Abstract
By analyzing 6,480 tree leaf samples from 57 sites within Brazilian biomes, we considered whether vegetation types in terrestrial ecosystems reflect biogeochemical diversity and whether they fit into a leaf economics spectrum (LES). To achieve this, we investigated the relations among leaf carbon (C) and nitrogen (N) concentrations, their isotope natural abundance and C:N ratio. In addition, we tested their correlations with mean annual temperature (MAT) and precipitation (MAP), as climatic factors. We found consistent differences in the C and N concentrations and their isotopic composition among the vegetation types. MAP is the main climatic driver of changes in N, C:N ratio, δ15N, and δ13C, correlating negatively with N and positively with C:N ratio. These relations show that these biomes follow an LES. The Caatinga had the highest δ15N values, suggesting that N residence time in soil is longer due to low leaching and plant uptake. We observed that MAP is not the only factor influencing δ13C values in different biomes; instead canopy effect probably explains the highest values observed in the Cerrado. Our results reinforce earlier findings that life diversity in the tropics reflects biogeochemistry diversity and leaf δ15N opens the possibility for investigating plant trade-offs dictated by the LES. Finally, we expect our findings to contribute to a better understanding of the tropics in global climate models.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The importance of terrestrial ecosystems for the modern carbon (C) cycle has been emphasized in several modelling exercises (Friedlingstein et al. 2006; Yue et al. 2020). Feedback from terrestrial ecosystems on increased atmospheric CO2 are key components in monitoring and mitigating the effects of on-going climate changes (Schimel 1995; Schimel et al. 2015a; Pugnaire et al. 2019). In terrestrial ecosystems, the importance of plants is paramount since the coupled photosynthesis-respiration processes modulate biosphere-atmosphere C exchanges. Recent simulations indicate that the land component of the global C budget has been an increasing C sink, equivalent in 2018 to roughly one-third of the total fossil fuel emissions due to CO2 and N fertilization (Friedlingstein et al. 2019). However, the uncertainty linked to this estimate is still large mainly because the spatiotemporal variability associated with the terrestrial gross primary production (GPP; Anav et al. 2015; Rödig et al. 2018). The largest values of GPP are not surprisingly observed in the tropics due to energy and water availability (Anav et al. 2015). At the same time, the role of the tropics in the modern global C cycle is also important due to the net balance of land-use changes, increasing the land-atmosphere CO2 flux (van der Werf et al. 2017; Duvert et al. 2020; Souza et al. 2020).
It is well recognized that availability of water and energy are important drivers of plant richness at the regional and continental scales (e.g., Currie and Paquin 1987; Field et al. 2005). Most of the Brazilian territory is located within the southern tropical zone between the Equator and the Tropic of Capricorn (23.5°S). Due to Earth’s sphericity, this is a region of the planet that receives year-round abundant solar energy, where the precipitation in most of the country is >1300 mm year-1. As a result, Brazil stands as a megadiverse country regarding its fauna and flora. According to the Brazil Flora Group (2015), more than 30,000 species of angiosperms alone have been cataloged. Amazonia (49% of the country area) and the Atlantic Forest (13%) biomes are well known for luxurious tropical rainforests (Morellato and Haddad 2000). Although in most of the country rainfall is abundant, some regions like the Cerrado (24%) display remarkable seasonality (Eiten 1972), while the Caatinga (10%) is characterized by a negative water balance (evapotranspiration higher than precipitation), being the only truly semiarid region of the country (Oliveira et al. 2019). In the Pantanal (2%), seasonal flooding of lowlands (80% of the area) hinders the development of dense forest formations over broad areas (Evans et al. 2014). The Pampas (2%) has a temperate and subtropical climate, having the lowest temperatures in the country, which together with fires and grazing prevents the development of extensive forests (Overbeck et al. 2007).
In each biome, there is an iconic vegetation type that covers most of the area. Rainforests are the iconic vegetation of Amazonia and Atlantic Forest, while different physiognomies of savannas, dry forests and grasslands are predominant in the Cerrado, Caatinga, Pampas, and Pantanal. However, other vegetation types also occur in each biome, like white-sand forest vegetation, locally called Campina, as well as patches of savanna-like vegetation encroaching into several areas of Amazonia. Savanna-like vegetation is also common in the Atlantic Forest. Even in the Cerrado, there are several savanna physiognomies types: campo limpo (open grasslands), campo sujo, cerrado and cerradão (woody-savanna). In the Pampas, there are the Araucaria forests, which are evergreen temperate forests dominated by the gymnosperm Araucaria angustifolia.
Brazilian biomes are under severe pressure by human-induced land use and land cover changes: in the last 30 years the country lost 71 Mha of natural vegetation (Souza et al. 2020). As a country characterized by an economy largely dependent on the production of raw commodities, Brazil pursues a fragile balance between the exploitation of natural resources and preservation (DeFries et al. 2004). Amazon deforestation is an iconic example that represents the dichotomy between agriculture expansion and conservation (Pereira et al. 2019). Those biomes near the coast (Atlantic Forest and Caatinga), the first to be settled by Europeans, suffered the most extensive changes (Souza et al. 2020). Only 15–20% of the original cover of the Atlantic Forest is left, leaving a highly fragmented landscape with several consequences to the biota and ecosystem functions (Tabarelli et al. 2004; Souza et al. 2020). Chronic land-use and cover changes in the Caatinga has already led to severe biological impoverishment of plant species (Ribeiro et al. 2015); and the Cerrado, considered an agriculture frontier in the 1960s and 1970s, has already lost 40–50% of its original vegetation cover (Sano et al. 2010; Souza et al. 2020).
Stein et al. (2014) have postulated that species richness is driven by “heterogeneity diversity”, and Díaz and Cabido (2001) have shown that life diversity generally translates into plant “functional diversity”, which in turn regulates ecosystem processes. Therefore, the widespread biological impoverishment occurring in Brazilian biomes may have severe consequences on several ecosystem services, including food production (Joly et al. 2019). Townsend et al. (2008) argue that in the tropics, life diversity coupled with environmental conditions generates an extraordinary “biogeochemical diversity”, and to capture such diversity, it is important to understand the functioning of tropical ecosystems across environmental gradients. Considering the high pace of land-use and cover changes in Brazil, a minimum understanding of such biogeochemical diversity is crucial before some Brazilian biomes reach the so-called “tipping point” as defined by Lovejoy and Nobre (2019).
By the same token, the Amazon region is also identified as a “tipping element” (Lenton et al. 2008), defined as a sub-continental area having a disproportional importance on global climate health (Cox et al. 2000; Huntingford et al. 2013; Brienen et al. 2015). Paradoxically, although tropical systems have long been recognized as key areas in modern C cycles, these regions of the globe are still very underrepresented in global climate models (Schimel et al. 2015b). Partially because of the complex task of reaching an adequate survey on tropical areas due to several logistic constraints, and due to their enormous plant biodiversity (Kreft and Jetz 2007; Schimel et al. 2015b).
The natural isotope abundance of N (δ15N) and C (δ13C) offer a powerful way to evaluate the “biogeochemical diversity” of terrestrial ecosystems as well. Over the leaf lifespan, the leaf δ15N integrates the isotopic signature of nitrogen (N) sources and its dynamics and availability in soil (e.g., Houlton et al. 2006; Nardoto et al. 2008; Craine et al. 2009), while leaf δ13C integrates plant C assimilation and transpiration (Farquhar et al. 1989a; Ehleringer et al. 1993). Therefore, these isotopic traits provide a way to integrate the large temporal and spatial variability inherent in point measurements and to scale N and C cycling and plant-water relations.
This study is an exploratory investigation on the “biogeochemical diversity”, considering C and N isotope ratios and associated elemental composition of vegetation types in the main Brazilian biomes using a data set composed of more than six thousand tree leaf samples from more than fifty sites across the country. Although this study is exploratory by design, we anticipated based on well-established trends that: (i) foliar N concentration will be higher in biomes with predominance of deciduous and semi-deciduous trees (Aerts 1996; Aerts and Chapin 1999; Han et al. 2005; Sardans et al. 2016), as a corollary, (ii) we expected that foliar C:N ratios will be also lower in these biomes (Zhao et al. 2016; Zhang et al. 2020). We also expected that (iii) foliar δ13C (Swap et al. 2004; Diefendorf et al. 2010; Kohn 2010; Basu et al. 2019) and δ15N (Schuur and Matson 2001; Nardoto et al. 2008) should be inversely correlated with precipitation.
Material and methods
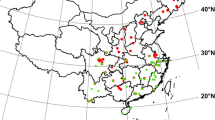
A total of 6,480 tree leaf samples were collected from 57 sites across the six Brazilian biomes (Fig. 1), encompassing the following main vegetation types according to IBGE (2012) classification: Forested Campinarana (Campina of the Amazon region, forest on sandy soils), Dense Ombrophilous Forest (evergreen non-flooded Amazon forest, evergreen coastal Atlantic Forest), Open Ombrophilous Forest (transition forest in Cerrado-Amazon regions), Mixed Ombrophilous Forests (Araucária forest, evergreen forest of the South region of Brazil with presence of A. angustifolia), Semideciduous Seasonal Forest (inland Atlantic Forest), Savanna (forest savanna of the Cerrado), Forested Savanna-Park of the Pantanal (periodically flooded), Savanna-Steppe (Caatinga of the Northeast region, xeric shrubland and thorny forest), Coastal Marine Vegetation (Restinga, forest formed on sandy, acidic, and nutrient-poor soils near the ocean), Fluvio-Marine Vegetation (Mangrove). Ombrophilous forests are located in areas where the dry season is shorter than four months, while seasonal forests occur in areas with a longer dry season.
Table 1 summarizes geographic coordinates and climatic data, as well as the type of vegetation in each of the 57 sampling sites. Fig. 1 shows the spatial distribution of these sites within the Brazilian biomes. Although some of the sampling sites are clearly located in transition zones, especially between Amazonia and the Cerrado, they were assigned to only one biome according to the plant community affiliation (forest vs. savanna).
This study analyzed original experimental data together with published ones. Therefore, there were slight differences in sampling protocols of different projects involved, and these are more prominent in the selection of species for sampling, especially in multispecies tropical forests. Several studies followed the abundance criteria, which gives preference to sampling the most common species, genera or family (e.g. Lins et al. 2016), while others selected species based on logistic facilities. The latter was applied in the Large-Scale Biosphere Atmosphere Project in the Amazon (LBA), more specifically, in the Tapajós National Forest, where a catwalk was installed in the middle of the canopy (e.g., Domingues et al. 2018). Most of the studies followed the protocol proposed by Cornelissen et al. (2003); therefore, preference was given to mature, fully expanded, healthy sunlit leaves from branches located in the middle of the canopy. However, it is perhaps naive to assume that almost 6,500 leaf samples were collected following this protocol, especially in tropical forest where access to the top of the canopy is challenging. For instance, the main goal of some samplings was botanical identification and not for elemental analysis or isotope ratio. In these cases, sampling of sunlit leaves was not mandatory. As it is well known, sun exposure strongly influences the C isotopic composition of the leaves (Buchmann et al. 1997; Ometto et al. 2006), which in turn is also affected by the sampling height, not recorded for each leaf sample. In tropical forests, there is a significant difference between δ13C of the leaves sampled from the lower canopy compared with the leaves from the upper canopy, the so-called canopy effect (Medina and Minchin 1980). Such gradient could be as high as 2.5% each 10 m of canopy height (Ometto et al. 2006). To minimize this bias in our analysis, leaf samples from the understory were removed from this study.
All samples included in the present work were analyzed at the Laboratory of Isotope Ecology of the Center for Nuclear Energy in Agriculture (CENA), University of São Paulo (USP) over the last 20 years. From 2 to 3 mg of sub-samples of dry leaves (which were previously oven-dried at 60°C for 72 h) were transfer to tin capsules, which were dry combusted in an elemental analyzer (model CHN1110; Carlo Erba, Milan, Italy) to determine N and C concentrations. The N2 and CO2 generated from the combustion was purified in a gas chromatographic column and passed directly to the inlet of a gas isotope ratio mass spectrometer (model Delta Plus, ThermoFisher Scientific, Bremen, Germany). The isotope ratios of C and N are expressed by the standard equation:
where, δX is the C or N stable isotopic composition and Rsample and Rstandard are the 13C:12C or the 15N:14N ratios of the samples and standards, respectively. The primary standard was Pee Dee Belemnite for C, and atmospheric air for N. The internal organic standard was BBOT (C26H26N2O2S). Experimental precision was measured as the mean ± standard error of the repeatability of BBOT analysis and was found to be equal to 0.2‰ and 0.3‰ for C and N, respectively.
We first presented foliar elemental concentration and isotope values considering all 6,480 leaf samples through statistical parameters like average, standard deviation and coefficient of variation, grouping vegetation types according to the IBGE (2012) classification (Table 2). Then we compared foliar elemental concentration and isotope values among vegetation types. In order to explore such differences, all Ombrophilous Forests were re-grouped as “Evergreen” forests, in contrast with the Seasonal Semideciduous Forest plot, labeled as “Seasonal”. The other vegetation types were kept under the IBGE (2012) classification, but we used local names such as Forested Savanna for Cerrado; Forested Savanna-Steppe for Caatinga; Campinarana for Campina; Forested Savanna-Park for Pantanal; Marine Vegetation for Restinga; Fluvio-Marine Vegetation for Mangrove. Following this re-grouping, we conducted statistical analysis and graphic display by averaging samples by plot, which we called “plot-average”. This was done because our database was not generated specifically for this purpose, and a large proportion of the data was generated from Ombrophilous Forests. Through averaging data by plot, we aimed to reduce a disproportional weight exerted from this type of vegetation within our data set.
Box-plots were used to represent this comparison, sorting vegetation types in the horizontal axis (x-axis) based on increasing order of foliar N concentration. To test the differences statistically, we applied a generalized linear model (GLM, “lme4” package on R platform), according to data distribution that was tested using the “fitdistrplus” package of R. After the GLM, we applied the Tukey post-hoc test to assess differences among pairs of vegetation types. Carbon concentration and C and N isotope ratios followed a normal distribution, while N concentration followed a gamma distribution. Therefore, data regarding foliar N concentration was log-transformed.
We investigated the correlations between plot-average C and N stable isotopic ratios and associated elemental composition across vegetation types through biplots. Finally, we tested the effect of mean annual precipitation (MAP) and mean annual temperature (MAT) on plot-averages elemental and isotopic compositions also using GLM fitting. Climatological data were obtained from Climate-Data (2020), considering the municipality of each sampling site as reference. There is a weak (r2adj = 0.05), but significant direct (P = 0.05) correlation between MAT and MAP (data not showed). Therefore, we did not apply statistical tests using a multiple regression with MAT and MAP in the same model.
Results
The complete data set used in this study can be found at https://doi.org/10.17632/38npddpnts.1. The GLM statistical parameters used in this study are presented in Table S1 of the electronic supplementary material.
Grouping data according to vegetation types revealed significant differences among them (Table 2). The highest foliar N concentrations and δ15N were observed for the Seasonal Forest and Caatinga, significantly higher than the others (Fig. 2a, c). Lower foliar N concentrations and δ15N were observed in the Campina, and Cerrado (Fig. 2a, c). Foliar C:N ratio followed the reverse trend of foliar N concentration, where vegetation types with the highest foliar N concentrations had the lowest foliar C:N ratio and vice-versa (Fig. 2b). Consequently, we found significant correlations between foliar δ15N and foliar N plot-average concentrations (Fig. 3a, r2adj = 0.34, P < 0.01), and between foliar δ15N and foliar C:N plot-average ratios (Fig. 3b, r2adj = 0.40, P < 0.01).
Leaves in the Campina had the highest C concentration, followed by the Cerrado (Fig. 2d). However, foliar C concentration in the Cerrado was not different from Evergreen, Seasonal and Restinga vegetation types. On the other hand, the lowest foliar C concentration was observed in the Mangrove, followed by the Caatinga (Fig. 2d). Finally, the Campina had the most negative foliar δ13C, significantly different from other vegetation types. On the other hand, the least negative foliar δ13C was observed in the Caatinga, followed by the Cerrado (Fig. 2e). We found a weak, but significant positive correlation between foliar δ13C and foliar δ15N plot-average values (Fig. 3c, r2adj = 0.20, P < 0.01).
We investigated if climatic factors such as MAP and MAT could influence plot-average foliar C and N concentrations or their isotopic ratios (Moles et al. 2014). There were no significant correlations between foliar traits and MAT (data not shown). On the other hand, all the investigated parameters showed significant correlations with MAP (Fig. 4) except foliar C plot-average concentration (P = 0.19). Foliar N plot-average concentration had a weak negative correlation with MAP (radj2 = 0.14, P < 0.01) (Fig. 4a). Conversely, foliar C:N plot-average ratio had a weak psotive correlation with MAP (radj2 = 0.13, P < 0.01) (Fig. 4b). Foliar δ13C and δ15N plot-average values were both negatively correlated with MAP (P < 0.01), with higher radj2 compared to foliar N concentration and C:N ratio (0.53 and 0.32, respectively; Fig. 4d and c).
Discussion
We extended the findings of Townsend et al. (2007) beyond foliar N:P ratios, by showing that life diversity is followed by a large variability of C and N elemental and isotopic composition (biogeochemistry diversity). Foliar N concentrations varied from 3 to > 70 mg g-1 [coefficient of variation (CV) = 40%], foliar C:N ratio from 6 to > 80 (CV = 40%), δ15N from −15 to 19‰ (CV = 120%), and δ13C from −40 to −23‰ (CV = 8%) (Table 2). Such variability is only comparable with the diversity found at the global scale (Craine et al. 2009; Diefendorf et al. 2010; Kattge et al. 2011; Zhang et al. 2020). Townsend et al. (2008), argue that such biogeochemistry diversity is a consequence of environmental heterogeneity (Stein et al. 2014), which in turn is the interplay between abiotic factors such as soil age, soil chemistry, landscape dynamics, and the richness of organisms, especially plant species.
The stereotype of tropical soils is that they generally seem to be in geomorphologically stable areas, where intense leaching by high rainfall has produced old deep soils (Porder et al. 2005). However, according to Sanchez and Buol (1974), soil temperature, which is higher in the tropics than in other areas is the major difference between soils of tropical and extra-tropical areas. Other than that, soils in tropical areas are as variable as in any other region of the globe, encompassing soils of different ages with a wide range of weathering states (Richter and Babbar 1991; Sanchez and Logan 1992).
The same variability observed in weathering states of tropical soils is also observed in their nutrient content (Sanchez and Logan 1992). For instance, due to low rainfall, soil pH and base saturation are generally higher in the Caatinga than in any other biome (Arruda et al. 2017). On the other hand, soils of white-sand vegetation like the Amazon Campina and coastal Restingas are poorer than in any other type of vegetation (Arruda et al. 2017; Bonilha et al. 2012; Mendonça et al. 2017). This variability in the soil nutrient pool was reflected in the foliar chemistry composition along gradients of soil fertility among areas of evergreen forests in the Amazon (Fyllas et al. 2009); and in areas of ecotones, such as Campina-Evergreen forests in the Central Amazon region (Mardegan et al. 2009; Mendonça et al. 2017), and Cerrado-Seasonal forests in the southeastern region of the country (Miatto et al. 2016).
On the regional to continental continuum, precipitation and temperature are probably the most important filter to select composition of plant communities (Currie 1991). However, on the local to regional continuum, landscape dynamics plays a key role in species sorting (Porder et al. 2005; Balzotti et al. 2016). For instance, in a toposequence located in the Central Amazon region, topography determine the soil N-availability, which is higher in the top flat areas, decreasing in the middle-slope and reaching a minimum in the lower parts of the terrain, where periodic flooding by small streams increase nutrient limitations (Luizão et al. 2004; Nardoto et al. 2008). Tiessen et al. (1994), have presented a similar example in a toposequence in the upper Negro River in the northern Amazon. In stark contrast, in Kaua’i, Hawai’i; Porder et al. (2005) found that most stable upland areas were nutrient-poor with nutrient availability increasing toward lowland areas.
In areas of high-gradient landscapes, erosion is an important process in reshuffling nutrient distribution by differential landscape stability, which in turn changes soil residence times across steeply dissected terrain (Weintraub et al. 2015). The effects of erosion have been detected not only for rock-derived nutrients, such as phosphorus (Porder and Hilley 2011), but also for N in subtropical (Hilton et al. 2013) and tropical areas (Weintraub et al. 2015). In Brazil, the Atlantic Forest, especially on coastal areas of southeastern Brazil, have steeply dissected terrain along the Serra do Mar rift system. In these areas, shallow soils predominate, with relatively short residence times (Furian et al. 1999; Martins et al. 2015), in sharp contrast with very deep soils that dominate the Amazon region.
All the above-mentioned abiotic factors (soil age and chemistry, and landscape dynamics) have a strong effect on the composition of vegetal species (Stein et al. 2014). In turn, species composition has a high degree of control over foliar N (Fyllas et al. 2009; Asner and Martin 2016; Balzotti et al. 2016). A clear example of this sort of influence is the foliar N higher concentrations found in leaves of Fabaceae specimen, regardless their N-fixing capabilities (Vitousek et al. 2002). Specimens of this family are widespread in the Amazon (Moreira et al. 1992); Atlantic Forest (Joly et al. 2012); Cerrado (Sprent et al. 1996); and Caatinga (Souza et al. 2012).
In our study, the biogeochemistry diversity was amplified by the inclusion of tropical forests in drier regions or regions with a prominent rainfall seasonality. In the Caatinga, MAP varies from 300 to 1,000 mm and drought events are recurrent (Oliveira et al. 2019); in sharp contrast with areas of the Amazon and Evergreen Atlantic Forest where MAP is higher than 2,000 mm. Besides the inclusion of biomes with lower precipitation, this study also includes the Seasonal Atlantic Forest, where MAP averages 1,500 mm, but rainfall seasonality is strong. Drier conditions or seasonality lead to establishing semi-deciduous species in the Seasonal Atlantic Forest (Morellato et al. 2000), and deciduous species in the Caatinga (Machado et al. 1997). As stated before, the highest foliar N concentration (Fig. 2a), and the lowest foliar C:N ratios (Fig. 2b) were observed in these vegetation types. This difference is likely explained by the higher N resorption efficiency in deciduous than in evergreen tree species (Aerts 1996).
Although Moles et al. (2014) showed that on a global level, MAP is a poor predictor of foliar traits, perhaps because MAP is not the best proxy of water availability. We found strong influence of MAP on plot averages δ15N and δ13C values, and to a lesser extent on plot averages foliar N concentration and C:N ratio (Fig. 4).
We found an inverse correlation between foliar δ15N and MAP (Fig. 4c), a commonly found trend (Heaton 1987; Austin and Vitousek 1998; Austin and Sala 1999; Handley et al. 1999; Amundson et al. 2003; Santiago et al. 2004; Houlton et al. 2006; Nardoto et al. 2008; Craine et al. 2009; Nardoto et al. 2014). Higher δ15N would suggest higher N-availability because excess-N not taken up by plants would be prone to volatilization and denitrification, which are highly fractionating processes, leaving an 15N-enriched substrate behind (Högberg 1997; Houlton et al. 2006). Therefore, the observed trend shown in Fig. 4c suggests that N-availability decreases with increasing precipitation (Schuur and Matson 2001; Aranibar et al. 2004; Swap et al. 2004; Pardo et al. 2006).
We also found a weak but significantly negative correlation between MAP and plot average foliar N concentration (Fig. 4a), and a positive weak correlation with foliar C:N ratio (Fig. 4b). These trends would reinforce the hypothesis of lower and higher N-availability in relatively wetter and drier sites, respectively (Austin and Vitousek 1998; Aranibar et al. 2004; Santiago et al. 2004). Additionally, the above trends fit well with the leaf economics spectrum (LES) theory, which states that in wetter areas, longer leaf lifespan, higher leaf mass per area and lower foliar N concentrations are expected (Wright et al. 2004). In turn, in drier sites, plants tend to have higher foliar N concentration as a strategy for maximizing photosynthesis during the shorter growing season (Reich et al. 1997, 1999), such as the Caatinga (Barros et al. 2020).
For tropical and subtropical rainforests, there is enough evidence showing that N availability is lower in wetter areas mainly because soil redox condition decreases decomposition rates of soil detritus, leading to N immobilization by the microorganisms in wetter sites (Schuur and Matson 2001). Nitrification rates also decreased in lowland wetter areas along a soil toposequence in Central Amazon as already mentioned (Luizão et al. 2004). Consequently, with low production of NO3-, chances of 15N-enrichment of the substrate are reduced (Houlton et al. 2006), leading to a soil and plant lower δ15N values in these sites. Houlton et al. (2007), observed that even if NO3- remains in the soil of wetter areas, plants prefer NH4+ which is 15N-depleted compared with this anion. Additionally, N-depleted ecosystems tend to rely strongly on mycorrhizal associations to increase their N content (Michelsen et al. 1996). Ectomycorrhizae is well known for delivering 15N-depleted N to plants (Hobbie et al. 1999), which results in lower δ15N in these N-depleted ecosystems (Hobbie and Högberg 2012). Typical examples of N-poor, and low foliar δ15N vegetation types in Brazil are the Cerrado and Campina (Fig. 2a), where mycorrhizal associations seem to contribute to lower foliar δ15N (Bustamante et al. 2004; Mardegan et al. 2009).
As rainfall decreases, but without causing higher water deficit, mineralization and nitrification rates increase leading to an increase in N-availability to plants and for N-gas losses (Högberg and Johannisson 1993; Austin and Vitousek 1998; Sousa-Neto et al. 2011). The highest foliar δ15N values were observed in the Caatinga, suggesting that N-availability is high in this biome (Freitas et al. 2010a). Certainly, the predominantly eutrophic soil of the Caatinga is one factor that contributes to this (Arruda et al. 2017) since there is a strong correlation between soil fertility and N-availability (Vitousek and Sanford Jr 1986). Although Fabaceae is an important family in Caatinga communities, it seems that most of them are not N-fixing or fix at low rates (Freitas et al. 2010b), which is also an indication of high N availability (Vitousek et al. 2002). On the other hand, Caatinga soil N2O emissions, which are a sensitive indicator of N availability (Davidson et al. 2007), have been reported to be extremely low, similar to the Cerrado (Ribeiro et al. 2016). Alternatively, Freitas et al. (2015), proposed that the high foliar δ15N values in the Caatinga are mostly a consequence of the lag between brief pulses of nitrification-denitrification and low N uptake by plants on the onset of the rainy season.
Precipitation also strongly influenced foliar δ13C (Fig. 4d), in line with several other studies that reported similar trends (Swap et al. 2004; Diefendorf et al. 2010; Kohn 2010; Basu et al. 2019). The most likely explanation for this trend is the decrease in leaf stomatal conductance with reduced water availability, which in turn leads to a decrease of the intercellular pCO2 (pi), if the demand for CO2 is kept higher than the supply of CO2 (Farquhar and Sharkey 1982; Farquhar et al. 1989a, b).
Despite the importance of water availability, we observed that plants in the Cerrado had higher foliar δ13C compared to forests at similar MAP (Fig. 4d). Therefore, in such cases, water availability is not the only factor invoked to explain these variations. The most likely explanation is the so-called canopy effect in tropical forests (Medina and Minchin 1980; Buchmann et al. 1997), which produces 13C-depleted plants and a gradient of foliar δ13C values along the canopy. The reasons behind this are the recycling of 13C-depleted CO2 in the forest (i.e., assimilation of biogenic CO2 derived from forest soil respiration; Van der Merwe and Medina 1991) and the increased fractionation due to photosynthesis taking place under low light conditions. Ometto et al. (2006), assessing four rainforests of the Amazon region, found an average increase of 0.2‰ per meter of canopy height; therefore, in a 30 m-height canopy, foliar δ13C would be increased by 6‰ at the top of the canopy compared to lower height foliage.
Conclusion
The results presented here reinforce earlier findings by Townsend et al. (2008) that high life diversity in the tropics, generated by abundance of water and energy, and enhance by environmental diversity, is followed by high biogeochemistry diversity. Such diversity, however, appears to respond in a synchronized way to changes in precipitation.
Finally, we expect that the database presented here can contribute to a better understanding of the tropics in global models, a fundamental step in helping mitigate effects of land-use and climatic changes that the tropics are facing with global consequences. We also expect that this database can help a broad community of plant ecologists understand regional and global patterns of plant trait variation.
Data availability
The complete data set used in this paper can be found at the following link: https://doi.org/10.17632/38npddpnts.1
Change history
30 December 2020
A Correction to this paper has been published: https://doi.org/10.1007/s10533-020-00735-x
References
Aerts R (1996) Nutrient resorption from senescing leaves of perennials: are there general patterns? J Ecol 84:597–608. https://doi.org/10.2307/2261481
Aerts R, Chapin FSIII (1999) The mineral nutrition of wild plants revisited: a re-evaluation of processes and patterns. Adv Ecol Res 30:1–67. https://doi.org/10.1016/S0065-2504(08)60016-1
Amundson R, Austin AT, Schuur EA, Yoo K, Matzek V, Kendall C, Uebersax A, Brenner D, Baisden WT (2003) Global patterns of the isotopic composition of soil and plant nitrogen. Glob Biogeochem Cycles 17:1031. https://doi.org/10.1029/2002GB001903
Anav A, Friedlingstein P, Beer C, Ciais P, Harper A, Jones C, Murray-Tortarolo G, Papale D, Parazoo NC, Peylin P, Piao S, Sitch S, Viovy N, Wiltshire A, Zhao M (2015) Spatiotemporal patterns of terrestrial gross primary production: a review. Rev Geophys 53:785–818. https://doi.org/10.1002/2015RG000483
Aranibar JN, Otter L, Macko AS, Feral CJW, Epstein HE, Dowty PR, Eckardt F, Shugart HH, Swap RJ (2004) Nitrogen cycling in the soil-plant system along a precipitation gradient in the Kalahari sands. Glob Chang Biol 10:359–373. https://doi.org/10.1046/j.1529-8817.2003.00698.x
Arruda DM, Fernandes-Filho EI, Solar RRC, Schaefer CEGR (2017) Combining climatic and soil properties better predicts covers of Brazilian biomes. Sci Nat 104:32. https://doi.org/10.1007/s00114-017-1456-6
Asner GP, Martin RE (2016) Convergent elevation trends in canopy chemical traits of tropical forests. Glob Chang Biol 22:2216–2227. https://doi.org/10.1111/gcb.13164
Austin AT, Sala OE (1999) Foliar delta15N is negatively correlated with rainfall along the IGBP transect in Australia. Aust J Plant Physiol 26:293–295
Austin AT, Vitousek PM (1998) Nutrient dynamics on a precipitation gradient in Hawaii. Oecologia 113:519–529. https://doi.org/10.1007/s004420050405
Balzotti CS, Asner GP, Taylor PG, Cleveland CC, Cole R, Martin RE, Nasto M, Osborne BB, Porder S, Townsend AR (2016) Environmental controls on canopy foliar nitrogen distributions in a Neotropical lowland forest. Ecol Appl 26:2451–2464. https://doi.org/10.1002/eap.1408
Barros V, Melo A, Santos M, Nogueira L, Frosi G, Santos MG (2020) Different resource-use strategies of invasive and native woody species from a seasonally dry tropical forest under drought stress and recovery. Plant Physiol Biochem 147:181–190. https://doi.org/10.1016/j.plaphy.2019.12.018
Basu S, Ghosh S, Sanyal P (2019) Spatial heterogeneity in the relationship between precipitation and carbon isotopic discrimination in C3 plants: inferences from a global compilation. Glob Planet Chang 176:123–131. https://doi.org/10.1016/j.gloplacha.2019.02.002
Bonilha RM, Casagrande JC, Soares MR, Reis-Duarte RM (2012) Characterization of the soil fertility and root system of restinga forests. R Bras Ci Solo 36:1804–1813. https://doi.org/10.1590/S0100-06832012000600014
Brazil Flora Group (2015) Growing knowledge: an overview of seed plant diversity in Brazil. Rodriguésia 66:1085–1113. https://doi.org/10.1590/2175-7860201566411
Brienen RJW, Phillips OL, Feldpausch TR, Gloor E, Baker TR, Lloyd J, Lopez-Gonzalez G, Monteagudo-Mendoza A, Malhi Y, Lewis SL, Vásquez Martinez R, Alexiades M, Álvarez Dávila E, Alvarez-Loayza P, Andrade A, Aragão LEOC, Araujo-Murakami A, Arets EJMM, Arroyo L, Aymard JAC, Bánki OS, Baraloto C, Barroso J, Bonal D, Boot RGA, Camargo JLC, Castilho CV, Chama V, Chao KJ, Chave J, Comiskey JA, Cornejo Valverde F, da Costa L, de Oliveira EA, Di Fiore A, Erwin TL, Fauset S, Forsthofer M, Galbraith DR, Grahame ES, Groot N, Hérault B, Higuchi N, Honorio Coronado EN, Keeling H, Killeen TJ, Laurance WF, Laurance S, Licona J, Magnussen WE, Marimon BS, Marimon-Junior BH, Mendoza C, Neill DA, Nogueira EM, Núñez P, Pallqui Camacho NC, Parada A, Pardo-Molina G, Peacock J, Peña-Claros M, Pickavance GC, Pitman NCA, Poorter L, Prieto A, Quesada CA, Ramírez F, Ramírez-Angulo H, Restrepo Z, Roopsind A, Rudas A, Salomão RP, Schwarz M, Silva N, Silva-Espejo JE, Silveira M, Stropp J, Talbot J, ter Steege H, Teran-Aguilar J, Terborgh J, Thomas-Caesar R, Toledo M, Torello-Raventos M, Umetsu RK, van der Heijden GMF, van der Hout P, Guimarães Vieira IC, Vieira SA, Vilanova E, Vos VA, Zagt RJ (2015) Long-term decline of the Amazon carbon sink. Nature 519:344–348. https://doi.org/10.1038/nature14283
Buchmann N, Guehl J, Barigah T, Ehleringer JR (1997) Interseasonal comparison of CO2 concentrations, isotopic composition, and carbon dynamics in an Amazonian rainforest (French Guiana). Oecologia 110:120–131. https://doi.org/10.1007/s004420050140
Bustamante MMC, Martinelli LA, Silva DA, Camargo PB, Klink CA, Domingues TF, Santos RV (2004) 15N natural abundance in woody plants and soils of the central brazilian savanas (cerrado). Ecol Appl 14:200–213. https://doi.org/10.1890/01-6013
Climate-Data (2020) Dados climáticos para cidades mundiais. ©Climate-Data.org. https://pt.climate-data.org/. Accessed 20 Apr 2020.
Cornelissen JHC, Lavorel S, Garnier E, Díaz S, Buchmann N, Gurvich DE, Reich PB, Ter Steege H, Morgan HD, Van Der Heijden MGA, Pausas JG, Poorter H (2003) A handbook of protocols for standardised and easy measurement of plant functional traits worldwide. Aust J Bot 51:335–380. https://doi.org/10.1071/BT02124
Cox PM, Betts RA, Jones CD, Spall SA, Totterdell IJ (2000) Acceleration of global warming due to carbon-cycle feedbacks in a coupled climate model. Nature 408:184–187. https://doi.org/10.1038/35041539
Craine JM, Elmore AJ, Aidar MPM, Bustamante M, Dawson TD, Hobbie EA, Kahmen A, Mack MC, McLauchlan KK, Michelsen A, Nardoto GB, Pardo LH, Pefiuelas J, Reich PB, Schuur EAG, Stock WD, Ternpler PH, Virginia RA, Welker JM, Wright IJ (2009) Global patterns of foliar nitrogen isotopes and their relationships with climate, mycorrhizal fungi, foliar nutrient concentrations, and nitrogen availability. New Phytol 183:980–992. https://doi.org/10.1111/j.1469-8137.2009.02917.x
Currie DJ (1991) Energy and large-scale patterns of animal- and plant speciesrichness. Am Nat 137:27–49
Currie DJ, Paquin V (1987) Large-scale biogeographical patterns of species richness of tree. Nature 329:326–327. https://doi.org/10.1038/329326a0
Davidson EA, Carvalho CJR, Figueira AM, Ishida FY, Ometto JPHB, Nardoto GB, Sabá RT, Hayashi SN, Leal EC, Vieira ICG, Martinelli LA (2007) Recuperation of nitrogen cycling in Amazonian forests following agricultural abandonment. Nature 447:995–999. https://doi.org/10.1038/nature05900
DeFries RS, Foley JA, Asner GP (2004) Land-use choices: balancing human needs and ecosystem function. Front Ecol Environ 2:249–257. https://doi.org/10.1890/1540-9295(2004)002[0249:LCBHNA]2.0.CO;2
Díaz S, Cabido M (2001) Vive la différence: plant functional diversity matters to ecosystem processes. Trends Ecol Evol 16:646–655. https://doi.org/10.1016/S0169-5347(01)02283-2
Diefendorf AF, Mueller KE, Wing SL, Koch PL, Freeman KH (2010) Global patterns in leaf 13C discrimination and implications for studies of past and future climate. Proc Natl Acad Sci USA 107(13):5738–5743. https://doi.org/10.1073/pnas.0910513107
Domingues TF, Ometto JPHB, Nepstad DC, Brando PM, Martinelli LA, Ehleringer JR (2018) Ecophysiological plasticity of Amazonian trees to long-term drought. Oecologia 187:933–940. https://doi.org/10.1007/s00442-018-4195-2
Duvert C, Hutley LB, Beringer J, Bird MI, Birkel C, Maher DT, Northwood M, Rudge M, Setterfield SA, Wynn JG (2020) Net landscape carbon balance of a tropical savanna: relative importance of fire and aquatic export in offsetting terrestrial production. Glob Chang Biol 26:5899–5913. https://doi.org/10.1111/gcb.15287
Ehleringer JR, Hall AE, Farquhar GD (1993) Stable isotopes and plant carbon: water relations. Academic Press, London
Eiten G (1972) The Cerrado vegetation of Brazil. Bot Rev 38:201–341. https://doi.org/10.1007/BF02859158
Evans TL, Costa M, Tomas WM, Camilo AR (2014) Large-scale habitat mapping of the Brazilian Pantanal wetland: a synthetic aperture radar approach. Remote Sens Environ 155:89–108. https://doi.org/10.1016/j.rse.2013.08.051
Farquhar GD, Sharkey TD (1982) Stomatal conductance and photosynthesis. Annu Rev Plant Physiol 33:317–345. https://doi.org/10.1146/annurev.pp.33.060182.001533
Farquhar GD, Ehleringer JR, Hubick KT (1989a) Carbon isotope discrimination and photosynthesis. Annu Rev Plant Phyiol 40:503–537. https://doi.org/10.1146/annurev.pp.40.060189.002443
Farquhar GD, Hubick KT, Condon AG, Richards RA (1989b) Carbon isotope fractionation and plant water-use efficiency. In: Rundel PW, Ehleringer JR, Nagy KA (eds) Stable isotopes in ecological research. Springer, New York, pp 21–40
Field R, O’Brien EM, Whittaker RJ (2005) Global models for prediction woody plant richness from climate: development and evaluation. Ecology 86:2263–2277. https://doi.org/10.1890/04-1910
Freitas ADS, Sampaio EVSB, Menezes RSC, Tiessen H (2010a) 15N natural abundance of non-fixing woody species in the Brazilian dry forest (caatinga). Isot Environ Healt S 56:210–218. https://doi.org/10.1080/10256016.2010.488805
Freitas ADS, Sampaio EVSB, Santos CERS, Fernandes AR (2010b) Biological nitrogen fixation in tree legumes of the Brazilian semi-arid caatinga. J Arid Environ 74:344–349. https://doi.org/10.1016/j.jaridenv.2009.09.018
Freitas ADS, Sampaio EVSB, Ramos APS, Barbosa MRV, Lyra RP, Araújo EL (2015) Nitrogen isotopic patterns in tropical forests along a rainfall gradient in Northeast Brazil. Plant Soil 391:109–122. https://doi.org/10.1007/s11104-015-2417-5
Friedlingstein P, Cox P, Betts R, Bopp L, von Bloh W, Brovkin V, Cadule P, Doney S, Eby M, Fung I, Bala G, John J, Jones C, Joos F, Kato T, Kawamiya M, Knorr W, Lindsay K, Matthews HD, Raddatz T, Rayner P, Reich C, Roeckner E, Schnitzler KG, Schnur R, Strassmann K, Weaver AJ, Yoshikawa C, Zeng N (2006) Climate Carbon cycle feedback analysis: results from the C4MIP model intercomparison. J Clim 19:3337–3353. https://doi.org/10.1175/JCLI3800.1
Friedlingstein P, Jones MW, O’Sullivan M, Andrew RM, Hauck J, Peters GP, Peters W, Pongratz J, Sitch S, Quéré CL, Bakker DCE, Canadell JG, Ciais P, Jackson RB, Anthoni P, Barbero L, Bastos A, Bastrikov V, Becker M, Bopp L, Buitenhuis E, Chandra N, Chevallier F, Chini LP, Currie KI, Feely RA, Gehlen M, Gilfillan D, Gkritzalis T, Goll DS, Gruber N, Gutekunst S, Harris I, Haverd V, Houghton RA, Hurtt G, Ilyina T, Jain AK, Joetzjer E, Kaplan JO, Kato E, Goldewijk KK, Korsbakken JI, Landschützer P, Lauvset SK, Lefèvre N, Lenton A, Lienert S, Lombardozzi D, Marland G, McGuire PC, Melton JR, Metz N, Munro DR, Nabel JEMS, Nakaoka SI, Neill C, Omar AM, Ono T, Peregon A, Pierrot D, Poulter B, Rehder G, Resplandy L, Robertson E, Rödenbeck C, Séférian R, Schwinger J, Smith N, Tans PP, Tian H, Tilbrook B, Tubiello FN, van der Werf GR, Wiltshire AJ, Zaehle S (2019) Global carbon budged 2019. Earth Syst Sci Data 11:1783–1838. https://doi.org/10.5194/essd-11-1783-2019
Furian S, Barbiéro L, Boulet R (1999) Organisation of the soil mantle in tropical southeastern Brazil (Serra do Mar) in relation to landslides processes. Catena 38:65–83. https://doi.org/10.1016/S0341-8162(99)00015-6
Fyllas NM, Patiño S, Baker TR, Nardoto GB, Martinelli LA, Quesada CA, Paiva R, Schwarz M, Horna V, Mercado LM, Santos A, Arroyo L, Jiménez EM, Luizão FJ, Neill DA, Silva N, Prieto A, Rudas A, Silveira M, Vieira ICG, Lopez-Gonzalez G, Malhi Y, Phillips OL, Lloyd J (2009) Basin-wide variations in foliar properties of Amazonian forest: phylogeny, soils and climate. Biogeosciences 6:2677–2708. https://doi.org/10.5194/bg-6-2677-2009
Han W, Fang J, Guo D, Zhang Y (2005) Leaf nitrogen and phosphorus stoichiometry across 753 terrestrial plant species in China. New Phytol 168:377–385. https://doi.org/10.1111/j.1469-8137.2005.01530.x
Handley LL, Austin AT, Robinson D, Scrimgeour CM, Raven JA, Heaton THE, Schmidt S, Stewart GR (1999) The 15N natural abundance (δ15N) of ecosystem samples reflects measures of water availability. Aust J Plant Physiol 26:185–199. https://doi.org/10.1071/PP98146
Heaton TH (1987) The 15N/14N ratios of plants in South Africa and Namibia: relationship to climate and coastal/saline environments. Oecologia 74:236–246. https://doi.org/10.1007/BF00379365
Hilton RG, Galy A, West AJ, Hovius N, Roberts GG (2013) Geomorphic control on the δ15N of mountain forests. Biogeosciences 10:1693–1705. https://doi.org/10.5194/bg-10-1693-2013
Hobbie EA, Högberg P (2012) Nitrogen isotopes link mycorrhizal fungi and plants to nitrogen dynamics. New Phytol 196:367–382. https://doi.org/10.1111/j.14698137.2012.04300.x
Hobbie EA, Macko SA, Shugart HH (1999) Insights into nitrogen and carbon dynamics of ectomycorrhizal and saprotrophic fungi from isotopic evidence. Oecologia 118:353–360. https://doi.org/10.1007/s004420050736
Högberg P (1997) Transley review no. 95 15N natural abundance in soil-plant systems. New Phytol 137:179–203. https://doi.org/10.1046/j.1469-8137.1997.00808.x
Högberg P, Johannisson C (1993) 15N abundance of forests is correlated with losses of nitrogen. Plant Soil 157:147–150. https://doi.org/10.1007/BF02390237
Houlton BZ, Sigman DM, Hedin LO (2006) Isotopic evidence for large gaseous nitrogen losses from tropical rainforests. Proc Natl Acad Sci USA 103:8745–8750. https://doi.org/10.1073/pnas.0510185103
Houlton BZ, Sigman DM, Schuur EAG, Hedin LO (2007) A climate-driven switch in plant nitrogen acquisition within tropical forest communities. Proc Natl Acad Sci USA 104:8902–8906. https://doi.org/10.1073/pnas.0609935104
Huntingford C, Zelazowski P, Galbraith D, Mercado LM, Sitch S, Fisher R, Lomas M, Walker AP, Jones CD, Booth BBB, Malhi Y, Hemming D, Kay G, Good P, Lewis SL, Phillips OL, Atkin OK, Lloyd J, Gloor E, Zaragoza-Castells J, Meir P, Betts R, Harris PP, Nobre C, Marengo J, Cox PM (2013) Simulated resilience of tropical rainforests to CO2-induced climate change. Nat Geosci 6:268–273. https://doi.org/10.1038/ngeo1741
IBGE – Instituto Brasileiro de Geografia e Estatística (2012) Manual técnico da vegetação brasileira. IBGE, Rio de Janeiro
Joly CA, Assis MA, Bernacci LC, Tamashiro JY, Campos MCR, Gomes JAMA, Lacerda MS, Santos FAM, Pedroni F, Pereira LS, Padgurschi MCG, Prata BEM, Ramos E, Torres RB, Rochelle A, Martins FR, Alvez LF, Vieira SA, Martinelli LA, Camargo PB, Aidar MPM, Eisenlohr PV, Simões E, Villani JP, Belinello R (2012) Floristic and phytosociology in permanent plots of the Atlantic Rainforest along an altitudinal gradiente in southeastern Brazil. Biota Neotrop 12. https://doi.org/10.1590/S1676-06032012000100012
Joly CA, Padgurschi MCG, Pires APF, Agostinho AA, Marques AC, Amaral AG, Cervone COFO, Adams C, Baccaro FB, Sparovek G, Overbeck GE, Espindola GM, Vieira ICG, Metzger JP, Sabino J, Farinaci JS, Queiroz LP, Gomes LC, da Cunha MMC, Piedade MTF, Bustamante MMC, May P, Fearnside P, Prado RB, Loyola RD (2019) Apresentando o Diagnóstico Brasileiro de Biodiversidade e Serviços Ecossistêmicos. In: Joly CA, Scarano FR, Seixas CS, Metzger JP, Ometto JP, Bustamante MMC, Padgurschi MCG, Pires APF, Castro PFD, Gadda T, Toledo P (eds.) 1° Diagnóstico Brasileiro de Biodiversidade e Serviços Ecossistêmicos, Editora Cubo, São Carlos, pp 351. . https://doi.org/10.4322/978-85-60064-88-5
Kattge J, Díaz S, Lavorel S, Prentice IC, Leadley P, Bönisch G, Garnier E, Westoby M, Reich PB, Wright IJ, Cornelissen JHC, Violle C, Harrison SP, van Bodegom PM, Reichstein M, Enquist BJ, Soudzilovskaia NA, Ackerly DD, Anand M, Atkin O, Bahn M, Baker TR, Baldocchi D, Bekker R, Blanco CC, Blonder B, Bond WJ, Bradstock R, Bunker DE, Casanove F, Cavender-Bares J, Chambers JQ, Chapin FD, Chave J, Coomes D, Cornwell CJM, Dobrin BH, Duarte L, Durka W, Elser J, Esser G, Estiarte M, Fagan WF, Fang J, Fernández Mendez F, Fidelis A, Finegan B, Flores O, Ford H, Frank D, Freschet GT, Fyllas NM, Gallagher RV, Green WA, Gutierrez AG, Hickler T, Higgins SI, Hodgson JG, Jalili A, Jansen S, Joly CA, Kerkhoff AJ, Kirkup D, Kitajima K, Kleyer M, Klotz S, Knops JMH, Kramer K, Kühn I, Kurokawa H, Laughlin D, Lee TD, Leishman M, Lens F, Lenz T, Lewis SL, Lloyd J, Llusià J, Louault F, Ma S, Mahecha MD, Manning P, Massad T, Medlyn BE, Messier J, Moles AT, Müller SC, Nadrowski K, Naeem S, Niinemets Ü, Nöllert S, Nüske A, Ogaya R, Oleksyn J, Onipchenko VG, Onoda Y, Ordoñez J, Overbeck G, Ozinga WA, Patiño S, Paula S, Pausas JG, Peñuelas J, Phillips OL, Pillar V, Poorter H, Poorter L, Poschlod P, Prinzing A, Proulx R, Rammig A, Reinsch S, Reu B, Sack L, Salgado-Negret B, Sardans J, Shiodera S, Shipley B, Siefert A, Sosinski E, Soussana JF, Swaine E, Swenson N, Thompson K, Thorton P, Waldram M, Weiher E, White M, White S, Wright SJ, Yguel B, Zaehle S, Zanne AE, Wirth C (2011) Try – a global database of plant traits. Glob Chang Biol 17:2905–2935. https://doi.org/10.1111/j.1365-2486.2011.02451.x
Kohn MJ (2010) Carbon isotope compositions of terrestrial C3 plants as indicators of (paleo)ecology and (paleo)climate. Proc Natl Acad Sci USA 107:19691–19695. https://doi.org/10.1073/pnas.1004933107
Kreft H, Jetz W (2007) Global patterns and determinants of vascular plant diversity. Proc Natl Acad Sci USA 104:5925–5930. https://doi.org/10.1073/pnas.0608361104
Lenton TM, Held H, Kriegler E, Hall JW, Lucht W, Rahmstorf S, Schellnhuber HJ (2008) Tipping elements in the Earth’s climate system. Proc Natl Acad Sci USA 105:1786–1793. https://doi.org/10.1073/pnas.0705414105
Lins SRM, Coletta LD, Ravagnani EC, Gragnani JG, Mazzi EA, Martinelli LA (2016) Stable carbon composition of vegetation and soils across an altitudinal range in the coastal Atlantic Forest of Brazil. Trees 30:1315–1329. https://doi.org/10.1007/s00468-016-1368-7
Lovejoy TE, Nobre C (2019) Amazon tipping point: last chance for action. Sci Adv 5:eaba2949. https://doi.org/10.1126/sciadv.aba2949
Luizão RCC, Luizão FJ, Paiva RQ, Monteiro TF, Sousa LS, Kruijt B (2004) Variation of carbon and nitrogen cycling processes along a topographic gradient in a central Amazonian forest. Glob Chang Biol 10:592–600. https://doi.org/10.1111/j.1529-8817.2003.00757.x
Machado ICS, Barros LM, Sampaio EVSB (1997) Phenology of Caatinga species at Serra Talhada, PE. Biotropica 29:57–68
Mardegan SF, Nardoto GB, Higuchi N, Moreira MZ, Martinelli LA (2009) Nitrogen availability patterns in white-sand vegetations of Central Brazilian Amazon. Trees 23:479–488. https://doi.org/10.1007/s00468-008-0293-9
Martins SC, Neto ES, Piccolo MC, Almeida DQA, Camargo PB, Carmo JB, Porder S, Lins SRM, Martinelli LA (2015) Soil texture and chemical characteristics along an elevation range in the coastal Atlantic forest of Southeast Brazil. Geoderma Reg 5:106–116. https://doi.org/10.1016/j.geodrs.2015.04.005
Medina E, Minchin P (1980) Stratification of δ13C values of leaves in Amazonian rain forests. Oecologia 45:377–378. https://doi.org/10.1007/BF00540209
Mendonça BAF, Filho EIF, Schaefer CEGR, Mendonça JGF, Vasconcelos BNF (2017) Soil-vegetation relationships and community structure in a “terra-firme”-white-sand vegetation gradient in Viruá National Park, northern Amazon, Brazil. An Acad Bras Cienc 89:1269–1293. https://doi.org/10.1590/0001-3765201720160666
Miatto RC, Wright IJ, Batalha MA (2016) Relationships between soil nutrient status and nutrient-related leaf traits in Brazilian cerrado and seasonal forest communities. Plant Soil 404:13–33. https://doi.org/10.1007/s11104-016-2796-2
Michelsen A, Schmidt IK, Jonasson S, Quarmby C, Sleep D (1996) Leaf 15N abundance of subartic plants provides field evidence that ericoid, ectomycorrhizal and non-and arbuscular mycorrhizal species access different sources of soil nitrogen. Oecologia 105:53–63. https://doi.org/10.1007/BF00328791
Moles AT, Perkins SE, Laffan SW, Flores-Moreno H, Awasthy M, Tindall ML, Sack L, Pitman A, Kattge J, Aarssen LW, Anand M, Bahn M, Blonder B, Cavender-Bares J, Cornelissen JHC, Cornwell WK, Díaz S, Dickie JB, Freschet GT, Griffiths JG, Gutierrez AG, Hemmings FA, Hickler T, Hitchcock TD, Keighery M, Kleyer M, Kurokawa H, Leishman MR, Liu K, Niinemets Ü, Onipchenko V, Onada Y, Penuelas J, Pillar VD, Reich PB, Shiodera S, Siefert A, Sosinski EE Jr, Soudzilovskaia NA, Swaine EK, Swenson NG, van Bodegom PM, Warman L, Weiher E, Wright IJ, Zhang H, Zobel M, Bonser SP (2014) Which is a better predictor of plant traits: temperature of precipitation? J Veg Sci 25:1167–1180. https://doi.org/10.1111/jvs.12190
Moreira FMS, Silva MF, Miana FS (1992) Occurrence of nodulation in legume species in the Amazon region of Brazil. New Phytol 121:563–570. https://doi.org/10.1111/j.1469-8137.1992.tb01126.x
Morellato LPC, Haddad CFB (2000) Introduction: the Brazilian Atlantic Forest. Biotropica 32:786–792. https://doi.org/10.1111/j.1744-7429.2000.tb00618.x
Morellato LPC, Talora DC, Takahasi A, Bencke CC, Romera EC, Zipparro VB (2000) Phenology of Atlantic rain forest trees: a comparative study. Biotropica 32:811–823. https://doi.org/10.1111/j.1744-7429.2000.tb00620.x
Nardoto GB, Ometto JPHB, Ehleringer JR, Higuchi N, Bustamante MMC, Martinelli LA (2008) Understanding the influences of spatial patterns on N availability within the Brazilian Amazon Forest. Ecosystems 11:1234–1256. https://doi.org/10.1007/s10021-008-9189-1
Nardoto GB, Quesada CA, Patiño S, Saiz G, Baker TR, Schwarz M, Schrodt F, Feldpausch TR, Domingues TF, Marimon BS, Marimon Junior BH, Vieira ICG, Silveira M, Bird MI, Phillips OL, Lloyd J, Martinelli LA (2014) Basin-wide variations in Amazon forest nitrogen cycling characteristics as inferred from plant and soil 15N:14N measurements. Plant Ecol Divers 7:173–187. https://doi.org/10.1080/17550874.2013.807524
Oliveira GC, Francelino MR, Arruda DM, Fernandes-Filho EI, Schaefer CEGR (2019) Climate and soils at the Brazilian semiarid and the forest-Caatinga problem: new insights and implications for conservation. Environ Res Lett 14:104007. https://doi.org/10.1088/1748-9326/ab3d7b
Ometto JPHB, Ehleringer JR, Domingues TF, Berry JA, Ishida FY, Mazzi E, Higuchi N, Flanagan LB, Nardoto GB, Martinelli LA (2006) The stable carbon and nitrogen isotopic composition of vegetation in tropical forests of the Amazon Basin, Brazil. Biogeochemistry 79:251–274. https://doi.org/10.1007/s10533-006-9008-8
Overbeck GE, Müller SC, Fidelis A, Pfadenhauer J, Pillar VD, Blanco CC, Boldrini I, Both R, Forneck ED (2007) Brazil’s neglected biome: the South Brazilian Campos. Perspect Plant Ecol 9:101–116. https://doi.org/10.1016/j.ppees.2007.07.005
Pardo LH, Templer PH, Goodale CL, Duke S, Groffman PM, Adams MB, Boeckx P, Boggs J, Campbell J, Colman B, Compton J, Emmett B, Gundersen P, Kjønaas J, Lovett G, Mack M, Magill A, Mbila M, Mitchell MJ, McGee G, McNulty S, Nadelhoffer K, Ollinger S, Ross D, Rueth H, Rustad L, Schaberg P, Schiff S, Schleppi P, Spoelstra J, Wessel W (2006) Regional assessment of N saturation using foliar and root δ15N. Biogeochemistry 80:143–171. https://doi.org/10.1007/s10533-006-9015-9
Pereira EJAL, Ferreira PJS, Ribeiro LCS, Carvalho TS, Pereira HBB (2019) Policy in Brazil (2016-2019) threaten conservation of the Amazon rainforest. Environ Sci Policy 100:8–12. https://doi.org/10.1016/j.envsci.2019.06.001
Porder S, Hilley GE (2011) Linking chronosequences with the rest of the world: predicting soil phosphorus content in denuding landscapes. Biogeochemistry 102:153–166. https://doi.org/10.1007/s10533-010-9428-3
Porder S, Asner GP, Vitousek PM (2005) Ground-based and remotely sensed nutrient availability across a tropical landscape. P Natl Acad Sci USA 102:10909–10912. https://doi.org/10.1073/pnas.0504929102
Pugnaire FI, Morillo JA, Peñuelas J, Reich PB, Bardgett RD, Gaxiola A, Wardle DA, van der Putten WH (2019) Climate change effects on plant-soil feedbacks and consequences for biodiversity and functioning of terrestrial ecosystems. Sci Adv 5:eaaz1834. https://doi.org/10.1126/sciadv.aaz1834
Reich PB, Walters MB, Ellsworth DS (1997) From tropics to tundra: global convergence in plant functioning. Proc Natl Acad Sci USA 94:13730–13734. https://doi.org/10.1073/pnas.94.25.13730
Reich PB, Ellsworth DS, Walters MB, Vose JM, Gresham C, Volin JC, Bowman WD (1999) Generality of leaf trait relationships: a test across six biomes. Ecology 80:1955–1969. https://doi.org/10.1890/0012-9658(1999)080[1955:GOLTRA]2.0.CO;2
Ribeiro EMS, Arroyo-Rodríguez V, Santos BA, Tabarelli M, Leal IR (2015) Chronic anthropogenic disturbance drives the biological impoverishment of the Brazilian Caatinga vegetation. J Appl Ecol 52:611–620. https://doi.org/10.1111/1365-2664.12420
Ribeiro K, Sousa-Neto ER, Carvalho Junior JÁ, Lima JRS, Menezes RSC, Duarte-Neto PJ, Guerra GS, Ometto JPHB (2016) Land cover changes and greenhouse gas emissions in two different soil covers in the Brazilian Caatinga. Sci Total Environ 571:1048–1057. https://doi.org/10.1016/j.scitotenv.2016.07.095
Richter DD, Babbar LI (1991) Soil diversity in the tropics. Adv Ecol Res 21:315–389. https://doi.org/10.1016/S0065-2504(08)60100-2
Rödig E, Cuntz M, Ramming A, Fischer R, Taubert F, Huth A (2018) The importance of forest structure for carbon fluxes of the Amazon rainforest. Environ Res Lett 13:054013. https://doi.org/10.1088/1748-9326/aabc61
Sanchez PA, Buol SW (1974) Soils of the tropics and the world food crisis. Science 188:598–603. https://doi.org/10.1126/science.188.4188.598
Sanchez PA, Logan TJ (1992) Myths and science about the chemistry and fertility of soils in the tropics. In: Lal R, Sanchez PA (eds) Myths and science of soil of the tropics, SSSA special publications no. 29. Soil Science Society of America/American Society of Agronomy, Madison, pp 35–46. https://doi.org/10.2136/sssaspecpub29.c3
Sano EE, Rosa R, Brito JLS, Ferreira LG (2010) Land cover mapping of the tropical savanna region in Brazil. Environ Monit Assess 166:113–124. https://doi.org/10.1007/s10661-009-0988-4
Santiago LS, Kitajima K, Wright SJ, Mulkey SS (2004) Coordinated changes in photosynthesis, water relations and leaf nutritional traits of canopy trees along a precipitation gradient in lowland tropical forest. Oecologia 139:495–502. https://doi.org/10.1007/s00442-004-1542-2
Sardans J, Alonso R, Carnicer J, Fernández-Martínez M, Vivanco MG, Peñuelas J (2016) Factors influencing the foliar elemental composition and stoichiometry in forest trees in Spain. Perspect Plant Ecol 18:52–69. https://doi.org/10.1016/j.ppees.2016.01.001
Schimel D (1995) Terrestrial ecosystems and the carbon cycle. Glob Chang Biol 1:77–91. https://doi.org/10.1111/j.1365-2486.1995.tb00008.x
Schimel D, Stephens BB, Fisher JB (2015a) Effect of increasing CO2 on the terrestrial carbon cycle. Proc Natl Acad Sci USA 112:436–441. https://doi.org/10.1073/pnas.1407302112
Schimel D, Pavlick R, Fisher JB, Asner GP, Saatchi S, Townsend P, Miller C, Frankenber C, Hibbard K, Cox P (2015b) Observing terrestrial ecosystems and the carbon cycle from space. Glob Chang Biol 21:1762–1776. https://doi.org/10.1111/gcb.12822
Schuur EAG, Matson PA (2001) Net primary productivity and nutrient cycling across a mesic to wet precipitation gradient in Hawaiian montane forest. Oecologia 128:431–422. https://doi.org/10.1007/s004420100671
Sousa-Neto E, Carmo JB, Keller M, Martins SC, Alves LF, Vieira SA, Piccolo MC, Camargo PB, Couto HTZ, Joly CA, Martinelli LA (2011) Soil-atmosphere exchange of nitrous oxide, methane and carbon dioxide in a gradient of elevation in the coastal Brazilian Atlantic forest. Biogeosciences 8:733–742. https://doi.org/10.5194/bg-8-733-2011
Souza LQ, Freitas ADS, Sampaio EVSB, Moura PM, Menezes RSC (2012) How much nitrogen is fixed by biological symbiosis in tropical dry forests? 1. Trees and shrubs. Nutr Cycl Agroecosyst 2:171–179. https://doi.org/10.1007/s10705-012-9531-z
Souza CM, Shimbo JZ, Rosa MR, Parente LL, Alencar AA, Rudorff BFT, Hasenack H, Matsumoto M, Ferreira LG, Souza-Filho PWM, de Oliveira SW, Rocha WF, Fonseca AV, Marques CB, Diniz CG, Costa D, Monteiro D, Rosa ER, Vélez-Martin E, Weber EJ, Lenti FEB, Paternost FF, Pareyn FGC, Siqueira JV, Vieira JL, Neto LCF, Saraiva MM, Sales MH, Salgado MPG, Vasconcelos R, Galano S, Mesquita VV, Azevedo T (2020) Reconstructing three decades of lande use and landa cover changes in Brazilian Biomes with Landsat archive and Earth engine. Remote Sens 12:2735. https://doi.org/10.3390/rs12172735
Sprent JI, Geoghegan IE, Whitty PW, James EK (1996) Natural abundance of 15N and 13C in nodulated legumes and other plants in the cerrado and neighbouring regions of Brazil. Oecologia 105:440–446. https://doi.org/10.1007/BF00330006
Stein A, Gerstner K, Kreft H (2014) Environmental heterogeneity as a universal driver os species richness across taxa, biomes and spatial scales. Ecol Lett 14:866–880. https://doi.org/10.1111/ele.12277
Swap RJ, Aranibar JN, Dowty PR, Gilhooly IIIWP, Macko SA (2004) Natural abundance of 13C and 15N in C3 and C4 vegetation of southern Africa: patterns and implications. Glob Chang Biol 10:350–358. https://doi.org/10.1046/j.1529-8817.2003.00702.x
Tabarelli M, Silva JMC, Gascon C (2004) Forest fragmentation, synergisms and the impoverishment of neotropical forests. Biodivers Conserv 13:1419–1425. https://doi.org/10.1023/B:BIOC.0000019398.36045.1b
Tiessen H, Chacon P, Cuevas E (1994) Phosphorus and nitrogen status in soils and vegetation along a toposequence of dystrophic rainforests on the Upper Rio Negro. Oecologia 99:145–150. https://doi.org/10.1007/BF00317095
Townsend AR, Cleveland CC, Asner GP, Bustamante MMC (2007) Controls over foliar N:P ratios in tropical rain forests. Ecology 88:107–118. https://doi.org/10.1890/00129658(2007)88[107:COFNRI]2.0.CO;2
Townsend AR, Asner GP, Cleveland CC (2008) The biogeochemical heterogeneity of tropical forests. Trends Ecol Evol 23:424–431. https://doi.org/10.1016/j.tree.2008.04.009
Van der Merwe NJ, Medina E (1991) The canopy effect, carbon isotope ratios and foodwebs in Amazonia. J Archaeol Sci 18:249–259. https://doi.org/10.1016/0305-4403(91)90064-V
Van der Werf GR, Randerson JT, Giglio L, van Leeuwen TT, Chen Y, Rogers BM, Mu M, van Marle MJR, Morton DC, Collatz GJ, Yokelson RJ, Kasibhatla PS (2017) Global fire emissions estimates during 1997–2016. Earth Syst Sci Data 9:697–720. https://doi.org/10.5194/essd-9-697-2017
Vitousek PM, Sanford RL Jr (1986) Nutrient cycling in moist tropical forest. Annu Rev Ecol Syst 17:137–167. https://doi.org/10.1146/annurev.es.17.110186.001033
Vitousek PM, Cassman K, Cleveland C, Crews T, Field CB, Grimm NB, Howarth RW, Marino R, Martinelli L, Rastetter EB, Sprent JI (2002) Towards an ecological understanding of biological nitrogen fixation. Biogeochemistry 57:1–54. https://doi.org/10.1023/A:1015798428743
Weintraub SR, Taylor PG, Porder S, Cleveland CC, Asner GP, Townsend AR (2015) Topographic controls on soil nitrogen availability in a lowland tropical forest. Ecology 96:1561–1574. https://doi.org/10.1890/14-0834.1
Wright IJ, Reich PB, Westoby M, Ackerly DD, Baruch Z, Bongers F, Cavender-Bares J, Chapin T, Cornelissen JHC, Diemer M, Flexas J, Garnier E, Groom PK, Gulias J, Hikosaka K, Lamont BB, Lee T, Lee W, Lusk C, Midgley JJ, Navas ML, Niinemets Ü, Oleksyn J, Osada N, Poorter H, Poot P, Prior L, Pyankov VI, Roumet C, Thomas SC, Tjoelker MG, Veneklaas EJ, Villar R (2004) The worldwide leaf economics spectrum. Nature 428:821–827. https://doi.org/10.1038/nature02403
Yue C, Ciais P, Houghton RA, Nassikas AA (2020) Contribution of land use to the interannual variability of the land carbon cycle. Nat Commun 11:3170. https://doi.org/10.1038/s41467-020-16953-8
Zhang J, He N, Liu C, Xu L, Chen Z, Li Y, Wang R, Yu G, Sun W, Xiao C, Chen HYH, Reich PB (2020) Variation and evolution of C:N ratio among different organs enable plants to adapt to N-limited environments. Glob Chang Biol 26:2534–2543. https://doi.org/10.1111/gcb.14973
Zhao N, Yu G, He N, Wang Q, Guo D, Zhang X, Wang R, Xu Z, Jiao C, Li N, Jia Y (2016) Coordinated pattern of multi-element variability in leaves and roots across Chinese forest biomes. Glob Ecol Biogeogr 25:359–367. https://doi.org/10.1111/geb.12427
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Responsible Editor: Robert W. Howarth.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original version of this article was revised: The initial online publication contained typesetting mistakes in the author information. The original article has been corrected.
This paper is an invited contribution to the 35th Anniversary Special Issue, edited by Sujay Kaushal, Robert Howarth, and Kate Lajtha.
The initial online publication contained typesetting mistakes in the author information. The original article has been corrected.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Martinelli, L.A., Nardoto, G.B., Soltangheisi, A. et al. Determining ecosystem functioning in Brazilian biomes through foliar carbon and nitrogen concentrations and stable isotope ratios. Biogeochemistry 154, 405–423 (2021). https://doi.org/10.1007/s10533-020-00714-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10533-020-00714-2