Abstract
Polymorphid acanthocephalans are parasites of marine mammals, waterfowl and ichthyophagous birds. Among these, the genus Profilicollis is known to use exclusively decapods as intermediate hosts. Here, we report the first record of living cystacanths of Profilicollis parasitizing the body cavity of a fish host, Oligosarcus jenynsii, inhabiting the freshwater section of an estuarial system, Mar Chiquita coastal lagoon, in south-east Buenos Aires Province, Argentina. In this environment, cystacanths of Profilicollis chasmagnathi have been previously recorded infecting decapod crabs and as transient accidental infections in the gut of some carcinophagous fishes. In the present study, larvae from the crab Neohelice granulata, from the intestine of the estuarine fish Odontesthes argentinensis and from the body cavity of O. jenynsii were morphologically and genetically compared, confirming their identity as P. chasmagnathi, a species characteristic of estuaries and marine coasts along Argentina, Uruguay and Chile. These findings can be interpreted as a possible case of incipient paratenicity for Profilicollis, and a colonization event of freshwater habitats, probably promoted by the highly variable conditions, typical of ecotonal environments. In addition, cystacanths of the genus Polymorphus were also found in O. jenynsii, representing the first record of this genus in Oligosarcus from Argentina.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Acanthocephalans of the family Polymorphidae Meyer, 1931 are parasites of marine mammals, waterfowl and fish-eating birds (Schmidt and Hugghins 1973). At present, fifteen polymorphid genera are recognized worldwide (Amin 2013; Presswell et al. 2020), whose life cycles typically include crustaceans as intermediate hosts and, in some cases, fish as paratenic hosts (Schmidt 1985). Among them, Profilicollis Meyer, 1931 was considered as a subgenus of Polymorphus Lühe, 1911 for many decades, until Nickol et al. (1999) reinstated its generic status based on ecological evidences. These differences are mainly related to life cycle characteristics; indeed, whereas members of Polymorphus use amphipods as intermediate hosts, those of Profilicollis use decapods (Nickol et al. 1999, 2002). Amphipods were the ancestral intermediate hosts, while the association with decapods represents episodes of secondary colonization as shown by phylogenetic studies that support the monophyly and the validity of the genus Profilicollis (García-Varela and Pérez-Ponce de León 2008; García-Varela et al. 2013).
An additional difference between Polymorphus and Profilicollis seems to be the use of fish as paratenic hosts by the former, a feature shared by other polymorphid genera, i.e. Andracantha Schmidt, 1975, Bolbosoma Porta, 1908 and Corynosoma Lühe, 1911 (Aznar et al. 2006). Indeed, cystacanths of Polymorphus have been reported parasitizing the internal organs of several freshwater fish in America (Amin et al. 1995; Santos et al. 2008; García-Prieto et al. 2010; Alcántar-Escalera et al. 2013; Rauque et al. 2018). On the other hand, cystacanths of Profilicollis have occasionally been found in the stomach and intestinal contents of fishes (Alarcos and Etchegoin 2010).
Profilicollis chasmagnathi (Holcman-Spector, Mañé-Garzón & Dei-Cas, 1977) is the only representative of the genus so far reported at adult stage along the southwestern Atlantic coast (Lorenti et al. 2018). This species has been recorded at the estuaries of Buenos Aires Province and Patagonian coasts, infecting the gut of several bird species (Martorelli 1989; Vizcaíno 1989; Diaz et al. 2011; La Sala et al. 2013; Lorenti et al. 2018). Cystacanths of this species are common parasites of different crab species inhabiting estuarine and rocky intertidal marine habitats in Uruguay and Argentina (Holcman-Spector et al. 1977; Martorelli 1989; Alda et al. 2011; La Sala et al. 2012; Méndez Casariego et al. 2016; Rodríguez et al. 2017). These cystacanths occur at high prevalence in two varunid crab species, Cyrtograpsus angulatus (Dana, 1851) and Neohelice granulata (Dana, 1851) in soft bottom intertidal areas and salt marshes of the Mar Chiquita coastal lagoon, Buenos Aires Province, Argentina (Martorelli 1989; Méndez Casariego et al. 2016). These crabs have also been found in the stomach and intestine, along with cystacanths of P. chasmagnati, of several marine fish species that use the estuary as feeding grounds (Alarcos and Etchegoin 2010).
During parasitological studies on estuarine and freshwater fishes from the Mar Chiquita basin, cystacanths referable to Profilicollis were found in the intestine of the silverside Odontesthes argentinensis (Valenciennes, 1835) (Atherinopsidae) from estuarine areas. Similar larvae were also found in the mesenteries of the dientudo Oligosarcus jenynsii (Günther, 1864) (Characidae) from a freshwater tributary of the lagoon, along with larvae of the genus Polymorphus in the same microhabitat. This was an unexpected finding, because members of Profilicollis have never been recorded infecting the internal organs of fishes.
To the best of our knowledge, there is a single previous record of polymorphid acanthocephalans in the genus Oligosarcus, namely of cystacanths of Polymorphus sp. in O. hepsetus (Cuvier, 1829) from Brazil (Abdallah et al. 2004). The closely related genera Profilicollis and Polymorphus can be very difficult to distinguish based only on their morphology because they display some degree of overlapping in diagnostic features (Amin 1992). Furthermore, species within Profilicollis are very similar to each other (Rodríguez and D’Elía 2016; Rodríguez et al. 2016). Consequently, the aim of this research was to identify those larval acanthocephalans found parasitizing O. jenynsii to get insight on the potential departures from the typical life cycle (two-host and marine-estuarine) of Profilicollis, which could be driven by the variability in the ecological conditions imposed by an ecotonal freshwater-estuarine environment.
Materials and Methods
Study area
Fish samples were obtained at three different locations of the Mar Chiquita basin. This is a coastal lagoon in south-east Buenos Aires Province, Argentina. It is an elongated water body, parallel to the coastline, separated from the sea by a barrier of dunes and only connected to it by an inlet that seasonally varies both in width and in position (Isla 1997). Mar Chiquita is characterized by a marked salinity gradient, with values ranging from 29.15 (at the inlet area) to 2.8 (at its innermost section) (Marcovecchio et al. 2019). These authors identified three different areas, an external area functioning as a coastal marine system, an intermediate estuarine system and an inland water system, whose extents vary inter-annually depending on the rainfall. For example, the extent of the marine section varied between 12 and 71% of the total surface across rainy and dry years (Marcovecchio et al. 2019). The limit between these sections is also variable daily and seasonally depending on the amplitude of the tides, the meteorological conditions and the volume of fresh water present in the lagoon (Reta et al., 2001). The lagoon, with a total area of ~60 km2 receives waters from numerous streams (Marcovecchio et al. 2019) being also in connection with two Pampean shallow lakes, Nahuel Rucá and Hinojales. One of its main tributaries is Arroyo Grande stream, to the north of the lagoon. This system is therefore a vast ecotonal region of great biodiversity that combines freshwater environments, wetlands and grasslands with marshes, coastal dunes and beaches (Fig 1).
Map of the study area showing Mar Chiquita coastal lagoon and sampling sites (black dots), its main tributary streams, and Nahuel Rucá and Hinojales lakes, in Buenos Aires Province, Argentina. Locality References: AG: Arroyo Grande stream, HL: Hinojales lake NR: Nahuel Rucá lake, MC: Mar Chiquita coastal lagoon, CC: estuarine area near Cangrejo creek discharge
Host samples
Fish were captured, by means of gillnets, at different locations during parasitological surveys in the lagoon. A total of 30 specimens of O. jenynsii were obtained at Arroyo Grande (AG: 37°31'14"S, 57°19'37" W; April 2018) and 40 O. argentinensis were caught inside the lagoon body (MC: 37°36'54"S, 57°23'26"W; April 2017-Agust 2018). Additionally, a sample of three decapod crabs, N. granulata, collected by hand in an estuarine area near the freshwater discharge of Cangrejo Creek (CC: 37°44'14"S, 57°26'18"W; March 2019), was used to obtain cysthacanths from the natural intermediate host. Also data from a sample of 214 O. jenynsii, caught at Nahuel Rucá lake (NR: 37°36'57"S, 57°25'29"W; July 2008-October 2012) was used for comparative purposes (Fig. 1).
Parasitological studies and morphology
All hosts were frozen at -20°C until examination. After thawing at room temperature, they were necropsied and the polymorphid acanthocephalans were collected. Prevalence and mean abundance (sensu Bush et al. 1997) were calculated for each parasite species in each host sample, with 95% bootstrap confidence intervals, following Rózsa et al. (2000), using Quantitative Parasitology software (QPweb) (Reiczigel et al. 2019). The microhabitat and state of preservation of each cystacanth was recorded, considering larvae as viable when entire and encysted, or as dead when partially reabsorbed (darkened and degraded).
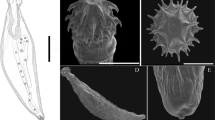
Complete individuals were kept in distilled water for several hours in order to allow the eversion of the proboscis prior to fixation; they were later identified at generic level following Amin (1992) and Presswell et al. (2020). A single Profilicollis sp. from each host species and one Polymorphus sp. found parasitizing O. jenynsii were reserved for genetic identification (fixed and preserved in 96% ethanol). The remaining fully extended specimens were fixed in 4% formalin to later perform morphological studies under a stereoscopic microscope. Measurements included total length, proboscis length and width, neck length and width, somatic spines area length and width and hind trunk length and width. In addition, the number of hook rows and hooks per row was recorded. These variables were used in the taxonomical identification of the cystacanths, to the lowest level possible.
DNA extraction, PCR amplification, sequencing and sequence analysis
DNA extractions were carried out using the DNeasy Blood and Tissue® kit (QIAGEN, Hilden, Germany) on whole specimens. A region of the mitochondrial DNA was amplified by polymerase chain reaction (PCR). Mitochondrial cytochrome c oxidase subunit 1 (mtDNA cox1) was amplified using the universal DNA primers LCO1490 (forward) and HCO2198 (reverse) described by Folmer et al. (1994). All PCR reactions were set up in 25 μl reactions using 5 μl of DNA (≥10 ng) as the template, 0.5 μl (0.5 mM) of each primer and 12.5 μl (2X) HotStarTaq Master Mix (QIAGEN). The PCR was carried out using the following conditions: initial step for enzyme activation and denaturation at 95 °C for 15 min, followed by 35 cycles of amplification at 94 °C for 30 s, 50 °C for 30 s and 72 °C for 1:45 min, followed by 10 min of post amplification at 72 °C. Each PCR product was purified using QIAquick spin columns (QIAquick Gel Extraction Kit, QIAGEN). Sequencing was performed using ABI 3730XLs automated sequencer (Applied Biosystems, Macrogen, South Korea).
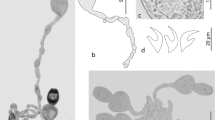
Sequences were edited and assembled in Proseq 3.5 (Filatov 2002). For identification, consensus sequences were compared against the NCBI database using the BLAST algorithm (Altschul et al. 1990). Curated contig sequences were deposited in GenBank (Accession numbers: MT580122, MT580123, MT580124, MT580125, Fig. 2)
Maximum likelihood tree inferred by mtDNA cox1 showing the taxonomic position of Profilicollis chasmagnathi and Polymorphus sp. from three host species (Neohelice granulata, Oligosarcus jenynsii and Odontesthes argentinensis). Nodal supports are indicated for ML, MP (1,000 replicates, only bootstrap values greater than 70% are shown) and BI (only posterior probabilities greater than 0.7 are shown). Names in bold correspond to sequences obtained in the present study. Asterisks indicate sequences deposited as P. antarticus that were posteriorly identified as P. chasmagnathi (KU928252, Rodríguez and D’Elia 2016; KX646756, Rodríguez et al. 2016)
The obtained fragments from the mtDNA cox1 gene were aligned with sequences from other members of the genera Profilicollis and Polymorphus, retrieved from GenBank. Sequences from Bolbosoma turbinella (Diesing, 1851) and Corynosoma australe Johnston, 1937 were also aligned and used as outgroup. The alignment was performed by ClustalW (Thompson et al. 1994) as implemented in MEGA 7.0 software package (Kumar et al. 2016), using default parameters. The reading frame for the mtDNA cox1 sequences was determined by translating the sequences, specifying the appropriate gene code (invmtDNA) and by starting at different positions in the alignment and inspecting for stop codons.
The estimation of intra and interspecific genetic divergence among specimens was conducted in MEGA7.0 software package (Kumar et al. 2016), using the Tamura-Nei model (Tamura and Nei 1993).
Hypothesis on the specific identity of the cystacanths found in different host species were tested using evolutionary three inference methods, based on analysis of character-state data, as recommended by Nadler and Pérez-Ponce de León (2011), which has the advantage of revealing the particular changes in states supporting individual species. For that purpose, three different inference methods, namely maximum parsimony (MP), maximum likelihood (ML) and Bayesian inference (BI), were used to construct trees in order to visualize relationships between the sequences obtained in the present study and those from cystacanths and adults retrieved from Genbank. MP analyses were performed using PAUP∗ 4.0b10 (Swofford 2001), using a heuristic search with tree-bisection-reconnection (TBR), branch swapping and random addition of sequence. All characters were treated as unordered. ML analyses were performed using PhyML 3.1 (Guindon and Gascuel 2003).
Reliabilities of phylogenetic relationships were evaluated using nonparametric bootstrap analysis (Felsenstein 1985) with 1,000 replicates for MP and ML trees. Bootstrap values exceeding 70 were considered well supported (Hillis and Bull 1993). Bayesian inference was performed with MrBayes 3.1.1 (Ronquist and Huelsenbeck 2003). The Bayesian posterior probability analysis was performed with the MCMC algorithm where the number of chains was 4, the temperature of heated chains was 0.2 with 1,000,000 generations while the sub-sampling frequency was 100, with a burn-in fraction of 0.25. JModelTest (Posada 2008) was run to determine the best-fit model for the obtained data set, as implemented in the Akaike information criterion (AIC) (Posada and Buckley 2004). The best-fit model GTR + I + G was used for BI analysis and ML.
Results
Larval acanthocephalans, referable to Profilicollis, were found encysted in the intestinal mesenteries of O. jenynsii caught at AG. Similar cystacanths were found free in the intestinal contents of O. argentinensis from MC and in the body cavity of N. granulata (the natural host) from CC. A relatively high prevalence and low mean abundance of Profilicollis were recorded in both fish species (Table 1). In addition, a specimen of O. jenynsii from AG had two cystacanths referable to Polymorphus encysted in its intestinal mesenteries. In comparison, those O. jenynsii from NR were parasitized only by Polymorphus sp., at lower prevalence and mean abundance than in AG (Table 1).
The cystacanths found in O. argentinensis were in most cases associated with remnants of the crab C. angulatus, present in the intestinal contents. All of them had their proboscis invaginated and were therefore considered as viable. Some O. argentinensis presented encapsulated lesions in their intestinal wall, with dark, degraded contents that, in some cases, contained remains of proboscis that resembled those of Profilicollis sp. These lesions could indicate previous attachment sites; however, they were not quantified as Profilicollis sp., since their identity could not be asserted. On the other hand, all cystacanths found on O. jenynsii were encysted in the mesenteries and evidenced a strong reaction by the host, represented by brownish encapsulations. Despite this, 46% of them were identified as viable.
The Profilicollis sp. collected from the three host species (O. argentinensis, O.jenynsii and N. granulata) were morphologically similar to each other, with a constant number of hook rows in the proboscis (18) and of hooks in each row (8), although larvae from O. jenynsii showed slightly smaller body measurements than those from crabs and O. argentinensis (Table 2). Morphological and morphometric data allowed determining them as P. chasmagnathi according to Amin, 1992 and Vizcaíno, 1989.
Further confirmation was accomplished through the genetic studies, which demonstrated that all specimens studied were in effect P. chasmagnathi. The mtDNA cox1 sequences (637 bp) from the cystacanths obtained from N. granulata crabs, O. jenynsii and O. argentinensis matched >99% with the mtDNA cox1 sequences of P. chasmagnathi deposited in GenBank. The genetic divergences between the P. chasmagnathi from the present study and those from GenBank were in average 1.0% (range: 0.9% - 1%). When compared with sequences from congeners available in GenBank (P. botulus (Van Cleave, 1916), P. altmani (Perry, 1942) and P. novaezelandensis Brockerhoff & Smales, 2002), the interspecific divergences ranged from 16 to 30%.
Regarding those larvae morphologically determined as Polymorphus sp., the closest match of a mtDNA cox1 sequence from the cystacanth found in O. jenynsii (631 bp) was 93.8% to a sequence of Polymorphus deposited en GenBank. The genetic divergence among species of Polymorphus retrieved from GenBank varied between 7.3, with P. brevis (Van Cleave, 1916), to 42%, with P. minutus (Zeder, 1800).
The results from all genetic analyses are summarized in a single tree with bootstrap and posterior probability values, as shown in Fig. 2. The ML analysis resulted in a single tree with -log likelihood: 4382.4844. The MP analysis revealed that 333 characters were constant; 293 were parsimony-informative, and 27 variable characters were parsimony-uninformative. For BI analysis, the average standard deviation of split frequencies was 3.29 × 10-3, after 1×106 generations. Trees based on ML, MP and BI analysis yielded similar topologies, as shown in the ML consensus tree (Fig. 2).
In summary, the mtDNA cox1 analysis allowed determining that the Profilicollis cystacanths sequenced in this study were in effect P. chasmagnathi, while the specific status of the Polymorphus sp. found in O. jenynsii could not be further asserted, although its sequence was included as a sister taxon to P. brevis in a well-supported clade.
Discussion
An integrative approach, combining morphological and genetic evidences allowed confirming that all cystacanths of the genus Profilicollis found in O. argentinensis from MC, O. jenynsii from AG and N. granulata from CC, are conspecific and belong to P. chasmagnathi. Those cystacanths of Polymorphus sp. found in O. jenynsii could not be morphologically determined beyond the genus level due to their larval stage, since the diagnostic characters at species level often rely on the structure and morphology of the reproductive system in both sexes (Presswell et al. 2020).
The genetic analysis showed that the relationships among Profilicollis and Polymorphus species, recovered in this study, are congruent with those from previous studies (García-Varela et al. 2013; Huston et al. 2020). Indeed, phylogenetic relationships among species of Polymorphus were poorly resolved, indicating that, as currently constituted, Polymorphus is not monophyletic (García-Varela et al. 2013), with the present material being placed genetically closer to P. brevis among the compared congeners. On the other hand, sequences of Profilicollis obtained in this study resulted in a well-supported clade with all sequences available of P. chasmagnathi (cystacanths and adults) from South American hosts. The low genetic divergence of P. chasmagnathi across host species was congruent with those values from previous studies in the region (Rodríguez et al. 2017; Lorenti et al. 2018). A diagnostic feature of the genus Profilicollis is the use of decapod crustaceans as intermediate hosts (Nickol et al. 1999, 2002) and up to the present, the lack of paratenic hosts, being directly transmitted to aquatic birds (its definitive hosts) through the consumption of crustaceans (Lorenti et al. 2018). Profilicollis chasmagnathi has been reported in several species of estuarine-dependent fishes (O. argentinensis, Paralichthys orbignyanus (Valenciennes, 1839), Micropogonias furnieri (Desmarest, 1823) and Pogonias cromis (Linnaeus, 1766)) from Mar Chiquita (Alarcos and Etchegoin 2010). The authors found these acanthocephalans in the gut of fishes, consequently, such infections should be considered as accidental and transient, being the result of predation on the decapod crabs inhabiting the lagoon. In fact, although crabs harbouring Profilicollis larvae are often predated by teleosts and elasmobranchs, it is considered that these fish do not play any role in the life cycle of the parasite (Oliva et al. 2008). In the present study, the presence of tissue lesions containing acanthocephalan hooks in the mucosa of some specimens of O. argentinensis, but not of attached worms, supports the idea of the transient nature of such infections in this hosts. This is supported by the morphometric similitude between worms from crabs and silversides, indicating that larvae were released from the crab’s body during digestion.
On the other hand, the presence of P. chasmagnathi in the mesenteries of O. jenynsii represents a long-term or permanent infection, with the fish acting as paratenic host, a symbiotic relationship that had not been reported yet for this genus. In the present study, crab remains were found neither in intestinal nor in stomach contents of any dientudo; however, the presence of cystacanths of P. chasmagnathi would indicate the previous consumption of infected crabs. Oligosarcus jenynsii is a generalist carnivorous and a freshwater species, tending to piscivory at larger sizes (Nunes and Hartz 2006), but freshwater decapods (shrimps and crabs) are frequently reported preys in other localities (Rodrigues et al. 2012). It is therefore possible that it feeds on estuarine crabs when this fish, visits myxo-oligohaline areas, at the north of the lagoon, mostly during winter (González-Castro et al. 2009). It is possible too that the infections occured in freshwater areas, thanks to the marked euryhalinity of the crabs N. granulata and C. angulatus, commonly parasitized by P. chasmagnathi, especially of the latter, which was abundant in Arroyo Grande (pers. obs.).
The absence of P. chasmagnathi in a large sample of O. jenynsii from Nahuel Rucá is therefore, explained by the rarity of both crab species in that lake. Indeed, only few specimens of C. angulatus were sporadically observed during fish sampling, all of them of a size too large to be prey of O. jenynsii (pers. obs.), supporting a crab-fish transmission in Arroyo Grande where small crabs can be eaten by the fish.
The generalized life-cycle pattern of acanthocephalans invariably includes an arthropod intermediate host (Kennedy 2006). In the case of polymorphids, these are crustaceans (García-Varela et al. 2013), which are infected by consuming the parasite’s eggs. After penetrating the gut wall of the crustacean host, the acanthor larvae develops into an acanthella and then into a cystacanth stage (Reish 1950; Rayski and Garden 1961). The acquisition of paratenic hosts, albeit facultative ones, is rare among acanthocephalans (Kennedy 2006); however, it enables them to ascend trophic levels and so move through food chains favouring transmission (Kennedy 2006) by bridging the trophic gap between intermediate and definitive hosts (Aznar et al. 2006). Beyond this evolutionary advantage, sometimes it is difficult to assess whether a host with acanthocephalans in the body cavity is actually a paratenic or an accidental host, in whose body cavity acanthocephalans may also occur (Kennedy 2006). A possible mechanism of the extra-intestinal infection by larval acanthocephalans in fish has been proposed for other genera; in fact, it is not uncommon to find partially excysted parasites in the body cavity and its organs of suitable definitive hosts (Kennedy 2006). According to this author, the age of cystacanths can play a role in the efficiency and site of infection. As examples, De Giusti (1949) and Nickol (1985) found that immature larvae could not effectively attach to their definitive fish hosts and would pass through the intestine wall to encyst (still as a cystacanth) in an extra-intestinal site, while older larvae are able to attach to the intestine and normally progress with their life cycle. This could be the case for those cystacanths found in dientudos, although they are not the definitive hosts, and could explain the smaller size of cystacanths infecting them relative to those found in crabs. Nevertheless, the fact that this fish could be a suboptimal host affecting the parasite’s development, as also indicated by the finding of dead worms, cannot be discarded.
Host capture, the colonization of new hosts by parasites (Holmes and Price 1980), seems to be an extended phenomenon in polymorphids, not only at the level of definitive (Kennedy 2006) and intermediate hosts (García-Varela et al. 2013), but also of paratenic hosts. Indeed, birds and mammals have captured Polymorphus species from fish, while marine mammals, may have captured Corynosoma and Bolbosoma species from birds (Kennedy 2006). Similarly, the acquisition of decapods as intermediate hosts for some genera (Profilicollis, Arhytmorhynchus Lühe, 1911, Ibirhynchus Garcia-Varela, Pérez-Ponce de León, Aznar & Nadler, 2011 and Hexaglandula Petrochenko, 1950) and of euphausiids for Bolbosoma, represent episodes of secondary colonization from amphipods, the ancestral intermediate hosts (García-Varela et al. 2013). Therefore, the incorporation of a paratenic host in the life cycle of Profilicollis, even in an apparently incipient stage, should not be surprising, since it has been frequent in related genera. The transmission of P. chasmagnathi cystacanths to a fish paratenic host implies their ability to penetrate the fish’s intestine and re-encyst in the mesenteries. This process is the same followed by the phylogenetically closely related genus Polymorphus (Alcántar-Escalera et al. 2013; Huston et al. 2020; Presswell et al. 2020), as well as by other polymorphids, such as Andracantha, Bolbosoma and Corynosoma (Aznar et al. 2006; García-Varela et al. 2013). On the other hand, the disability of P. chasmagnathi to parasitize the internal organs or tissues of other fish species, in whose guts is frequently found (Alarcos and Etchegoin 2010; present study), could represent a kind of host specificity and requires further research, although differential host defence mechanisms could be involved. The finding of dark lesions in the intestines of some silversides containing proboscis remains could be indicative of such defences. For example, the polymorphid Corynosoma strumosum (Rudolphi, 1802) may or may not respond secreting a protective thick layer of glycocalyx on its tegument (Skorobrekhova and Nikishin 2017) depending on the nature of the encapsulation defence mounted by the different species of paratenic fish hosts (varying from fibroblastic to leukocytal). In consequence, C. strumosum shows variable degrees of adaptation to disparate hosts (Skorobrekhova and Nikishin 2017; Nikishin and Skorobrekhova 2019).
Beyond the possible role of O. jenynsii as host of P. chasmagnathi could be considered as accidental or as an incipient paratenicity phenomenon, a high proportion of these larvae found were alive at the time of capture of these hosts. This implies that they could be viable for the infection of ichthyophagous birds, which would potentially enable P. chasmagnathi to widen its host range. Such facts are relevant, especially considering the low specificity of this parasite for its definitive hosts. Indeed, P. chasmagnathi has been reported in six families of birds, belonging to five orders (Martorelli 1989; Vizcaíno 1989; Torres et al. 1993; Diaz et al. 2011; La Sala et al. 2013; Lorenti et al. 2018), including podicipedids and phalacrocoracids, which are primarily piscivorous (Petracci et al. 2009; Josens et al. 2010) and abundant in Mar Chiquita basin (Favero et al. 2001; Ferrero and Iribarne 2001).
The capture of a fish paratenic host challenges the phylogenetic conservatism of the genus Profilicollis. However, host specificity cannot be considered a fixed trait, because in spite of being phylogenetically constrained to a large extent, it is strongly influenced by local environmental conditions (Mouillot et al. 2006), which cause considerable variation in realized host specificity (Wells and Clark 2019). Indeed, it has been proposed that estuaries and coastal-brackish lagoons are environments physically variable enough to select generalist genotypes of fish, in order they can adjust their morphology, physiology and behaviour to a wide range of conditions (Bamber and Henderson 1988). This selected plasticity would pre-adapt estuarine populations to invade, colonize and radiate into vacant niches in freshwater (Bamber and Henderson 1988), an eco-evolutionary mechanism that could explain a new host-parasite system, such as P. chasmagnathi-O. jenynsii.
The present findings represent the first record of the incorporation of a paratenic host in the life cycle of a member of the genus Profilicollis, and consequently an exception to the phylogenetic conservatism characteristic of this genus. Regarding the ecology of Profilicollis it would also imply a possible mechanism for the colonization of both a freshwater host and the freshwater environment, by an acanthocephalan genus ‘exclusive’ of marine and brackish habitats. This transition is probably promoted by the highly variable environmental conditions, typical of ecotonal environments between marine and freshwater realms, such as Mar Chiquita coastal lagoon.
References
Abdallah VD, Azevedo RK, Luque JL (2004) Metazoários Parasitos dos lambaris Astyanax bimaculatus (Linnaeus, 1758), A. parahybae Eigenmann, 1908 e Oligosarcus hepsetus (Cuvier, 1829) (Osteichthyes: Characidae), do Rio Guandu, Estado do Rio de Janeiro, Brasil. Rev Bras Parasitol Vet 13:57–63
Alarcos AJ, Etchegoin JA (2010) Parasite assemblages of estuarine-dependent marine fishes from Mar Chiquita coastal lagoon (Buenos Aires Province, Argentina). Parasitol Res 107:1083–1091. https://doi.org/10.1007/s00436-010-1974-z
Alcántar-Escalera FJ, García-Varela M, Vázquez Domínguez E, Pérez-Ponce de León G (2013) Using DNA barcoding to link cystacanths and adults of the acanthocephalan Polymorphus brevis in central Mexico. Mol Ecol Resour 13:1116–1124. https://doi.org/10.1111/1755-0998.12090
Alda P, La Sala L, Marcotegui P, Martorelli SR (2011) Parasites and epibionts of grapsid crabs in Bahía Blanca estuary, Argentina. Crustaceana 84:559–571
Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ (1990) Basic local alignment search tool. J Mol Biol 215:403–410. https://doi.org/10.1016/S0022-2836(05)80360-2
Amin OM (1992) Review of the genus Polymorphus Luhe, 1911 (Acanthocephala: Polymorphidae), with the synonymization of Hexaglandula Petrochenko, 1950, and Subcorynosoma Hoklova, 1967, and a key to the species. Qatar Univ Sci J 12:115–123
Amin OM (2013) Classification of the Acanthocephala. Folia Parasitol 60:273–305. https://doi.org/10.14411/fp.2013.031
Amin OM, Heckmann RA, Mesa R, Mesa E (1995) Description and host relationships of cystacanths of Polymorphus spindlatus (Acanthocephala: Polymorphidae) from their paratenic fish hosts in Perú. J Helminthol Soc Wash 62:249–253
Aznar FJ, Pérez-Ponce de León G, Raga JA (2006) Status of Corynosoma (Acanthocephala: Polymorphidae) based on anatomical, ecological and phylogenetic evidence, with the erection of Pseudocorynosoma n. gen. J Parasitol 92:548–564. https://doi.org/10.1645/GE-715R.1
Bamber RN, Henderson PA (1988) Pre-adaptative plasticity in atherinids and the estuarine seat of teleost evolution. J Fish Biol 33:17–23. https://doi.org/10.1111/j.1095-8649.1988.tb05554.x
Bush AO, Lafferty KD, Lotz JM, Shostak AW (1997) Parasitology meets ecology on its own terms: Margolis et al. revisited. J Parasitol 83:575–583. https://doi.org/10.2307/3284227
De Giusti DL (1949) The life cycle of Leptorhynchoides thecatus (Linton), an acanthocephalan of fish. J Parasitol 35:437–460. https://doi.org/10.2307/3273647
Diaz JI, Cremonte F, Navone GT (2011) Helminths of the kelp gull, Larus dominicanus, from the northern Patagonian coast. Parasitol Res 109:1555–1562. https://doi.org/10.1007/s00436-011-2396-2
Favero M, Bachmann S, Copello S, Mariano-Jelicich R, Silva MP, Ghys M, Khatchikian C, Mauco L (2001) Aves marinas del sudeste bonaerense. In: Iribarne O (ed) Reserva de Biósfera Mar Chiquita: Características Físicas, Biológicas y Ecológicas. Editorial Martín, Mar del Plata, Argentina, pp 251–267
Felsenstein J (1985) Confidence Limits on Phylogenies: An Approach Using the Bootstrap. Evolution 39:783–791. https://doi.org/10.1111/j.1558-5646.1985.tb00420.x
Ferrero L, Iribarne O (2001) Avifauna de Mar Chiquita. Síntesis del trabajo de Mariano Manuel Martínez. In: Iribarne O (ed) Reserva de Biósfera Mar Chiquita: Características Físicas, Biológicas y Ecológicas. Editorial Martín, Mar del Plata, Argentina, pp 227–250
Filatov DA (2002) ProSeq: A software for preparation and evolutionary analysis of DNA sequence data sets. Mol Ecol Notes 2:621–624. https://doi.org/10.1046/j.1471-8286.2002.00313.x
Folmer O, Black M, Hoeh W, Lutz R, Vrijenhoek R (1994) DNA primers for amplification of mitochondrial cytochrome c oxidase subunit I from diverse metazoan invertebrates. Mol Mar Biol Biotechnol 3:294–299
García-Prieto L, García-Varela M, Mendoza-Garfias B, Pérez-Ponce de León G (2010) Checklist of the Acanthocephala in wildlife vertebrates of Mexico, vol 1419. Zootaxa, pp 1–50. https://doi.org/10.11646/zootaxa.2419.1.1
García-Varela M, Pérez-Ponce de León G (2008) Validating the systematic position of Profilicollis Meyer, 1931 and Hexaglandula Petrochenko, 1950 (Acanthocephala: Polymorphidae) using cytochrome c oxidase (cox 1). J Parasitol 94:212–217. https://doi.org/10.1645/GE-1257.1
García-Varela M, Pérez-Ponce de León G, Aznar FJ, Nadler SA (2013) Phylogenetic relationship among genera of Polymorphidae (Acanthocephala), inferred from nuclear and mitochondrial gene sequences. Mol Phylogenet Evol 68:176–184. https://doi.org/10.1016/j.ympev.2013.03.029
González-Castro M, Díaz de Astarloa JM, Cousseau MB, Figueroa DE, Delpiani SM, Bruno DO, Guzzoni JM, Blasina GE, Deli Antoni MY (2009) Fish composition in a south-western Atlantic temperate coastal lagoon: spatial–temporal variation and relationships with environmental variables. J Mar Biol Assoc UK 89:593–604. https://doi.org/10.1017/S0025315409003002
Guindon S, Gascuel O (2003) A simple, fast, and accurate algorithm to estimate large phylogenies by maximum likelihood. Syst Biol 52:696–704. https://doi.org/10.1080/10635150390235520
Hillis DM, Bull JJ (1993) An empirical test of bootstrapping as a method for assessing confidence in phylogenetic analysis. Syst Biol 42:182–192. https://doi.org/10.1093/sysbio/42.2.182
Holcman-Spector B, Mañé-Garzón F, Dei-Cas E (1977) Una larva cystacantha (Acanthocephala) de la cavidad general de Chasmagnathus granulata Dana, 1851. Rev Biol Uruguay 5:67–76
Holmes JC, Price PW (1980) Parasite communities: the roles of phylogeny and ecology. Syst Zool 29:203–213. https://doi.org/10.2307/2412650
Huston DC, Cribb TH, Smales LR (2020) Molecular characterisation of acanthocephalans from Australian marine teleosts: proposal of a new family, synonymy of another and transfer of taxa between orders. Syst Parasitol 97:1–23. https://doi.org/10.1007/s11230-019-09896-2
Isla FI (1997) Seasonal behaviour of Mar Chiquita tidal inlet in relation to adjacent beaches, Argentina. J Coast Res 13:1221–1232
Josens ML, Bó MS, Favero M (2010) Foraging ecology of the Great Grebe Podicephorus major in Mar Chiquita Lagoon (Buenos Aires, Argentina). Ardeola 57:133–141
Kennedy CR (2006) Ecology of the Acanthocephala. Cambridge University Press, Cambridge, UK
Kumar S, Stecher G, Tamura K (2016) MEGA7: molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol Biol Evol 33:1870–1874. https://doi.org/10.1093/molbev/msw054
La Sala LF, Perez AM, Martorelli SR (2012) Epidemiology of acanthocephalan infections in crabs from the Bahía Blanca Estuary, Argentina. J Helminthol 86:446–452. https://doi.org/10.1017/S0022149X11000678
La Sala LF, Perez AM, Smits JE, Martorelli SR (2013) Pathology of enteric infections induced by the acanthocephalan Profilicollis chasmagnathi in Olrog’s gull, Larus atlanticus, from Argentina. J Helminthol 87:17–23. https://doi.org/10.1017/S0022149X11000721
Lorenti E, Rodríguez SM, Cremonte F, D'Elía G, Diaz JI (2018) Life cycle of the parasite Profilicollis chasmagnathi (Acanthocephala) on the Patagonian coast of Argentina based on morphological and molecular data. J Parasitol 104:479–485. https://doi.org/10.1645/17-134
Marcovecchio JE, De Marco SG, Magani F, Spetter CV, Beltrame MO, Cionchi JL (2019) Hydraulic stopper effect as a regulator of inorganic nutrients distribution in Mar Chiquita coastal lagoon (Argentina). Ecohydrol Hydrobiol 19: 629–641. https://doi.org/10.1016/j.ecohyd.2019.04.005
Martorelli SR (1989) El rol de Cyrtograpsus angulatus (Crustacea; Brachyura) en los ciclos de vida de Microphallus szidati (Digenea; Microphallidae) y Falsificollis chasmagnathi (Acanthocephala: Filicollidae). Algunos aspectos de su ecología parasitaria. Mem Inst Oswaldo Cruz 84:567–574. https://doi.org/10.1590/S0074-02761989000400016
Méndez Casariego A, Merlo M, Etchegoin J (2016) Spatial variability of larval parasites harboured by two crab species in an estuarine environment in Argentina. J Mar Biol Assoc UK 96:633–637. https://doi.org/10.1017/S0025315415000594
Mouillot D, Krasnov BR, Shenbrot GI, Gaston KJ, Poulin R (2006) Conservatism of host specificity in parasites. Ecography 29:596–602. https://doi.org/10.1111/j.0906-7590.2006.04507.x
Nadler SA, Pérez-Ponce de León G (2011) Integrating molecular and morphological approaches for characterizing parasite cryptic species: implications for parasitology. Parasitology 138:1688–1709. https://doi.org/10.1017/S003118201000168X
Nickol BB (1985) Epizootiology. In: Crompton DWT, Nickol BB (eds) Biology of the Acanthocephala. Cambridge University Press, Cambridge UK, pp 307–346
Nickol BB, Crompton DWT, Searle DW (1999) Reintroduction of Profilicollis Meyer, 1931, as a genus in Acanthocephala: Significance of the intermediate host. J Parasitol 85:716–718. https://doi.org/10.2307/3285748
Nickol BB, Heard RW, Smith NF (2002) Acanthocephalans from crabs in the southeastern US, with the first intermediate hosts known for Arhythmorhynchus frassoni and Hexaglandula corynosoma. J Parasitol 88:79–83. https://doi.org/10.1645/0022-3395(2002)088[0079:AFCITS]2.0.CO;2
Nikishin VP, Skorobrekhova EM (2019) Two Strategies of acanthocephalan interrelations with paratenic hosts. Biol Bull Russ Acad Sci 46:814–822. https://doi.org/10.1134/S1062359019080090
Nunes DM, Hartz SM (2006) Feeding dynamics and ecomorphology of Oligosarcus jenynsii (Gunther, 1864) and Oligosarcus robustus (Menezes, 1969) in the lagoa Fortaleza, Southern Brazil. Braz J Biol 66:121–132. https://doi.org/10.1590/S1519-69842006000100016
Oliva ME, Barrios I, Thatje S, Laudien J (2008) Changes in prevalence and intensity of infection of Profilicollis altmani (Perry, 1942) cystacanth (Acanthocephala) parasitizing the mole crab Emerita analoga (Stimpson, 1857): an El Niño cascade effect? Helgol Mar Res 62:S57–S62. https://doi.org/10.1007/s10152-007-0082-7
Petracci PF, Cereghetti J, Martín J, Obed S (2009) Dieta del Biguá (Phalacrocorax olivaceus) durante la primavera en el estuario de Bahía Blanca, Buenos Aires, Argentina. El Hornero 24:73–78
Posada D (2008) jModelTest: phylogenetic model averaging. Mol Biol Evol 25:1253–1256. https://doi.org/10.1093/molbev/msn083
Posada D, Buckley TR (2004) Model selection and model averaging in phylogenetics: advantages of akaike information criterion and bayesian approaches over Likelihood Ratio tests. Syst Biol 53:793–808. https://doi.org/10.1080/10635150490522304
Presswell B, Bennett JD, Smales LR (2020) Morphological and molecular characterisation of a new genus and species of acanthocephalan, Tenuisoma tarapungi n.g., n. sp. (Acanthocephala: Polymorphidae) infecting red-billed gulls in New Zealand, with a key to the genera of the Polymorphidae Meyer, 1931. Syst Parasitol 97:25–39. https://doi.org/10.1007/s11230-019-09898-0
Rauque C, Viozzi G, Flores V, Vega R, Waicheim A, Salgado-Maldonado G (2018) Helminth parasites of alien freshwater fishes in Patagonia (Argentina). Int J Parasitol 7:369–379. https://doi.org/10.1016/j.ijppaw.2018.09.008
Rayski C, Garden EA (1961) Life-cycle of an acanthocephalan parasite of the eider duck. Nature 192:185–186. https://doi.org/10.1038/192185a0
Reiczigel J, Marozzi M, Fabian I, Rozsa L (2019) Biostatistics for parasitologists – a primer to Quantitative Parasitology. Trends Parasitol 35:277–281. https://doi.org/10.1016/j.pt.2019.01.003
Reish DJ (1950) Preliminary note on the life cycle of the acanthocephalan, Polymorphus kenti Van Cleave, 1947. J Parasitol 36:496. https://doi.org/10.2307/3273182
Reta R, Martos P, Perillo G, Piccolo M, Ferrante A (2001) Características hidrográficas del estuario de la laguna Mar Chiquita. In: Iribarne O (ed) Reserva de Biósfera Mar Chiquita: Características Físicas, Biológicas y Ecológicas. Editorial Martín, Mar del Plata, Argentina, pp 31–52
Rodrigues LR, Fontoura NF, da Motta Marques D (2012) Feeding dynamics of Oligosarcus jenynsii (Günther, 1864) in a subtropical coastal lake assessed by gut-content analysis and stable isotopes. IJPAES 2:126–134
Rodríguez SM, D’Elía G (2016) Pan-American marine coastal distribution of Profilicollis altmani based on morphometric and phylogenetic analysis of cystacanths from the mole crab Emerita brasiliensis. J Helminthol 91:371–375. https://doi.org/10.1017/S0022149X16000237
Rodríguez SM, D’Elía G, Valdivia N (2016) The phylogeny and life cycle of two species of Profilicollis (Acanthocephala: Polymorphidae) in marine hosts off Pacific coast of Chile. J Helminthol 91:589–596. https://doi.org/10.1017/S0022149X16000638
Rodríguez SM, Diaz JI, D’Elía G (2017) Morphological and molecular evidence on the existence of a single estuarine acanthocephalan species of the genus Profilicollis along the Atlantic and Pacific coasts of southern South America. Syst Parasitol 94:527–533. https://doi.org/10.1007/s11230-017-9716-6
Ronquist F, Huelsenbeck JP (2003) MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics 19:1572–1574. https://doi.org/10.1093/bioinformatics/btg180
Rózsa L, Reiczigel J, Majoros G (2000) Quantifying parasites in samples of hosts. J Parasitol 86:228–232. https://doi.org/10.1645/0022-3395(2000)086[0228:QPISOH]2.0.CO;2
Santos CP, Gibson DI, Tavares LER, Luque JL (2008) Checklist of the Acanthocephala associated with the fishes of Brazil. Zootaxa 1938:1–22. https://doi.org/10.5281/zenodo.184999
Schmidt GD (1985) Development and life cycles. In: Crompton DWT, Nickol BB (eds) Biology of the Acanthocephala. Cambridge University Press, Cambridge, UK, pp 273–305
Schmidt G, Hugghins E (1973) Acanthocephala of South American Fishes. Part 2. Palaeacanthocephala. J Parasitol 59(5):836–838. https://doi.org/10.2307/3278419
Skorobrekhova EM, Nikishin VP (2017) The morphological peculiarities of the acanthocephalan Corynosoma strumosum (Rudolphi, 1802) (Polymorphidae) in paratenic hosts, the eelpout Zoarces elongatus (Kner, 1868) (Zoarcidae) and the halibut Hippoglossus stenolepis (Schmidt, 1904) (Pleuronectidae). Russ J Mar Biol 43:49–56. https://doi.org/10.1134/S1063074017010126
Swofford DL (2001) PAUP*: phylogenetic analysis using parsimony (and other methods) 4. 0:b5
Tamura K, Nei M (1993) Estimation of the number of nucleotide substitutions in the control region of mitochondrial DNA in humans and chimpanzees. Mol Biol Evol 10:512–526. https://doi.org/10.1093/oxfordjournals.molbev.a040023
Thompson JD, Higgins DG, Gibson TJ (1994) CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res 22:4673–4680. https://doi.org/10.1093/nar/22.22.4673
Torres P, Schlatter R, Montefusco A, Gesche W, Ruiz E, Contreras A (1993) Helminth parasites of piscivorous birds from lakes in the south of Chile. Mem Inst Oswaldo Cruz 88:341–343
Vizcaíno SI (1989) Acanthocephalan parasites of argentine birds I. Morphological complements to the knowledge of Polymorphus (Profilicollis) chasmagnathi comb. nov. (Polymorphydae). Stud Neotrop Fauna E 24:189–192. https://doi.org/10.1080/01650528909360790
Wells K, Clark NJ (2019) Host specificity in variable environments. Trends Parasitol 29:203–213. https://doi.org/10.1016/j.pt.2019.04.001
Acknowledgements
The authors would like to thank Lic. M. Graziano (Laboratorio de Biología Molecular, Instituto de Investigaciones Marinas y Costeras (IIMyC), UNMdP-CONICET) for her help and advice during the molecular studies, Dr. M. González-Castro (IIMyC) for his help in the capture and determination of the fish in Mar Chiquita and Arroyo Grande, and Mr. P. Urrutia for allowing the samplings to be carried out in his property (Nahuel Rucá lake). Finally, Mr. J. Levy, Mr. S. Cruz and Mrs. L. Fito for their help in the collection of samples.
Funding
This study was financed by the Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET) (PIP No. 112-201501-00973), the Fondo para la Investigación Científica y Tecnológica (PICT No. 2013) and the Universidad Nacional de Mar del Plata (EXA 1016/20).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
All applicable institutional, national and international guidelines for the care and use of animals were followed.
Conflicts of interest/Competing interests
The authors declare no conflict of interests.
Additional information
Section Editor: Shokoofeh Shamsi
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Levy, E., Rossin, M., Braicovich, P. et al. Profilicollis chasmagnathi (Acanthocephala) parasitizing freshwater fishes: paratenicity and an exception to the phylogenetic conservatism of the genus?. Parasitol Res 119, 3957–3966 (2020). https://doi.org/10.1007/s00436-020-06825-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00436-020-06825-x