Abstract
Cryptosporidium is one of the most important parasitic protozoa of concern within the food production industry, worldwide. This review describes the evolution and its development, and it monitors the methodology that has been used for Cryptosporidium in food material since 1984, when the first publication appeared regarding the detection of Cryptosporidium parvum in food materials. The methods that are currently being used for the detection of Cryptosporidium oocysts in food material (mainly vegetables) and all of the other available published methods are discussed in this review. Generating more consistent and reliable data should lead to a better understanding of the occurrence, transport and fate of the oocysts in food material. Improvements in monitoring and developing effective methodology, along with food security, offer more practical possibilities for both the developed and developing worlds.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Foodborne outbreaks of Cryptosporidium
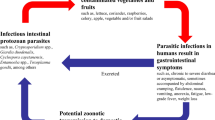
Cryptosporidium is a protozoan parasite genus in which its species can infect humans and animals. Humans can become infected via the faecal-oral route, via direct or indirect contact with animals, or via person-person contact. Infection via the faecal-oral route occurs through the immediate ingestion of infectious oocysts (excreted in faeces) that contaminate food or water (Hancock-Allen et al. 2017). Cryptosporidium spp. is considered to be one of the most important foodborne parasites. It is responsible for several well-documented foodborne outbreaks (Table 1), as well as waterborne outbreaks (Baldursson and Karanis 2011; Efstratiou et al. 2017; Karanis et al. 2007a; Mahon and Doyle 2017; Widerström et al. 2014) and occupational outbreaks (CDC 2014; Hancock-allen et al. 2017).
The shift of consumer’s dietary habits towards fresh produce is associated with the occurrence of cryptosporidial foodborne outbreaks (Macarisin et al. 2010a). Cold-pressed non-alcoholic apple cider, ozonated apple cider, milk, béarnaise sauce, raw meat, raw liver, chicken salad, pre-cut mixed salad leaves (bagged salad) and frisée salad are all reported as the main causes of foodborne Cryptosporidium outbreaks. Outbreaks are usually associated with food service and catering industries. Fresh produce that is consumed after minimal preparation is highly likely to be a vehicle for the transmission of the Cryptosporidium infection (Amorós et al. 2010; Cook et al. 2007). Other food matrices (specifically fruit juice, molluscs and sprouted seeds) have also been reported as vehicles for the transmission of this protozoan parasite (Iqbal et al. 2015; Marquis et al. 2015; Mosteo et al. 2016; Robertson 2014; Robertson and Gjerde 2001a; Tei et al. 2016).
Cryptosporidium spp. are well-known causative agents in gastrointestinal disease in humans (particularly children under 2 years old) and in animals worldwide (Bouzid et al. 2013; Checkley et al. 2015; Feng and Xiao 2011). The fact that this parasite will most likely not be destroyed by chemicals or sanitizing treatments suggests its ubiquitous distribution (Dixon et al. 2013; Fletcher et al. 2012; Karanis et al. 2007a). Scientists are motivated to focus on the development of the detection methods that are currently being used for Cryptosporidium oocysts as they are small in size, they have a low infective dose, they can be distributed differently according to climatic changes, and they have the ability to cause waterborne and foodborne outbreaks (Efstratiou et al. 2017; Baldursson and Karanis 2011; Karanis et al. 2007a; Table 1) (Efstratiou et al. 2017; Kotloff 2017; Rosado-García et al. 2017; Squire and Ryan 2017).
It has been proposed that there are three ways for Cryptosporidium to enter the food production process:
-
Food contamination at farms as Cryptosporidium oocysts can be found in soil.
-
Exposure of food to water that has been contaminated (by humans or animals) with faeces (from a surface or from the ground, at any stage in processing).
-
Transfer through infected food handlers (Amorós et al. 2010; Dawson 2005).
The direct diagnosis of Cryptosporidium in a stained stool sample preparation is a general diagnostic method that is used in clinical laboratories, however, in food its isolation requires a more complex procedure which could indicate unequal distributions of the parasite (a small number of organisms relative to the amount of the interfering material).
Elution, extraction, isolation and detection of Cryptosporidium species in or on items of food are not an easy job; the differences in the properties of the various food matrices could be the main cause of this (Lalonde and Gajadhar 2016a). Cryptosporidium will therefore most likely remain an underdiagnosed food-related problem.
Despite the above, the ISO method ‘immunomagnetic separation technique (IMS) and immunofluorescent assay (IFA)’, being the gold standard for the detection and enumeration of Cryptosporidium and Giardia in/on food (ISO 18744:2016(en) 2016), is still unable to determine the species or genotypes/assemblages of the Cryptosporidium oocysts and Giardia cysts. The ISO method would therefore need to be combined with molecular detection tools (PCR, RT-PCR) and sequencing.
Single detection assays alone are not sufficient to identify Cryptosporidium oocysts as a minimum of three screening methods should be used for its identification (Ortega et al. 1997). Procedures such as filtration, centrifugation and immunomagnetic separation, alone or in combination, may help to concentrate and purify Cryptosporidium oocysts from the food debris (Iqbal et al. 2015). Inside each procedure, several technical steps and reagents are utilized.
There are many risky food materials that have been infected with Cryptosporidium (Table 2) (via different methods) within the food and catering industry.
This review will highlight the most commonly used methods for the detection of oocysts within the different food matrices (Fig. 1). We will also discuss the favourable and unfavourable characteristics of these methods.
Elution (i.e., extraction/wash) of food material to purify Cryptosporidium oocysts
Extraction is the first step in any protocol for detecting Cryptosporidium oocysts that have been suspected in food. The success of all the subsequent steps will be dependent upon the efficiency of this first one. It is therefore important to maximize recovery because even a small number of oocysts (less than 10 oocysts for Cryptosporidium parvum) could cause illness (Dawson 2005; Shields et al. 2012) and constitute a major risk for food consumers (immunocompetent and immunocompromized). Simply washing vegetables fail to remove most of the Cryptosporidium oocysts because the oocysts are highly resistant and are firmly attached to the peel (Macarisin et al. 2010b; Ortega et al. 1997), hence the elution process being required. The elution process will depend on the extraction of oocysts from the food. The choice of the elution buffer (extractant) and the elution method will influence the oocyst recovery from the food. It is also important to note that the extraction methods that have been proven suitable for one specific food matrix could be unsuitable for the others (Cook et al. 2006a, 2007; Hohweyer et al. 2016; Robertson and Gjerde 2000).
Elution buffers
Different buffers were used for the elution of Cryptosporidium oocysts from different food matrices (Cook et al. 2006a, 2007; Robertson and Gjerde 2000, 2001a; Shields et al. 2012) (Table 3). ‘0.1 M HEPES, pH 5.5’, ‘1 M sodium bicarbonate, pH 6.0’, ‘1 M glycine, pH 5.5’, ‘1 M bicine, pH 5.6’, ‘1% lauryl sulphate’, ‘EB’, ‘0.1 M tricine, pH 5.4’ and ‘PBS, pH 7.2’ were compared to establish their ability to recover Cryptosporidium from lettuce and raspberries. Three of them EB, 0.1 M tricine pH 5.4 and PBS were excluded at the preliminary evaluation due to low oocyst recovery (Cook et al. 2006a). A glycine wash buffer ‘glycine 1 M’ was the cheapest extractant and was proven to be the most effective, compared to the previous ones, with optimal pH and no excess debris formation. Furthermore, the glycine wash does not influence oocysts recovery in IMS, and it has satisfactorily performance that suits both lettuce and raspberries (Cook et al. 2006a). A prolonged, intensive washing (up to 12 h) in 1 M glycine pH 5.5 elution buffer has been used for oocysts recovery from Spinacia oleracea (Macarisin et al. 2010a) and apples (Macarisin et al. 2010b); however, it was unable to separate all of the Cryptosporidium oocysts from their matrix (Macarisin et al. 2010a,b).
EB composed of 0.01 M Tris pH 7.4, 0.1% lauryl sulphate, 0.005 M EDTA and 150 ppm antifoam A generated broad recovery efficiencies of Cryptosporidium from water, using IMS (Smith 1998); however, it was suboptimal for extracting oocysts from lettuce and raspberries. This may have been due to solubility issues with the Laureth 12 detergent, low molarity and neutral pH that were not correct for the oocysts extraction from both lettuce and raspberries. Equally, 1 M sodium bicarbonate extractant produced an excessive amount of debris with raspberries, resulting in the prevention of oocysts identification and enumeration on the microscope slides (Cook et al. 2006a).
PBS pH 7 with ‘0.01% polysorbate surfactant’ and ‘Tween 20’ were used to elute Cryptosporidium from vegetable and apple surfaces, and it failed to completely remove the oocysts from both surfaces (Macarisin et al. 2010b). The ‘Tris-SDS’ buffer was only used to elute apple, and it allowed for the best recovery compared to the others; however, it also failed to recover all of the oocysts (Macarisin et al. 2010b). The failure of the recovery of the oocysts could be because the apple surface was not scrubbed by Macarisin et al. (2010b).
Shields et al. (2012) compared ‘deionized water’, ‘1 M glycin pH 5.5’, ‘modified detachment solution’ and ‘0.1% Alconox®’ buffers, without a clarification method to recover Cryptosporidium from lettuce, herbs and raspberries. A modified detachment solution gave slightly better recovery rates, with no significant difference between the deionized water and ‘1 M glycine, pH 5.5’, while ‘Alconox®’ gave the best recovery rate.
Deionized water formed air bubbles across the individual drupes of berries, causing it to float and leafy greens remained limp upon removal. ‘Alconox®, 0.1%’ contains both synthetic detergent surfactant agents and food additives with emulsifying and dispersing properties that were useful in completely saturating the food and assisting in the removal of the oocysts from the surface. It prevented air bubble formation with the berries, causing them to sink; more importantly, it also caused swelling of the leafy greens, resulting in the surface area being more exposed to the wash solution (Shields et al. 2012). Considering the above results, Alconox®, 0.1% was superior to all the other reagents.
However, another researcher claimed that buffers containing a surfactant or detergent were observed to be unsuitable for produce types due to the excessive bubble production that interferes with oocyst recovery during the stomaching process (Lalonde and Gajadhar 2016b). Shields et al. (2012) did not use stomaching in their experiment, while Lalonde and Gajadhar (2016b) did. This could explain the difference in the appropriateness of surfactant as an elution buffer.
‘PET’ (sodium pyrophosphate as a dispersing agent and Tween 80) and ‘TBS’ elution buffers were compared on milk, for eluting the membrane filter under different temperatures, achieving optimal recovery with the PET solution/40 °C (Minarovičová et al. 2011).
In meat, ‘1 M glycine’ and a ‘detergent-based buffer’ were used for the elution of seeded oocysts because detergent-based buffers provide the best recovery (Robertson and Huang 2012; Fig. 1).
Elution methods
Scientists used many proposed elution methods to extract Cryptosporidium oocysts from various food matrices. Stomaching, orbital shaking, rolling, rotating drum, sonication, kneading and pulsification are all the reported methods used to elute oocysts (Cook et al. 2006a, 2007; Frazar and Orlandi 2007; Gottfries 2011; Lalonde and Gajadhar 2016a, b; Robertson and Gjerde 2000, 2001a, b, 2008; Robertson and Huang 2012). Other alternative approaches (adhesive tape and pepsin digestions) were also used; however, they were not used as elution methods, whereas adhesive tape recovered the oocysts from selected fresh produce surfaces (Fayer et al. 2013). Pepsin and protein digestion turned the shellfish mixture into a slurry for further extraction methodologies (Robertson and Gjerde 2008; Willis et al. 2012). It would be very difficult to compare all these methods in order to select the most suitable one for the tested food matrix. The most important goal is to optimize the method and reach the maximum oocyst recovery; however, during the optimization process, some results will still not provide an accurate decision of selection.
According to the food matrix, scientists also recorded some remarks concerning each tested method (Table 4). Vegetables and fruits are the most tested food matrices for oocysts recovery. Experiments used to recover oocysts from their surfaces have provided us with background information regarding the methods examined.
Stomaching is considered as an effective method to extract oocysts from the surface of different food (lettuce, raspberries) (Cook et al. 2006a, b); however, some conditions (duration of stomaching, volume of used buffer, type of food material) should be considered before using this method. It is a matter of how to ensure the balance between maximum oocysts recovery and minimum formation of debris (that will be suspended later in the elution buffer) (Robertson and Huang 2012).
Pulsification (rapid beating of the sample in a pulsifier) was used in parallel to stomaching, in an experiment performed on lettuce. It provided the same recovery efficiency of Cryptosporidium oocysts. Despite this, stomaching was selected to be used in the continuation of the experiment due to the fact that it is commonly used by other laboratories in food analysis, allowing collaborative trials (Cook et al. 2006a). For the apple, pulsification in a stomacher bag homogenized the fruit, causing inability to detect Cryptosporidium oocysts (Macarisin et al. 2010b).
Shaking and rolling methods allowed for gentle handling of raspberries and did not display any difference in oocysts recovery. Shaking allows for a gentler treatment, compared to heavy handed methods that may cause the excessive production of matrix and affect the process of elution. Nevertheless, rolling was retained for further processing, and capped containers were used in its manoeuvre, reducing the risks of spillage and cross-contamination (Cook et al. 2006a).
For the kneading method, it performed differently with the lettuce and raspberries by different laboratory workers which makes it an unfavourable method to use (Gottfries 2011).
Rotating drum is another method affected by duration when used with lettuce, bean sprouts and strawberries. Different time periods 1 and 5 min significantly differ the efficiency of oocysts recovery. Although, higher numbers were recovered with the shortest time (1 min), it was not consistent for all sample types; in addition, the longer washing process accumulates large amounts of debris and cells in the samples (Robertson and Gjerde 2000).
Other researchers have explored the use of alternative approaches, using adhesive tape to remove Cryptosporidium oocysts from the surfaces of selected fresh produce (apple, peach, cucumber, tomato), and it was found to be successful in extracting levels of 10 oocysts per application area on the fresh produce’s surface. It was, however, very much dependent on the surface of the produce with incomplete oocysts removal (Fayer et al. 2013; Robertson 2014).
In milk processing, other scientists favoured shaking, being the most effective compared to stomaching (Minarovičová et al. 2011).
For the analysis of shellfish, pepsin digestion is used as a cheaper method, compared to the other isolation techniques that are used to survey shellfish for the Cryptosporidium parasite. Pepsin digestion has a relatively high recovery efficiency (70–80%) of Cryptosporidium oocysts using IMS (Robertson and Gjerde 2008). In 2012, this method was modified to use protein digestion without including IMS. It was then followed by the concentration (centrifugation) and washing (detergent) of the sample, to avoid the high costs relating to the IMS procedure. As a result, formation of a large pellet impeded the completion of the analysis in some samples (Willis et al. 2012).
With regard to the previous information, the following could be concluded: No single standardized developed elution technique could ensure complete Cryptosporidium oocyst recovery from variable food matrices.
Concentration and purification techniques
Concentration is made possible through simple centrifugation. This process is mostly used in the majority of the publications that deal with different food matrices (vegetables, fruits, milk, juice, shell fish and meat) (Cook et al. 2006a,b; Frazar and Orlandi 2007; 2016b; Moriarty et al. 2005; Robertson and Gjerde 2000, 2001b; Robertson and Huang 2012; Shields et al. 2012). Concentration was used in experiments and was either followed by purification (Sheather flotation and/or others) methods (Deng and Cliver 2000; El Sherbini et al. 2016; Li et al. 2006; Machado et al. 2006; Miller et al. 2006) or followed by IMS (Cook et al. 2006a; Hohweyer et al. 2016; Li et al. 2006; Utaaker et al. 2015).
Centrifugation is a direct, simple and rapid technique aiming to sediment the eluate from the food samples by using different centrifugal velocity and different time periods. Centrifugal velocities and time periods would need to be adjusted according to the aim of study and the type of food material being used.
Seven centrifugal flotation solutions (sheather, sodium chloride, magnesium sulphate, zinc sulphate, aluminium sulphate, ammonium sulphate 40% and ammonium sulphate 80%) were appraised for Cryptosporidium oocysts recovery in milk, using concentration and re-concentration processes. The highest oocysts recovery (11.6%) was achieved by using ammonium sulphate (40% w/v) (Machado et al. 2006; Fig. 1). However, in the suspended milk clot analysis, magnesium sulphate obtained 42.5% oocysts recovery. These results indicate that the process of re-concentration is more successful when using a high-density solution (Machado et al. 2006).
To detect C. parvum oocysts in apple juice, Sheather’s flotation and formalin ethyl acetate sedimentation were used to evaluate the effectiveness of the different procedures (modified acid-fast staining (AFS); direct immunofluorescence assay (DIFA); DIFA following immunomagnetic capture of oocysts; polymerase chain reaction (PCR), following flotation or flotation with immunomagnetic capture). Sheather was more efficient than sedimentation in oocyst recovery as it presented with higher sensitivity to all the methods of detection following it thereafter (AFS, DIFA and PCR) (Deng and Cliver 2000). Sheather also played a role in reducing the large quantity of extraneous matter in the samples; however, it was claimed that the excessive plant debris interferes with the efficiency of Sheather’s flotation, reducing oocysts recovery (Lalonde and Gajadhar 2016b).
For surveying Cryptosporidium oocysts in shellfish, Sheather’s flotation helped researchers to concentrate oocysts for further identification in its homogenate (Li et al. 2006; Miller et al. 2006).
Isolation (separation) methods of the oocysts from the food material
Immunomagnetic separation
IMS is incorporated into standardized and validated methods for the detection of Cryptosporidium in food (Smith and Nichols 2010). It has been used for the separation of Cryptosporidium from the eluates, homogenates and digestates of different food matrices such as mussels (Li et al. 2006), lettuce (Cook et al. 2006a; Utaaker et al. 2015), raspberries (Cook et al. 2006a; Hohweyer et al. 2016) and basil (Hohweyer et al. 2016).
ISO 18744 method ‘immunomagnetic separation technique and immunofluorescent assay for the detection of Cryptosporidium and Giardia in leavy greens and berry fruits’ contains the IMS step (ISO 18744:2016(en) 2016). IMS is considered to be the most expensive step out of the ISO protocol (Utaaker et al. 2015). Utaaker et al. (2015) modified the ISO method in a trial in order to reduce its cost, by adapting the IMS step throughout reducing the quantity of IMS reagents ‘using only 20% of the amount of beads’ and modifying the buffering system. The reduction in magnetic beads used for the IMS step leads to a cheaper modified method, compared to the standard ISO method.
From the economic view, using IMS with mussel samples ensured that larger tissue volumes will be analysed, making it a cost-efficient test. Additionally, it also decreased the time and the number of tests required for the sample processing, and it reduced the amount of sediment and inhibitors facing the detection techniques (Miller et al. 2006).
IMS increases the efficiency of the recovery of oocysts within the different food matrices, and it also enhances the concentration of oocysts from the samples that contain a low abundance of oocysts (Gottfries 2011). Preceding the detection methods with IMS, increased detection rates and higher sensitivity production are achieved when used with apple juice, mussel homogenates and cockles (Deng and Cliver 2000; Miller et al. 2006).
IMS effectiveness is, however, dependent upon maximizing the capture and release of oocysts, which are influenced by the affinity of the antibody paratope for the oocyst epitope and also by the physio-chemical influences of the surrounding matrix (turbidity, divalent cation concentration and pH variability) (Cook et al. 2006a; Smith and Nichols 2010).
Furthermore, the oocyst capture-antibody reagents have been developed against a small number of C. parvum isolates, and they do not recognize all of the Cryptosporidium species/genotypes/subgenotypes, especially the C. hominis isolates. The IMS antibody affinity also differs amongst the available commercial kits (Smith and Nichols 2010).
Some scientists also objected, considering it as an expensive tool because the cost per test increased (up to $50) (Miller et al. 2006; Robertson 2014). Besides, it did not perform well when parasites were isolated from an oyster tissue homogenate, and its effectiveness was hampered by the nature of the food (MacRae et al. 2005).
Detection/identification methods of the oocysts
Non-molecular approaches
Acid fast stains
Acid fast stains (Ziehl Neelsen (ZN) and Auramine O (AuO) staining techniques) were firstly used to identify acid-fast organisms, mainly Mycobacterium tuberculosis. They were then modified and adapted by Henriksen and Pohlenz (1981) ‘ZN’ and Payne et al. (1983) ‘AuO’ to be used in the identification of Cryptosporidium . Most countries started to identify Cryptosporidium oocysts 35 years ago by using AFS.
AFS stains are considered to be one of the most widely used techniques for Cryptosporidium identification. It has been used globally to identify Cryptosporidium in stools (Ahmed et al. 2014; Aly Shalash et al. 2016; Baron et al. 1989; Hanscheid et al. 2008; Henriksen and Pohlenz 1981; Ignatius et al. 1997, 2016; Ley et al. 1988); however, it has only been used occasionally for the detection of Cryptosporidium in water (Rayan et al. 2009; Rodríguez-Hernandez et al. 1994) and in food (different vegetables) (El Sherbini et al. 2016; Ortega et al. 1997) as well as in oocysts visualization and identification by microscopy (Cook et al. 2006a).
In the ZN technique, the cryptosporidial oocysts appeared as bright rose pink spherules on a pale green background when stained by fresh fruits and vegetables (El Sherbini et al. 2016). It also only requires an ordinary light microscope which is available in almost every lab.
Unlike ZN, the AuO technique stains the Cryptosporidium oocysts as fluoresced bright yellow-green round bodies, which can be easily differentiated from extraneous material (Hanscheid et al. 2008; Khurana et al. 2012; Ley et al. 1988). It also requires a fluorescent microscope, and it seems to have a higher yield than carbolfuchsin (Hanscheid et al. 2008; Ley et al. 1988). AuO is known to be highly sensitive and specific; it would use lower screening time per smear (30 s vs. 7 min) and needs to be screened at a low magnification (×400 vs. ×100) (Khurana et al. 2012).
AFS are proven to be easy to perform, highly practicable and inexpensive, and they allow a rapid approach for the detection of Cryptosporidium spp. oocysts. Moreover, researchers will have the chance to investigate the presence of oocysts with clear identification in the smears (Hanscheid et al. 2008; Khurana et al. 2012; Ley et al. 1988; Ignatius et al. 1997, 2016).
AFS, however, still seems to be a time-consuming approach and tedious, if multiple samples are examined (Deng and Cliver 2000). A fluorescent microscope that is required for AuO is also not always available in smaller laboratories (Pacheco et al. 2013). Furthermore, Cryptosporidium species are indistinguishable by this approach (Deng and Cliver 2000; Ignatius et al. 2016).
Other scientists considered AFS to be unreliable, and they considered it as the least sensitive with detection limits of 3000 oocysts, compared to DIFA and PCR, where less than 10 oocysts can cause infection in a healthy individual (Chappell et al. 2006).
Direct immunofluorescent assay
DIFA is the most commonly used method in oocysts detection for the various food matrices, and it is also stipulated in the ISO 18744 method (ISO/TC 34/SC 9/WG) (Ortega et al. 1997; Robertson 2014). It is usually performed by drying the final post IMS concentrate on a microscope slide that is mixed with a fluorescent marker (fluoresce in isothiocyanateconjugated anti-Cryptosporidium monoclonal antibody (FITC-CMAb)) on the monoclonal antibody (Robertson 2014).
DIFA can be used on formalin-preserved samples that provide quantitative data (Miller et al. 2006). The combination of immunofluorescence, size and the morphology allow visual identification of the oocysts, and it distinguishes them from the other organisms (Keserue et al. 2012; Miller et al. 2006). DIFA was found to be the most sensitive staining technique with the ability to recognize oocysts, when compared to AFS and PCR as it requires less expertise in examining the apple juice and haemolymph of oysters (Deng and Cliver 2000).
However, the auto fluorescence of mussel haemocytes made DIFA unsuitable for examining haemolymph (Miller et al. 2006). In addition, the process of labelling C. parvum oocysts fluorescently can sometimes cause large clumps of oocysts to form (Shields et al. 2012). Besides, it was also claimed to be very tedious and time consuming (Keserue et al. 2012).
DIFA requires microscopy expertise with a fluorescence microscope, which is highly expensive, and it does not provide genotype data unless its combined with PCR methods (Deng and Cliver 2000; Keserue et al. 2012; Miller et al. 2006; Robertson 2014; Shields et al. 2013).
An additional approach that improves the detection by providing additional visual markers for identification is the 4′6 diamindino-2-phenylindole (DAPI) (Robertson 2014). It is an enhanced fluorescent morphology method, using the fluorogen DAPI, which intercalates with nuclei in the sporulated oocysts, in conjunction with (FITC-CMAb) and fluorescence microscopy to visualize oocyst’s nuclei.
Microscopy-based methods in general are a fully quantitative approach as they directly detect oocysts contamination in/on food matrices with no need for signal interpretation. In DIFA with or without DAPI, immunofluorescence microscopy is the method used for investigation; it offers increased sensitivity over conventional ones (Iqbal et al. 2015), and both the intact and empty oocysts can be detected (Gottfries 2011; Smith and Nichols 2010).
The size of the pellet and the nature of debris after many food washes may act as a technological limitation in front of the fluorescent microscope (Shields et al. 2013). Underestimation of contamination will therefore occur, creating confusion for the investigator regarding the detection and enumeration through the inconsistent staining of oocysts (Lalonde and Gajadhar 2016b).
Flow cytometry
Flow cytometry (FCM) is another detection method considered for Cryptosporidium that combines both concentration and detection with food matrices (Iqbal et al. 2015; Keserue et al. 2012). It has been used in other types of samples (faeces, water, soil extract and HCT-8 cells) (Barbosa et al. 2008; Hsu et al. 2005; Valdez et al. 1997). FCM can detect Cryptosporidium as a discrete particle if labelled with the appropriate fluorescent tag (Valdez et al. 1997). Superiority of FCM was found to be 4–34 times sensitive, compared to conventional DIFA (Valdez et al. 1997).
It can evaluate morphological and functional characteristics of Cryptosporidium in which the number of oocysts could be precisely determined; however, it remains underutilized (Barbosa et al. 2008; Hsu et al. 2005). It has the capability to identify C. parvum from other microorganisms present in the debris (no cross-reaction) (Barbosa et al. 2008) by using cell sorting and isolating properties (Hsu et al. 2005).
This approach appeared to be very convenient, simple and efficient as it does not rely on slides or manual counting, allowing easy detection, fast performance, cost-effectiveness, and it has potential for automation. Furthermore, larger sample volumes can be rapidly analysed without human assistance, and its specificity can be improved by using multilabels (Rohde et al. 2015; Valdez et al. 1997). It offers the prospect of real-time analysis of microorganisms (Hsu et al. 2005). However, FCM has some disadvantages depending on the nature of the parasite examined, with the potential of presenting false positive and false negative results; the large equipment costs a lot, and it requires a well-trained technician (Keserue et al. 2012; Robertson 2014). It is affected by the amount of staining as insufficient amounts will not allow clear separation, and excessive amounts provide non-specific staining (Barbosa et al. 2008). It has been noted that large particles can clog FCM and they must therefore be removed or disaggregated (Valdez et al. 1997). Due to all of the previous flaws, it has not been fully accepted to be included in any of standard methods as of yet (Robertson 2014).
Laser scanning confocal microscope
Laser scanning confocal microscope (LSCM) is an approach used to study the interaction of microorganisms with food, providing three-dimensional information, without physically sectioning the specimen (Anguish and Ghiorse 1997; Takeuchi and Frank 2001).
In conventional fluorescent microscopes, photobleaching is a respected problem (Anguish and Ghiorse 1997; Takeuchi and Frank 2001), while LSCM reduces the photobleaching by illuminating only the focal point in the specimen (Takeuchi and Frank 2001).
It has been used in visualizing Cryptosporidium in different matrices (food, soil and faeces) (Anguish and Ghiorse 1997; Macarisin et al. 2010a) being an improved method for oocysts visualization, providing automatic stage position. Moreover, it can accurately analyse the oocyst structure, even if it is obscured by the other surrounded matrices (Anguish and Ghiorse 1997).
This approach creates difficulties in the detection of sensitivity and the viability assessment when there are a small number of oocysts present. It also lacks the ability to differentiate between species of Cryptosporidium (Anguish and Ghiorse 1997). LSCM has been claimed to be time consuming, requiring high experience with expensive instruments and being labour intensive (Fayer et al. 2013; Karanis pers. communication).
The type of food matrix can affect LSCM’s clarity, limiting tissue visualization by the extreme auto-fluorescence property that some food produce (pigments) (Takeuchi and Frank 2001). Taking these investigations into consideration, LSCM is not a preferred method of choice for the detection of Cryptosporidium in food matrices.
Electron microscope (EM)
Many researchers used an electron microscope with Cryptosporidium; however, each of whom had his own aim. Some researchers focused on confirming its presence and location by electron microscope (EM) magnification on fresh produce (apple and cucumber) (Fayer et al. 2013; Macarisin et al. 2010b; Ortega et al. 1997), and others focused on observing its developmental stages in culture (Aldeyarbi and Karanis 2016a, b).
Oocysts presence on fresh produce has been observed using SEM (Fayer et al. 2013; Macarisin et al. 2010b; Ortega et al. 1997), while observation of the Cryptosporidium developmental stages in culture has been achieved using SEM and TEM alone or combined (Aldeyarbi and Karanis 2016a, b). The ideal system of EM combines high resolution with high information content (Pomeranz 1976).
For Cryptosporidium, the presence of oocysts on the surface of vegetables (lettuce, cilantro, radish, etc.) after washing was observed using SEM, with no determination if recovered oocysts are infectious or not (Ortega et al. 1997). Low-temperature SEM (LT-SEM) identified Cryptosporidium oocysts on apple, with high magnification and great field depth, without dissolving its structure or denaturing the sample (Macarisin et al. 2010b). Similarly, working on seeded fresh produce (apples, peaches, cucumbers and tomatoes), LT-SEM was used to identify oocysts on their skin and on adhesive tape that contained the oocysts that were removed from the fresh produce. It was able to portray its adherence clearly and locate oocysts within the microscopic fissures present on the skin of the fresh produce (Fayer et al. 2013).
However, EM was considered to be time-consuming and labour-intensive, requiring expensive equipment and requiring a high level of expertise (Fayer et al. 2013). There are many preparation steps that are required for food samples to ensure that they are ready for SEM, producing mechanical damage, osmotic stress and surface tension that ends with significant harmful changes in the structure of the specimen (Macarisin et al. 2010b).
Molecular approaches
PCR
DNA extraction methods for various food matrices have varied widely, using manual extraction or kit extraction methods. DNA extraction methods are central to efficient PCR amplification. Cryptosporidium oocysts detection levels in food are much lower than 108 oocysts, and using IMS-pre-DNA extraction preparation will increase its recovery (Gottfries 2011). Additionally, DNA from stressed and damaged oocysts may not always amplify (Smith and Nichols 2010).
DNA extraction is mentioned to be affected by the technique that is used to prepare the DNA template, with superiority of some over others (Frazar and Orlandi 2007; Shields et al. 2013). A DNA extraction kit was used with orange juice, apple cider, whole milk, strawberries, parsley and lettuce), being less time-consuming, with less sample manipulation in comparison to the less sensitive filter-based method ‘FTA concentrator-PS filter’ (Frazar and Orlandi 2007) that caused contact lysis of microorganisms and trapped the released nucleic acids within the fibre matrix of the filter (Orlandi and Lampel 2000). Regardless of the used technique, the quality of the obtained DNA template is influenced by the type of food matrix where the oocysts were derived from (Frazar and Orlandi 2007).
Heat lysis, a simple DNA isolation method, was used to isolate oocyst DNA from milk and apple juice. It was found to be totally inapplicable and insufficient, ensuring that the inhibitors in the food components take its role in abrogating the PCR reaction. On the other hand, high-quality DNA was obtained using QIAamp DNA Mini Kit (Minarovičová et al. 2007).
Four DNA kits (FastDNA, MoBio, QIAamp and modified UNEX) have been assessed for the difference in DNA detection of Cryptosporidium and Cyclospora cayetanensis in raspberries, basil and pesto. UNEX was the most successful one to extract oocysts DNA from all 3 food matrices, taking the shortest time. Nevertheless, FastDNA and QIAamp were equal in their success of effective extraction. Taking in regard, FastDNA required less time to process and QIAamp overcame the inhibitor propriety. MoBio produced the poorest results which returned us to the crushed garnet’s unsuitability with food matrices that could be solved by changing the disruption matrix (Shields et al. 2013).
Scientists develop molecular methods for genotyping-detected oocysts and to also instil more confidence in the identification process. PCR has already been proven effective in the analysis of food samples for Cryptosporidium, and several PCR assays are being applied using different primers, providing a new generation of diagnostics (Minarovičová et al. 2007). Cool and moist conditions are favourable in order to ensure a prolonged shelf life for leafy greens and for the survival of oocysts in the environment. A positive PCR result of protozoan oocyst detection in fresh produce should therefore be considered as an indication of risk to public health (Lalonde and Gajadhar 2016a).
PCR equipment is becoming a cost-effective approach (Robertson 2014), with it being simple, broadly used, having high throughput, and it has been validated and implemented by many researchers (Rohde et al. 2015). PCR is more specific, and it is less dependent on human factors than microscopy. Despite this, it cannot distinguish between the infective and non-infective oocysts, and it is often affected by inhibitors; however, it provides the opportunity for species identification, unlike DIFA (Robertson 2014; Smith and Nichols 2010). In addition, it is more sensitive and discriminatory (Cook et al. 2006a; Minarovicová et al. 2009) to species/genotype/subtype levels (Shields et al. 2013; Smith and Nichols 2010). To date, Cryptosporidium genus is considered as a multispecies parasite, based on the molecular genetic analysis at limited and highly conserved loci. Thirty-four Cryptosporidium species and more than 60 genotypes have been recognized.
Different PCR types were tested in order to choose the best PCR condition for each food matrix (Frazar and Orlandi 2007; Lalonde and Gajadhar 2016b; Miller et al. 2006; Minarovičová et al. 2007; Shields et al. 2013). Nested PCR, using nested primers, performing a secondary round of amplifications has been mentioned to increase sensitivity and specificity of Cryptosporidium detection in water, milk and apple juice (Minarovičová et al. 2007). It has a higher specificity compared to the conventional PCR, as well as a high correlation with DIFA in the case of Cryptosporidium. PCR-RFLP is currently considered as a widely applied method that is used in obtaining faecal and water samples, with the advantages of it differentiating between the major parasite genotypes by subsequent sequencing.
Real-time PCR assay is known to be a sensitive molecular detection method for Cryptosporidium in food and in environmental samples (Minarovicová et al. 2009). The real-time PCR assay with melt curve analysis (qPCR MCA) is well suited for detecting Cryptosporidium oocysts in leafy greens and berries, with high-throughput screening. It can provide an estimate of the quantity of the oocysts detected. It is also capable of detecting DNA from as few as 10 oocysts (Lalonde and Gajadhar 2016a).
Nevertheless, for ensuring optimal assay sensitivity, a cool storage temperature for samples should be maintained. In addition, if molecular epidemiology of positive samples will be investigated, further molecular assessment should be combined to qPCR MCA (sequencing, species specific PCR, etc.) (Lalonde and Gajadhar 2016a), that can be performed directly from its product (Lalonde and Gajadhar 2011).
Bivalve TaqMan PCR is another rapid real-time PCR system with a high throughput in detecting Cryptosporidium from fresh or frozen tissue samples (mussels), using total RNA which provides a correlation to oocysts viability. Moreover, it provides semi-quantitative results with the detection of multiple Cryptosporidium species (Miller et al. 2006).
PCR was, however, claimed to be less sensitive than DIFA (Gómez-Couso et al. 2006a,b). Sensitivity and reproducibility of PCR could be affected by the lack of identified intrinsic, high sensitivity capability of Cryptosporidium genomic targets, mixtures of species/genotypes/subgenotypes, hardness to lyses the oocysts, low numbers of it and its stressed and damaged nature (non-nucleated) in food concentrates (Gottfries 2011; Robertson 2014; Smith and Nichols 2010).
Wide range of inhibitory substances like poly-phenolics can also abrogate the efficiency of the PCR. Differences in the type of food matrices may require adjustment of PCR conditions/matrix (e.g., apple juice and apple cedar) in order to form another inhibitory factor (Deng and Cliver 2000; Frazar and Orlandi 2007; Robertson 2014; Rohde et al. 2015). Different processing methods such as stomaching that damages more plant tissue and releases more PCR inhibitors are inappropriate to be used in combination with the molecular assays (Lalonde and Gajadhar 2016b). However, washing of the oocysts before DNA extraction and the addition of BSA to the PCR reaction could help eliminate such inhibitors or completely replace the washing step with orbital shaking (Lalonde and Gajadhar 2016b).
PCR does not assess viability, virulence or infectivity (Rohde et al. 2015) so it is considered a risky indicator, making it difficult to interpret the results and accurately determine the significance of its positivity, with respect to the risks caused by contamination (Lalonde and Gajadhar 2016a).
Effective molecular methods that maximize DNA extraction and minimize the PCR inhibitors in samples are required. Molecular tools such as loop-mediated isothermal amplification-PCR (LAMP-PCR) (Karanis et al. 2007b; Bakheit et al. 2008; Karanis and Ongerth 2009) or droplet digital PCR (ddPCR) (Yang et al. 2014) should increase the sensitivity of detection for Cryptosporidium in food samples in the future.
Fluorescent in situ hybridization
Florescent in situ hybridization (FISH) utilizes fluorescently labelled cDNA oligonucleotide probes that target specific cellular rRNA sequences for direct identification of microorganisms (Amann et al. 1995). Its specificity and sensitivity is dependent on the probe design (Takeuchi and Frank 2001) and with its hybridization, various probes can be used (DNA (PNA and LNA), RNA, multilocus and double-labelled probes) (Rohde et al. 2015).
FISH represents a promising alternative method to PCR in visualizing the target ribosomal RNAs in food samples as it is specifically capable of identifying two species and it is able to differentiate the viable organisms from the dead (Alagappan et al. 2008; Rohde et al. 2015).
Furthermore, FISH can be used to accurately identify C. parvum, making it a good method for both epidemiological and outbreak investigations. When it is combined with Cryptosporidium-specific antibody, rapid detection of C. parvum in infectious samples is easily achievable, with the capacity of targeting several species (Alagappan et al. 2008; Takeuchi and Frank 2001).
Implementation of the FISH technique in food safety monitoring had favourable characterizations due to it being simple, specific, sensitive, reliable, robust, quick, less cross-reactive and having high-throughput testing, with economic feasibility (Alagappan et al. 2008; Rohde et al. 2015).
Food samples that are investigated by FISH go through general methodical procedures ending with observation by either microscopy or flow cytometry (Amann et al. 1995; Rohde et al. 2015; Takeuchi and Frank 2001). Combination of flow cytometry with FISH (flow-FISH), instead of fluorescence microscopy, demonstrated rapid and high-throughput potential (Manti et al. 2011; Rohde et al. 2015).
All of the FISH steps, however (hybridization time, temperature, permeabilization and fixation conditions and specific probe design), necessitate efforts for optimization (Amann et al. 1995; Rohde et al. 2015). Besides, no FISH probes that differentiate C. parvum from C. hominis have been available (Alagappan et al. 2008). Cryptosporidium oocysts could lose their viability due to aging, prolonged exposure to the environment and/or being inactivated (application of heat, disinfectants and/or radiation on the oocysts). Although FISH has the ability to retrospectively determine oocyst viability, a problem may still arise. Such inactivated oocysts will appear viable with FISH. This might return to certain degree of permeabilization that occurs due to the method applied to inactivate the oocysts, allowing RNAs to penetrate the cyst wall and fluoresce at the background level (Dorsch and Veal 2001).
Oligonucleotide probes used for FISH have the ability to bind non-specifically with debris in water samples. The non-specific binding will result in extrabackground fluorescence (Dorsch and Veal 2001) as well; same scenario will happen with food eluates. This will become a problem if the FISH signal coming from the target oocysts is weak.
Loop-mediated isothermal amplification
This approach has been the first used for detecting Cryptosporidium in water, in faecal samples and in environmental samples (Bakheit et al. 2008; Karanis et al. 2007b; Plutzer et al. 2010); it was then widely used for the detection of other pathogens (Karanis and Ongerth 2009). It has also recently been used for detection of plant species in food matrices, spices (caraway, cumin, seeds of mustard and celery powder) and white bread mix, being an alternative method to achieve food safety and food control (Focke et al. 2013).
Some researchers agreed with LAMP unaccountable advantages either compared with PCR or not compared with it (Gallas-Lindemann et al. 2013; Karanis and Ongerth 2009; Plutzer et al. 2010). LAMP is an isothermal nucleic acid amplification test that produces highly specific and sensitive amplification of DNA or RNA, being insensitive to extraneous materials other than the target and can differentiate in between organisms by single nucleotide polymorphism (Karanis and Ongerth 2009). In addition, its reaction is carried out at a constant temperature without requiring a thermal cycler, in contrast to PCR that required a series of alternating temperature cycles, making it a simple, rugged and low-cost technique.
In LAMP, the target gene needs four different primers which are used to identify six distinct regions that reach high specificity. Furthermore, in addition to this, a pair of ‘loop primers’ accelerates the reaction which provides the primers with a specific nature that will increase the amount of DNA produced compared to PCR (Nagamine et al. 2002).
LAMP reagents characterized by relative stability at 25 and 37 °C, being able to hold the potential in tropical countries (Ocker et al. 2016; Thekisoe et al. 2009). However, it is only useful as a diagnostic and detection technique, and it cannot be used in cloning. Obtaining many PCR molecular applications and designing primer sets for LAMP are difficult issues, and it also has less ability to freely choose the target site than in PCR. The multiplexing approaches for LAMP that are currently in use are less developed than the ones used for PCR. LAMP has proven to be an alternative rapid tool to PCR for the detection of Cryptosporidium in environmental samples by using specific designed primers (Karanis et al. 2007b; Bakheit et al. 2008; Plutzer et al. 2010).
For Cryptosporidium species, LAMP has high specificity and sensitivity in its identification and differentiation (Karanis et al. 2007b; Bakheit et al. 2008; Plutzer et al. 2010). It could be used as a useful diagnostic tool for Cryptosporidium epidemiological studies without being affected by inhibitors that stand as obstacles in front of PCR (Bakheit et al. 2008; Karanis and Ongerth 2009; Plutzer et al. 2010). Hence, it can skip the most expensive step (IMS) for removing such inhibitors (Plutzer et al. 2010).
However, a risk for contamination of restriction digestion after LAMP could occur, which should always be avoided (Karanis and Ongerth 2009); besides, LAMP available sequences do not cover all Cryptosporidium species as they have a limited range (Bakheit et al. 2008), and it still has not been used for the viability assessment through RNA.
Aptamers
DIFA and PCR have been very useful in testing food samples; however, low concentration of oocysts in food, difficulty in eluting oocysts from some food matrices, lack of enrichment methods and the presence of PCR inhibitors are respected obstacles facing them. Therefore, a highly sensitive and specific aptamer was selected for detecting the presence of Cryptosporidium on pineapple and mango (Iqbal et al. 2015).
Aptamers are synthetic nucleic acids that fold into a unique three-dimensional amendment, having the capability of specific binding in order to target with incredible affinity (Ellington and Szostak 1990; Iqbal et al. 2015). This approach has a low oocysts detection limit with a high level of discrimination against the disorganized background, suggesting that it is a useful standby method to immunofluorescence microscopy. Additionally, it is less expensive to produce, compared to monoclonal antibodies because of its single-stranded oligonucleotide component (Iqbal et al. 2015). Testing its ability to distinguish Cryptosporidium to species/genotype/subtype level is still a future inquiry.
Considered factors for method optimization and efficiency of oocysts recovery
There are many factors affecting the recovery efficiency of Cryptosporidium oocysts from different food matrices; this is due to both the method being used and the food material’s nature (Table 5).
Factors related to the food matrix
Nature of the food sample
Each food matrix displays specific characteristics (trapping and adhesion force) that may interfere with oocyst removal and elution (Cook et al. 2006a; Hohweyer et al. 2016).
Various leafy greens (e.g., lettuce) have a large surface area to weight ratio, and rosettes of leaves are held together in nubs of roots. Such characteristics provide protected leaf areas and form pockets for contaminants, ‘soil and debris’ to gather, resulting in a loss of oocysts. It will make the washing method more effective for detecting widely distributed low numbers of oocysts (El Sherbini et al. 2016; Frazar and Orlandi 2007; Robertson 2014). Furthermore, leafy greens contain phenols, polysaccharides and pectin compounds that inhibit PCR and may reduce the sensitivity of molecular assays (Lalonde and Gajadhar 2016b). Contrary, vegetables with flat surfaces such as cucumbers, tomatoes and carrots reduce the rate of parasitic attachment (Damen et al. 2007).
For berries, delicacy of the fruit, sensitivity to temperature and irregular surface area are the main problems (Lalonde and Gajadhar 2016b; Robertson 2014). Delicacy make the fruit break easily, inducing fragments that remain in the elution buffer and impede subsequent analysis steps (Robertson 2014). In this respect, using gentle orbital shaking method was favourable (Cook et al. 2006a; Lalonde and Gajadhar 2016b).
Temperature is very important to maintain the integrity of the plant tissue and ensure minimal deterioration during processing; it might affect the integrity of the oocysts as well (Lalonde and Gajadhar 2016b). Oocysts were best removed from smooth-skinned blueberries using a glycine buffer, but raspberries, strawberries and blackberries were more effectively washed with surfactant containing elution solution (sodium pyrophosphate), to allow complete wetting of the irregular surface areas (Lalonde and Gajadhar 2016b; Shields et al. 2012).
Hair-like projections and courgette surfaces in some vegetables and fruits (apples, peaches, cucumbers, zucchini and tomatoes) are other physical barriers facing recovery, detection and identification of Cryptosporidium oocysts (Robertson 2014), making standard household washing and adhesive tape approaches unsuitable methods for elution (Fayer et al. 2013; Robertson 2014).
Sprouted seeds with some food materials (e.g., bean sprouts) often treated as a raw agricultural product, being highly liable to contamination, and affects the oocysts recovery efficiency during its material removal in the washing step. Besides this, it also directly interferes with the IMS technique and forms a coating over the beads (Robertson and Gjerde 2000).
Milk is a complex physicochemical emulsion, composed of proteins, mineral salts, lipids and vitamins (Machado et al. 2006). This viscous matrix makes the separation of Cryptosporidium from it very difficult (Minarovicová et al. 2009). Mixing milk with sulphate solutions (MgSO4, ZnSO4, AlSO4, NH4SO4 40% and NH4SO4 80%) causes coagulation when centrifuged, forming a clot. This clot will remain and be fixed in the intermediate layer of the tube, requiring further separate transfers for both the sediment and the clot to two tubes (ponderous). While, mixing milk with sucrose and sodium chloride lacks clot formation. Coagulation of milk favours the increase of Cryptosporidium spp. oocysts concentration (Machado et al. 2006). Furthermore, heating milk and the equipment used for separation could ease the filtration process (Minarovicová et al. 2009). Different types of orange juice matrix’s interfere between beads and magnet, causing poor recovery of oocysts from it (Frazar and Orlandi 2007). Apple cider has also been proven to be a difficult matrix (Frazar and Orlandi 2007). Pesto sauce is also characterized by it containing multiple ingredients that may interfere with the DNA extraction process and downstream applications such as PCR (Shields et al. 2013).
Shellfish is an interesting reservoir of Cryptosporidium oocysts and a source of infection to humans (Downey and Graczyk 2007). The consistency of the shellfish tissue affects the IMS making it less effective in the more mucoid ones. There is also a debate regarding which part of tissue should be used in the analysis; however, most researchers agree that tissue homogenates are more useful than gill samples (homogenate or washings) or hemolymph for the detection of Cryptosporidium (MacRae et al. 2005; Robertson 2014). However, it is important to note that oocysts could be lost during tissue homogenate preparation, especially if the sieving step was used before the analysis. Additionally, hemocytes often obscure Cryptosporidium oocysts and interfere with accurate oocysts visualization using DIFA (Miller et al. 2006). Furthermore, separating hemolymph during homogenization and later adding it to the pellet provided the best results for oocysts recovery from oysters (Downey and Graczyk 2007).
During recovery, some of the non-specific purification methods (flotation on caesium chloride (CsCl2), sucrose gradients or lipid extraction) should be considered (Robertson 2014). Besides depuration time is very important in the total removal of parasitic stages from molluscs (Gómez-Couso et al. 2003).
Resinous components of rosemary herb with other compounds of polyphenolic and polysaccharide affect its molecular detection, causing PCR inhibition. This originates from the plant itself or its chemical properties, which makes the effective detection of oocysts an impossible mission; it might therefore require treatment during the extraction of DNA to eliminate inhibitors (Lalonde and Gajadhar 2016b).
Fatty components of cured meat products become dissolved in the buffer during stomaching which has been modified with using ice-cold buffers. It coats the paramagnetic beads with limiting IMS efficiency and causes background fluorescence to stand as an obstacle in front of DIFA detection. To minimize such effect, adding Evans Blue (0.1%) (quenching agent that shifts the auto fluorescent spectrum to be in a longer wavelength) will be beneficial. Furthermore, the fat globules’ small size (2–3 μm) and cluster appearance resemble Cryptosporidium oocysts causing microscopic distraction (Robertson and Huang 2012).
Another disadvantage is cutting the meat that has formed hard edges as this could pierce the stomacher bag resulting in the loss of the sample. To reduce the potential of sample loss, soaking it for 30 min, will soften it (Robertson and Huang 2012).
All previous characteristics confirm that methods used to increase the recovery efficiency of Cryptosporidium spp. oocysts in food depend on the nature and composition of the food sample and on the methodology used for oocysts concentration (Table 5) (Machado et al. 2006).
Quantity of the food sample
The quantity of the analysed sample is an important factor affecting the efficiency of oocysts recovery (Robertson and Gjerde 2001a; Robertson 2014). It is demonstrated that the greater the sample size, the greater the quantity of debris in the eluate, making the method less efficient at parasite recovery and it may hinder other subsequent steps (Robertson and Gjerde 2001a).
The use of a small amount of sample in inoculation experiments allows greater contamination per gram, increasing washing space, using a small amount of elution buffer and reducing the interfering matter from the sample.
Significant increase in oocysts recovery (70%) was recorded using 30 g lettuce compared to 100 g (Robertson and Gjerde 2001a). Similarly, more material of bean sprouts induced reducible recovery efficiencies (Robertson and Gjerde 2001a).
However, for the un-inoculated sample, the decrease in the amount of the food sample used will decrease the detection rate of oocysts; this is most probably due to usual low contamination (Robertson and Gjerde 2001a). Therefore, attention should be given to the sample size selection, not only to maximize recovery efficiency but to also provide a chance for the detection of low-level contamination (Robertson and Gjerde 2001a, 2001b). For liquid samples like milk, it followed the same concept that increasing the sample volume decreased the recovery efficiency by causing clots on the filter used (Minarovicová et al. 2009).
Considering the above, it can be concluded that while weight to volume is important, there is also a limit (Shields et al. 2012).
Age of the food sample
Sample freshness is a parameter of importance, and the food sample should be as fresh as possible (Robertson and Gjerde 2000). It is expected that older samples will produce more interfering matters (cellular debris, microflora and excretory products of microflora and biofilms, specifically bacterial exo-polysaccharides) in the eluate, which by extension will interfere with the subsequent purification and detection procedures (Robertson and Gjerde 2001a,b).
For example, bean sprouts produce copious amounts of interfering debris, even when in its freshest form. This interfering material might mostly be due to its method of cultivation that encourages bacterial growth (Robertson and Gjerde 2000; Robertson 2014). Using the same IMS kits with 10 days old resulted in a largely reduced recovery rate when compared with bean sprout samples that were 7 days old (30–18%) (Robertson and Gjerde 2001a).
Factors related to the method
Different food matrices (fruits, vegetables, meat, shellfish, dairy products and beverages) vary significantly in both biochemical and physical characteristics. Properties that affect the choice of elution are approached in a trial to optimize parasite removal and minimize contamination (Robertson 2014). Thus, a method appropriate for one type of food is unsuitable for another, omitting the concept of ‘one method suits all’ (Robertson 2014) (Table 5).
Factors related to elution buffer
Different buffers have been tested in order to try to increase oocysts recovery because different food matrices differ in their pH; in respect of this reason, optimizing the buffer pH, molarity and ratio is essential to overcome the pH extremes of the food matrices (Cook et al. 2006a).
Higher recovery rates will be achieved when the pH value is in between 7.0 and 8.1 (Frazar and Orlandi 2007). The pH is critical for extracting oocysts from the food materials, concentrating the oocysts via IMS and keeping the integrity of the food samples (Cook et al. 2006a). Decreasing the pH below 4 causes lysis of lettuce cells and forms particles in the eluate that hamper the oocysts extraction (Cook et al. 2006a).
A membrane filter elution buffer was used in oocysts extraction by Robertson and Gjerde with the dilution of 1:4 (Robertson and Gjerde 2000). Other researchers faced some difficulty with the preparation of this elution buffer, especially with dissolving the Laureth 12 detergent. It is difficult to use because it forms different batches, generating different recovery efficiencies (Cook et al. 2006a; Gottfries 2011). In addition, neutral pH and low molarity of the membrane filter elution buffer made it inferior to other tested buffers (Cook et al. 2006a). The pH of the glycine buffer (1 M) was critical for a high Cryptosporidium oocysts recovery from lettuce (Cook et al. 2006a), explaining that the pH dependence may be related to different non-covalent interactions between the Cryptosporidium oocysts and the lettuce (Cook et al. 2007).
Time frame between seeding and washing
Many studies handled the time between the seeding of the food with oocysts and the initiation of the washing step, differently (Cook et al. 2006a, b; Robertson and Gjerde 2000; Shields et al. 2012). The time span used by researchers was varied between 3 h (Amorós et al. 2010; Cook et al. 2006a, b; Robertson and Gjerde 2000), 12 h (Ripabelli et al. 2004) and 24 h (Ortega et al. 1997; Shields et al. 2012).
The time span that should be used to ensure good recovery for all food matrices has still not been determined yet but it mainly depends on how long the food samples will take to arrive for processing after being contaminated, favouring ‘1–3-h’ time span as the freshness of the food samples will still be preserved (Shields et al. 2012).
Addition of rinsing steps added to the main washing step
The rinsing step is the second step in the washing process of the food matrix; it has been added to the main washing step, along with the normal completion of the other subsequent steps. It was recorded that the rinsing step should occur immediately after the main washing step, as it resulted in a great improvement in the recovery rates by 4.3–10% with raspberries and 12.5–33.0% with spinach, with no mention of the washing solution used (Shields et al. 2012).
The second wash step participated in the increase of recovery (even though the amount was dependent upon the food matrix and the wash solution being used), recommending that its incorporation with all further washing experiments would be preferable.
Selection of IMS kits
Many IMS kits were used by researchers for the isolation of Cryptosporidium; Dynal anti-Cryptosporidium, ImmuCell Cryptoscan and Aureon Biosystems are compared IMS kits used in isolation processing of food samples (Cook et al. 2006a; Robertson and Gjerde 2000, 2001a). Using Dynal anti-Cryptosporidium kit (DB) or the ImmuCell Cryptoscan (IC) with lettuce and bean sprouts provided no significant difference in oocysts recovery (Robertson and Gjerde 2001a). Aureon Biosystems provided a prototype anti-Cryptosporidium IMS kit, showing significant superior recovery efficiency amongst the previous ones when used with bean sprouts (Robertson and Gjerde 2001a). Superior beads, antibody, buffering system or all of them were proposed characteristics that explained superiority of Aureon Biosystems kits (Robertson and Gjerde 2001a).
Over the development of time, new commercially IMS kits will keep on appearing so it is important to keep in mind that improved IMS techniques have the potential to increase the recovery efficiency from the food samples.
Strength of magnet used in IMS
The magnet used in IC kits is stronger than magnet in DB kits, which were compared to test their effect on the oocysts recovery rate (Robertson and Gjerde 2001a). Using a stronger magnet unfortunately did not result in a significant improvement in the oocysts recovery (Robertson and Gjerde 2001a). Strong magnet collects more beads; however, it also collects other associated materials with it, coating the paramagnetic beads (Robertson and Gjerde 2000), reducing antibody-parasite binding and hampering the IMS procedure ‘specifically were noticed with analysis of bean sprouts’ (Robertson and Gjerde 2001a). Therefore, selecting a suitable IMS system with superior magnetic beads and/or buffering system will improve the recovery rate.
Types of PCR assays in use
Using one system of PCR or combining it with another affects the efficiency of Cryptosporidium oocysts recovery. When two PCR systems (1ry conventional + 2ry nested real time) were combined to detect Cryptosporidium oocysts in milk, the detection rate was improved. Furthermore, the Cryptosporidium detection limit in milk varied according to different PCR systems used, conventional PCR, real-time PCR and nested PCR (1 × 103, 1 × 102 and 1 × 10 oocysts/ml) (Minarovičová et al. 2007).
Other researchers cleared that TaqMan PCR and conventional PCR were similar in sensitivity for their detection of Cryptosporidium oocysts in mussels; however, conventional PCR caused cross-reaction with dinoflagellates, while the Cryptosporidium TaqMan system was more specific, returning to its automated testing with high-throughput and semi-quantitative results. Finally, combining both, with DNA sequence analysis, we will obtain detailed molecular data (Miller et al. 2006).
Conclusions
Cryptosporidium is a foodborne and waterborne intestinal parasite, which poses a threat to human health, not only in developing countries with poor hygiene but also in the industrialized and developed world, where the criteria for fresh food preparation are of high standards. The criteria cause problems for the food chain industries. It should be possible to guarantee that food industries will be able to offer uncontaminated food to their customers at the places even where there are few numbers of oocysts present.
We presented detailed information regarding the total procedure of the concentration, separation and identification of Cryptosporidium oocysts from the food material and took any known and significant factor into consideration from the primary step (concentration), the intermediate step (isolation) and final detection step (identification) that could possibly influence the detection of the oocysts in a negative or positive way. This is the first technical and comprehensive review of its kind that discusses all of the available methods utilized within the period of almost 35 years, covering methodological monitoring and the procedures for Cryptosporidium in food material worldwide.
Standard detection methods do not exist, and they require skilled personnel to secure the analysis as well as the detection of the oocysts in the target food material. Most of the methods described here have been adapted from those used in water analysis, and they include both conventional and molecular-based detection. Considerable efforts are, however, contributing towards the improvements of the methods that are available for certain ‘high-risk’ foods (notably ready-to-eat salads and fresh produce). The FDA Bacteriological Analytical Manual (BAM) provides a protocol that has been designed to detect Cryptosporidium oocysts in fresh produce washes and in filterable samples such as juices.
Detection methods for the food industry require effective concentration procedures to detect the existing numbers of protozoan oocysts that are likely to be present in the food material. Food is a difficult material to process for the detection and identification of Cryptosporidium oocysts. Many steps are required to be followed (elution, concentration, purification and isolation) just to prepare the food sample for the detection step. The nature of the food material can vigorously affect the success of the investigation for Cryptosporidium presence. Size, consistency, shape and surfaces are all different features relating to the food type that needs to be taken in account. Food age and quantity can also provoke further meditation. Moreover, other factors related to the selected method should be kept in consideration. These different aspects could create unanswered questions that the researcher would need to take into consideration before initiating the first step of the process.
Due to the lack of standard methods, automating instruments and high-throughput systems being available, laboratory work is severely limited. Furthermore, different techniques should be used as they could complement each other, and at the same time, it is important to acknowledge the limitation characteristics of each technique. This knowledge would also be beneficial to researchers later on, as they would then be aware that selecting a standard, reliable, validated method, that suits different food matrices will ensure the quality of the data that is essential for establishing food safety surveillance.
To enhance Cryptosporidium surveillance in the food chain, techniques are required to improve our current understanding of the significance of Cryptosporidium in primary food production and its significance throughout the various food chains. This also includes sectors and food industries in which the fresh fruit or vegetables appear to be sold as ready-to-eat products that are not required to be further heat-processed or cooked by consumers. DNA-based detection methods are more sensitive, faster and easier to perform than microscopy, and they offer the possibility of the differentiation of species and genotypes. The lack of availability of sensitive detection methods for Cryptosporidium represents a large challenge for the food industries that are called to support research in their areas for the development of more precise available methods.
References
Åberg R, Sjöman M, Hemminki K, Pirnes A, Räsänen S, Kalanti A, Pohjanvirta T, Caccio SM, Pihlajasaari A, Toikkanen S, Huusko S, Rimhanen-Finne R (2015) Cryptosporidium parvum caused a large outbreak linked to frisée salad in Finland, 2012. Zoonoses Public Health 62(8):618–624. https://doi.org/10.1111/zph.12190
Ahmed SA, Ali IH, Jianhua L, Fang W, Pengtao G, Fan L, Xichen Z (2014) Zoonotic Cryptosporidium parvum in diarhea patients in Changchun, China. Trends Life Sci 3:2319–4731
Alagappan A, Tujula NA, Power M, Ferguson CM, Bergquist PL, Ferrari BC (2008) Development of fluorescent in situ hybridisation for Cryptosporidium detection reveals zoonotic and anthroponotic transmission of sporadic cryptosporidiosis in Sydney. J Microbiol Methods 75(3):535–539. https://doi.org/10.1016/j.mimet.2008.08.007
Aldeyarbi HM, Karanis P (2016a) Electron microscopic observation of the early stages of Cryptosporidium parvum asexual multiplication and development in in vitro axenic culture. Eur J Protistol 52:36–44. https://doi.org/10.1016/j.ejop.2015.07.002
Aldeyarbi HM, Karanis P (2016b) The ultra-structural similarities between Cryptosporidium parvum and the Gregarines. J Eukaryot Microbiol 63(1):79–85. https://doi.org/10.1111/jeu.12250
Aly Shalash IR, Zalat R, El-Enain G, EL-Mohandes M, EL-Faramawy M, Aly E (2016) Comparison between modified acid fast staining and antigen detection assay as diagnostic techniques for Cryptosporidium parvum. World J Med Sci 13:72–78
Amann RI, Ludwig W, Schleifer KH (1995) Phylogenetic identification and in situ detection of individual microbial cells without cultivation. Microbiol Rev 59(1):143–169
Amorós I, Alonso JL, Cuesta G (2010) Cryptosporidium oocysts and Giardia cysts on salad products irrigated with contaminated water. J Food Prot 73(6):1138–1140. https://doi.org/10.4315/0362-028X-73.6.1138
Anguish LJ, Ghiorse WC (1997) Computer-assisted laser scanning and video microscopy for analysis of Cryptosporidium parvum oocysts in soil, sediment, and feces. Appl Environ Microbiol 63(2):724–733
Avazpoor M, Yousefipoor M, Dusty M, Mehdipour M, Seifipour F, Gholam Z (2015) Determination of the level of parasitic infection (Cryptosporidium and Giardia) of the vegetables marketed in Ilam city. Environ Heal Eng Manag J 2:37–40
Bakheit MA, Torra D, Palomino LA, Thekisoe OMM, Mbati PA, Ongerth J, Karanis P (2008) Sensitive and specific detection of Cryptosporidium species in PCR-negative samples by loop-mediated isothermal DNA amplification and confirmation of generated LAMP products by sequencing. Vet Parasitol 158(1-2):11–22. https://doi.org/10.1016/j.vetpar.2008.09.012
Baldursson S, Karanis P (2011) Waterborne transmission of protozoan parasites: review of worldwide outbreaks—an update 2004-2010. Water Res 45(20):6603–6614. https://doi.org/10.1016/j.watres.2011.10.013
Barbosa JMM, Costa-de-Oliveira S, Rodrigues AG, Hanscheid T, Shapiro H, Pina-Vaz C (2008) A flow cytometric protocol for detection of Cryptosporidium spp. Cytom Part A 73:44–47
Baron EJ, Schenone C, Tanenbaum B (1989) Comparison of three methods for detection of Cryptosporidium oocysts in a low prevalence population. J Clin Microbiol 27(1):223–224
Blackburn BG, Mazurek JM, Hlavsa M, Park J, Tillapaw M, Parrish M, Salehi E, Franks W, Koch E, Smith F, Xiao L, Arrowood M, Hill V, da Silva A, Johnston S, Jones JL (2006) Cryptosporidiosis associated with ozonated apple cider. Emerg Infect Dis 12(4):684–686. https://doi.org/10.3201/eid1204.050796
Bouzid M, Hunter PR, Chalmers RM, Tyler KM (2013) Cryptosporidium pathogenicity and virulence. Clin Microbiol Rev 26:115–134
Calvo M, Carazo M, Arias ML, Chaves C, Monge R, Chinchilla M (2004) Prevalence of Cyclospora sp., Cryptosporidium sp, Microsporidia and fecal coliform determination in fresh fruit and vegetables consumed in Costa Rica. Arch Latinoam Nutr 54:428–432
Caradonna T, Marangi M, Del Chierico F, Ferrari N, Reddel S, Bracaglia G, Normanno G, Putignani L, Giangaspero A (2017) Detection and prevalence of protozoan parasites in ready-to-eat packaged salads on sale in Italy. Food Microbiol 67:67–75. https://doi.org/10.1016/j.fm.2017.06.006
Casemore DP, Jessop EG, Douce D, Jackson FB (1986) Cryptosporidium plus Campylobacter: an outbreak in a semi-rural population. J Hyg (Lond) 96(01):95–105. https://doi.org/10.1017/S0022172400062586
CDC (1996) Foodborne outbreak of diarrheal illness associated with Cryptosporidium parvum—Minnesota, 1995. Morb Mortal Wkly Rep 45:783–784
CDC (1997) Outbreaks of Escherichia coli O157:H7 infection and cryptosporidiosis associated with drinking unpasteurized apple cider—Connecticut and New York, October 1996. Morb Mortal Wkly Rep 46:4–8
CDC (1998) Foodborne outbreak of cryptosporidiosis—Spokane, Washington, 1997. Morb Mortal Wkly Rep 47:565–567
CDC (2011) Cryptosporidiosis outbreak at a summer camp—North Carolina, 2009. Morb Mortal Wkly Rep 60:918–922
CDC (2014) Outbreak of cryptosporidiosis among responders to a rollover of a truck carrying calves—Kansas, April 2013. Morb Mortal Wkly Rep 63:1185–1188
CDC (2015) Cryptosporidiosis associated with consumption of unpasteurized goat milk—Idaho, 2014. Morb Mortal Wkly Rep 64:194–195
Chappell CL, Okhuysen PC, Langer-Curry R, Widmer G, Akiyoshi DE, Tanriverdi S, Tzipori S (2006) Cryptosporidium hominis: experimental challenge of healthy adults. Am J Trop Med Hyg 75(5):851–857
Checkley W, White AC, Jaganath D, Arrowood MJ, Chalmers RM, Chen X-M, Fayer R, Griffiths JK, Guerrant RL, Hedstrom L, Huston CD, Kotloff KL, Kang G, Mead JR, Miller M, Petri WA, Priest JW, Roos DS, Striepen B, Thompson RCA, Ward HD, Van Voorhis WA, Xiao L, Zhu G, Houpt ER (2015) A review of the global burden, novel diagnostics, therapeutics, and vaccine targets for Cryptosporidium. Lancet Infect Dis 15(1):85–94. https://doi.org/10.1016/S1473-3099(14)70772-8
Cook N, Paton CA, Wilkinson N, Nichols RAB, Barker K, Smith HV (2006a) Towards standard methods for the detection of Cryptosporidium parvum on lettuce and raspberries. Part 1: development and optimization of methods. Int J Food Microbiol 109(3):215–221. https://doi.org/10.1016/j.ijfoodmicro.2005.12.015
Cook N, Paton CA, Wilkinson N, Nichols RAB, Barker K, Smith HV (2006b) Towards standard methods for the detection of Cryptosporidium parvum on lettuce and raspberries. Part 2: validation. Int J Food Microbiol 109(3):222–228. https://doi.org/10.1016/j.ijfoodmicro.2005.12.014
Cook N, Nichols RAB, Wilkinson N, Paton CA, Barker K, Smith HV (2007) Development of a method for detection of Giardia duodenalis cysts on lettuce and for simultaneous analysis of salad products for the presence of Giardia cysts and Cryptosporidium oocysts. Appl Environ Microbiol 73(22):7388–7391. https://doi.org/10.1128/AEM.00552-07
Damen JG, Banwat EB, Egah DZ, Allanana JA (2007) Parasitic contamination of vegetables in Jos, Nigeria. Ann Afr Med 6:115–118
Danyluk MD, Goodrich-Schneider RM, Schneider KR, Harris LJ, Worobo RW (2012) Outbreaks of foodborne disease associated with fruit and vegetable juices. Univ. Florida IFAS Ext. 1–7
Dawson D (2005) Foodborne protozoan parasites. Int J Food Microbiol 103:207–227
Deng MQ, Cliver DO (2000) Comparative detection of Cryptosporidium parvum oocysts from apple juice. Int J Food Microbiol 54:155–162
Dixon B, Parrington L, Cook A et al (2013) Detection of Cyclospora, Cryptosporidium, and Giardia in ready-to-eat packaged leafy greens in Ontario, Canada. J Food Prot 76:307–313. https://doi.org/10.4315/0362-028X.JFP-12-282
Dorsch MR, Veal DA (2001) Oligonucleotide probes for specific detection of Giardia Lamblia oocyst by fluorescent in situ hybridization. J Appl Microbiol 90:836–842. https://doi.org/10.1046/j.1365-2672.2001.01325.x
Downey AS, Graczyk TK (2007) Maximizing recovery and detection of Cryptosporidium parvum oocysts from spiked eastern oyster (Crassostrea virginica) tissue samples. Appl Environ Microbiol 73:6910–6915
EFSA (2007) The community summary report on trends and sources of zoonoses, zoonotic agents, antimicrobial resistance and foodborne outbreaks in the european union in 2006. EFSA J 130:1–352
Efstratiou A, Ongerth JE, Karanis P (2017) Waterborne transmission of protozoan parasites: review of worldwide outbreaks—an update 2011-2016. Water Res 114:14–22. https://doi.org/10.1016/j.watres.2017.01.036
El Sherbini GT, Kamel NO, Geneedy MR, Temsah AG (2016) A comparative study of the occurrence of Cryptosporidium parvum oocysts found on fresh fruits and vegetables sold in supermarkets and open-aired markets. Int J Curr Microbiol App Sci 5(8):760–768. https://doi.org/10.20546/ijcmas.2016.508.085
Ellington AD, Szostak JW (1990) In vitro selection of RNA molecules that bind specific ligands. Nature 346:818–822
Elsser KA, Moricz M, Proctor EM (1986) Cryptosporidium infections: a laboratory survey. CMAJ 135:211–213
Ethelberg S, Lisby M, Vestergaard LS, Enemark HL, Olsen KEP, Stensvold CR, Nielsen HV, Porsbo LJ, Plenser A-M, MØlbak K (2009) A foodborne outbreak of Cryptosporidium hominis infection. Epidemiol Infect 137(03):348–356. https://doi.org/10.1017/S0950268808001817
Fayer R, Graczyk TK, Lewis EJ, Trout JM, Farley ACA (1998) Survival of infectious Cryptosporidium parvum oocysts in seawater and eastern oysters (Crassostrea virginica) in the Chesapeake Bay. Appl Environ Microbiol 64(3):1070–1074
Fayer R, Lewis EJ, Trout JM, Graczyk TK, Jenkins MC, Higgins J, Xiao L, Lal AA (1999) Cryptosporidium parvum in oysters from commercial harvesting sites in the Chesapeake Bay. Emerg Infect Dis 5(5):706–710. https://doi.org/10.3201/eid0505.990513
Fayer R, Trout J, Lewis E, Xiao L, Lal A, Jenkins M, Graczyk T (2002) Temporal variability of Cryptosporidium in the Chesapeake Bay. Parasitol Res 88(11):998–1003. https://doi.org/10.1007/s00436-002-0697-1
Fayer R, Santin M, Macarisin D, Bauchan G (2013) Adhesive-tape recovery combined with molecular and microscopic testing for the detection of Cryptosporidium oocysts on experimentally contaminated fresh produce and a food preparation surface. Parasitol Res 112(4):1567–1574. https://doi.org/10.1007/s00436-013-3305-7
Feng Y, Xiao L (2011) Zoonotic potential and molecular epidemiology of Giardia species and giardiasis. Clin Microbiol Rev 24(1):110–140. https://doi.org/10.1128/CMR.00033-10
Fletcher SM, Stark D, Harkness J, Ellis J (2012) Enteric protozoa in the developed world: a public health perspective. Clin Microbiol Rev 25:420–449. https://doi.org/10.1128/CMR.05038-11
Focke F, Haase I, Fischer M (2013) Loop-mediated isothermal amplification (LAMP): methods for plant species identification in food. J Agric Food Chem 61:2943–2949. https://doi.org/10.1021/jf304295b
Frazar C, Orlandi P (2007) Evaluation of two DNA template preparation methods for post-immunomagnetic separation detection of Cryptosporidium parvum in foods and beverages by PCR. Appl Environ Microbiol 73(22):7474–7476. https://doi.org/10.1128/AEM.01652-07
Freire-Santos F, Oteiza-López AM, Vergara-Castiblanco CA, Ares-Mazás E, Álvarez-Suárez E, García-Martín O (2000) Detection of Cryptosporidium oocysts in bivalve molluscs destined for human consumption. J Parasitol 86(4):853–854.
Gallas-Lindemann C, Sotiriadou I, Mahmoodi MR, Karanis P (2013) Detection of Toxoplasma gondii oocysts in different water resources by loop mediated isothermal amplification (LAMP). Acta Trop 125(2):231–236. https://doi.org/10.1016/j.actatropica.2012.10.007
Gelletlie R, Stuart J, Soltanpoor N, Armstrong R, Nichols G, AnonymousFreidank H, Kist M, Angus K, Scott C, Smith H, Mtambo M, Gibbs H, Harp J, Frayer R, Pesch B, Jackson G (1997) Cryptosporidiosis associated with school milk. Lancet (London, England) 350(9083):1005–1006. https://doi.org/10.1016/S0140-6736(05)64071-8
Giangaspero A, Molini U, Iorio R, Traversa D, Paoletti B, Giansante C (2005) Cryptosporidium parvum oocysts in seawater clams (Chamelea gallina) in Italy. Prev Vet Med 69(3-4):203–212. https://doi.org/10.1016/j.prevetmed.2005.02.006
Gomez-Bautista M, Ortega-Mora LM, Tabares E, Lopez-Rodas V, Costas E (2000) Detection of infectious Cryptosporidium parvum oocysts in mussels (Mytilus galloprovincialis) and cockles (Cerastoderma edule). Appl Environ Microbiol 66(5):1866–1870. https://doi.org/10.1128/AEM.66.5.1866-1870.2000
Gómez-Couso H, Freire-Santos F, Martínez-Urtaza J, García-Martín O, Ares-Mazás ME (2003) Contamination of bivalve molluscs by Cryptosporidium oocysts: the need for new quality control standards. Int J Food Microbiol 87(1-2):97–105. https://doi.org/10.1016/S0168-1605(03)00057-6
Gómez-Couso H, Méndez-Hermida F, Ares-Mazás E (2006a) Levels of detection of Cryptosporidium oocysts in mussels (Mytilus galloprovincialis) by IFA and PCR methods. Vet Parasitol 141:60–65
Gómez-Couso H, Méndez-Hermida F, Castro-Hermida JA, Ares-Mazás E (2006b) Cryptosporidium contamination in harvesting areas of bivalve molluscs. J Food Prot 69(1):185–190
Gottfries C (2011) Detection of Cryptosporidium oocysts on lettuce and raspberries 1–13. Dissertation, University of Upssala
Graczyk TK, Fayer R, Lewis EJ, Trout JM, Farley CA (1999) Cryptosporidium oocysts in Bent mussels (Ischadium recurvum) in the Chesapeake Bay. Parasitol Res 85(7):518–521. https://doi.org/10.1007/s004360050590
Graczyk TK, Marcogliese DJ, de Lafontaine Y, Da Silva AJ, Mhangami-Ruwende B, Pieniazek NJ (2001) Cryptosporidium parvum oocysts in zebra mussels (Dreissena polymorpha): evidence from the St Lawrence river. Parasitol Res 87(3):231–234. https://doi.org/10.1007/s004360000293
Guiguet Leal DA, Pereira MA, Bueno Franco RM, Branco N, Neto R (2008) First report of Cryptosporidium spp. oocysts in oysters (Crassostrea rhizophorae) and cockles (Tivela mactroides) in Brazil. J Wat Health 6:527–532
Hancock-Allen J, Alden ÃNB, Cronquist AB (2017) Cryptosporidiosis outbreak at an academic animal research laboratory―Colorado, 2014. Am J Ind Med 214:208–214
Hanscheid T, Cristino JM, Salgado MJ (2008) Screening of auramine-stained smears of all fecal samples is a rapid and inexpensive way to increase the detection of coccidial infections. Int J Infect Dis 12(1):47–50. https://doi.org/10.1016/j.ijid.2007.04.008
Harper CM, Cowell NA, Adams BC, Langley AJ, Wohlsen TD (2002) Outbreak of Cryptosporidium linked to drinking unpasteurised milk. Commun Dis Intell Q Rep 26(3):449–450
Henriksen S, Pohlenz J (1981) Staining of Cryptosporidia by a modified Ziehl-Neelsen technique. Acta Vet Scand 22(3-4):594–596
Hohweyer J, Cazeaux C, Travaillé E, Languet E, Dumètre A, Aubert D, Terryn C, Dubey JP, Azas N, Houssin M, Loïc F, Villena I, La Carbona S (2016) Simultaneous detection of the protozoan parasites Toxoplasma, Cryptosporidium and Giardia in food matrices and their persistence on basil leaves. Food Microbiol 57:36–44. https://doi.org/10.1016/j.fm.2016.01.002
Hong S, Kim K, Yoon S, Park W-Y, Sim S, Yu J-R (2014) Detection of Cryptosporidium parvum in environmental soil and vegetables. J Korean Med Sci 29(10):1367–1371. https://doi.org/10.3346/jkms.2014.29.10.1367
Hsu BM, Wu NM, Jang HD, Shih FC, Wan MT, Kung CM (2005) Using the flow cytometry to quantify the Giardia cysts and Cryptosporidium oocysts in water samples. Environ Monit Assess 104(1-3):155–162. https://doi.org/10.1007/s10661-005-1608-6
Ignatius R, Lehmann M, Miksits K, Regnath T, Arvand M, Engelmann E, Futh U, Hahn H, Wagner J (1997) A new acid-fast trichrome stain for simultaneous detection of Cryptosporidium parvum and microsporidial species in stool specimens. J Clin Microbiol 35:446–449
Ignatius R, Klemm T, Zander S, Bosco Gahutu J, Kimmig P, Mockenhaupt FP, Regnath T (2016) Highly specific detection of Cryptosporidium spp. oocysts in human stool samples by undemanding and inexpensive phase contrast microscopy. Parasitol Res 115(3):1229–1234. https://doi.org/10.1007/s00436-015-4859-3
Insulander M, de Jong B, Svenungsson B (2008) A food-borne outbreak of cryptosporidiosis among guests and staff at a hotel restaurant in Stockholm county, Sweden, September 2008. Euro Surveill 13
Insulander M, Silverlås C, Lebbad M, Karlsson L, Mattsson J, Svenungsson B (2013) Molecular epidemiology and clinical manifestations of human cryptosporidiosis in Sweden. Epidemiol Infect 141(05):1009–1020. https://doi.org/10.1017/S0950268812001665
Iqbal A, Labib M, Muharemagic D, Sattar S, Dixon BR, Berezovski MV, Chalmers R, Katzer F, Ryan U, Fayer R, Xiao L, Chalmers R, Scallan E, Hoekstra R, Angulo F, Tauxe R, Widdowson M, Roy S, Budu-Amoako E, Greenwood S, Dixon B, BarkemaH MCJ, Dixon B, Coklin T, Farber J, Parrington L, Kingombe C, Bin Ross W, Dixon B, Savioli L, Smith H, Thompson A, Labib M, Berezovski M, Tuerk C, Gold L, Ellington A, Szostak J, Berezovski M, Lechmann M, Musheev M, Mak T, Krylov S, Xiao Y, Lai R, Plaxco K, Sefah K, Shangguan D, Xiong X, O’Donoghue M, Tan W, Daniels D, Chen H, Hicke B, Swiderek K, Gold L (2015) Detection of Cryptosporidium parvum oocysts on fresh produce using DNA aptamers. PLoS One 10(9):e0137455. https://doi.org/10.1371/journal.pone.0137455
ISO 18744:2016(en) (2016) Microbiology of the food chain—detection and enumeration of Cryptosporidium and Giardia in fresh leafy green vegetables and berry fruits
Karanis P, Ongerth J (2009) LAMP – a powerful and flexible tool for monitoring microbial pathogens. Trends Parasitol 25:498–499. https://doi.org/10.1016/j.pt.2009.07.010
Karanis P, Kourenti C, Smith H (2007a) Waterborne transmission of protozoan parasites: a worldwide review of outbreaks and lessons learnt. J Water Health 5:1–38
Karanis P, Thekisoe O, Kiouptsi K, Ongerth J, Igarashi I, Inoue N (2007b) Development and preliminary evaluation of a loop-mediated isothermal amplification procedure for sensitive detection of Cryptosporidium oocysts in fecal and water samples. Appl Environ Microbiol 73:5660–5662
Keserue H-A, Füchslin HP, Wittwer M, Nguyen-Viet H, Nguyen TT, Surinkul N, Koottatep T, Schürch N, Egli T (2012) Comparison of rapid methods for detection of Giardia spp. and Cryptosporidium spp. (oo)cysts using transportable instrumentation in a field deployment. Environ Sci Technol 46(16):8952–8959. https://doi.org/10.1021/es301974m
Khurana S, Sharma P, Sharma A, Malla N (2012) Evaluation of Ziehl-Neelsen staining, auramine phenol staining, antigen detection enzyme linked immunosorbent assay and polymerase chain reaction, for the diagnosis of intestinal cryptosporidiosis. Trop Parasitol 2(1):20–23. https://doi.org/10.4103/2229-5070.97234
Kotloff KL (2017) The burden and etiology of diarrheal illness in developing countries. Pediatr Clin North Am 64:799–814
Lalonde LF, Gajadhar AA (2011) Detection and differentiation of coccidian oocysts by real-time PCR and melting curve analysis. J Parasitol 97:725–730. https://doi.org/10.1645/GE-2706.1
Lalonde LF, Gajadhar AA (2016a) Detection of Cyclospora cayetanensis, Cryptosporidium spp., and Toxoplasma gondii on imported leafy green vegetables in Canadian survey. Food Waterborne Parasitol 2:8–14
Lalonde LF, Gajadhar AA (2016b) Optimization and validation of methods for isolation and real-time PCR identification of protozoan oocysts on leafy green vegetables and berry fruits. Food Waterborne Parasitol 2:1–7. https://doi.org/10.1016/j.fawpar.2015.12.002
Ley DH, Levy MG, Hunter L, Corbett W, Barnes HJ (1988) Cryptosporidia-positive rates of avian necropsy accessions determined by examination of auramine O-stained fecal smears. Avian Dis 32(1):108–113. https://doi.org/10.2307/1590957
Li X, Guyot K, Dei-Cas E, Mallard J-P, Ballet JJ, Brasseur P (2006) Cryptosporidium oocysts in mussels (Mytilus edulis) from Normandy (France). Int J Food Microbiol 108(3):321–325. https://doi.org/10.1016/j.ijfoodmicro.2005.11.018
Lowery CJ, Nugent P, Moore JE, Millar BC, Xiru X, Dooley JS (2001) PCR-IMS detection and molecular typing of Cryptosporidium parvum recovered from a recreational river source and an associated mussel (Mytilusedulis) bed in Northern Ireland. Epidemiol Infect 127(3):545–553
Macarisin D, Bauchan G, Fayer R (2010a) Spinacia oleracea L. leaf stomata harboring Cryptosporidium parvum oocysts: a potential threat to food safety. Appl Environ Microbiol 76:555–559
Macarisin D, Santín M, Bauchan G, Fayer R (2010b) Infectivity of Cryptosporidium parvum oocysts after storage of experimentally contaminated apples. J Food Prot 73(10):1824–1829. https://doi.org/10.4315/0362-028X-73.10.1824
Machado ECL, Stamford TLM, Alves LC, Melo RG, Shinohara NKS (2006) Effectiveness of Cryptosporidium spp. oocysts detection and enumeration methods in water and milk samples. Arq Bras Med Vet Zootec 58(3):432–439. https://doi.org/10.1590/S0102-09352006000300023
MacRae M, Hamilton C, Strachan NJC, Wright S, Ogden ID (2005) The detection of Cryptosporidium parvum and Escherichia coli O157 in UK bivalve shellfish. J Microbiol Methods 60(3):395–401. https://doi.org/10.1016/j.mimet.2004.10.017
Mahon M, Doyle S (2017) Waterborne outbreak of cryptosporidiosis in the South East of Ireland: weighing up the evidence Ir J Med Sci 186(4):989–994
Maikai BV, Baba-Onoja EBT, Elisha IA (2013) Contamination of raw vegetables with Cryptosporidium oocysts in markets within Zaria metropolis, Kaduna State, Nigeria. Food Control 31(1):45–48. https://doi.org/10.1016/j.foodcont.2012.09.032
Manti A, Boi P, Amalfitano S, Puddu A, Papa S (2011) Experimental improvements in combining CARD-FISH and flow cytometry for bacterial cell quantification. J Microbiol Methods 87(3):309–315. https://doi.org/10.1016/j.mimet.2011.09.003
Marquis ND, Record NR, Fernández Robledo JA (2015) Survey for protozoan parasites in Eastern oysters (Crassostrea virginica) from the Gulf of Maine using PCR-based assays. Parasitol Int 64(5):299–302. https://doi.org/10.1016/j.parint.2015.04.001
McKerr C, Adak GK, Nichols G, Gorton R, Chalmers RM, Kafatos G, Cosford P, Charlett A, Reacher M, Pollock KG, Alexander CL, Morton S (2015) An outbreak of Cryptosporidium parvum across England and Scotland associated with consumption of fresh pre-cut salad leaves, May 2012. PLoS One 10:1–13
Millard PS, Gensheimer KF, Addiss DG, Sosin DM, Beckett GA, Houck-Jankoski A, Hudson A (1994) An outbreak of cryptosporidiosis from fresh-pressed apple cider. JAMA 272(20):1592–1596. https://doi.org/10.1001/jama.1994.03520200048034
Miller WA, Miller MA, Gardner IA, Atwill ER, Harris M, Ames J, Jessup D, Melli A, Paradies D, Worcester K, Olin P, Barnes N, Conrad PA (2005) New genotypes and factors associated with Cryptosporidium detection in mussels (Mytilus spp.) along the California coast. Int J Parasitol 35:1103–1113
Miller WA, Gardner IA, Atwill ER, Leutenegger CM, Miller MA, Hedrick RP, Melli AC, Barnes NM, Conrad PA (2006) Evaluation of methods for improved detection of Cryptosporidium spp. in mussels (Mytilus californianus). J Microbiol Methods 65(3):367–379. https://doi.org/10.1016/j.mimet.2005.08.011
Minarovičová J, Kaclikova E, Krascsenicsova K, Siekel P (2007) Detection of Cryptosporidium parvum by polymerase chain reaction. J Food Nutr Res 46:58–62
Minarovicová J, Kaclíková E, Krascsenicsová K, Siekel P, Kuchta T (2009) A single-tube nested real-time polymerase chain reaction for sensitive contained detection of Cryptosporidium parvum. Lett Appl Microbiol 49:568–572
Minarovičová J, Lopašovská J, Valík Ľ, Kuchta T (2011) A method for the detection of Cryptosporidium parvum oocysts in milk based on microfiltration and real-time polymerase chain reaction. Food Anal Methods 4:116–120
Monge R, Arias ML (1996) Presence of various pathogenic microorganisms in fresh vegetables in Costa Rica. Arch Latinoam Nutr 46(4):292–294
Mongej R, Chinchilla M (1996) Presence of Cryptosporidium oocysts in fresh vegetables. J Food Prot 59(2):202–203. https://doi.org/10.4315/0362-028X-59.2.202
Moriarty EM, Duffy G, McEvoy JM et al (2005) The effect of thermal treatments on the viability and infectivity of Cryptosporidium parvum on beef surfaces. J Appl Microbiol 98:618–623. https://doi.org/10.1111/j.1365-2672.2004.02498.x
Mosteo R, Goñi P, Miguel N, Abadías J, Valero P, Ormad MP (2016) Bioaccumulation of pathogenic bacteria and amoeba by zebra mussels and their presence in watercourses. Environ Sci Pollut Res 23:1833–1840
Nagamine K, Hase T, Notomi T (2002) Accelerated reaction by loop-mediated isothermal amplification using loop primers. Mol Cell Probes 16:223–229
Negm AY (2003) Human pathogenic protozoa in bivalves collected from local markets in Alexandria. J Egypt Soc Parasitol 33:991–998
Ocker R, Prompunjai Y, Chutipongvivate S, Karanis P (2016) Malaria diagnosis by loop-mediated isothermal amplification (LAMP) in thailand. Rev Inst Med Trop Sao Paulo 58:27
Orlandi PA, Lampel KA (2000) Extraction-free, filter-based template preparation for rapid and sensitive PCR detection of pathogenic parasitic protozoa. J Clin Microbiol 38:2271–2277
Ortega YR, Roxas CR, Gilman RH, Miller NJ, Cabrera L, Taquiri C, Sterling CR (1997) Isolation of Cryptosporidium parvum and Cyclospora cayetanensis from vegetables collected in markets of an endemic region in Peru. Am J Trop Med Hyg 57:683–686
Pacheco FT, Silva RK, Martins AS, Oliveira RR, Alcântara-Neves NM, Silva MP, Soares NM, Teixeira MC (2013) Differences in the detection of Cryptosporidium and Isospora (Cystoisospora) oocysts according to the fecal concentration or staining method used in a clinical laboratory. J Parasitol 99:1002–1008
Payne P, Lancaster LA, Heinzman M, McCutchan JA (1983) Identification of Cryptosporidiumin patients with the acquired immunodeficiency syndrome. New Engl J Med 309:613–614
Petersen TB, Petersen HH, Abaidoo RC, Enemark H, Dalsgaard A (2014) Occurrence of Cryptosporidium spp. oocysts in low quality water and on vegetables irrigated with low quality water in Kumasi, Ghana. In: The 5th International Giardia and Cryptosporidium Conference. Sweden, p. 116
Plutzer J, Törökné A, Karanis P (2010) Combination of ARAD microfibre filtration and LAMP methodology for simple, rapid and cost-effective detection of human pathogenic Giardia duodenalis and Cryptosporidium spp. in drinking water. Lett Appl Microbiol 50:82–88
Pomeranz Y (1976) Scanning electron microscopy in food science and technology. Adv Food Res 22:205–307
Pönka A, Kotilainen H, Rimhanen-Finne R, Hokkanen P, Hänninen ML, Kaarna A, Meri T, Kuusi M (2009) A foodborne outbreak due to Cryptosporidium parvum in Helsinki, November 2008. Euro Surveill. 14. https://doi.org/10.2807/ese.14.28.19269-en
Quiroz ES, Bern C, MacArthur JR, Xiao L, Fletcher M, Arrowood MJ, Shay DK, Levy ME, Glass RI, Lal A (2000) An outbreak of cryptosporidiosis linked to a foodhandler. J Infect Dis 181(2):695–700. https://doi.org/10.1086/315279
Rahman J, Islam Talukder A, Hossain F, Mahomud S, Atikul Islam M, Shamsuzzoha S (2014) Detection of Cryptosporidium oocyts in commonly consumed fresh salad vegetables. Am J Microbiol Res 2:224–226
Ranjbar-Bahadori S, Mostoophi A, Shemshadi B (2013) Study on Cryptosporidium contamination in vegetable farms around Tehran. Trop Biomed 30(2):193–198
Rayan HZ, Eida OM, El-hamshary EM, Ahmed SA (2009) Detection of human Cryptosporidium species in surface water sources in ismailia using polymerase chain reaction. Parasitol United J 2:119–126
Rimseliene G, Vold L, Robertson L, Nelke C, Soli K, Johansen OH, Thrana FS, Nygard K (2011) An outbreak of gastroenteritis among schoolchildren staying in a wildlife reserve: thorough investigation reveals Norway’s largest cryptosporidiosis outbreak. Scand J Public Health 39:287–295
Ripabelli G, Leone A, Sammarco ML, Fanelli I, Grasso GM, McLauchlin J (2004) Detection of Cryptosporidium parvum oocysts in experimentally contaminated lettuce using filtration, immunomagnetic separation, light microscopy, and PCR. Foodborne Pathog Dis 1(4):216–222. https://doi.org/10.1089/fpd.2004.1.216
Robertson LJ (2014) Approaches to detecting Cryptosporidium oocysts in different food matrices, In: Cryptosporidium as a foodborne pathogen. Springer New York, New York, pp. 25–38
Robertson LJ, Gjerde B (2000) Isolation and enumeration of Giardia cysts, Cryptosporidium oocysts, and Ascaris eggs from fruits and vegetables. J Food Prot 63(6):775–778. https://doi.org/10.4315/0362-028X-63.6.775
Robertson LJ, Gjerde B (2001a) Occurrence of parasites on fruits and vegetables in Norway. J Food Prot 64(11):1793–1798. https://doi.org/10.4315/0362-028X-64.11.1793
Robertson LJ, Gjerde B (2001b) Factors affecting recovery efficiency in isolation of Cryptosporidium oocysts and Giardia cysts from vegetables for standard method development. J Food Prot 64(11):1799–1805. https://doi.org/10.4315/0362-028X-64.11.1799
Robertson LJ, Gjerde B (2008) Development and use of a pepsin digestion method for analysis of shellfish for Cryptosporidium oocysts and Giardia cysts. J Food Prot 71(5):959–966. https://doi.org/10.4315/0362-028X-71.5.959
Robertson LJ, Huang Q (2012) Analysis of cured meat products for Cryptosporidium oocysts following possible contamination during an extensive waterborne outbreak of cryptosporidiosis. J Food Prot 75(5):982–988. https://doi.org/10.4315/0362-028X.JFP-11-525
Robertson LJ, Johannessen GS, Gjerde BK, Loncarevic S (2002) Microbiological analysis of seed sprouts in Norway. Int J Food Microbiol 75(1-2):119–126. https://doi.org/10.1016/S0168-1605(01)00738-3
Rodríguez-Hernandez J, Canut-Blasco A, Ledesma-Garcia M, Martín-Sánchez AM (1994) Cryptosporidium oocysts in water for human consumption. Comparison of staining methods. Eur J Epidemiol 10(2):215–218. https://doi.org/10.1007/BF01730373
Rohde A, Hammerl JA, Appel B et al (2015) FISHing for bacteria in food--a promising tool for the reliable detection of pathogenic bacteria? Food Microbiol 46:395–407. https://doi.org/10.1016/j.fm.2014.09.002
Romanova TV, Shkarin VV, Khazenson LB (1992) Group cryptosporidiosis morbidity in children. Med Parazitol (Mosk) 3:50–52
Rosado-García FM, Guerrero-Flórez M, Karanis G, Hinojosa MDC, Karanis P (2017) Water-borne protozoa parasites: the Latin American perspective. Int J Hyg Environ Health 220:783–798
Said DES (2012) Detection of parasites in commonly consumed raw vegetables. Alex J Med 48:345–352
Schets FM, van den Berg HHJL, Engels GB, Lodder WJ, de Roda Husman AM (2007) Cryptosporidium and Giardia in commercial and non-commercial oysters (Crassostrea gigas) and water from the Oosterschelde, the Netherlands. Int J Food Microbiol 113:189–194
Sharma KP, Chattopadhyay UK (2015) Isolation and identification of Cryptosporidium spp. from raw meat amples sold in open markets of the city of Kolkata. IOSR J Agric Vet Sci Ver I 8:2319–2372
Shields JM, Lee MM, Murphy HR (2012) Use of a common laboratory glassware detergent improves recovery of Cryptosporidium parvum and Cyclospora cayetanensis from lettuce, herbs and raspberries. Int J Food Microbiol 153:123–128
Shields JM, Joo J, Kim R, Murphy HR (2013) Assessment of three commercial DNA extraction kits and a laboratory-developed method for detecting Cryptosporidium and Cyclospora in raspberry wash, basil wash and pesto. J Microbiol Methods 92:51–58
Smith HV (1998) Detection of parasites in the environment. Parasitology 117(Suppl):S113–S141
Smith HV, Nichols RAB (2010) Cryptosporidium: detection in water and food. Exp Parasitol 124:61–79
Squire SA, Ryan U (2017) Cryptosporidium and Giardia in Africa: current and future challenges. Parasit Vectors 10:195
Takeuchi K, Frank JF (2001) Confocal microscopy and microbial viability detection for food research. J Food Prot 64(12):2088–2102. https://doi.org/10.4315/0362-028X-64.12.2088
TDH (2015) Disease investigations linked to increased raw milk consumption. TDH Invetigating Cases Gastrointest. Dis. http://outbreakdatabase.com/reports/2015_Raw_Milk_Crypto_Tennessee.pdf
Tedde T, Piras G, Salza S, Nives RM, Sanna G, Tola S, Culurgioni J, Piras C, Merella P, Garippa G, Virgilio S (2013) Investigation into Cryptosporidium and Giardia in bivalve mollusks farmed in Sardinia region and destined for human consumption. Ital J Food Saf 2(2):26. https://doi.org/10.4081/ijfs.2013.e26
Tei FF, Kowalyk S, Reid JA, Presta MA, Yesudas R, Mayer DCG (2016) Assessment and molecular characterization of human intestinal parasites in bivalves from Orchard Beach, NY, USA. Int J Environ Res Public Health 13(4):381. https://doi.org/10.3390/ijerph13040381
Thekisoe OMM, Bazie RSB, Coronel-Servian AM, Sugimoto C, Kawazu S-I, Inoue N (2009) Stability of loop-mediated isothermal amplification (LAMP) reagents and its amplification efficiency on crude trypanosome DNA templates. J Vet Med Sci 71(4):471–475. https://doi.org/10.1292/jvms.71.471
Ugwoke EV, Umoh JU, Okolocha EC, Lawal IA (2013) Cryptosporidium oocysts in Anodonta sp. (bivalve mollusc) as indicators of pollution of Tiga Lake ecosystem in Kano State, Nigeria. J Parasitol Vector Biol 5:77–82
Utaaker KS, Huang Q, Robertson LJ (2015) A reduced-cost approach for analyzing fresh produce for contamination with Cryptosporidium oocysts and/or Giardia cysts. Food Res Int 77:326–332
Utaaker KS, Kumar A, Joshi H, Chaudhary S, Robertson LJ (2017) Checking the detail in retail: occurrence of Cryptosporidium and Giardia on vegetables sold across different counters in Chandigarh, India. Int J Food Microbiol 263:1–8. https://doi.org/10.1016/j.ijfoodmicro.2017.09.020
Valdez LM, Dang H, Okhuysen PC, Chappell CL (1997) Flow cytometric detection of Cryptosporidium oocysts in human stool samples. J Clin Microbiol 35(8):2013–2017
Vojdani JD, Beuchat LR, Tauxe RV (2008) Juice-associated outbreaks of human illness in the United States, 1995 through 2005. J Food Prot 71(2):356–364. https://doi.org/10.4315/0362-028X-71.2.356
Widerström M, Schönning C, Lilja M, Lebbad M, Ljung T, Allestam G, Ferm M, Björkholm B, Hansen A, Hiltula J, Långmark J, Löfdahl M, Omberg M, Reuterwall C, Samuelsson E, Widgren K, Wallensten A, Lindh J (2014) Large outbreak of Cryptosporidium hominis infection transmitted through the public water supply, Sweden. Emerg Infect Dis 20:581–589
Willis JE, Greenwood S, Spears J, Davidson J, McClure C, McClure JT (2012) Ability of oysters (Crassostrea virginica) to harbour zoonotic parasites Cryptosporidium parvum and Giardia duodenalis during constant or limited exposures in a static tank system. Oral Presentation, IV International Giardia & Cryptosporidum Conference, Wellington, New Zealand. Page 126 of Abstract book
Yang R, Paparini A, Monis P, Ryan U (2014) Comparison of next-generation droplet digital PCR (ddPCR) with quantitative PCR (qPCR) for enumeration of Cryptosporidium oocysts in faecal samples. Int J Parasitol 44(14):1105–1113. https://doi.org/10.1016/j.ijpara.2014.08.004
Yoshida H, Matsuo M, Miyoshi T, Uchino K, Nakaguchi H, Fukumoto T, Teranaka Y, Tanaka T (2007) An outbreak of cryptosporidiosis suspected to be related to contaminated food, October 2006, Sakai City, Japan. Jpn J Infect Dis 60(6):405–407
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Ahmed, S.A., Karanis, P. An overview of methods/techniques for the detection of Cryptosporidium in food samples. Parasitol Res 117, 629–653 (2018). https://doi.org/10.1007/s00436-017-5735-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00436-017-5735-0