently inserted in a coupled continuous flow isotope ratio mass spectrometer (Delta Plus; Thermo Scientific; Bremen, Germany) to evaluate the sample isotopic composition. During this process, carbon was transformed into CO2 and nitrogen into N2. Therefore, the mass spectrometer provided isotopic values expressed in delta (δ) per thousand (per mille, ‰) obtained from the equation ‘δ13C or δ15N = [(Rsample/Rstandard) − 1], where R is the corresponding ratio 13C/12C or 15N/14N. Carbon and nitrogen international standards [Vienna Pee Dee Belemnite (V-PDB) and atmospheric air, respectively] were used as a reference for our samples. Reference materials (RM) in a two-point anchorage strategy were used to calibrate the machine (NBS-19 and NBS-22 for carbon and IAEA-N1 and IAEA-N2 for nitrogen), in addition to lab materials in each run for quality control. We replicated the same individual material for 10% of the samples and estimated the precision of the analytical method measuring replicates of internal standards in all batches, which was 0.09‰ for carbon and nitrogen.
Statistical analysis
Decades of input of anthropogenic CO2 into the atmosphere has an expressive effect on δ13C values of ecosystems and organisms (i.e., the Suess effect; Revelle and Suess 1957). Considering the large time span of our data (~ 20 years), we calculated and employed annual correction factors, ranging from − 0.75 to − 0.05‰, to the individual δ13C values of birds (Long et al. 2005).
An organism tissue is composed of elements which come from food consumed. Therefore, its isotopic values would be the same of food sources plus a trophic discrimination factor (TDF) resulting from multiple factors inherent to the tissue (DeNiro and Epstein 1978, 1981). Each tissue (e.g., feather, claw, and blood) may have different TDF depending on the species metabolism (Hobson and Clark 1992). TDF vary not only among tissues but also among species, as each species has specific physiological and anatomical characteristics that influence the route isotopes take from food to consumer’s tissue (Caut et al. 2009; Pecquerie et al. 2010). We used two approaches to obtain TDF values for stable carbon and nitrogen isotopes, and compared them to see if they produced similar results. We used the SIDER package in R (“Stable Isotope Discrimination Estimation in R”) that estimates TDFs based on a phylogenetic regression model, so providing values for each species (Healy et al. 2017). However, we also used TDFs provided in a study that applied these factors for trophic guilds composed by terrestrial passerine species, so each TDF was relative to guild and not species-specific (Ferger et al. 2013). As both approaches yielded the same patterns regarding niche width and differences in δ13C and δ15N between HMLs and NLs, we used the TDFs provided by SIDER (Supplementary Table S5).
After correcting the δ13C and δ15N values considering the Suess effect and TDFs, we calculated the isotopic niche width using the SIBER package in R (“Stable Isotope Bayesian Ellipses in R”) (Jackson et al. 2011) for each of the five trophic guilds in HMLs and NLs. We used the niche width measure named Bayesian Standard Ellipse Area (SEAb) for each group (i.e., HMLs and NLs), especially due to the unbalanced number of samples in each landscape type. This measure fitted a Bayesian multivariate normal distribution to each group based on 10,000 iterations using Gibbs Sampling technique, and its results are given per mille squared (‰2). We used the posterior SEAb values generated by this analysis to run a Student’s t test to determine whether the means of SEAbNL and SEAbHML differed significantly. In addition, we created a histogram of SEAb posterior values and estimated the tail probability (two-tailed; significance level, < 0.05) under the mean of each group. We also calculated the Bayesian ellipse overlap between the two groups, which is based on the posterior distributions of the means and covariance matrices of each group. Then, we calculated the percentage of ellipse overlap between HMLs and NLs. Niche overlap indicates if guilds in HMLs and NLs have similar requirements of habitat and food resources. In addition, we employed the same analysis of niche overlap among trophic guilds for each landscape type.
We used the same SEAb calculation in a species-specific approach, so that we could reinforce niche width differences between individuals from HMLs and NLs. On this approach, we only considered species that had more than five individuals sampled for each group, applying to at least one species of each guild, except granivores. Considering that we had a different pool of species in each group (HMLs and NLs) within the trophic guilds, we also calculated the SEAb selecting the same pool of species for each group when it was possible (it was the case for insectivores, nectarivores, and omnivores). Thus, we ensured that the differences in niche width between groups were not a result derived from the differences in communities’ composition.
We employed Bayesian mixing model analyses (Moore and Semmens 2008) for all trophic guilds in HMLs and NLs to quantify the contribution of food sources to birds’ diet in each landscape type. We considered the three following groups of sources for all guilds’ models: (1) C3 plants, for which we combined plant isotopic values collected during field expeditions as HMLs sources (δ13C = − 30.1‰ ± 2.6 and δ15N = 2.1‰ ± 1.6) and values of C3 plant samples from Galetti et al. (2016) as NLs sources (δ13C = − 30.4‰ ± 2.9 and δ15N = 1‰ ± 2.2); (2) invertebrates, for which we combined isotopic values similarly as the previous group (HML: δ13C = − 26.7‰ ± 2.9 and δ15N = 4.6‰ ± 3.1; NL: δ13C = − 26.1‰ ± 3.4 and δ15N = 5.1‰ ± 3.7); and (3) C4 plants, for which we combined isotopic values from the literature (δ13C = − 13‰ ± 2 and δ15N = 7.8‰ ± 1.5) (Martinelli et al. 2009; Augusto et al. 2015; Carvalho et al. 2015). We used the MixSIAR package in R to run the analyses using the Markov chain Monte Carlo (MCMC) sampling for three replicate chains with a lenght of 100,000 iterations, a burn-in with 50,000 iterations and thin by 50 (Stock et al. 2018). We performed all mixing models separately for each trophic guild and also for each landscape type. We used informative priors for frugivores and nectarivores to certify that models would not consider invertebrates as the main source, as these guilds only complement the diet with this source group. We also employed informative priors for insectivores since invertebrates are the main component of their diet. We used non-informative priors for granivores and omnivores. Considering that omnivores have a varied diet composed of plants and invertebrates, we employed a concentration-dependent mixing model for this guild to account for disparities in carbon and nitrogen concentrations between the resources (Hopkins and Ferguson 2012; Phillips et al. 2014). Both [C] and [N] for food resources were obtained from the literature (Jesus et al. 2015; González‐Carcacía et al. 2020; Martinelli et al. 2020); for plants we employed [C] = 46.1% and [N] = 2.5%, and for invertebrates [C] = 43.9% and [N] = 12.7%. The convergence of model results were characterized by the deviance information criterion score (DIC) and Gelman–Rubin and Geweke diagnostics.
We used a permutation test with 10,000 iterations of the original data (Strasser and Weber 1999), as we had different numbers of samples in each group, to compare the mean and variances of δ13C and δ15N values between HMLs and NLs for each trophic guild. To compare isotopic values of food resources (invertebrates and plants) between HMLs and NLs, we used a Student’s t test and Wilcoxon signed-rank test depending on data distribution. All analyses were performed in R software version 4.0.3 (R Core Team 2020).
Bias evaluation
To ensure that δ13C and δ15N values of birds, obtained in a time span of ~ 20 years, did not show a strong influence of time, we analyzed the landscape composition in the year that occurred the sampling and compared with the currently available landscape composition, using MapBiomas land use maps (collection 5; Souza et al. 2020). Thus, we confirmed that no significant change in the land use and cover happened through the years. To assess if there were temporal trends in the isotopic individual values, we conducted a linear regression model on the time span (~ 20 years) of δ13C and δ15N data. In addition, we employed a model selection based on maximum likelihood evaluations to measure the potential influence of time and diversity of land uses in the composition of landscapes to the δ13C and δ15N values of birds. We calculated the diversity of each landscape through the Simpson’s Diversity Index, a popular diversity measure borrowed from community ecology (Farina 2006), since our HMLs and NLs had different compositions in land uses besides the predominance of agriculture or forest, respectively. Therefore, in the model selection, we considered the following global models as predictors to run the analysis: δ13C or δ15N ~ Percentage of forest cover + Simpson’s Diversity Index + Trophic guild + Year of sample collection. We selected the best models based on Akaike’s information criterion corrected for small sample sizes (AICc), considering models with AICc difference from the best model (Δ) lower than 2 and measured their probabilities by using the Akaike’s weight of evidence (Weight) (Burnham and Anderson 2003). If more than one model was selected by the set of parameters stipulated, we ran an averaging analysis to generate a single fit model. All model selection analyses were performed using the MuMIn package in R (Barton 2020). In addition, we used Pearson’s correlation coefficients to evaluate which land uses that compose the study sites were more correlated to δ13C or δ15N values using the ‘lares’ package in R (Lares 2020).
Results
The isotopic niches (SEAb) of all trophic guilds were significantly different between landscape types (t test, P < 0.05; Fig. 2). Nectarivores and omnivores had the greatest reduction in niche width from NLs to HMLs (44 and 41%, respectively), while insectivores had a reduction of 24% and frugivores of 9%. In contrast, granivores had an expansion of 26% from NLs to HMLs. Moreover, the distribution of posterior SEAb values for each guild was also significantly different within each landscape type (P < 0.05), corroborating the differences in SEAbNLs and SEAbHMLs means for all guilds (Supplementary Fig. S6). Overall, the niche overlap of HMLs over NLs was high for all trophic guilds (Fig. 2). The niche overlap among the five guilds in NLs was higher than in HMLs for almost all the comparisons, except between frugivores and granivores (Table 1).
Isotopic niche of bird trophic guilds (frugivores, granivores, insectivores, nectarivores and omnivores) sampled in the south-eastern Atlantic Forest, Brazil. Points represent isotopic individual values of birds that compose each guild, also separated by landscape type (human-modified landscapes, HMLs—purple squares; natural landscapes, NLs—green triangles). The value of isotopic niche width (SEAb—‰2) for each guild is located next to each landscape type in the graph (SEAbHMLs and SEAbNLs). The values of isotopic overlap of SEAbHMLs over SEAbNLs are also shown. Student’s t test values and degrees of freedom refer to the comparison between SEAbNL and SEAbHML mean values (for all guilds P < 0.05)
Isotopic niche widths between individuals from HMLs and NLs also varied when compared using a species-specific approach (Table 2). Nine out of 10 species analyzed with this approach had their niche widths reduced from NLs to HMLs, corroborating our major results for trophic guilds, even though one nectarivore species (Amazilia versicolor) showed an expansion in its niche in HMLs. In addition, when comparing guilds composed by the same pool of species (the case for insectivores, nectarivores, and omnivores) we also observed that niche widths were substantially reduced from NLs to HMLs (Table 2), corroborating that our major results of guild niche reduction were not derived from differences in the species composition of assemblages.
The mixing model results of all guilds showed that, in general, bird diet is mainly composed of C3 plants in NLs, while birds from HMLs increased the consumption of C4 plants (Table 3). Frugivores and nectarivores had a diet based mostly in C3 plants in both landscape types, although an increase in the consumption of C4 plants occurred in HMLs. As expected, insectivores had a diet based on invertebrates, but showed a change in the contribution of C3 and C4 plants depending on the landscape type. Omnivores showed substantial changes in diet between landscapes, with individuals from NLs showing a diet dominated by C3 plants, while in HMLs they were equally dependent on C3 plants and invertebrates. Granivores showed that in HMLs its diet is composed mostly of C4 plants, whereas in NLs the composition is predominantly of C3 plants.
The mean δ13C and δ15N values for almost all trophic guilds were significantly different between HMLs and NLs (P < 0.05), except from δ15N values of granivores (P = 0.14; Fig. 3, Supplementary Table S7). The comparison of mean δ13C and δ15N values for food resources (invertebrates and plants) between HMLs and NLs did not show a significant difference (P > 0.05; Supplementary Table S7), emphasizing that the differences showed by guilds are not a reflection of changes in the isotopic values of baselines. Birds in NLs had lower δ13C values, indicating a restricted use of forest habitat resources, whereas birds in HMLs had higher δ13C, which possibly results from the use of resources from both forest and anthropogenic habitats. This pattern was also observed for the δ15N values, i.e., individuals from NLs had lower values than those from HMLs. The distribution of individual values of δ13C of almost all guilds did not vary according to landscape type (P > 0.05), except for granivores (P = 0.04; Fig. 3). The distribution of individual values of δ15N showed different variances according to landscape type for frugivores, insectivores, and omnivores (P < 0.05). These δ15N values were homogeneously distributed in NLs, whereas in HMLs the values were concentrated near the central tendency (Fig. 3).
Violin plot of δ13C and δ15N values for each of the bird trophic guilds (frugivores, granivores, insectivores, nectarivores and omnivores) showing data distribution and density probabilities of individuals in each landscape type (human-modified landscapes, HMLs—purple; natural landscapes, NLs—green). Wider sections represent a higher probability of individuals within that range of values, while skinnier sections represent a lower probability. Violin plot also shows δ13C and δ15N means (diamonds), wherein asterisks indicate a significant difference between the means of individuals from NLs and HMLs (permutation test; P < 0.05)
Neither δ13C nor δ15N values showed a significant or strong linear trend across the years of data collection (δ13C: R2 = 0, P > 0.05; δ15N: R2 = 0.02, P < 0.05; Supplementary Fig. S8). In model selection results, we also observed that the time span between sample collection was not a strong variable to predict the δ13C and δ15N values of birds (Supplementary Table S9), therefore, not influencing our results. Nevertheless, the Simpson’s Diversity Index variable, which measures the diversity of land uses in each landscape, had a strong influence to predict the δ13C and δ15N values of birds (Supplementary Table S9). Since more than one model was found to be adequate (Δ < 2) to explain δ13C values, we built an average model, though for δ15N values we obtained only one model with a good fit (Supplementary Table S9). Despite the high influence of the Simpson’s Diversity Index to both models, the percentage of forest cover is also strongly related to δ13C and δ15N values. In addition, Pearson’s correlation coefficients indicated that forest cover and anthropogenic areas are the land uses most correlated to isotopic values, supporting our comparisons between deforested (HMLs) and forested (NLs) landscapes (Supplementary Fig. S10).
Discussion
Anthropogenic impacts on bird diet and habitat use
Results indicated the existence of a non-random reduction in isotopic niche width for bird trophic guilds in HMLs, corroborating our hypothesis of differences in isotopic niches influenced by deforestation. This reduction may be related to a depletion in food availability as a consequence of habitat loss, which leads to a decline of richness and abundance of numerous organisms (Fahrig 2003), many of them serve as food for birds. Niche width reduction rates from NLs to HMLs ranged from 9 to 44%, a variation expected as each guild experiences the implications of fragmentation and habitat loss differently (Bregman et al. 2014). In NLs, isotopic niches were wider, probably because of the greater diversity and availability of food resources and forested habitat.
The isotopic niches of nectarivores and omnivores in NLs were almost twice as great as in HMLs (44 and 41% of reduction, respectively), demonstrating the importance of natural forests for the provision of habitat and food resources to these birds (Ferger et al. 2013). Although insectivores and frugivores did not show reductions as high as the above guilds, also had a decrease in niche width (24 and 9%, respectively). Even though frugivores had the lowest niche decrease, this guild is negatively influenced by changes in vegetation complexity and food availability at the landscape scale (Morante-Filho et al. 2018). Thus, our results emphasize that, although guilds had experienced niche reduction differently, all of them demonstrate the impact of deforestation on the diversity of resources used. The observed high overlap of 53–77% between HMLs and NLs also shows that species in HMLs depend mostly on the same food resources available in NLs. Moreover, niche overlaps among guilds in NLs were higher than in HMLs, suggesting that individuals in NLs may share some resources, probably due to a greater abundance and availability in this landscape type, as individuals do not have to compete for scarce resources as in HMLs.
The niche of granivores expanded in HMLs, being 26% larger than in NLs. As granivores are commonly adapted to open areas and forest edges (Giraudo et al. 2008), they may have benefited from human activities, resulting in niche expansion. Besides forest loss, the ‘savannization’ and degradation of tropical forests favor the increase of grass cover, especially exotic species used in pastures (e.g., Silvério et al. 2013; Scarano and Ceotto 2015). This land use and cover seems to promote a niche width amplification for granivores in HMLs, as this guild seems to prefer seeds from grasses in terms of nutrient composition (Ríos and Manuel 2012), and many of the studied species usually eat exotic grasses. Although anthropogenic impacts are directly related to biodiversity loss in general, it is somehow expected that each species experience these impacts differently since each one depends on a distinct set of ecological variables (Newbold et al. 2013, 2015). Only one nectarivore (Amazilia versicolor) had its niche expanded from NLs to HMLs, requiring further studies to investigate which and how anthropogenic impacts might be influencing this expansion. We acknowledge that we could not depict the reason to the aforementioned result, but it can be explained by an increase in the species plasticity to incorporate other food resources that are not specific to its diet, as invertebrates, which are occasionally eaten by nectarivores (Wilman et al. 2014).
The impacts of anthropogenic activities related to land use changes lead individual birds to forage more frequently for resources in agricultural areas, whereas in pristine forests, C3 plants exert a greater influence on their diets (Boesing et al. 2021). The mean δ13C values were higher in HMLs compared to NLs for all guilds, indicating that birds in landscapes with low forest cover forage more frequently for resources in forest edges, clearings or even in the agricultural matrix, corroborating our hypothesis. Although landscapes in the study region are dominated by pastures and sugarcane crops (Souza et al. 2020), mean δ13C values and mixing models results showed that diets in almost all guilds (except granivores) are predominantly composed of forest resources (C3 plants) in both landscape types, but individuals from HMLs have a greater reliance on C4 plants. The turnover of forest habitats to savanna, mainly lead by anthropogenic land use changes, is directly influencing forest-specialist species in tropical regions (Sales et al. 2020). Although some individuals may tolerate the ‘savannization’ process (i.e., increase of C4 plants in landscapes) and forage for a mixture of food items from different sources (i.e., C3 and C4 plants), most birds prefer food resources from forests. Conversely, granivores are probably adapting to this increase in ‘savannization’ since this guild is changing the proportion of C3 in favor of C4 plants from NLs to HMLs.
Organisms that consume more than one type of resource (e.g., C3 and C4 plants) express these resources’ isotopic signatures in their tissues, but the δ13C value reveals the resource that is most consumed (Boecklen et al. 2011). Plants have different isotopic values according to their photosynthetic pathways. C3 plants, which mainly compose the tropical Atlantic Forest, have δ13C between − 24 and − 34‰ (Martinelli et al. 2009; Vitória et al. 2018). C4 plants have δ13C values between − 11 and − 15‰ (Martinelli et al. 2009; Powell et al. 2012), and are usually grasses associated with savannas and agricultural areas. Besides the main influence of plant photosynthetic pathways, δ13C values may also vary according to abiotic variables (e.g., soil and climate). Nevertheless, our study sites do not show significant differences in δ13C isoscape values (Powell et al. 2012; Sena-Souza et al. 2019), which enables the association of birds δ13C values with C3–C4 plants.
The mean δ15N values between landscapes varied for almost all guilds (except granivores), being higher in HMLs compared to NLs, again corroborating our hypothesis that δ15N values would differ between landscape types. This is possibly a result of the high anthropogenic influence on HMLs, considering that agricultural activities cause variations in δ15N values of organisms (Rubenstein and Hobson 2004; Magioli et al. 2019). The range of δ15N values was wider in NLs, probably a consequence of greater diversity of food resources in these landscapes, which may promote higher levels of among individual diet variation or individual diet specialization (Araújo et al. 2011). Conversely, the narrower range of δ15N values in HMLs may be explained by a simplification of the habitat with a reduction of food availability, which increases resource sharing between individuals, consequently resulting in similar isotopic values.
We acknowledge that, besides the great influence of forest cover on δ13C and δ15N values, the diversity of land uses in landscapes also influenced isotopic values, which is somewhat expected since different land uses (e.g., silviculture, urbanization) can act or not as habitat and source of food depending on the bird species (Lopes et al. 2015; Piratelli et al. 2017). We could not exactly evaluate how each of the land uses (except from the dichotomy forest–agriculture) may influence δ13C and δ15N values of birds, so we recommend that further studies focus on this topic.
Consequences for the future
Our results show that most trophic niches are declining in HMLs, thus, posing the question: how long will these species tolerate the reduction of their habitat and food resources? More conservation actions are necessary to allow ecological processes—intrinsically linked to bird species of all guilds (Sekercioglu 2006)—to operate normally in both NLs and HMLs, as impacts such as fragmentation and habitat loss are harmful to birds and their resources (Rigueira et al. 2013; Morante-Filho et al. 2015; Pfeifer et al. 2017). Although many species of birds persist in HMLs of tropical regions and increase their consumption of C4 plants in this landscape type, most individuals are dependent on C3 plants, which are typical from forests. Thus, it is crucial to maintain the relictual NLs and support further heterogenization actions (e.g., increase forest cover and promote diversified agriculture fields) in HMLs to sustain the diversity of birds and their food resources, consequently preserving many ecological processes (Sirami et al. 2019; Hendershot et al. 2020). Considering that bird niches represent their use of habitat and food resources, such conservation actions in HMLs may also promote a stabilization or even an increase in niche widths of the different bird guilds.
Data availability
All data produced from this study are provided in this manuscript and its supplementary material.
Code availability
Not applicable.
References
Aguiar AP, Chiarello AG, Mendes SL, Matos ED (2003) The central and Serra do Mar corridors in the Brazilian Atlantic forest. In: Galindo-Leal C, Câmara IG (eds) The Atlantic forest of South America: biodiversity status, threats and outlook center for applied biodiversity science at conservation international. Island Press, Washington, EUA, pp 118–132
Alexandrino ER, Buechley ER, Karr JR et al (2017) Bird based index of biotic integrity : assessing the ecological condition of Atlantic forest patches in human-modified landscape. Ecol Indic 73:662–675. https://doi.org/10.1016/j.ecolind.2016.10.023
Araújo MS, Bolnick DI, Layman CA (2011) The ecological causes of individual specialisation. Ecol Lett 14:948–958. https://doi.org/10.1111/j.1461-0248.2011.01662.x
Augusto FG, Filho MT, Ferreira A et al (2015) Land use change in the Atlantic forest affects carbon and nitrogen sources of streams as revealed by the isotopic composition of terrestrial invertebrates. Biota Neotrop 15:e20140188. https://doi.org/10.1590/1676-06032015018814
Barros FM, Peres CA, Pizo MA, Ribeiro MC (2019) Divergent flows of avian-mediated ecosystem services across forest-matrix interfaces in human-modified landscapes. Landsc Ecol 34:879–894. https://doi.org/10.1007/s10980-019-00812-z
Barton K (2020) Package ‘MuMIn’: multi-model inference. R package version 1.43.17. https://cran.r-project.org/package=MuMIn. Accessed 10 Nov 2020
Boecklen WJ, Yarnes CT, Cook BA, James AC (2011) On the use of stable isotopes in trophic ecology. Annu Rev Ecol Evol Syst 42:411–440
Boesing AL, Marques TS, Martinelli LA et al (2021) Conservation implications of a limited avian cross-habitat spillover in pasture lands. Biol Conserv 253:108898
Bogoni JA, da Silva PG, Peres CA (2019) Co-declining mammal–dung beetle faunas throughout the Atlantic forest biome of South America. Ecography 42:1803–1818. https://doi.org/10.1111/ecog.04670
Bregman TP, Sekercioglu CH, Tobias JA (2014) Global patterns and predictors of bird species responses to forest fragmentation: implications for ecosystem function and conservation. Biol Conserv 169:372–383. https://doi.org/10.1016/j.biocon.2013.11.024
Burnham KP, Anderson DR (2003) Model selection and multimodel inference: a practical information-theoretic approach, second. Springer, Fort Collins
Carrara E, Arroyo-Rodríguez V, Vega-Rivera JH et al (2015) Impact of landscape composition and configuration on forest specialist and generalist bird species in the fragmented Lacandona rainforest, Mexico. Biol Conserv 184:117–126. https://doi.org/10.1016/j.biocon.2015.01.014
Carvalho DR, Castro D, Callisto M et al (2015) Isotopic variation in five species of stream fishes under the influence of different land uses. J Fish Biol 87:559–578. https://doi.org/10.1111/jfb.12734
Caut S, Angulo E, Courchamp F (2009) Variation in discrimination factors (Δ15N and Δ13C): the effect of diet isotopic values and applications for diet reconstruction. J Appl Ecol 46:443–453. https://doi.org/10.1111/j.1365-2664.2009.01620.x
Chase JM, Leibold MA (2003) Ecological niches: linking classical and contemporary approaches. The University of Chicago Press, Chicago, IL
da Silva BG, Koch I, Piratelli AJ (2020) Fruit and flower availability affect bird assemblages across two successional stages in the Atlantic forest. Stud Neotrop Fauna Environ 00:1–13. https://doi.org/10.1080/01650521.2020.1743550
DeNiro MJ, Epstein S (1978) Influence of diet on the distribution of carbon isotopes in animals. Geochim Cosmochim Acta 42:495–506
DeNiro MJ, Epstein S (1981) Influence of diet on the distribution of nitrogen isotopes in animals. Geochim Cosmochim Acta 45:341–351
Devictor V, Julliard R, Jiguet F (2008) Distribution of specialist and generalist species along spatial gradients of habitat disturbance and fragmentation. Oikos 117:507–514. https://doi.org/10.1111/j.2008.0030-1299.16215.x
Estavillo C, Pardini R, da Rocha PLB (2013) Forest loss and the biodiversity threshold: an evaluation considering species habitat requirements and the use of matrix habitats. PLoS ONE 8:e82369. https://doi.org/10.1371/journal.pone.0082369
Estes JA, Terborgh J, Brashares JS et al (2011) Trophic downgrading of planet earth. Science 333:301–306. https://doi.org/10.1126/science.1205106
Fahrig L (2003) Effects of habitat fragmentation on biodiversity. Annu Rev Ecol Evol Syst 34:487–515. https://doi.org/10.1146/132419
Farina A (2006) Principles and methods in landscape ecology. Springer, Dordrecht, The Netherlands
FBDS (2019) The Brazilian foundation for sustainable development. https://www.fbds.org.br/article.php3?id_article=594. Accessed 13 Aug 2019
Ferger SW, Bohning-Gaese K, Wilcke W et al (2013) Distinct carbon sources indicate strong differentiation between tropical forest and farmland bird communities. Oecologia 171:473–486. https://doi.org/10.1007/s00442-012-2422-9
Galetti M, Rodarte RR, Neves CL et al (2016) Trophic niche differentiation in rodents and marsupials revealed by stable isotopes. PLoS ONE 11:1–15. https://doi.org/10.1371/journal.pone.0152494
Giraudo AR, Matteucci SD, Alonso J et al (2008) Comparing bird assemblages in large and small fragments of the Atlantic forest hotspots. Biodivers Conserv 17:1251–1265. https://doi.org/10.1007/s10531-007-9309-9
González-Carcacía JA, Herrera MLG, Nassar JM (2020) Dietary importance of C3 and CAM food pathways for birds in a neotropical semiarid zone. Biotropica 00:1–8. https://doi.org/10.1111/btp.12798
Haddad NM, Brudvig LA, Clobert J et al (2015) Habitat fragmentation and its lasting impact on earth’s ecosystems. Sci Adv 1:e1500052. https://doi.org/10.1126/sciadv.1500052
Healy K, Guillerme T, Kelly SBAA et al (2017) SIDER: an R package for predicting trophic discrimination factors of consumers based on their ecology and phylogenetic relatedness. Ecography 41:1–7. https://doi.org/10.1111/ecog.03371
Hebert CE, Wassenaar LI (2001) Stable nitrogen isotopes in waterfowl feathers reflect agricultural land use in western Canada. Environ Sci Technol 35:3482–3487. https://doi.org/10.1021/es001970p
Hendershot JN, Smith JR, Anderson CB et al (2020) Intensive farming drives long-term shifts in avian community composition. Nature 579:393–396. https://doi.org/10.1038/s41586-020-2090-6
Hobson KA (2011) Isotopic ornithology: a perspective. J Ornithol 152:S49–S66. https://doi.org/10.1007/s10336-011-0653-x
Hobson KA, Clark RG (1992) Assessing avian diets using stable isotopes II: factors influencing diet-tissue fractionation. Condor 94:189–197
Hopkins JB, Ferguson JM (2012) Estimating the diets of animals using stable isotopes and a comprehensive Bayesian mixing model. PLoS ONE. https://doi.org/10.1371/journal.pone.0028478
Inger R, Bearhop S (2008) Applications of stable isotope analyses to avian ecology. Ibis 150:447–461. https://doi.org/10.1111/j.1474-919X.2008.00839.x
Jackson AL, Inger R, Parnell AC, Bearhop S (2011) Comparing isotopic niche widths among and within communities: SIBER–Stable Isotope Bayesian Ellipses in R. J Anim Ecol 80:595–602. https://doi.org/10.1111/j.1365-2656.2011.01806.x
Jesus FM, Pereira MR, Rosa CS et al (2015) Preservation methods alter carbon and nitrogen stable isotope values in crickets (Orthoptera: Grylloidea). PLoS ONE 10:e0137650. https://doi.org/10.1371/journal.pone.0137650
Lares B (2020) Package ‘lares’: analytics, data mining and machine learning sidekick. R package version 4.9.8. https://github.com/laresbernardo/lares. Accessed 18 Nov 2020
Long ES, Sweitzer RA, Diefenbach DR, Ben-David M (2005) Controlling for anthropogenically induced atmospheric variation in stable carbon isotope studies. Oecologia 146:148–156. https://doi.org/10.1007/s00442-005-0181-6
Lopes IT, Gussoni COA, Demarchi LO et al (2015) Diversity of understory birds in old stands of native and Eucalyptus plantations. Restor Ecol 23:662–669. https://doi.org/10.1111/rec.12216
Magioli M, Moreira MZ, Fonseca RCB et al (2019) Human-modified landscapes alter mammal resource and habitat use and trophic structure. Proc Natl Acad Sci. https://doi.org/10.1073/pnas.1904384116
Martensen AC, Ribeiro MC, Banks-Leite C et al (2012) Associations of forest cover, fragment area, and connectivity with neotropical understory bird species richness and abundance. Conserv Biol 26:1100–1111. https://doi.org/10.1111/j.1523-1739.2012.01940.x
Martinelli LA, Ometto JPHB, Ferraz ES et al (2009) Desvendando questões ambientais com isótopos estáveis. Oficina de textos, São Paulo, Brazil
Martinelli LA, Nardoto GB, Soltangheisi A et al (2020) Determining ecosystem functioning in Brazilian biomes through foliar carbon and nitrogen concentrations and stable isotope ratios. Biogeochemistry 117:26842–26848. https://doi.org/10.1007/s10533-020-00714-2
Mittermeier RA, Turner WR, Larsen FW et al (2011) Global biodiversity conservation: the critical role of hotspots. In: Zachos FE, Habel JC (eds) Biodiversity hotspots. Springer Publishers, London, UK, pp 3–22
Moore JW, Semmens BX (2008) Incorporating uncertainty and prior information into stable isotope mixing models. Ecol Lett 11:470–480. https://doi.org/10.1111/j.1461-0248.2008.01163.x
Morante-Filho JC, Faria D, Mariano-Neto E, Rhodes J (2015) Birds in anthropogenic landscapes: the responses of ecological groups to forest loss in the Brazilian Atlantic forest. PLoS ONE 10:1–18. https://doi.org/10.1371/journal.pone.0128923
Morante-Filho JC, Arroyo-Rodríguez V, de SouzaPessoa M et al (2018) Direct and cascading effects of landscape structure on tropical forest and non-forest frugivorous birds. Ecol Appl 28:2024–2032. https://doi.org/10.1002/eap.1791
Motta Junior JC (1990) Estrutura trófica e composição das avifaunas de três habitats terrestres na região central do estado de São Paulo. Ararajuba 1:65–71
Newbold T, Scharlemann JPW, Butchart SHM et al (2013) Ecological traits affect the response of tropical forest bird species to land-use intensity. Proc R Soc B 280:1–8. https://doi.org/10.1098/rspb.2012.2131
Newbold T, Hudson LN, Hill SLL et al (2015) Global effects of land use on local terrestrial biodiversity. Nature 520:45–50. https://doi.org/10.1038/nature14324
Newsome SD, del Rio CM, Bearhop S, Phillips DL (2007) A niche for isotopic ecology. Front Ecol Environ 5:429–436. https://doi.org/10.1890/060150.01
Pagani-Núñez E, Liang D, He C et al (2019) Niches in the Anthropocene: passerine assemblages show niche expansion from natural to urban habitats. Ecography (Cop) 42:1–10. https://doi.org/10.1111/ecog.04203
Pardini R, Faria D, Accacio GM et al (2009) The challenge of maintaining Atlantic forest biodiversity: a multi-taxa conservation assessment of specialist and generalist species in an agro-forestry mosaic in southern Bahia. Biol Conserv 142:1178–1190. https://doi.org/10.1016/j.biocon.2009.02.010
Pecquerie L, Nisbet RM, Fablet R et al (2010) The impact of metabolism on stable isotope dynamics: a theoretical framework. Philos Trans R Soc B Biol Sci 365:3455–3468. https://doi.org/10.1098/rstb.2010.0097
Pfeifer M, Lefebvre V, Peres CA et al (2017) Creation of forest edges has a global impact on forest vertebrates. Nature 551:187–191. https://doi.org/10.1038/nature24457
Phillips DL, Inger R, Bearhop S et al (2014) Best practices for use of stable isotope mixing models in food-web studies. Can J Zool 92:823–835. https://doi.org/10.1139/cjz-2014-0127
Piratelli AJ, Franchin AG, Marín-Gómez OH (2017) Urban conservation: toward bird-friendly cities in Latin America. In: MacGregor-Fors I, Escobar-Ibáñez JF (eds) Avian ecology in Latin American cityscapes. Springer International Publishing, Cham, Germany, pp 143–158
Pizo MA (2007) Frugivory by birds in degraded areas of Brazil. In: Dennis AJ, Schupp EW, Green RJ, Westcott DA (eds) Seed dispersal: theory and its application in a changing world. CABI, Wallingford, UK, pp 615–627
Post DM (2002) Using stable isotopes to estimate trophic position: models, methods, and assumptions. Ecology 83:703. https://doi.org/10.2307/3071875
Powell RL, Yoo E-H, Still CJ (2012) Vegetation and soil carbon-13 isoscapes for South America: integrating remote sensing and ecosystem isotope measurements. Ecosphere 3:109. https://doi.org/10.1890/ES12-00162.1
QGIS (2018) Geographic information system. Open source geospatial foundation project. http://qgis.osgeo.org. Accessed 15 Dec 2018
R Core Team (2020) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria
Revelle R, Suess HE (1957) Carbon dioxide exchange between atmosphere and ocean and the question of an increase of atmospheric CO2 during the past decades. Tellus 9:18–27. https://doi.org/10.1111/j.2153-3490.1957.tb01849.x
Rezende CL, Scarano FR, Assad ED et al (2018) From hotspot to hopespot: an opportunity for the Brazilian Atlantic forest. Perspect Ecol Conserv 16:208–214. https://doi.org/10.1016/j.pecon.2018.10.002
Rigueira DMG, da Rocha PLB, Mariano-Neto E (2013) Forest cover, extinction thresholds and time lags in woody plants (Myrtaceae) in the Brazilian Atlantic forest: resources for conservation. Biodivers Conserv 22:3141–3163. https://doi.org/10.1007/s10531-013-0575-4
Ríos A, Manuel J (2012) Effects of nutritional and anti-nutritional properties of seeds on the feeding ecology of seed-eating birds of the Monte desert, Argentina. Condor 114:44–55. https://doi.org/10.1525/cond.2012.110043
Rubenstein DR, Hobson KA (2004) From birds to butterflies: animal movement patterns and stable isotopes. Trends Ecol Evol 19:256–263. https://doi.org/10.1016/j.tree.2004.03.017
Sales LP, Galetti M, Pires MM (2020) Climate and land-use change will lead to a faunal “savannization” on tropical rainforests. Glob Chang Biol 26:7036–7044. https://doi.org/10.1111/gcb.15374
Scarano FR, Ceotto P (2015) Brazilian Atlantic forest: impact, vulnerability, and adaptation to climate change. Biodivers Conserv 24:2319–2331. https://doi.org/10.1007/s10531-015-0972-y
Scheele BC, Foster CN, Banks SC, Lindenmayer DB (2017) Niche contractions in declining species: mechanisms and consequences. Trends Ecol Evol 32:346–355. https://doi.org/10.1016/j.tree.2017.02.013
Sekercioglu CH (2006) Increasing awareness of avian ecological function. Trends Ecol Evol 21:464–471. https://doi.org/10.1016/j.tree.2006.05.007
Sena-Souza JP, Costa FJV, Nardoto GB (2019) Background and the use of isoscapes in the Brazilian context: essential tool for isotope data interpretation and natural resource management. Ambient Agua Interdiscip J Appl Sci 14:1. https://doi.org/10.4136/ambi-agua.2282
Sexton JP, Montiel J, Shay JE et al (2017) Evolution of ecological niche breadth. Annu Rev Ecol Evol Syst 48:183–206. https://doi.org/10.1146/annurev-ecolsys-110316-023003
Silvério DV, Brando PM, Balch JK et al (2013) Testing the Amazon savannization hypothesis: fire effects on invasion of a neotropical forest by native cerrado and exotic pasture grasses. Philos Trans R Soc B Biol Sci 368:20120427. https://doi.org/10.1098/rstb.2012.0427
Sirami C, Gross N, Baillod AB et al (2019) Increasing crop heterogeneity enhances multitrophic diversity across agricultural regions. Proc Natl Acad Sci 116:16442–16447. https://doi.org/10.1073/pnas.1906419116
Souza CM, Shimbo JZ, Rosa MR et al (2020) Reconstructing three decades of land use and land cover changes in Brazilian biomes with landsat archive and earth engine. Remote Sens 12:2735. https://doi.org/10.3390/rs12172735
Steffen W, Sanderson A, Tyson PD et al (2005) Global change and the earth system: a planet under pressure, 2nd edn. Springer, Berlin, Germany
Stock BC, Jackson AL, Ward EJ et al (2018) Analyzing mixing systems using a new generation of Bayesian tracer mixing models. PeerJ 6:e5096. https://doi.org/10.7717/peerj.5096
Strasser H, Weber C (1999) On the asymptotic theory of permutation statistics. Math Methods Stat 8:220–250
Uezu A, Metzger JP (2011) Vanishing bird species in the Atlantic forest: relative importance of landscape configuration, forest structure and species characteristics. Biodivers Conserv 20:3627–3643. https://doi.org/10.1007/s10531-011-0154-5
Vellend M, Baeten L, Becker-Scarpitta A et al (2017) Plant biodiversity change across scales during the Anthropocene. Annu Rev Plant Biol 68:563–586. https://doi.org/10.1146/annurev-arplant-042916-040949
Victor MAM, Cavalli AC, Guillaumon JR, Serra Filho R (2005) Cem anos de devastação: revisitada 30 anos depois. Ministério do Meio Ambiente, Brasília, Brazil
Vitória AP, Ávila-Lovera E, De Oliveira VT et al (2018) Isotopic composition of leaf carbon (δ13C) and nitrogen (δ15N) of deciduous and evergreen understorey trees in two tropical Brazilian Atlantic forests. J Trop Ecol 34:145–156. https://doi.org/10.1017/S0266467418000093
Wiley AE, Ostrom PH, Stricker CA et al (2010) Isotopic characterization of flight feathers in two pelagic seabirds: sampling strategies for ecological studies. Condor 112:337–346. https://doi.org/10.1525/cond.2010.090186
Willis EO (1979) The composition of avian communities in remanescent woodlots in southern Brasil. Pap Avulsos Zool 33:1–25
Wilman H, Belmaker J, Simpson J et al (2014) EltonTraits 1.0: species-level foraging attributes of the world’s birds and mammals. Ecology 95:2027–2027. https://doi.org/10.1890/13-1917.1
Acknowledgements
We sincerely thank the people that helped this research, without knowing they were doing it, through depositing specimens of birds in MZUSP collections (Museum of Zoology of the University of São Paulo, Brazil). All the authors thank São Paulo Research Foundation (FAPESP), Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), and Conselho Nacional de Pesquisa e Desenvolvimento Científico e Tecnológico (CNPq) for the financial support. We thank the reviewers who provided helpful feedback to improve our manuscript.
Funding
São Paulo Research Foundation (FAPESP) provided financial support that allowed the data collection (#2010/05343-5, #2011/06782-5 and #2011/04046-0). ABN was financed by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior—Brasil (CAPES)—Finance Code #88882.328664/2019-01). ABN is supported by a doctoral scholarship from FAPESP (process #2020/07619-0). JAB is supported by a postdoctoral fellowship grant from FAPESP (#2018-05970-1). ERA is founded by Conselho Nacional de Pesquisa e Desenvolvimento Científico e Tecnológico (CNPq) research grant (#300744/2020-0—Programa de Capacitação Institucional). ERA also was supported by CAPES during his postdoctoral research (Finance Code 001—CAPES PNPD 2013/1723). CNPq provided research grants for KMPMBF (#308632/2018-4), LFS (#302291/2015-6 and #308337/2019-0) and MAP (#304742/2019-8).
Author information
Authors and Affiliations
Contributions
ABN, KMPMBF, MZM, LFS, MAP and WRS conceived the ideas and designed methodology; ABN, LFS, ERA, DTAL, MAP, VCO, RJD, AVC and AJP collected the data; ABN, MM and JAB analyzed the data; ABN led the writing of the manuscript. All authors contributed critically to the drafts and gave final approval for publication.
Corresponding author
Ethics declarations
Conflict of interest
Not applicable.
Ethical approval
Ethics approval for this study was waived by ICMBio according to IN 3/2014 and RN 18/2014.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Additional information
Communicated by Seth Newsome.
In memoriam Daniela Tomasio Apolinario da Luz.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Navarro, A.B., Magioli, M., Bogoni, J.A. et al. Human-modified landscapes narrow the isotopic niche of neotropical birds. Oecologia 196, 171–184 (2021). https://doi.org/10.1007/s00442-021-04908-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00442-021-04908-9