Abstract
Acanthocephalans display a two-host life cycle that involves arthropods as intermediate hosts and vertebrates as definitive hosts. Some species also use paratenic hosts to bridge the trophic gap between both obligatory hosts. However, the relative role of these paratenic hosts in the transmission to definitive hosts has seldom been assessed quantitatively. We report on infection patterns of cystacanths of Corynosoma australe Johnston, 1937 in 20 common teleost species and the Argentine shortfin squid Illex argentinus (Castellanos) from the Patagonian shelf of Argentina. We also explore the role of different fish species in the transmission of C. australe to the most important definitive host in the area, i.e. the South American sea lion Otaria flavescens Shaw. Cystacanths of C. australe were found in all host species except Heliconus lahillei Norman, Merluccius hubbsi Marini and I. argentinus. In eight fish species, the prevalence of C. australe was > 50% and mean intensity > 4, i.e. Acanthistius patachonicus (Jenyns), Nemadactylus bergi (Norman), Paralichthys isosceles Jordan, Percophis brasiliensis Quoy & Gaimard, Prionotus nudigula Ginsburg, Scomber colias Gmelin, Raneya brasiliensis (Kaup) and Xystreurys rasile (Jordan). Two surveys on the trophic ecology of South American sea lions in the study area consistently found a generalist diet dominated by M. hubbsi, and data on the frequency of occurrence and number of other fish and cephalopod species in stomach contents strongly suggest that only R. brasiliensis may play a prominent role in the transmission of C. australe. This result raises interesting questions on the costs of paratenicity.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Acanthocephalans display a two-host life cycle that involves arthropods as intermediate hosts and vertebrates as definitive hosts (Kennedy 2006). Some acanthocephalans also use paratenic hosts (usually teleosts, reptiles or small mammals) as complementary pathways to bridge the trophic gap between the arthropods and the vertebrate definitive hosts (Schmidt 1985). After being recruited into the paratenic host, cystacanths (the infective larval stage) pass from the intestinal wall to extra-intestinal sites, e.g. mesenteries, where they are encapsulated (Kennedy 2006; Taraschewski 2000).
Paratenic transmission seems to occur in 10–20% of the more than 1298 acanthocephalan species described (Parker et al. 2009; Amin 2013). However, little is known about the specific identity of paratenic hosts for most acanthocephalan species (Kennedy 2006). Available evidence suggests that paratenicity can be facultative in many cases (Schmidt 1985; Taraschewski 2000), but for species that reproduce in carnivorous mammals or birds (e.g. species of Corynosoma Lühe, 1904, Centrorhynchus Lühe, 1911 or Oncicola Travassos, 1916), transmission often depends on paratenic hosts to reach the definitive host (Taraschewski 2000).
Much of the research on acanthocephalans that use paratenic hosts has been carried out with species of Corynosoma, a group of cosmopolitan marine acanthocephalans that use pinnipeds as primary definitive hosts and, to a lesser extent, cetaceans, aquatic birds, sea otters and semiaquatic rats (Aznar et al. 2006, 2012; Hernández-Orts et al. 2017 and references therein). The complete life cycle is relatively well-known only for few species of Corynosoma, and it involves benthic amphipods as intermediate hosts (e.g. Hoberg 1986; Sinisalo and Valtonen 2003) and a wide array of fish as potential paratenic hosts (e.g. Valtonen 1983; Laskowski and Zdzitowiecki 2005).
Studies on cystacanths of Corynosoma spp. in fish have provided valuable information about species records (e.g. Laskowski and Zdzitowiecki 2005), taxonomic issues (e.g. Sardella et al. 2005), host–parasite interactions (e.g. Skorobrechova and Nikishin 2011), development (e.g. Hernández-Orts et al. 2012) and ecology (e.g. Valtonen 1983; Sinisalo and Valtonen 2003). However, there is little quantitative research on the relative role of several paratenic fish hosts from a local community in the transmission to definitive hosts (Valtonen 1983). To assess such relative role in transmission, quantitative data on both prey composition in the definitive hosts and infection levels in both prey and non-prey fish hosts are required.
In Patagonia, Argentina, Corynosoma australe Johnston, 1937 is a common acanthocephalan that reproduces in the intestine of the South American sea lion Otaria flavescens (Shaw) and the South American fur seal Arctocephalus australis (Zimmermann) (Hernández-Orts et al. 2013). Gravid females of C. australe were recently found also in the intestine of the Magellanic penguin Spheniscus magellanicus (Forster) from Brazil (Hernández-Orts et al. 2017), but the parasite is generally unable to reproduce in non-pinniped predators such as elasmobranchs (Knoff et al. 2001), fish-eating birds (Hoberg and Ryan 1989) or cetaceans (Aznar et al. 2012). Corynosoma australe is known to use marine teleosts as paratenic hosts, and cystacanths have been commonly reported in many marine teleosts collected off the coast of Argentina (e.g. Timi et al. 2011; Vales et al. 2011; Soares et al. 2018 and references therein). However, to our knowledge, there have been not attempt to evaluate the role of these fish species in the transmission of C. australe to definitive hosts. In this study, we firstly report on infection parameters of C. australe in one species of cephalopod and 20 common teleost species inhabiting the Patagonian shelf of Argentina. Then, we use previous dietary data in the same area from one of the main definitive hosts, the South American sea lion (see Koen-Alonso et al. 2000; Romero et al. 2011), to investigate the role of different fish species in the transmission of C. australe.
Material and methods
Sample collection
A total of 27 Argentine shortfin squids Illex argentinus (Castellanos) and 542 individuals of 20 species of fish were examined (Table 1). Squids and fishes were caught by commercial bottom trawling vessels during 2006–2007 in two areas of the Patagonian shelf of Argentina: north (42° 45′–42° 59′ S, 61° 09′–62° 58′ W) and central Patagonia (47° 00′–47° 19′ S, 61° 59′–64° 25′ W). Hosts were selected based on their abundance in the sampling areas (Bezzi et al. 2000) and according to their body size, i.e. fish whose size was within the range of those reported in the diet of sea lions from the study area (Koen-Alonso et al. 2000; Marine Mammal Laboratory, CESIMAR – CCT CONICET – CENPAT, unpublished data). Collected specimens were kept on ice on board and, after arrival to the laboratory, were identified following Brunetti et al. (1999) for squids and Menni et al. (1984) for fishes. Scientific names for fish species were validated following Froese and Pauly (2018). Individual hosts were then either examined fresh or were frozen in plastic bags at − 20 °C for later examination. Fresh or thawed specimens were dissected, and internal organs were separated in Petri dishes and examined under a stereomicroscope (up to 40×) to detect encapsulated cystacanths. Worms were placed in physiological saline, removed from their capsule and fixed in 70% ethanol. All the cystacanths were examined using a stereomicroscope (up to 80×) and identified following Sardella et al. (2005).
Infection patterns
Ecological terms follow Bush et al. (1997) and Rózsa et al. (2000). The 95% confidence interval (CI) for prevalence was set with Sterne’s exact method (Reiczigel 2003), whereas the 95% CIs for mean abundance and mean intensity were estimated with the bias-corrected and accelerated bootstrap using 20,000 replications (Reiczigel and Rózsa 2005). A preliminary analysis indicated that there were no significant differences in the abundance of cystacanths of C. australe between the two sampling localities for any fish species with a sample size ≥ 12 individuals (Mann–Whitney tests, P > 0.05). Therefore, infection parameters and statistical analyses were calculated for pooled data.
Relationship between infection patterns and South American sea lion diet
To explore the relative importance of different fish species in the transmission of C. australe to South American sea lions, we used dietary data from sea lions obtained in the study area. Two independent data sets were dealt with, namely, that from Koen-Alonso et al. (2000), including additional data from Koen-Alonso (1999), which was based on stomach contents obtained from 59 sea lions (28 males, 31 females) in northern and central Patagonia between 1982 and 1998, and that from Romero et al. (2011), which was based on 33 sea lions (17 males, 16 females) collected in northern Patagonia (San Matías Gulf) between 2006 and 2009.
We used two parameters to measure the relative importance of each fish species in the diet, which were gathered from the dietary surveys mentioned above:
-
1)
Percentage by number (%N), which is calculated as per cent of prey individuals of fish species i in the overall sample of individual prey regardless of prey species. This parameter is somehow analogous to a ‘relative abundance’ of fish species per sea lion and is relevant from a parasitological point of view because each individual fish represents a potential ‘packet’ of C. australe recruits.
-
2)
Index of relative importance (IRI) (Hart et al. 2002), which is calculated as (%Ni + %Wi) × %FOi, where %N is as defined above, %W is the percentage wet weight of fish species i in the overall sample (a measure of biomass) and FO is the frequency of occurrence of fish species i in the sample of sea lions (analogous to a ‘prevalence’). The IRI reduces bias in the description of animal dietary data and it is also important as it combines at least two parameters that potentially have a direct positive relationship with the likelihood of infection, i.e. %N and %FO.
The association between ‘%N’ or ‘IRI’ and prevalence or mean abundance of C. australe were investigated with one-tailed Spearman’s correlation tests. Note that precision of the mean abundance is more dependent on sample size because of the aggregated nature of parasite populations (Rózsa et al. 2000). We attempted to gather the most accurate estimation on infection levels of C. australe, and therefore, the parasitological data obtained in this study was completed with published information for five fish species, i.e. Engraulis anchoita Hubbs & Marini, Merluccius hubbsi Marini, Pinguipes brasilianus Cuvier, Pseudopercis semifasciata (Cuvier) and Raneya brasiliensis (Kaup) from the same area (MacKenzie and Longshaw 1995; Timi and Poulin 2003; Sardella and Timi 2004; Timi et al. 2008; Timi and Lanfranchi 2009; Vales et al. 2011). For these fish species, weighted averages of mean or median abundance per survey based on fish sample size were used.
Statistical software
Confidence intervals for infection parameters and bootstrap replications were calculated with the free software Quantitative Parasitology 3.0 (Reiczigel and Rózsa 2005). Other statistical analyses were carried out with SPSS v19. Statistical significance was set at p < 0.05.
Results
Infection patterns
A total of 1367 cystacanths of C. australe were collected from 18 of 20 marine fish species examined (Table 2). None of the Argentine shortfin squids I. argentinus were infected with acanthocephalans. Eight fish species, i.e. Acanthistius patachonicus (Jenyns), Brama brama (Bonnaterre), Congiopodus peruvianus (Cuvier), Cottoperca gobio (Günther), Genypterus blacodes (Forster), Patagonotothen ramsayi (Regan), Seriolella porosa Guichenot and Stromateus brasiliensis Fowler represent new host records for C. australe. The smallest fish infected was an individual of P. ramsayi 14.7 cm long (intensity, 1), whereas the largest was an individual of Macruronus magellanicus Lönnberg 83.0 cm long (intensity, 1). A specimen of Paralichthys isosceles Jordan 33.2 cm long presented the highest infection level (intensity, 138).
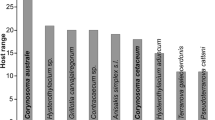
Corynosoma australe showed notable differences in the prevalence and abundance between fish species from Patagonia (Table 2). Prevalence (P) values were high (> 75%) in three benthic fishes, i.e. Raneya brasiliensis (Kaup) (P = 100%), Paralichthys isosceles Jordan (P = 80.0%) and Xystreurys rasile (Jordan) (P = 79.3%). The highest mean abundance (MA) values of C. australe were also observed in benthic fishes, i.e. P. isosceles (MA = 26.5), A. patachonicus (MA = 13.9) and X. rasile (MA = 6.9). In contrast, C. peruvianus and C. gobio and S. brasiliensis harboured much less cystacanths, and Helicolenus lahillei Norman and Merluccius hubbsi Marini were uninfected (Table 2). The eight fish species for which the prevalence of C. australe was > 50%, and mean intensity > 3 (Table 2) accounted for 90.7% of total number of worms found in the sample.
In six of the 14 fish species with n ≥ 10, there was a significant positive correlation between the abundance of cystacanths of C. australe and host body size: A. patachonicus (Spearman rank correlation: rs = 0.668, n = 16, one-tailed p = 0.005), G. blacodes (rs = 0.558, n = 44, one-tailed p < 0.001), Nemadactylus bergi (Norman) (rs = 0.609, n = 32, one-tailed p < 0.001), P. isosceles (rs = 0.817, n = 15, one-tailed p < 0.001), P. ramsayi (rs = 0.259, n = 84, one-tailed p = 0.018) and P. semifasciata (rs = 0.381, n = 31, one-tailed p = 0.034).
Relationship between infection patterns and sea lion diet
In the studies of Koen-Alonso et al. (2000) and Romero et al. (2011), M. hubbsi was, by far, the most important prey of South American sea lions, in terms of number and biomass (%IRI were 39.7% and 44.4% in each study, respectively), even including non-fish prey such as cephalopods. However, infections of C. australe in M. hubbsi were rare in the study area (Fig. 1; Table 2). In the present study, of the eight fish for which the prevalence of C. australe was > 50% and mean intensity > 3 (Table 2), two, i.e. A. patachonicus and S. colias, were not reported as prey of South American sea lions in any survey, and five seldom occurred in stomach contents (%FO < 8%): N. bergi, P. isosceles, P. brasiliensis, P. nudigula and X. rasile (Koen-Alonso et al. 2000; Romero et al. 2011). However, R. brasiliensis was the second fish species in importance in the diet of sea lions (%IRI were 7.3% and 15.1%, respectively in these studies). The most important cephalopod species in the diet of sea lion was I. argentinus (%IRI were 13.9% and 4.6%, respectively); however, cystacanths of C. australe were not detected in this squid species.
Relationship between infection levels of cystacanths of Corynosoma australe obtained in the present study (open symbols) or from other fish parasitological surveys (solid symbols) and percentage by number (%N) or index of relative importance (IRI) of individual fish species in the diet of South American sea lions Otaria flavescens. Dietary data were obtained from Koen-Alonso et al. (2000) (squares) and Romero et al. (2011) (circles)
None of the correlations between infection levels of C. australe in fish species and the relative importance of each fish in the diet of sea lions was statistically significant. Results using Koen-Alonso’s et al. (2000) dietary data set were as follows (all p values are one-tailed): %N and prevalence: rs = 0.003, n = 13, p = 0.50; %N and mean abundance: rs = 0.28, p = 0.536; IRI and prevalence: rs = − 0.094, p = 0.62; IRI and mean abundance: rs = − 0.171, p = 0.712. Results for Romero’s et al. (2011) dietary dataset were as follows: %N and prevalence: rs = − 0.350, n = 9, p = 0.82; %N and mean abundance: rs = − 0.576, p = 0.95; IRI and prevalence: rs = −0.267, p = 0.76; IRI and mean abundance: rs = − 0.559, p = 0.94.
Discussion
Results from the present study showed that cystacanths of C. australe are widely distributed in teleosts from Patagonia and suggest that not all fish species consumed by sea lions are heavily infected with C. australe. At least over a period of 27 years (1982–2009), the diet of sea lions was dominated by three fish species, i.e. E. anchoita, R. brasiliensis and, especially, M. hubbsi (Koen-Alonso et al. 2000; Romero et al. 2011). Two fish species, E. anchoita and M. hubbsi, are incidental hosts for C. australe in the southwestern Atlantic (MacKenzie and Longshaw 1995; Timi and Poulin 2003; present study). Therefore, two key prey for sea lions seems to be of little importance and would hardly contribute in the transmission of C. australe in this region. In contrast, the intake of R. brasiliensis by sea lions would likely promote a continuous recruitment of C. australe (see Vales et al. 2011). Indeed, R. brasiliensis could play a primary link in the transmission of C. australe throughout the trophic web since it has also been reported as an important prey item in wide array of marine birds and fishes (Gosztonyi et al. 2007), including other pinniped species inhabiting the study area (Vales et al. 2011).
The fact that the bulk of the sea lions’ diet is derived from only a few species agrees with previous studies on other pinniped species (Naya et al. 2002). However, sea lions are, above all, opportunistic and broad-spectrum feeders that can shift their food habits depending on prey availability and distribution (Koen-Alonso et al. 2000). Just in the study area alone, 23 fish species and 7 cephalopod species have been reported as prey (Koen-Alonso et al. 2000; Romero et al. 2011). Strong short-term dietary plasticity has been reported in otariid species in response to changes in prey availability (e.g. Naya et al. 2002). Thus, the ecological ubiquity of C. australe in the trophic web would ensure the chances of transmission to sea lions regardless of potential dietary changes (Aznar et al. 2004) and would explain the heavy infections reported in sea lions in the study area (Hernández-Orts et al. 2013).
Our results also showed a significant correlation between the abundance of cystacanths of C. australe and the size of at list six species of marine fish. Cystacanths of Corynosoma spp. are likely to have long life spans, surviving encapsulated in fish paratenic hosts for several years (Valtonen 1983; Comiskey and Mackenzie 2000). Although larger fish, heavily parasitized, will significantly contribute to the transmission of C. australe, there are physical limits to the size of fish that sea lions can consume. In fact, sea lions apparently consume mainly small fish (< 35 cm TL), occasionally medium-sized fish (> 50 cm TL) and rarely large fish (> 65 cm TL) (Koen-Alonso et al. 2000; Marine Mammal Laboratory, CESIMAR – CCT CONICET – CENPAT, unpublished data). Accordingly, most infections in large fish will probably be dead ends in the life cycle of C. australe.
Cephalopods are other important preys of sea lions in Patagonia that were not included in our study except for a single species, i.e. the Argentine shortfin squid I. argentinus. However, this species seems to be unsuitable host for C. australe (Nigmatullin and Shukhgálter 1990; Sardella et al. 1990; González and Kroeck 2000; present study). Other species, like the red octopus Enteroctopus megalocyathus (Gould) and the Patagonian squid Loligo gahi (Orbigny), are important preys of sea lions, since they have %IRI ranging from 1.2 to 25.6 and 0.2 to 5.7, respectively (Koen-Alonso et al. 2000; Romero et al. 2011). Unfortunately, the helminth fauna of the red octopus and the Patagonian squid remains unknown. Available evidence suggests that acanthocephalan infections in cephalopods are infrequent and usually represent accidental or transitory infections (Pascual and Hochberg 1996).
In Patagonian waters, Magellanic penguins S. magellanicus and especially South American fur seals A. australis are also readily infected with C. australe and act as definitive host for this acanthocephalan (Hernández-Orts et al. 2013; Hernández-Orts JS unpublished data). However, the diet of fur seals and penguins is dominated by small pelagic fishes (e.g. M. hubbsi, R. brasiliensis, E. anchoita or silverside Odontesthes spp.) and cephalopod (e.g. Illex spp. and Loligo spp.) (Scolaro et al. 1999; Vales et al. 2011; Marine Mammal Laboratory, CESIMAR – CCT CONICET – CENPAT, unpublished data). As noted above, most of these prey species are rarely infected with cystacanths of C. australe (MacKenzie and Longshaw 1995; González and Kroeck 2000; Timi and Poulin 2003; Carballo et al. 2011; present study). Future studies, addressing the role of fur seals and penguins as definitive hosts of C. australe are necessary to elucidate the transmission dynamics of this acanthocephalan in Patagonia.
The transmission patterns of C. australe in the study area underlie the potential trade-off between spreading infections through the trophic web, which would result in many paratenic host species being slightly infected, or infecting specific taxa that are likely consumed by definitive hosts, which would result in more reduced set of species but more heavily infected. In this context, the present study suggests that significant potential losses of infective stages may occur through many infected fish that are non-prey for sea lions. Although paratenic fish hosts are an integral element in the life cycle of C. australe, it would be interesting to quantify losses due to infection of non-prey species and to explore the conditions that allow the long-term stability of transmission rates to definitive hosts.
References
Amin OM (2013) Classification of the Acanthocephala. Folia Parasitol 60:273–305. https://doi.org/10.14411/fp.2013.031
Aznar FJ, Cappozzo HL, Taddeo D, Montero FE, Raga JA (2004) Recruitment, population structure, and habitat selection of Corynosoma australe (Acanthocephala) in South American fur seals, Arctocephalus australis, from Uruguay. Can J Zool 82:726–733. https://doi.org/10.1139/z04-044
Aznar FJ, Pérez-Ponce de León G, Raga JA (2006) Status of Corynosoma (Acanthocephala: Polymorphidae) based on anatomical, ecological, and phylogenetic evidence, with the erection of Pseudocorynosoma n. gen. J Parasitol 92(3):548–564. https://doi.org/10.1645/GE-715R.1
Aznar FJ, Hernández-Orts JS, Suárez AA, García-Varela M, Raga JA, Cappozzo HL (2012) Assessing host-parasite specificity through coprological analysis: a case study with species of Corynosoma (Acanthocephala: Polymorphidae) from marine mammals. J Helminthol 86(2):156–164. https://doi.org/10.1017/S0022149X11000149
Bezzi S, Akselman R, Boschi EB (2000) Síntesis del estado de las pesquerías marítimas argentinas y de la Cuenca del Plata. Años 1997-1998, con la actualización de 1999. Publicaciones especiales. INIDEP, Mar del Plata
Brunetti NE, Ivanovic ML, Sakai M (1999) Calamares de importancia comercial en la Argentina: Biología, distribución, pesquerías, muestreo biológico. Japan International Cooperation Agency and Instituto Nacional de Investigación y Desarrollo Pesquero, Mar del Plata
Bush AO, Lafferty KD, Lotz JM, Shostak AW (1997) Parasitology meets ecology on its own terms: Margolis et al. revisited. J Parasitol 83(4):575–583. https://doi.org/10.2307/3284227
Carballo MC, Navone GT, Cremonte F (2011) Parasites of the silversides Odontesthes smitti and Odontesthes nigricans (Pisces: Atherinopsidae) from Argentinean Patagonia. Comp Parasitol 78(1):95–103. https://doi.org/10.1654/4445.1
Comiskey P, Mackenzie K (2000) Corynosoma spp. may be useful biological tags for saithe in the northern North Sea. J Fish Biol 57(2):525–528. https://doi.org/10.1111/j.1095-8649.2000.tb02190.x
Froese R, Pauly D (2018) FishBase. http://www.fishbase.org. Accessed 20 June 2018
González RA, Kroeck MA (2000) Enteric helminths of the shortfin squid Illex argentinus in San Matias Gulf (Argentina) as stock discriminants. Acta Parasitol 45(2):89–93
Gosztonyi AE, Kuba L, Mansur LE (2007) Estimación de la talla utilizando relaciones morfométricas de huesos del cráneo, de la cintura escapular y de la distancia precaudal ósea en Raneya brasiliensis (Kaup, 1856) (Pisces, Ophidiiformes, Ophidiidae) de aguas patagónicas. Rev Biol Mar Oceanogr 42(1):1–5. https://doi.org/10.4067/S0718-19572007000100001
Hart RK, Calver MC, Dickman CR (2002) The index of relative importance: an alternative approach to reducing bias in descriptive studies of animal diets. Wildl Res 29:415–421. https://doi.org/10.1071/WR02009
Hernández-Orts JS, Timi JT, Raga JA, García-Varela M, Crespo EA, Aznar FJ (2012) Patterns of trunk spine growth in two congeneric species of acanthocephalan: investment in attachment may differ between sexes and species. Parasitology 139(7):945–955. https://doi.org/10.1017/S0031182012000078
Hernández-Orts JS, Montero FE, Juan-García A, García NA, Crespo EA, Raga JA, Aznar FJ (2013) Intestinal helminth fauna of the South American sea lion Otaria flavescens and fur seal Arctocephalus australis from northern Patagonia, Argentina. J Helminthol 87:336–347. https://doi.org/10.1017/S0022149X12000454
Hernández-Orts JS, Brandão M, Georgieva S, Raga JA, Crespo EA, Luque JL, Aznar FJ (2017) From mammals back to birds: host-switch of the acanthocephalan Corynosoma australe from pinnipeds to the Magellanic penguin Spheniscus magellanicus. PLoS One 12(10):e0183809. https://doi.org/10.1371/journal.pone.0183809
Hoberg EP (1986) Aspects of ecology and biogeography of Acanthocephala in Antarctic seabirds. Ann Parasitol Hum Comp 61(2):199–214
Hoberg EP, Ryan PG (1989) Ecology of helminth parasitism in Puffinus gravis (Procellariiformes) on the breeding grounds at Gough Island. Can J Zool 67:220–225. https://doi.org/10.1139/z89-030
Kennedy CR (2006) Ecology of the Acanthocephala. Cambridge University Press, Cambridge
Knoff M, São Clemente SC, Pinto RM, Gomes DC (2001) Digenea and Acanthocephala of elasmobranch fishes from the southern coast of Brazil. Mem Inst Oswaldo Cruz 2001(96):1095–1101. https://doi.org/10.1590/S0074-02762001000800012
Koen-Alonso M (1999) Estudio comparado de la alimentación entre algunos predadores de alto nivel trófico de la comunidad marina del norte y centro de Patagonia, PhD Thesis, Universidad Nacional de Buenos Aires, Buenos Aires, Argentina
Koen-Alonso M, Crespo EA, Pedraza SN, García NA, Coscarella MA (2000) Food habits of the South American sea lion, Otaria flavescens, off Patagonia, Argentina. Fish B-NOAA 98(2):250–263
Laskowski Z, Zdzitowiecki K (2005) The helminth fauna of some notothenioid fishes collected from the shelf of Argentine Islands, West Antarctica. Pol Polar Res 26(4):315–324
MacKenzie K, Longshaw M (1995) Parasites of the hakes Merluccius australis and M. hubbsi in the waters around the Falkland Islands, southern Chile, and Argentina, with an assessment of their potential value as biological tags. Can J Fish Aquat Sci 52(S1):213–224. https://doi.org/10.1139/f95-529
Menni RC, Ringuelet RA, Arámburu RH (1984) Peces marinos de la Argentina y Uruguay. Reseña histórica. Clave de familia, géneros y especies. Catálogo crítico. Editorial Hemisferio Sur, Buenos Aires
Naya DE, Arim M, Vargas R (2002) Diet of South American fur seals (Arctocephalus australis) in Isla de Lobos, Uruguay. Mar Mammal Sci 18(3):734–745
Nigmatullin CM, Shukhgálter OA (1990) Helmintofauna y aspectos ecológicos de las relaciones parasitarias del calamar (Illex argentinus) en el Atlántico sudoccidental. Frente Maritimo 7:57–68
Parker GA, Ball MA, Chubb JC (2009) To grow or not to grow? Intermediate and paratenic hosts as helminth life cycle strategies. J Theor Biol 258(1):135–147. https://doi.org/10.1016/j.jtbi.2009.01.016
Pascual S, Hochberg FG (1996) Marine parasites as biological tags of cephalopod hosts. Parasitol Today 12(8):324–327. https://doi.org/10.1016/0169-4758(96)40004-7
Reiczigel J (2003) Confidence intervals for the binomial parameter: some new considerations. Stat Med 22(4):611–621. https://doi.org/10.1002/sim.1320
Reiczigel J, Rózsa L (2005) Quantitative parasitology 3.0. http://www.zoologia.hu/qp/qp.html
Romero MA, Dans S, González R, Svendsen G, García N, Crespo E (2011) Trophic overlap between the South American sea lion Otaria flavescens and the demersal trawl fishery in San Matías Gulf, Patagonia, Argentina. Lat Am J Aquat Res 39:344–358. https://doi.org/10.4067/S0718-560X2011000200016
Rózsa L, Reiczigel J, Majoros G (2000) Quantifying parasites in samples of hosts. J Parasitol 86:228–232. https://doi.org/10.1645/0022-3395(2000)086[0228:QPISOH]2.0.CO;2
Sardella NH, Timi JT (2004) Parasites of Argentine hake in the Argentine Sea: population and infracommunity structure as evidence for host stock discrimination. J Fish Biol 65(6):1472–1488. https://doi.org/10.1111/j.0022-1112.2004.00572.x
Sardella NH, Roldán MI, Tanzola D (1990) Helmintos parásitos del calamar (Illex argentinus) en la subpoblación bonaerense-norpatagónica. Frente Maritimo 7:53–56
Sardella NH, Mattiucci S, Timi JT, Bastida RO, Rodríguez DH, Nascetti G (2005) Corynosoma australe Johnston, 1937 and C. cetaceum Johnston & Best, 1942 (Acanthocephala: Polymorphidae) from marine mammals and fishes in Argentinian waters: allozyme markers and taxonomic status. Syst Parasitol 61(2):143–156. https://doi.org/10.1007/s11230-005-3131-0
Schmidt GD (1985) Development and life cycle. In: Crompton DWT, Nickol BB (eds) Biology of the Acanthocephala. Cambridge University Press, Cambridge, pp 273–305
Scolaro JA, Wilson RP, Laruenti S, Kierspel M, Gallelli H, Upton JA (1999) Feeding preferences of the Magellanic penguin over its breeding range in Argentina. Waterbirds 22(1):104–110. https://doi.org/10.2307/1521999
Sinisalo T, Valtonen ET (2003) Corynosoma acanthocephalans in their paratenic fish hosts in the northern Baltic Sea. Parasite 10(3):227–233
Skorobrechova EM, Nikishin VP (2011) Structure of capsule surrounding acanthocephalans Corynosoma strumosum in paratenic hosts of three species. Parasitol Res 108(2):467–475. https://doi.org/10.1007/s00436-010-2088-3
Soares IA, Lanfranchi AL, Luque JL, Haimovici M, Timi JT (2018) Are different parasite guilds of Pagrus pagrus equally suitable sources of information on host zoogeography? Parasitol Res 117(6):1865–1875. https://doi.org/10.1007/s00436-018-5878-7
Taraschewski H (2000) Host-parasite interactions in acanthocephala: a morphological approach. Adv Parasitol 46:1–179. https://doi.org/10.1016/S0065-308X(00)46008-2
Timi JT, Lanfranchi AL (2009) The metazoan parasite communities of the Argentinean sandperch Pseudopercis semifasciata (Pisces: Perciformes) and their use to elucidate the stock structure of the host. Parasitology 136(10):1209–1219. https://doi.org/10.1017/S0031182009990503
Timi JT, Poulin R (2003) Parasite community structure within and across host populations of a marine pelagic fish: how repeatable is it? Int J Parasitol 33(12):1353–1362. https://doi.org/10.1016/S0020-7519(03)00203-0
Timi JT, Lanfranchi AL, Etchegoin JA, Cremonte F (2008) Parasites of the Brazilian sandperch Pinguipes brasilianus Cuvier: a tool for stock discrimination in the Argentine Sea. J Fish Biol 72(6):1332–1342. https://doi.org/10.1111/j.1095-8649.2008.01800.x
Timi JT, Rossin MA, Alarcos AJ, Braicovich PE, Cantatore DMP, Lanfranchi AL (2011) Fish trophic level and the similarity of non-specific larval parasite assemblages. Int J Parasitol 41(3–4):309–316. https://doi.org/10.1016/j.ijpara.2010.10.002
Vales DG, García NA, Crespo EA, Timi JT (2011) Parasites of a marine benthic fish in the Southwestern Atlantic: searching for geographical recurrent patterns of community structure. Parasitol Res 108(2):261–272. https://doi.org/10.1007/s00436-010-2052-2
Valtonen ET (1983) Relationships between Corynosoma semerme and C. strumosum (Acanthocephala) and their paratenic fish hosts in the Bothnian Bay, Baltic Sea. Acta Univ Oul A 21:1–32
Acknowledgments
We would like to thank Barbara Berón-Vera, Marina Aversa and Soledad Leonardi for their technical assistance. We specially thank to Prefectura Naval Argentina and ALPESCA S.A. for allowing us to collect our material on the hake trawlers. Institutional support was given by the Centro Nacional Patagónico (CONICET, Argentina).
Funding
This study was supported by projects BBVA Project No. BIOCON 04, CGL2012-39545 from the Ministry of Economy and Competitiveness, Spain, PROMETEOII/2015/018 of the Valencian Government, Spain, and the Mohamed bin Zayed Species Conservation Fund (Project No. 0925516).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Ethical approval
All applicable institutional, national and international guidelines for the care and use of animals were followed. Fish were collected under the collecting permit No. 24/06 and 23/07 issued by the Dirección de fauna y flora Silvestre, Gobierno del Chubut, Argentina, to EAC.
Additional information
Section Editor: Simonetta Mattiucci
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Hernández-Orts, J.S., Montero, F.E., García, N.A. et al. Transmission of Corynosoma australe (Acanthocephala: Polymorphidae) from fishes to South American sea lions Otaria flavescens in Patagonia, Argentina. Parasitol Res 118, 433–440 (2019). https://doi.org/10.1007/s00436-018-6177-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00436-018-6177-z