Abstract
Morphology of capsules surrounding acanthocephalan Corynosoma strumosum in paratenic hosts (sea fishes of three species from the northern part of the Sea of Okhotsk) was studied. A thick layer of glycocalyx is formed on the surface of acanthocephalan’s tegument in smelts Osmerus mordax dentex and Hypomesus olidus; the surrounding capsule is formed by fibroblasts and collagen fibers and do not include inflammatory cells. Besides fibroblasts, capsule of the sole Limanda aspera consists also of macrophages, granulocytes, “dark” cells, and once of erythrocytes that indicate obvious inflammatory response of the host’s organism to invasion; glycocalyx on the surface of acanthocephalans from the sole is weakly developed. The obtained results allow considering the smelts as the most suitable paratenic hosts and the yellow-finned sole as unsuitable paratenic host for the studied acanthocephalans.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In the life cycle of acanthocephalans, paratenic hosts are not considered to be obligatory, but very often, they represent “… important ecological bridges between intermediate and definitive hosts…” (Schmidt 1985). Acanthocephalans are also supposed to have no development in such hosts (Petrochenko 1956), though this opinion has been doubted during the recent years (Sharpilo and Salamatin 2005).
Morphological aspects of relationships between acanthocephalans and obligatory hosts (namely intermediate and definitive ones) have been rather fully studied by the present time (Taraschewski 2000; Nikishin 2004). Particularly, cellular response of intermediate host to invasion (hemocyte attack) was shown to be overcome by forming of acellular cyst by parasite (Rotheram and Crompton 1972; Nikishin 1992); a thick layer of glycocalyx on the surface of cystacanth possibly also has protective function (Nikishin 2004). A structure of host–parasite interface of the adult acanthocephalans and definitive host was proved to depend on systematic position of parasite and host, and besides on the mode of attachment of acanthocephalans to the intestine wall (Taraschewski 2000). At the same time, morphology of interactions of acanthocephalans with paratenic hosts is poorly studied. It is only known that after penetration of acanthocephalan into such host, it is surrounded either with capsule consisting of the host’s cells (Bogitsh 1961) or with hyaline envelope (Amin et al. 1995); however, there is no any detailed description of the host’s cells participating in encapsulation. Summing up not numerous data on host–parasite interface of acanthocephalans and paratenic hosts, Taraschewski (2000) emphasized that “…any further discussion on the subject of paratenic hosts and postcyclic transmission has to be postponed until we know more about… host–parasite interface of acanthocephalans belonging to genera like Corynosoma…, which obviously depend in their transmission on the use of paratenic hosts….”. Besides, there is no information about peculiarities of capsule construction surrounding acanthocephalans of the same species in different hosts and in cases of different locations of acanthocephalans, in one host. In this connection, micro- and ultrastructure of capsules surrounding acanthocephalans Corynosoma strumosum in the three sea fishes: smelts Osmerus mordax dentex and Hypomesus olidus and yellow-finned sole Limanda aspera have been studied.
Materials and methods
Acanthocephalan C. strumosum is a dominating species of corynosomes in the Sea of Okhotsk and includes in its life cycle a lot of sea fishes serving as paratenic hosts (Atrashkevich 2008). In the study, we used one specimen of arctic rainbow smelt and pond smelt and three specimens of yellow-finned sole caught in Nagaevo Bay and Gertner Bay (Magadan). Encapsulated acanthocephalans occurred at the surfaces of internals and in the tissues of mesentery of the studied fishes. The collected capsules containing acanthocephalans were fixed in 2% of glutaraldehyde on 0.1 M phosphate buffer during 2 days, postfixed in 2–4% osmium tetroxide on 0.2 M phosphate buffer for 2 h, dehydrated in graded ethanol, and embedded in mixture of Epon and Araldite. During dehydration, the specimens were stained with 1% uranyl acetate on 70% ethanol during one night. The obtained blocks were cut into semithin (for light microscopy) and ultrathin (for electron microscopy) sections. Semithin sections were stained with methylene blue or mixture of methylene blue and crystal violet and viewed through the light microscopes Biomed-2 and Olympus CX41. The ultrathin sections were contrasted with lead citrate and viewed through the electron microscopes JEM-7 and JEM-1011. In general, three acanthocephalans from arctic rainbow smelt, one from pond smelt, and 11 from yellow-finned soles were studied.
Results
On the surface of tegument of all studied acanthocephalans, a layer of glycocalyx of wide range thickness depending on the species of paratenic host was found. Capsule structures surrounding corynosomes in the hosts of different species were also different.
Capsule surrounding acanthocephalans from the smelts
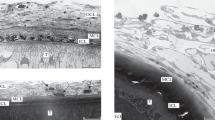
Scheme of the capsule structure is shown on Fig. 1. Tegument surface of acanthocephalans is covered with a layer of glycocalyx, 0.5–1.3 μm thick (Fig. 2 (3 and 4)). By morphological and size characteristics, it is similar with that described at the formed cystacanths (Nikishin 2000).
2 Host capsule and tegument (t) of acanthocephalan Corynosoma strumosum from arctic rainbow smelt. mlc middle layers of capsule, olc outer layers of capsule. Bar indicates 30 μm. 3 Layer of glycocalyx (g) on the surface of tegument (t) of acanthocephalan Corynosoma strumosum from smelts. Bar indicates 1 μm. 4 Fibrous material (arrows) and fragments of the destroyed cell (asterisk) in the space between tegument (t) of acanthocephalan Corynosoma strumosum and host capsule in smelts. sp spine. Arrowheads indicate glycocalyx. Bar indicates 1 μm
Three layers can be identified in the structure of capsules surrounding acanthocephalans from the smelts: inner one, which is the thinnest and densest (this layer is absent in two acanthocephalan capsules from arctic rainbow smelt); middle one, which is less dense; and outer one, which is the most friable (Figs. 1 and 2 (2)). Thickness of capsule varies from 17 to 72 μm that is determined by uneven thickness of each layer. Two specimens of corynosomes have capsules closely adjacent to glycocalyx at the surface of acanthocephalans (Fig. 2 (2)), other specimens have a space of 5–40 μm thick between parasite and capsule, filled with narrow and long chaotically directed electron-lucent fibers (Fig. 2 (4)).
Inner layer of capsule consists of lamellar elements of high electron density, sometimes the structures resembling nuclei are revealed in them (Fig. 3 (5)). Most probably this layer is formed by the fragments of destroyed host cells.
5 Structure of the inner (ilc) and middle (mlc) layers of capsule of acanthocephalan Corynosoma strumosum from smelts. n nuclei. Arrowheads indicate desmosomes. Bar indicates 2 μm. 6 Fibroblast (fb) from the middle layer of capsule of acanthocephalan Corynosoma strumosum in smelts. n nucleus, nu nucleolus, m mitochondria, ger granular endoplasmic reticulum. Arrowheads indicate finger-like protrusions. Bar indicates 2 μm
Middle layer of capsule is represented by flattened cells and their numerous processes directed parallel to acanthocephalan’s surface (Fig. 3). Nuclei of these cells are oval and possess a light karyoplasm, small prominent nucleolus, and moderate amounts of chromatin distributed along the nuclear envelope. Cytoplasm has low electron density and includes mitochondria, innumerous channels of granular endoplasmic reticulum (GER), free ribosomes and polysomes and many small vesicles. The cells are located close to each other and connected by multiple desmosomes (Fig. 3 (5)) and finger-like protrusions of cytoplasmic membrane (Fig. 3 (6)).
In the outer layer of capsule two sublayers can be distinguished, which are morphologically different (Fig. 1). Inner sublayer represents closely located highly compacted cells (Fig. 4 (7)). In the zone adjacent to the middle layer of capsule, these cells have a high nucleo–cytoplasmic ratio, the nuclei are characterized by high electron density, and cytoplasm contains mitochondria and free ribosomes. Intercellular space is filled with tightly located fibrils whose cross striation indicates their collagen nature. In the outer zone of this sublayer, cells become larger in size, electron density decreases, and bundles of collagen fibrils located in intercellular space have more loose organization. The outer sublayer of the outer capsule’s layer consists of large rounded or elongated loosely arranged cells with pronounced borders (Fig. 4 (8)). Nuclei of these cells are electron-lucent with prominent dense nucleolus. Large mitochondria, dilated cisternae of GER, Golgi complex, ribosomes, and numerous filaments occur in cytoplasm. The cells have connections resembling tight junctions (Fig. 4 (8)).
7 Fibrocytes (fc), fibroblasts (fb), and collagen fibers (arrows) in the proximal part of the outer layer of capsule of acanthocephalan Corynosoma strumosum from smelts. n nuclei. Bar indicates 2 μm. 8 Loosely arranged fibroblasts (fb) in the distal part of the outer layer of capsule of acanthocephalan Corynosoma strumosum from smelts. n nuclei, nu nucleolus, cf collagen fibers. Arrows indicate intercellular contacts. Bar indicates 2 μm
Capsule surrounding acanthocephalans from the yellow-finned sole
Glycocalyx layer on the surface of acanthocephalan’s tegument is considerably thinner than that at acanthocephalans from the smelts, not exceeding 0.1 μm.
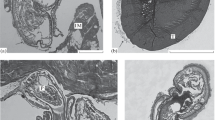
Among capsules surrounding corynosomes in the yellow-finned sole, one can recognize two varieties differing by their structure. Capsules of the first variety were found four times (Figs. 5 and 6 (10)). They are one-layered, 8–57 μm thick. Cells of two types can be distinguished in their structure. The first type cells dominate; they are flattened with numerous processes and connected with each other by many desmosomes (Fig. 6 (11)). Nuclei are elongated and relatively light. Cell cytoplasm possesses a short GER channels, mitochondria, and lipid droplets. In the outer part of capsule, the cells are loosely situated and they have more rounded shapes (Fig. 6 (10)); in intercellular space bundles of collagen fibers are observed, which is evidenced by their cross striation (Fig. 7 (12)). Some cells surrounded by fibers have dilated GER cisternae with electron-lucent content (Fig. 7 (12)). Along the inner surface of plasmatic membrane, a lot of vesicles are revealed, which possibly indicate process of exocytosis by which synthesized substances brought out from cytoplasm into intercellular space (Halton and Johnston 1982). According to all characteristics, these cells are fibroblasts.
10 Semithin section of capsule (c) of the first modification surrounding acanthocephalan Corynosoma strumosum in the yellow-finned sole. t acanthocephalan’s tegument, ld lipid droplets. Bar indicates 30 μm. 11 Ultrastructure of capsule (c) of the first modification in the yellow-finned sole. t acanthocephalan’s tegument, n nuclei of fibroblasts. Arrows indicate desmosomes. Bar indicates 1 μm
12 Capsule of the first modification in the yellow-finned sole: fibroblast. n nucleus, ger granular endoplasmic reticulum, cf collagen fibers. Bar indicates 1 μm. 13 Capsule of the first modification in the yellow-finned sole: macrophage. n nucleus, phs phagosome, rb residual body. Bar indicates 2 μm
Cells of the second variety can be observed close to surface of parasite, particularly in tegument folds and among fibroblasts (Fig. 7 (13)). They contain rounded and eccentric nuclei with diffuse chromatin structure. Cytoplasm contains short GER channels, Golgi complex, mitochondria, bodies of different forms with dense content and large electron-dense bodies resembling phagosomes, many residual bodies, and “empty” vacuoles. The mentioned features allow considering these cells as macrophages. In the outer part of capsule, these cells sometimes form small aggregations and differ from those of the inner part by the presence within the cytoplasm of the most numerous large electron-dense phagosomes, rarer occurrence of residual bodies, and large vacuoles (Fig. 5). Outer part of capsule often contains blood capillaries.
Capsules of the second modification were found at seven corynosomes. They are much thicker (17–115 μm) and clearly divided into two layers (Figs. 8 and 9). Inner layer of capsule contains three types of cells. Cells of the first type differ by occurrence of numerous spherical or almond-shaped electron-dense, membrane-bound granules (Figs. 10 and 11). Besides granules in their cytoplasm Golgi complex, GER channels and mitochondria are observed. We consider them as granulocytes.
The most numerous are the cells of the second type, which are morphologically identical to the cells of the second type, namely macrophages, described in capsule structure of the first modification (Figs. 10 and 11). Cells of the third type are innumerous and characterized by moderate or high nucleo–cytoplasmic ratio (Figs. 10 and 12 (18)). Nuclei are spherical with scattered electron-dense chromatin. Within the cytoplasm mitochondria with locally widened cristae, ribosomes and fine vesicles with the content of moderate density are found. At present, we do not interpret these cells and due to their high general electron density, call them “dark.”
18 “Dark” cell from the inner layer of capsule of the second modification in the yellow-finned sole. n nucleus, m mitochondria. Bar indicates 1 μm. 19 Fibroblast from the outer layer of capsule of the second modification in the yellow-finned sole. n nucleus, nu nucleolus, ger granular endoplasmic reticulum. Arrows indicate collagen fibers. Bar indicates 1 μm
Among cells of the inner capsule’s layer numerous bodies of rounded shape of 1.4–8.0 μm are dispersed (Fig. 9). Absence of surrounding membrane, high electron density and homogenous character of their material allow considering these bodies as lipid droplets.
Outer layer of capsule always has more intense staining on the light-microscope preparations comparing to that of the inner layer (Fig. 9). Besides the cells, which were analogously described in the inner layer, it contains fibroblasts easily identified by flattened shape and presence of numerous dilated GER cisternae (Fig. 12). Their nuclei have dispersed chromatin and indistinct nucleolus; perinuclear space is extended in places. Besides GER elements, cytoplasm includes mitochondria and free ribosomes; clusters of collagen fibrils can be viewed in intercellular space. In the distal part of this layer, innumerous blood capillaries are situated.
Once in the inner layer of capsule, sparse erythrocytes were found. Twice inner and outer layers of capsule were weakly marked and were hardly determined by light microscopy (Fig. 10). Besides, these capsules differ by low content of lipid droplets in the inner layer.
Discussion
The obtained results show significant differences in morphology of glycocalyx on the tegument surface of C. strumosum infesting hosts of different species and in structure of surrounding capsules. First, one should note the presence of a thick layer of glycocalyx on the tegument surface of acanthocephalans from smelts, which by specifications mostly resemble glycocalyx at the developed cystacanths. Glycocalyx both on the surface of helminthes and on the surface of any other cell is known to consist mainly of mucopolysaccharides (Ito 1969; Lumsden 1975), hence, it should be inevitably digested when passing through the initial sections of digestive system. Thus, its occurrence at the studied acanthocephalans parasitizing in smelts is supposed to be the evidence of its new formation in these hosts. Probably, the fact of new formation of glycocalyx was mistakenly interpreted as phenomenon of acanthocephalan encystment in paratenic host (Marchand and Grita-Timoulali 1992).
At the same time, acanthocephalans infesting the yellow-finned sole have glycocalyx iteratively less thickness and mostly resembling it covering metasoma of acanthocephalans in definitive host (Nikishin 2000). Mentioned difference in degree of glycocalyx development can be explained either by different age of the studied acanthocephalans or by species peculiarities of reaction of paratenic host to invasion. We incline to keep to the second variant with regard to more aggressive organism respond of the yellow-finned sole to invasion comparing to analogous organism respond of the smelts.
By total of morphological features all cells of inner, middle, and outer layers of capsules from smelts represent the same cell type situated at different stages of functional activity. Relying on morphological characteristics of cells, we suppose that medium layer and inner part of the outer layer of capsule are formed with fibrocytes and distal part of the outer layer contains mature fibroblasts synthesizing collagen fibrils. Thus, capsule surrounding corynosomes in smelts can be considered as totally fibroblastic. Capsules of similar structure were described around acanthocephalans Neoechinorhynchus cylindraceus from fish liver of genus Lepomis (Bogitsh 1961). Author underlines fibroblastic character of capsule indicating falseness of the earlier conceptions claiming that the outer capsule’s layer is formed with modified liver cells (Ward 1940). At the same time, difference between capsules around N. cylindraceus and C. strumosum consists in the fact that collagen fibers of the former one are located in the inner capsule’s layer and of the latter one, in the outer capsule’s layer. At present interpretation of this difference seems to be problematic; probably, it can be connected with the age of the studied acanthocephalans and reflects different stages of encapsulation process.
Capsules formed only by fibroblasts and collagen fibrils were observed around metacercariae of many digenetic trematodes using fishes as second intermediate hosts. For instance at Bucephaloides gracilescens (Halton and Johnston 1982), Uvulifer ambloplitis and Neascus pyriformis (Wittrock et al. 1991) and Bolbogonotylus corkumi (Walker and Wittrock 1992), together with corynosomes from the smelts, collagen fibrils are located in the median and outer parts of capsule and its inner part contains fragments of destroyed cells. In the capsule structure of Ascocotyle tenuicollis, only collagen fibers were described (Hicks and Steele 2003). At Ornithodiplostomum ptychocheilus, on the contrary, only fibroblasts were found, and collagen was not identified (So and Wittrock 1982). Relatively thin capsule of Opisthorchis felineus with insignificant amount of collagen fibrils in its structure is considered to be the high level parameter of parasite specificity to the host (Be’er 2005). It is important to underline that despite some differences in the given examples and in case with corynosomes from the smelts, no other cells, including inflammatory elements, were found in the capsule’s structure.
Other situation can be observed in case with the yellow-finned sole. Both modifications of capsule surrounding corynosomes in this paratenic host contain in their structure macrophages and granulocytes accumulating in the inner part of capsule and fibroblasts and collagen fibers occupying their outer part. Such distribution of cells allows the supposition that organism response of the yellow-finned sole to invasion starts with the expressed inflammatory reaction; migration of fibroblasts to the capsule’s wall and formation of fibrous material occur a few later and represent the second stage of capsule’s formation. If this supposition is correct, some differences in structures of capsules of the first and the second modifications can be connected with the invasion age: capsules of the first modification with larger amount of fibroblasts and fibrous material and with macrophages and granulocytes occur mainly in the body folds of acanthocephalans are older comparing to the capsules of the second modification with dominating inflammatory cells.
Thus, capsules surrounding acanthocephalans C. strumosum in paratenic hosts of different species significantly differ in their structures. Capsules from the smelts exclusively consist of fibroblasts and their derivatives (collagen fibrils), whereas capsules from the yellow-finned sole have evident inflammatory character. Such essential differences do not connect with localization peculiarities, since capsules, obtained from different organs of the same host, had similar structures. We suppose that differences in capsule structures reflect differences in response to paratenic hosts of various species to acanthocephalans’ invasion.
Examples of dependence of capsule structure on relating to host species are known at helminthes of other taxonomic groups, for instance, plerocercoids of some cestodes from different fishes (Pronina and Pronin 1988). According to these authors in obligatory hosts (sculpin, river perch, burbot), a thin fibroblastic capsule is formed around developing plerocercoids Triaenophorus nodulosus during first 2 or 3 weeks; whereas during infestation of optional hosts (pike, grayling, lenok, dace), plerocercoid is relatively slow surrounded by a thick two-layered capsule with dominating leucocytes and macrophages in its inner layer; at that, inflammatory process can involve the whole organ with localizing helminth. Authors suppose that weak cell reaction of organism of obligatory hosts, which indirectly expects the presence of anti-immune mechanisms at parasites, shows “…relative equilibrium in such host–parasitic systems and mutual adaptation of parasite and host…” (Pronina and Pronin 1988). Unfortunately, we do not know the age of the studied capsules; nevertheless, in general, our results are similar with the data of these authors. Some difference consists in the fact that in capsule of plerocercoids from optional hosts, namely in its inner part, one can observe degeneration of inflammatory cells and their replacement by fresh cellular elements (Pronina and Pronin 1988). We do not observe such process in capsules from the yellow-finned sole, though both forms of capsules from this host were different including number of inflammatory cells; and this is a fact, which can be caused by different age of the studied acanthocephalans. However, degeneration of fibroblasts was noted also in capsules from the smelts that shows definite counteraction of helminthes to the cell response of host.
Analysis of the obtained results and literary data let us consider the smelts as more suitable paratenic host for acanthocephalans C. strumosum and the yellow-finned sole as unsuitable paratenic host. Our further work will include more widened spectrum of the studied fishes and other animals aiming at determination of their affiliation to this or that group of paratenic host of this acanthocephalan.
References
Amin OM, Heckmann RA, Mesa R, Mesa E (1995) Description and host relationships of cystacanths of Polymorphus spindlatus (Acanthocephala: Polymorphidae) from their paratenic fish hosts in Peru. J Helminthol Soc Wash 62:249–253
Atrashkevich G (2008) Spiny-head worms genus of Corynosoma Lühe, 1904 (Acanthocephala: Polymorphidae) in the Okhotsk Sea and parasitic system with dominant species of the C. strumosum (Rudolphi, 1819). In: Galaktionov & Dobrovolskij (eds) Proceedings of the IV Congress of the Russian Society of Parasitologists—Russian Academy of Sciences, “Parasitology in XXI century—problems, methods, solutions”, V. 1. Lema, St Petersburg, pp 38–42
Be’er S (2005) Biology of the agent of opisthorchiasis. KMK Scientific Press Ltd, Moscow
Bogitsh BJ (1961) Histological and histochemical observations on the nature of the cyst of Neoechinorhynchus cylindratus in Lepomis sp. Proc Helminthol Soc Wash 28:75–81
Halton DW, Johnston BR (1982) Functional morphology of the metacercarial cyst of Bucephaloides gracilescens (Trematoda: Bucephalidae). Parasitology 85:45–52
Hicks T, Steele E (2003) Histological effect of Ascocotyle tenuicollis (Digenea: Heterophyidae) metacercarial infection on the heart of Fundulus heteroclitus (Teleostei: Cyprinodontidae). J South Carolina Academy Sci 1:10–18
Ito S (1969) Structure and function of the glycocalyx. Fed Proc 28:12–25
Lumsden RD (1975) Surface ultrastructure and cytochemistry of parasitic helminthes. Exp Parasitol 37:267–339
Marchand B, Grita-Timoulali Z (1992) Comparative ultrastructural study of the cuticle of larvae and adults of Centrorhynchus milvus Ward, 1956 (Acanthocephala, Centrorhynchidae). J Parasitol 78:355–359
Nikishin VP (1992) Formation of the capsule around Filicollis anatis in its intermediate host. J Parasitol 78:127–137
Nikishin VP (2000) Ultrastructural morphology of tegument in acanthocephalan. Parazitologia 34:125–143
Nikishin V (2004) Cytomorphology of the acanthocephala. Geos, Moscow
Petrochenko V (1956) Acanthocephala of domestic and wild animals. Izdatel’stvo Akademii Nauk SSSR, Moscow
Pronina S, Pronin N (1988) Interactions in systems helminthes–fishes. Nauka, Moscow
Rotheram S, Crompton DWT (1972) Observations on the early relationship between Moniliformis dubius (Acanthocephala) and the haemocytes of the intermediate host, Periplaneta Americana. Parasitology 64:15–21
Sharpilo V, Salamatin R (2005) Paratenic parasitism: origins and development of the concept. Logos, Kiev
Schmidt G (1985) Development and life cycles. In: Crompton D, Nickol B (eds) Biology of the Acanthocephala. Cambridge University Press, Cambridge, pp 273–305
So FW, Wittrock DD (1982) Ultrastructure of the metacercarial cyst of Ornithodiplostomum ptychocheilus (Trematoda: Diplostomatidae) from the brains of fathead minnows. Trans Am Microsc Soc 101:181–185
Taraschewski H (2000) Host-parasite interactions in Acanthocephala: a morphological approach. Adv Parasitol 46:1–179
Walker DJ, Wittrock DD (1992) Histochemistry and ultrastructure of the metacercarial cyst of Bolbogonotylus corkumi (Trematoda: Cryptogonimidae). J Parasitol 78:725–730
Ward HL (1940) Studies on the life history of Neoechinorhynchus cylindratus (Van Cleave, 1913) (Acanthocephala). Trans Am Microsc Soc 59:327–347
Wittrock DD, Bruce CS, Johnson AD (1991) Histochemistry and ultrastructure of the metacercarial cysts of blackspot trematodes Uvulifer ambloplitis and Neascus pyriformis. J Parasitol 77:454–460
Acknowledgments
The work was supported by grants of RFBR—FEB RAS (no. 09-04-98523) and FEB RAS (no. 09-III-A-06-218 and 10-III-Б-06-139). The authors thank Mrs. E. Schetinina for help in preparing the English version of the article.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Skorobrechova, E.M., Nikishin, V.P. Structure of capsule surrounding acanthocephalans Corynosoma strumosum in paratenic hosts of three species. Parasitol Res 108, 467–475 (2011). https://doi.org/10.1007/s00436-010-2088-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00436-010-2088-3