Abstract
The Atlantic Forest (AF) is one of the five most threatened and megadiverse world hotspots. It is arguably the most devastated and highly threatened ecosystem on the planet. The vast scope of habitat loss and extreme fragmentation in the AF hotspots has left intact very few extensive and continuous forested fragments. We compared bird assemblages between small (<100 ha) and large (>6,000 ha) forest remnants, in one of the largest AF remnants in Argentina. We performed 84 point-counts of birds in four large fragments (LF) and 67 points in 25 small fragments (SF). We recorded 4,527 bird individuals belonging to 173 species; 2,632 belonging to 153 species in LF and 1,897 in 124 species in SF. Small fragments suffered a significant loss of bird richness, mainly forest dependent species, but the birds abundance did not decrease, due to an increase in abundance of forest independent and semi-dependent bird species (edge and non forest species) that benefit from forest fragmentation. The bird guilds of frugivores, undestory, terrestrial and midstory insectivores, nectarivores and raptors, and the endemic species of AF were area sensitive, decreasing significantly in richness and abundance in the SF. Terrestrial granivores were the only guild positively affected by forest fragmentation, containing mainly edge species, which forage in open areas or borders including crops. Our first observations on fragmentation effects on bird assemblages in the southernmost Argentinean Atlantic Forests did not validate the hypothesis on pre-adaptation to human disturbances in the bird communities of AF. On the contrary, we observed that forest dependent, endemic and several sensitive bird guilds were strongly affected by fragmentation, putting in evidence the vulnerability to the fragmentation process and the necessity to conserve large remnants to avoid reduction of the high biodiversity of AF birds.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The Atlantic Forest (AF) is one of the world’s 25 recognized biodiversity hotspots, and it is considered between the five most threatened and megadiverse ecoregions (Galindo-Leal and Câmara 2003; Myers et al. 2000). The AF hotspots are arguably the most devastated and highly threatened ecosystems on the planet, occupying originally between 1 and 1.5 million km2 in southeast Brazil, eastern Paraguay, and northeastern Argentina; and at present only 7–8% of the original forest remain (Galindo-Leal and Câmara 2003). The area of remnant forest differs in each country. In Brazil and Paraguay it was estimated in 7–12% of the original area, and in Argentina 50% (Fragano and Clay 2003; Galindo-Leal and Câmara 2003; Giraudo et al. 2003b; Silva and Castelei 2003). The vast scope of habitat loss and extreme fragmentation in the AF hotspots has left intact very few extensive and continuous forested fragments. The two last largest AF remnants that reach near 10,000 km2 each are located in the Serra do Mar in São Paulo and Paraná states in Brazil, and spanning through most of Misiones province in Argentina (Galindo-Leal and Câmara 2003; Giraudo et al. 2003b). The conservation of Argentinean remnants of Atlantic Forest is a key issue to maintain species with large area requirements, as well as complete species assemblages, where ecological and evolutionary processes are proceeding unabated (Galindo-Leal and Câmara 2003; Giraudo et al. 2003b; Giraudo and Povedano 2004). Additionally, Marini and Garcia (2005) showed that the AF of southeastern Brazil lowlands, which are represented in Argentina too, contain the highest richness of threatened species and endemic birds at risk in all the ecoregion. However, the major factors causing habitat fragmentation and loss in Argentina have not diminished (Matteucci et al. 2004; Morello and Matteucci 1999; Giraudo et al. 2003b). A law on natural heritage protection (Law N° 3631 of Green Corridor) was approved in Misiones in 1999, which creates a legal policy for planning and management of these AF remnants in Argentina. The law establishes an “Integral Area for Conservation and Sustainable Development” formed by the major forest fragments and the protected areas connected by them. It lists among its priorities the creation of corridors, the support of sustainable development, and the conservation of forest remnants in Misiones (Rey 2003). In order to achieve these purposes, a thorough understanding of fragmentation effects on biodiversity is needed. This knowledge will allow the urgent design and establishments of patches and corridors to preclude the irreversible isolation of the major forest remnants and protected areas (Giraudo et al. 2003a, b).
Few studies have been developed in the Neotropical region in fragmented landscapes (Turner 1996; Marini 2001), and most of them were carried out in the Amazonian ecosystem in the framework of the Biological Dynamics of Forest Fragments Project, developed during more than two decades and producing relevant knowledge on fragmentation processes (p. e. Bierregaard and Stouffer 1997; Gascon et al. 1999; Laurance and Bierregaard 1997; Laurance et al. 2002). The few studies on the effects of fragmentation in the AF have been done in Brazil (see Galindo-Leal 2003 for a review), and there are no studies in Argentina. Due to its large latitudinal range, the Atlantic Forest region is remarkably heterogeneous (Galindo-Leal and Câmara 2003); thus, research results cannot be extrapolated, and local studies should be performed in each subregion. For example, the predation rates on artificial nests do not increase with decreasing fragment size in southeastern Brazilian forest, contrary to what has been proved in several regions of the world (Duca et al. 2001; Marini 2001).
Fragmentation produces a set of negative, complex and synergic consequences (see Saunder et al. 1995; Laurance and Bierregaard 1997; Tabarelli et al 2004 for a review), and it is considered the most important cause of biodiversity loss in the Neotropical region (Bierregaard and Lovejoy 1989). Many factors interact with habitat and population reduction, and diversity diminishes and community composition is changed as a consequence (Skole and Tucker 1993; Wiens 1994; Saunder et al. 1995). A review of research on fragmentation of Atlantic Forests (Galindo-Leal 2003) shows that small fragments contain less species, and that the high diverse original communities have been replaced by communities with a few dominant species (e. g. Willis 1979; Anjoz and Boçon 1999).
AF fragmentation has reached extreme magnitudes in Brazil; for example, in the south east of Pernambuco, 1839 forest fragments were studied in a sugar cane matrix (Ranta et al. 1998). Fragment sizes ranged from less than a hectare to 1,539 ha, with a mean extension of 34 ha. However, half of them measured less than 10 ha, and only 7% were larger than 100 ha. In San Pablo, Botucatu District, most of the patches were small (less than 20 ha) and very few occupied large extensions (over 60 ha), with a mean size of 11 ha (Blanco and Garcia 1997). It has been shown that the extreme fragmentation rate resulted in a reduction in diversity, changes of community and guild composition, and the reduction or local extinction of species of several groups, such as birds (Willis 1979; Anjos & Boçon 1999; Marini 2000), mammals (Chiarello 1999; 2000; Cullen et al. 2000), and trees (Tabarelli et al. 1999). In some cases, species and communities that are important for the ecosystem functioning have been damaged, such as large frugivorous birds including toucans and guans (D’angelo Neto et al. 1998; Chiarello 1999, 2000), large mammals such as tapirs, jaguars, giant armadillos, pumas, peccaries and the giant anteater (Galindo-Leal 2003; Giraudo and Abramson 1998; Chiarello 1999; Leite 2001); and fruit trees and shrubs of the highest canopy layer (Tabarelli et al. 1999). These changes in species assemblages, and the reduction or disappearance of key species and guilds, may have serious consequences in ecological processes and in functioning of the tropical forests altering their structure and their regeneration capacity (Terborgh 1988; Dirzo and Miranda 1990; Carrillo et al. 2000; Cuaron 2000).
The aim of this study is to compare bird assemblages between small and large forest remnants, a typical landscape pattern of fragmentation in the southernmost portion of Atlantic Forest in Argentina.
Methods
Study area and sampling sites
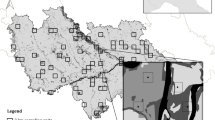
The study was done in fragments of native vegetation of AF in central of Misiones province (Argentina) near Aristóbulo del Valle, Argentina (27° 05′ 55″ S, 54° 53′ 41″ W, elevation between 141 and 549 m osl). Fragments were delimited by visual digitalization on screen using as backdrop a three band infrared composite (RGB 432) satellite image (Landsat 7ETM, May 25, 2002), with ArcView (ESRI 1996) and CartaLinx (Clark Labs 1998).
We studied 25 small fragments (SF) ranging in size from 3.5 to 99.3 ha (mean = 30.9, SD = 25.3) and four large fragments (LF) of between 6,547.6 and 33,151.9 ha (mean = 15,752.4, SD = 11,847.9). The large fragments sum a total area of 63,009.5 ha, and they are separated by pavement roads 15–20 m wide. The large fragments include two protected areas (Giraudo et al. 2003a), the provincial park Salto Encantado del Valle del Cuñapirú, created in 1993 (13,338 ha); and the private reserve of La Plata National University, Valle del Cuñapirú, created in 2000 (of 6,035 ha).
The forests in all the study area and the sampled fragments were invariably disturbed by selective logging and hunting, including the large fragments and protected areas (the latter were exploited by selective logging and hunting before their creation, and these illegal activities were recently detected in the reserves).
The fragments in the study area are mainly surrounded by perennial crops of yerba mate(Ilex paraguayensis) and tea (Thea sinensis). In minor proportion, some areas have annual crops of tobacco (Nicotiana tabacum), pine (Pinusspp.) plantations, and rarely eucalyptus (Eucalyptusspp.) plantations and introduced cultivated pastures.
Bird recording methods
The surveys were carried out between October 2004 and September 2006. We searched the birds by fixed -radius point-counts of 100 m. In each point we counted birds seen and heard for a fixed sampling of 20 min (Anjos 2004; Anjos and Boçon 1999). Each point was between 300 and 500 m of each other and at least 100 m of the forest edge. Independence among points was improved by the sampling of neighboring points by two observers simultaneously. We performed 151 point-counts, 84 in LF and 67 in SF, never during periods of rain or strong wind. The counts were performed during the morning, approximately between 06:00 and 11:00 h. In Atlantic Forest ecosystems, most of the contacts are heard but not seen due to its high vegetation complexity. In consequence the ability to acoustically identify bird species has a quite important role (Anjos 2004). All observers that sampled birds had a previous experience of more than 10 years recording and identifying birds in the Atlantic Forest. Nevertheless, some unidentified birds’ vocalizations were tape-recorded and identified subsequently. The point-count method was proved and used effectively in previous studies on Atlantic Forest bird assemblages (e.g. Aleixo 1999; Anjos 2004; Anjos and Boçon 1999; Protomastro 1999).
Data analyses
One of the strongest criticisms of specie-area studies is that organisms are considered independently of their association with the habitat under consideration (Marini 2001). In consequence, the species were categorized by: (a) their forest dependence (dependent, semi-dependent and independent), following Marini (2001) and field observations; (b) their guilds based on feeding habits, preferred foraging strata of vegetation and foraging substrate, according to Lopez de Casenave et al. (1998), Parker et al. (1996), Protomastro (1999), Sick (1988), and field observations, as follow: Aerial granivore (AG), terrestrial granivore (TG), frugivore (F), aerial insectivore (AI), bark insectivore (BI), canopy insectivore (CI), midstory insectivore (MI), terrestrial insectivore (TI), understory insectivore (UI), nectarivore (N), arboreal omnivore (AO), generalized omnivore (GO), terrestrial omnivore (TO), undestory omnivore (UO), and raptor (R) bird categories; and (c) their endemism to the Atlantic Forest region, according to Parker et al. (1996) and Ridgely and Tudor (1989, 1994).
The comparisons were performed using all bird, forest dependent species, guild and endemic assemblages. We compared differences in bird richness (number of species by point-count) and abundance (number of individuals by point-count) between LF and SF. We performed parametric (Student t) and non-parametric (Mann–Whitney U) univariate statistical tests, depending on the normality and homogeneity of the variables. The univariate normality assumptions were verified using Shapiro-Wilks test, while homogeneity of variance was verified with the F test.
All statistical analyses were performed using Infostat (2002) version 1.6. We used a significance level of 0.05 and 0.1 when interpreting our results, because some of the categories (for example raptors guild), showed a small sample size which resulted in an increase of Type II error. This type of error can be more costly than Type I in environmental and conservation research (Lopez de Casenave et al. 1998).
Results
We recorded 4,527 bird individuals belonging to 173 species, of which 2,632 belonging to 153 species were found in LF, and 1,897 in 124 species were in SF (Appendix 1). The difference in total richness between large and small fragments was statistically significant (U = 4520.5, P = 0.032). In average, the species number was higher in LF (mean richness = 20.3, range 7–43 species, n = 84) than in SF (mean = 17.5, range = 4–31, n = 67). The differences in abundance between large and small fragments was not statistically significant for all bird assemblages (U = 4793, P = 0.2623; mean for LF = 31.3 individuals, range 10–62, n = 84; mean for SF = 28.3, range = 6–46, n = 67). Nevertheless, both richness and abundance of forest dependent birds was significantly higher in LF than in SF (Fig. 1). On the contrary, the forest independent and semi-dependent birds showed a significantly lower richness and abundance in LF (Fig. 1).
Large fragments showed a higher proportion of forest dependent species (115 species, 75% of its avifauna) than small fragments (82 species, 66%). The inverse occurred with forest independent species, with lower proportion in large fragments (15 species, 10%) than in small fragments (19 species, 15%). The forest semi-dependent species showed a slightly higher fraction in small fragments (23 species, 19%) than in large fragments (23 species, 15%).
Only one guild, the terrestrial granivore, was statistically higher in richness and abundance in small fragments. Six out of the 13 defined guilds were statistically lower in small fragments in number of species, number of individuals or both (Table 1). Terrestrial insectivores, undestory insectivores and nectarivores showed a significant (95% of confidence level) higher richness and abundance in large fragments. Midstory insectivores showed significantly higher richness (95% of confidence) in large fragments, but the difference in abundance was not statistically significant. Frugivores showed a significant higher richness and abundance in the 95 and 90% confidence levels, respectively. Finally, the raptors showed significantly higher richness and abundance in large fragments at the 90% confidence level (Table 1).
We recorded 1,374 individuals (30% of the total) belonging to 52 endemic species of AF, representing a 30% of the 173 species observed, of which 886 individuals of 50 endemic species were in large fragments and 469 of 33 taxa in small fragments. Endemic species were area sensitive, they showed higher richness and abundance in LF (mean of 7.3 species and 10.7 individuals) than in SF (mean of 4.6 species and 7.0 individuals), and the difference was statistically significant (test U = 3773.5, P < 0.0001 for richness, and U = 3935.5, P < 0.0001 for abundance).
Discussion
According to Protomastro (1999) and Aleixo (1999), the Atlantic Forest remnants studied by us showed a high richness of birds (173 species), including an important number of endemic species (52), and some threatened and rare species (e. g. Platirynchus leucoryphus), confirming the high value for conservation of secondary and tropical forest fragments subjected to selective logging (Giraudo et al. 2003b; Turner and Corlett 1996).
In coincidence with several authors (e. g. MacArthur and Wilson 1967; Marini 2001; Willis 1979) small fragments suffered a significant loss of bird richness, mainly forest dependent species in the southernmost Atlantic Forest of Argentina, such as was observed by Anjos and Boçon (1999) in neighboring areas of Paraná, Brazil. Nevertheless, the bird abundance did not decrease significantly in SF, due to an increase in abundance of forest independent and semi-dependent bird species (Fig. 1). According to Anjos (2004), species that benefit from forest fragmentation tend to increase their relative abundance in the smallest fragments, mainly due to (1) the increase in area of habitat for edge and non forest species, (2) the loss of species in small fragments may result in reduced interspecific competition allowing persisting species to achieve unusually high densities, analogous to density compensation on islands (MacArthur et al. 1972; Laurance et al. 1997). This change in density of some species was recorded in Araucarian Brazilian forests (Anjos and Boçon 1999), in Amazonian forests (Bierregaard and Lovejoy 1989) and in Brazilian Atlantic Forest (Willis 1979), including some of the same species that increased in abundance in our study, mainly edge species (e.g. Pitangus sulphuratus, Turdus leucomelas, T. amaurochalinus, Troglodytes aedon, see abundance in Appendix 1) or open habitat species that used the border of SF (see Crypturellus parvirostris and Colaptes campestris, Appendix 1).
Frugivores richness and abundance decreased in small fragments studied by us, in coincidence with observations in patches of Amazonian forest (Bierregaard and Stouffer 1997) and Atlantic Forest (Aleixo and Vielliard 1995; Willis 1979). This guild depends on scattered trees of different species at difference seasons, and probably large woodlots have enough tree diversity to support them (Anjos and Boçon 1999; Willis 1979). Melo et al. (2006) observed, in an Atlantic Forest fragment, that the creation of forest edge may alter some attributes of seed rain, mainly its content of large-seeded plants and of those dispersed by vertebrates, which were lower in the forest edge than in the patch core. The small fragments have a higher area of forest edge and a smaller proportion of interior forest.
In agreement with other studies (e. g. Bierregaard and Stouffer 1997 for the Amazonian forest, Aleixo and Vielliard 1995 for the Atlantic Forest) undestory, terrestrial and midstory insectivores decreased in richness and abundance in SF (midstory insectivores only in richness). Terrestrial forest insectivores were markedly affected by fragmentation, detecting 11 species in LF and only 4 in SF, and several of the commonest ground species in LF, such as one Tyrannidae solitary terrestrial feeders (Corythopis delalandi) and two Formicariidae (Grallaria varia and Hylopezus ochroleucus), were never recorded in SF (see Appendix 1). Bierregaard and Stouffer (1997) mentioned a decrement of birds species which forage in the leaf litter in small fragments in Amazonian forest.
In contrast to observations by Bierregaard and Stouffer (1997) in Amazonia, our data showed a significant reduction of nectarivores in SF of Atlantic Forest. These differences may be explained by variations in flower production between the tropical Amazonian forest (more predictable and developed too in secondary habitats, Bierregaard and Stouffer 1997) and the southernmost subtropical Atlantic Forest of Argentina (the flower production is markedly seasonal and is probably affected by fragmentation and the reduction of plant diversity).
The raptors decreased in SF (significant to 90% of confidence level, due probably to a small sample size effect) as it is expected because these guilds contain rare species that have large area requirements and are more vulnerable to fragmentation (Laurance et al. 1997).
The bark insectivore, a guild described as vulnerable to fragmentation in AF (Anjos and Boçon 1999; Willis 1979), showed no significant differences between SF and LF in our research. We suggest that a density compensation, mentioned above, probably occurred in this guild, with some species increasing in abundance in SF (see Colaptes campestris in Appendix 1), and others decreasing or absent in SF (see Lepidocolaptes fuscus and Melanerpes Xavifronsin flavifrons in Appendix 1).
In contrast to that observed by Marini (2001) in forest fragments of the Brazilian Cerrado, the endemic bird species were area sensitive, decreasing significantly in richness and abundance in the Atlantic Forest SF. This is an important consideration due to the fact that endemic species become globally extinct when extirpated from their restricted range (Marini 2001), and the fragmentation of Atlantic Forest is extreme, affecting progressively all the biome (Galindo Leal and Câmara 2003).
Finally, terrestrial granivores were positively affected by forest fragmentation, increasing in richness and abundance in SF. Two dove species (Columba picazuro and Leptotila verreauxi) influenced this result, increasing their abundance in SF (Appendix 1), as they are mainly edge species, which forage in open areas or borders including crops. Some authors suggested that granivores are abundant and probably increasing near forest fragments (Leck 1979 in Marini 2001).
Our first observations on fragmentation effects on bird assemblages in the southernmost Argentinean Atlantic Forests, did not validate the hypothesis on pre-adaptation to human disturbances in the bird community of Atlantic Forest, suggested by Brown and Brown (1992) and supported by Protomastro (1999) based on a study in the Argentinean Atlantic Forest in the Iguazú National Park, Argentina. On the contrary, we observed that forest dependent, endemic and several sensitive bird guilds were strongly affected by fragmentation, putting in evidence the vulnerability to the fragmentation process and the necessity to conserve large remnants to avoid reduction of the high biodiversity of AF birds.
These first results on fragmentation effects in the Argentine Atlantic Forest, may be useful to help in regional conservation policymaking. For example, trying to avoid connection rupture between the Provincial Cuñapiru Park and the northern large forest fragment, which are at present connected by a 400 m corridor.
References
Aleixo A (1999) Conservação da avifauna da floresta Atlântica: efeitos da fragmentação e a importância de florestas seundárias. In Albuquerque JLB, Cândido JF, Straube FC, Roos AL (eds) Ornitología e conservação. Da ciência ás stratégias. Editora Unisul, Tubarão
Aleixo A, Vielliard JME (1995) Composição e dinâmica da avifauna da mata de Santa Genebra, Campinas, São Paulo, Brasil. Rev Brasil Zool 12:493–511
Altman A, Swift B (1993) Checklist of the birds of South America. Third Edition. BookMasters, Inc, Ashland
Anjos LD (2004) Species richness and relative abundante of birds in natural and anthropogenic fragments of Brazilian Atlantic forest. An Acad Bras Cienc 76:429–434
Anjos LD, Boçon R (1999) Bird communities in natural forest patches in southern Brazil. Wilson Bull 11:397–414
Bierregaard RO, Lovejoy TE (1989) Effects of forest fragmentation on Amazonian understory bird communities. Acta Amazonica 1:215–241
Bierregaard RO, Stouffer PC (1997) Understory birds and dynamic habitat mosaics in Amazonian rainforests. In: Laurance WF, Bierregaard RO (eds.) Tropical forest remnants. Ecology, management, and conservation of fragmented communities. University of Chicago Press, Illinois.
Blanco JLA, Garcia GJ (1997) A study of habitat fragmentation in Southeastern Brazil using remote sensing and geographic information systems (GIS). For Ecol Manage 98:35–47
Brown KS, Brown GG (1992) Habitat alteration and species loss in Brazilian forest. In: Whitmore TC, Sayer JA (eds) Tropical deforestation and species extinction. Chapman and Hall, London
Carrillo E, Wong G, Cuaron AD (2000) Monitoring mammal populations in Costa Rican protected areas under different hunting restrictions. Conserv Biol 14:1580–1591
Chiarello AG (1999) Effects of fragmentation of the Atlantic forest mammal communities in southeastern Brazil. Biol Conserv 89:71–82
Chiarello AG (2000) Density and population size of mammals in remanants of Brazilian Atlantic Forest. Conserv Biol 14:1649–1657
Clark Labs (1998) The spatial data builder, Cartalinks, Version 1.1. http://www.clarklabs.org
Cuaron A (2000) A global perspective on habitat disturbance and tropical rainforest mammals. Conserv Biol Special Sect 14:1574–1579
Cullen LJr, Bodmer RE, Valladares-Padua C (2000) Effects of hunting in habitat fragments of the Atlantic forests, Brazil. Biol Conserv 95:49–56
D’angelo Neto S, Venturin N, Oliveira Filho AT, Frieiro Costa FA (1998) Avifauna de quatro fisionomias florestais de pequeno tamanho (5–8 ha) no campus Da Ufla. Rev Bras Biol 58:1–7
Dirzo R, Miranda A (1990) Contemporary neotropical defaunation and forest structure, function, and diversity – A sequel to John Terborgh. Conserv Biol 4:443–445
Duca C, Gonçalves J, Marini MA (2001) Predação de ninhos artificiais emem fragmentos de matas de Minas gerais, Brasil. Ararajuba 9:113–117
ESRI (1996) ArcView GIS 3.2, Environmental Systems Research Institute, Inc. Redlands, CA. http://www.esri.com
Fragano F, Clay R (2003) Biodiversity status of the interior Atlantic forest of Paraguay. In: Galindo-Leal C, IG Câmara IG (eds) Atlantic Forest of the South America. Biodiversity status, threats, and outlook. Island Press, Washington DC
Galindo-Leal C (2003) Putting the pieces back togethers: fragmentation an landscape conservation. In: Galindo-Leal C, Câmara IG (eds) Atlantic Forest of the South America. Biodiversity status, threats, and outlook. Island Press, Washington DC
Galindo-Leal C, Câmara IG (2003) Atlantic Forest hotspot status: an overview. In: Galindo-Leal C, Câmara IG (eds) Atlantic Forest of the South America. Biodiversity status, threats, and outlook. Island Press, Washington DC
Gascon C, Lovejoy TE, Bierregaard RO, Malcolm JR, Stouffer PC, Vasconcelos HL, Laurence WF, Zimmerman B, Tocher M, Borges S (1999) Matrix habitat and species richness in tropical forest remnants. Biol Conserv 91:223–229
Giraudo AR, Abramson RR (1998) Usos de la fauna silvestre por los pobladores rurales en la selva paranaense de Misiones: tipos de uso, influencia de la fragmentación y posibilidades de manejo sustentable. Boletín Técnico de la Fundación Vida Silvestre Argentina 47:1–41
Giraudo AR, Krauczuk E, Arzamendia V, Povedano H (2003a) Critical analysis of protected areas in the Atlantic Forest of Argentina. In: Galindo-Leal C, Câmara IG (eds) Atlantic Forest of the South America. Biodiversity status, threats, and outlook. Island Press, Washington DC
Giraudo AR, Povedano H, Belgrano MJ, Pardyñas U, Miquelarena A, Ligier D, Krauczuk E, Baldo D, Castelino M (2003b) Biodiversity status of the Interior Atlantic Forest of Argentina. In: Galindo-Leal C, Câmara IG (eds) Atlantic Forest of the South America. Biodiversity status, threats, and outlook. Island Press, Washington DC
Giraudo AR, Povedano H (2004) Avifauna de la región biogeográfica Paranaense o Atlántica Interior de Argentina: biodiversidad, estado de conocimiento y de conservación. Insugeo, Miscelánea 12:5–12
Infostat (2002) Data analysis software system, version 1.1. Software produced by InfoStat group, Nacional University of Córdoba, Argentina. http://www.infostat.com.ar
Laurance WF, Bierregaard RO (eds) (1997) Tropical forest remnants. Ecology, management, and conservation of fragmented communities. The University Chicago Press, Chicago
Laurance WF, Bierregaard RO, Gascon C, Didham RK, Smith AP, Lynam AJ, Viana VM, Lovejoy TE, Sievin KE, Sites JW, Andersen M, Tocher MD, Kramer EA, Restrepo C, Moritz C (1997) Tropical forest fragmentation: synthesis of a diverse and dinamyc discipline. In: Laurence WF, Bierregaard RO (eds) Tropical forest remnants ecology, management, and conservation of fragmented communities. The University Chicago Press, Chicago
Laurance WF, Lovejoy TE, Vasconcelos HL, Bruna EM, Didham RK, Stouffer PC, Gascon C, Bierregaard RO, Laurance SG, Sampaio E (2002) Ecosystem decay of Amazonian forest fragments: a 22-year investigation. Conserv Biol 16:605–618
Leck CF (1979) Avian extinctions in an isolated tropical wet-forest preserve, Ecuador. Auk 96:343–352
Leite MRP (2001) Jaguar, puma, and local people in three protected areas, Atlantic coastal forest, Parana state, Brazil. In: Medellín RA, Chetkiewicz C, Rabinowitz A, Redford KH, Robinson JG, Sanderson E, Taber A (eds) El Jaguar en el nuevo milenio: Una evaluación de su estado, detección de prioridades y recomendaciones para la conservación de los jaguares en América. Universidad Nacional Autónoma de México/Wildlife Conservation Society, Mexico DF
Lopez de Casenave J, Pelotto JP, Caziani SM, Mermoz M, Protomastro J (1998) Reponses of avian assemblages to a natural edge in a Chaco semiarid forest in Argentina. Auk 115:425–435
MacArthur RH, Diamond JM, Karr JR (1972) Density compensation in island faunas. Ecology 53:330–342
MacArthur RH, Wilson EO (1967) The theory of island biogeography. Evolution Princeton University Press, Princeton
Marini MA (2000) Efeitos de fragmentação florestal sobre as aves em Minas Gerais. In: Santos Alves MA, Silva JMC, van Sluys M, Godoy Bergallo H, Rocha CFD (eds) O Ornitología no Brasil Pesquisa Atual e Perspectivas. Editorial Universidade Estadual do Rio de Janeiro, Brasil
Marini MA (2001) Effects of forest fragmentation on birds of the cerrado region, Brazil. Bird Conserv Intern 11:13–25
Marini MA, Garcia FI (2005) Bird conservation in Brazil. Conserv Biol 19:665–671
Matteucci SD, Morello J, Rodríguez A, Mendoza N (2004) Mosaicos de paisaje y conservación regional: el Alto Paraná Encajonado argentino-paraguayo. Editorial de la Facultad de Arquitectura, Diseño y Urbanismo. Universidad de Buenos Aires, Argentina
Melo FPL, Dirzo R, Tabarelli M (2006) Biased seed rain in forest edges: evidence form the Brazilian Atlantic forest. Biol Coserv 132:50–60
Morello J, Matteucci SD (1999) Biodiversidad y fragmentación de los bosques en la Argentina. In: Matteucci SD, Solbrig O, Morello J, Halffter G (eds) Biodiversidad y uso de la tierra. Conceptos y ejemplos en Latinoamérica. Colección CEA 24. Eudeba, Buenos Aires
Myers N, Mittermeier RA, Mittermeier CG, Da Fonseca GAB, Kent J (2000) Biodiversity hotspots for conservation priorities. Nature 403:853–858
Parker IIITA, Stotz DF, Fitzpatrick JW (1996) Ecological and distributional data base. In: Stotz DF, Fitzpatrick JW, Parker IIITA, Moskovits DK (eds) Neotropical birds. Ecology and conservation. The University Chicago Press, Chicago
Protomastro JJ (1999) A test for preadaptation to human disturbances in the bird community of the Atlantic forest. In: Albuquerque JLB, Cândido JF, Straube FC, Roos AL (eds) Ornitología e conservaçao. Da ciência ás stratégias. Editora Unisul, Tubarão
Ranta P, Blom T, Niemela J, Joensuu E, Siitonen M (1998) The fragmented Atlantic rain forest of Brazil: size, shape and distribution of forest fragments. Biodivers Conserv 7:385-403
Rey LA (2003) Last opportunity for the Atlantic Forest. In: Galindo-Leal C, Câmara IG (eds) Atlantic Forest of the South America biodiversity status, threats, and outlook. Island Press, Washington DC
Rigdely RS, Tudor G (1989) The birds of South America, vol 1. The Oscines passerines Univ Texas Press, Austin
Rigdely RS, Tudor G (1994) The birds of South America, vol 2. The Suboscines passerines Univ Texas Press, Austin
Saunders DA, Hobbs RJ, Margules CR (1995) Biological consequences of ecosystem fragmentation: a review. In: Ehrenfeld D (ed) Readings from conservation biology, pp 1–15
Sick H (1988) Ornitología brasileira: uma introduçao, vol 1 and 2. 3a ed. Universidade de Brasília, Brasília
Silva JMC, Castelei CHM (2003) Status of the biodiversity of the Atlantic Forest of Brazil. In: Galindo-Leal C, Câmara IG (eds) Atlantic Forest of the South America biodiversity status, threats, and outlook. Island Press, Washington DC
Skole D, Tucker C (1993) Tropical deforestation and habitat fragmentation in the Amazon: satellite data from 1978 to 1988. Science 260:1905–1910
Tabarelli M, Matovani W, Peres CA (1999) Effects of habitat fragmentation on plant guild structure in the montane Atlantic Forest of southeastern Brazil. Biol Conserv 91:119–127
Tabarelli M, Silva JMC, Gascon C (2004) Forest fragmentation, synergisms and the impoverishment of neoropical forest. Biodivers Conserv 13:2567–2586
Terborgh J (1988) The big things that run the world: as sequel to E. O. Wilson. Conserv Biol 2:402–403
Turner IM, Corlett RT (1996) The value of small, isolated fragments of lowland tropical rain forest. Tree 11:330–333
Turner IM (1996) Species loss in fragments of tropical rain forest: review of the evidence. J Appl Ecol 33:200–209
Wiens JA (1994) Habitat fragmentation: island vs landscape perspectives on birds conservation. Ibis 137:97–104
Willis EO (1979) The composition of avian communities in remanescent woodlots in southern Brazil. Pap Avul Zool S Paulo 33:1–25
Acknowledgements
We thank specially to Dr. Jorge Morello for his constant and selfless support and guidance during this research. Ramón Regner, Esteban Creus, Lionel Mehaudy, Andrés Bortoluzzi, from the National Institute of Limnology, and Ernesto Krauczuk, Ricardo Escobar, Julio Do Santos, Fabio Maloch y Eustaquio Rodríguez, from de Ministerio de Ecología de Misiones, are thanked for their collaboration and assistance in the field works; the CONAE for providing satellite images. This investigation was supported by the following institutions and projects: PICT 2002 N° 01-12831 (ANPCyT), PIP 6487 (CONICET) and Olrog Grant (AOP).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Giraudo, A.R., Matteucci, S.D., Alonso, J. et al. Comparing bird assemblages in large and small fragments of the Atlantic Forest hotspots. Biodivers Conserv 17, 1251–1265 (2008). https://doi.org/10.1007/s10531-007-9309-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10531-007-9309-9