Abstract
Parasite communities of Raneya brasiliensis are described and its parasites used as biological tags to discriminate its populations. Fish were caught in two zones of the Argentine Sea: one sample from San Jorge Gulf (Patagonian Region) and three samples from off the coast of Buenos Aires (Bonaerense Region). A total of 183 fish were examined for parasites and 11 species were found. Host body size and its ecology are pointed out as drivers of the paucity of taxa found. Multivariate similarity analyses allowed the identification of three stocks: one in the San Jorge Gulf, and two other in the Bonaerense Region. The parasite species that contributed most to the separation of the samples were generally those identified as biological markers in previous studies in the area. Patterns of distance decay in similarity among communities in R. brasiliensis were found; with dissimilarity values between distant localities being higher than between close ones. Whereas the composition and structure of parasite assemblages in Bonaerense waters reflect those of other fish species in this region, being mainly determined by the composition of the compound community, no repeatable patterns were found in the composition of parasites assemblages when R. brasiliensis was compared with other hosts species in Patagonia.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Parasite communities are highly complex ecological systems, driven by multiple ecological and evolutionary processes that interact across different spatial and temporal scales to create intricate assemblages with many interrelating entities. These features make recurrent patterns and general mechanisms less likely or difficult to identify (Poulin 2007). Furthermore, many parasite species can live in many different hosts and the dynamics of parasite populations and of communities they form can be quite different depending on the host characteristics. Moreover, parasite species richness and abundance can vary geographically for the same host species. The distribution patterns of marine parasites are mainly determined by temperature-salinity profiles and their association with specific masses of water (Esch and Fernández 1993) and therefore the size of the pool of available species must differ from one geographical area to the next, limiting the number of parasite species that a host can acquire over time, regardless of the characteristics of this host species (Luque et al. 2004); moreover, their abundance can be influenced by the characteristics of the local ecosystem and its trophic web (Luque and Poulin 2004; Marcogliese 2001, 2002). However, parasite community ecology has concentrated mainly on local processes instead of on large spatial scales (Guégan et al. 2005), and much of the available evidence derives from studies carried out without replication in both space and time (González and Poulin 2005; Poulin and Valtonen 2002; Timi and Poulin 2003; Vidal-Martínez and Poulin 2003).
On the other hand, the existence of spatial variability in the composition and abundance of fish parasite assemblages constitutes the basis for the use of parasites as biological tags for fish stock discrimination (Begg and Waldman 1999; MacKenzie 2002; MacKenzie and Abaunza 1998; Power et al. 2005). This methodology focuses on the differences between parasite populations/communities between zones, but their similarities are commonly ignored. Similarity in species composition among parasite communities is expected to decrease with increasing distance between them (Poulin 2003; Poulin and Morand 1999). This negative relationship is the outcome of ecological and evolutionary phenomena shaping spatial patterns in biodiversity and biogeography (Nekola and White 1999; Soininen et al. 2007), and has been recently recorded for parasite communities in fish from the Southwestern Atlantic (Timi et al. 2010), with fish samples from the coasts of the northern Argentine Sea (Bonaerense zone) being more similar each other than to fish from Patagonian waters, whereas all samples from Argentina showed higher similarity than in relation to those from Brazil.
In Patagonian waters, the oceanographic conditions are different to those from the Bonaerense zone (Acha et al. 2004; Bakun and Parrish 1991), and have been suggested as responsible for the differences found in parasitological studies of all fish species studied comparatively between these two areas (Timi 2003; Timi and Poulin 2003; Sardella and Timi 2004; Braicovich and Timi 2008; Timi and Lanfranchi 2009a, b; Timi et al. 2008, 2009). In Bonaerense coastal waters, parasitological studies on small sized fish species with a low level in the food chain have shown that parasite communities are dominated, in terms of prevalence and abundance, by non-specific larval stages (mainly nematodes, cestodes, and acanthocephalans; Lanfranchi et al. 2009; Timi and Lanfranchi 2009a, b; Timi et al. 2009). This pattern was also found in this area for others host fishes belonging to different ecological groups (Sardella and Timi 1996; Cremonte and Sardella 1997; Timi 2003; Timi and Poulin 2003; Sardella and Timi 2004; Timi et al. 2005, 2008; Braicovich and Timi 2008) and these parasites have been proposed as regional tags not only for fish populations but also for fish communities (Timi 2007). On the other hand, no such studies have been carried out in Patagonian waters, except for Pinguipes brasilianus, which shows parasite assemblages dominated by adult endoparasites in north Patagonian gulfs (Timi et al. 2010). Therefore, a comparison of the structure and composition of fish parasite communities between these areas could reveal the existence of recurrent patterns allowing the evaluation of the process underlying them.
The existence of repeatable spatial patterns in community structure is evaluated for the parasites of the banded cusk-eel (local name: raneya), Raneya brasiliensis (Kaup 1856) (Pisces, Ophidiiformes, Ophidiidae). It is a demersal-benthic fish (Nielsen et al. 1999), that inhabits coastal waters, at depth of 40 to 150 m, from Southern Brazil, about 23°S (Carvalho-Filho 1999) to San Jorge Gulf, around 46°S (Gosztonyi et al. 2007). This fish is caught as a part of the by-catch of the hake and the shrimp fisheries (Graça-Lopes et al. 2002; Menni and Lopez 1974). Although commercially unimportant, it constitutes a key link in the food web of the Southwestern Atlantic Ocean (Gosztonyi et al. 2007). In the Argentine Sea, it has been cited as an important prey item in a variety of marine mammals, marine birds and fishes (Gosztonyi et al. 2007 and reference therein). It preys mainly on benthic invertebrates (polychaetes, ophiuroides, molluscs, and crustaceans), as well as teleost fishes (Vera and Soares 2008). Hence, the raneya occupies the position of secondary consumer in the food chain (Vera and Soares 2008).
The aim of this study is therefore threefold: (1) to describe the parasite communities of R. brasiliensis in the southern boundaries of its distribution (off Buenos Aires and Patagonian coasts); (2) to identify the existence of different stocks of raneya in these areas by using its parasites as biological tags; and (3) to establish whether the parasite assemblages of raneya show the spatial structure previously observed for other sympatric fish species in the region.
Materials and methods
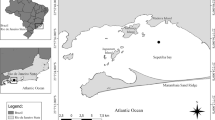
A total of 183 specimens of R. brasiliensis were examined for parasites. Fish were caught by trawl in two zones of the Argentine Sea (Fig. 1): one sample from the area of San Jorge Gulf, Chubut Province in the Patagonian region and three samples from off the coast of Buenos Aires Province (Bonaerense region), landed at the Port of Mar del Plata (38°03′S–57°32′W; Table 1); one of these obtained from a fish market, and therefore with no information on its geographical origin. Fish were either kept fresh or deep frozen at −18°C until examination. After defrosting fish was measured for total length (cm) and sexed. Body surface, gills, branchial and body cavity, viscera (stomach, intestine, liver, gall bladder, spleen, heart, gonads and mesenteries), gas bladder, kidneys and musculature were examined with the aid of a stereoscopic microscope.
The prevalence, mean abundance and its standard deviation were calculated for each parasite species. For those species with prevalence higher than 10% in a given sample (component species sensu Bush et al. 1990), Chi-squared analyses and a posteriori multiple comparisons, with previous angular transformation of each value, and Kruskall–Wallis and a posteriori Dunn’s tests were used to test for significant differences of prevalence or abundance, respectively between samples (Zar 1996).
The potential effect of host sex on prevalence and mean abundance of parasites was tested using Chi-squared analysis and the Mann–Whitney test, respectively and the relationship between host size and abundance was analyzed by Spearman’s rank correlation coefficients (Zar 1996).
For each individual fish harboring two or more species the following community descriptors were calculated at infracommunity level (Bush et al. 1997): total abundance (the number of parasites per host, N), species richness (S), Brillouin’s index of diversity (HB) and Berger–Parker’s index of dominance (BP; Magurran 1988). The potential variation of the infracommunity descriptors in relation to host sex (Mann–Whitney test) and size (Spearman’s rank correlation coefficients) was also tested.
Multivariate analyses applied to component species were performed using the PRIMER package V6 (Clarke and Gorley 2006; Clarke and Warwick 2001). As Scolex polymorphus is a complex of larval cestode species (Chambers et al. 2000) that can have different geographical distributions and cannot be used as reliable indicators of similarity among host populations (Braicovich and Timi 2008), this species was excluded from multivariate analyses.
Parasite community composition was compared among sampling localities by means of a permutation-based one-way analysis of similarity (ANOSIM, Clarke 1993). The statistical significance of the differences among zones was assessed after 10,000 permutations on abundance data.
Non-metric multidimensional scaling (MDS) was used to visualize differences in the composition of parasite infracommunities between samples, as well as to identify which parasite species were responsible for such differences; in this case the abundance matrix was transposed and parasite species assumed as samples, whereas individual hosts were analyzed as variables; the fit of the MDS ordinations were quantified by a value of stress. Hierarchical agglomerative clustering was performed for both the samples and the parasite species using group-average linking and resemblance levels were overlaid on the MDS plot.
Similarity percentages (SIMPER, Clarke 1993), were calculated within and between areas and used to determine which species characterized (typical species contributing substantially to the average similarity and doing it consistently by displaying a high ratio between that contribution and its standard deviation) and discriminated (discriminator species contributing largely to the average dissimilarity and displaying a high ratio between that contribution and its standard deviation) between parasite assemblages (Clarke and Gorley 2006). All multivariate procedures were based on the analysis of Bray–Curtis similarity matrices and applied to both untransformed abundance values and square-root-transformed data in order to down-weight the importance of very abundant species so that the less dominant species played some roles in determining similarity among samples (Clarke and Gorley 2006).
Results
General results
Mean host body lengths differed significantly among samples (F = 6.37, P < 0.01), with fish from San Jorge Gulf (SJG) and Mar del Plata—October (MDPoct) being significantly larger than those from Mar del Plata—June and Mar del Plata—November (MDPjun and MDPnov, respectively; all P < 0.05).
With the exception of four fish from SJG, all the rest were parasitized by at least 1 of the 11 metazoan parasite species found (Table 2), all of them at larval stage; except for Cucullanus genypteri, for which immature adults were also found encysted in the liver and mesenteries or free in the gut. Spores of Ceratomyxa sp. (Myxosporea) were found in the gall bladder of hosts from MDP, this microparasite was not included in further analyses. Prosorhynchus sp. was found in the head musculature of hosts, this tissue was not examined in the sample from SJG, therefore prevalence and abundance were compared among the three Bonaerense samples only and this species was excluded from other analyses. A total of 4.618 (5.135 including Prosorhynchus sp.) metazoan parasites were found in the overall sample.
Parasite populations
With the exception of Pseudoterranova sp., Corynosoma cetaceum and the unidentified plerocercoids, most parasite species reached the status of component species (prevalence > 10%) in one or more samples (Table 2). Most of them were present in all samples, except for Anisakis simplex s.l., which was only found in SJG. Corynosoma australe was the most prevalent and abundant parasite in the whole sample, representing 62.6% of all individual parasites found.
Parasites showed differential distributions among hosts from Patagonian and Bonaerense zones, and in a lesser extent among samples landed at Mar del Plata, as evidenced by comparisons of prevalence (Table 3) and abundance (Table 4) of component species. Prosorhynchus sp., compared among Bonaerense samples only, was similar between October and November and more prevalent than in June. Only C. australe showed similar values of prevalence in all samples; the rest of component species showed significant variations, with: Grillotia carvajalregorum, Nybelinia sp., S. polymorphus, and C. genypteri showing lower prevalence in SJG, whereas the inverse relationship was observed for A. simplex s.l. and Hysterothylacium aduncum. No differences were observed for any species among samples from Mar del Plata for comparisons involving those of October, and only Nybelinia sp. differed between samples from June and November. Regarding abundance, all species varied among samples, but a posteriori tests failed in finding the samples responsible for such differences for A. simplex s.l. The highest number of differences was observed in those comparisons involving fish from SJG. Among Bonaerense samples, those of June harbored more abundant populations of C. australe and less number of Nybelinia sp. and Prosorhynchus sp. than the other two, finally no differences were observed between fish from October and November for any species.
No effect of host sex on prevalence or abundance was observed for any parasite species from SJG and MDPoct (all P > 0.05). In MDPjun and MDPnov, only four female hosts were present in each sample and no comparisons between sexes were made.
No relationships between parasite abundance and host size were observed in fish from SJG (all P > 0.05) whereas three species, G. carvajalregorum, S. polymorphus, and C. genypteri showed values of abundance significantly higher in larger hosts from MDPjun (Rs = 0.69, P < 0.01; Rs = 0.49, P < 0.01, and Rs = 0.42, 0.01 < P < 0.05, respectively). As no differences in prevalence or abundance were found for any species between fish from MDPoct and MDPnov, hosts were pooled for correlation analyses, in this case two parasite species, C. australe and G. carvajalregorum increased significantly their abundance with host size (Rs = 0.34, P < 0.01 and Rs = 0.33, 0.01 < P < 0.05, respectively).
Parasite communities
As no differences in prevalence or abundance were found for any species between fish from MDPoct and MDPnov, hosts were pooled for community analyses (MDP hereafter). Twenty-eight hosts (27 from SJG and 1 from MDP) harbored monospecific infections (93% of them represented by C. australe). The maximum infracommunity species richness (six species) was found in only three hosts from the Bonaerense zone, with fish from this zone showing a higher proportion of richer infracommunities than those from SJG (Fig. 2). Significant differences were observed for all infracommunity indices, S (H = 71.91, P < 0.01), N (H = 91.27, P < 0.01), HB (H = 56.58, P < 0.01) and BP (H = 35.88, P < 0.01), when only hosts harboring two or more parasite species were considered (Fig. 3). A posteriori tests showed that S, N, and HB were lower in SJG than in Bonaerense samples, with only N differing between MDPjun and MDP (all P < 0.01); dominance, in turn was similar between SJG and MDPjun, and higher than in MDP (P < 0.01).
Similarity analyses
Similarity-based multivariate analysis based on untransformed data showed that fish from the three samples differed significantly in their parasite species composition (Global R ANOSIM = 0.72, P < 0.01), indicating that within-zone similarity exceeded among-zone similarity. In fact, all pair-wise comparisons between samples were significantly different (all P < 0.01). The same analyses, but considering fish from MDP (October + November) as different samples yielded similar results (Global R ANOSIM = 0.69 P < 0.01), with pair-wise comparisons between samples significantly different (all P < 0.01), except that comparing fishes from October and November (R ANOSIM = 0.07, P > 0.05).
Multidimensional scaling revealed an apparent pattern of separation between sampling sites, and the stress level (0.13) indicated that community structure was substantially different from random (Fig. 4a). Fish from Mar del Plata October and November, although analyzed as a single locality, are discriminated in the MDS plot, showing a high overlapping in the bidimensional space. The MDS applied to parasite species as samples and hosts as variables showed a clustering of species in relation to their importance in each zone, with a low stress level (0.0). Those species associated to the Bonaerense zone (C. genypteri, G. carvajalregorum, and Nybelinia sp.) clustered together in the plot and separated from SJG representatives (H. aduncum and A. simplex s.l.), C. australe, which was distributed in almost all fish, was ordered far apart although cluster analyses showed it as slightly more similar to species characteristic of Mar del Plata (Fig. 5a).
a Non-metric two-dimensional ordination plot using Bray–Curtis similarity based on untransformed parasite abundance data for each of the six most representative species across 179 individual R. brasiliensis from three samples from the Argentine sea: Mar del Plata June (triangles); Mar del Plata October + November (squares) and San Jorge Gulf (circles). Fish from Mar del Plata October (black squares) and November (empty squares) are discriminated in the figure, but analyzed as a single locality. b Idem after down-weighting the importance of very abundant species
a Non-metric 2-dimensional ordination plot using Bray–Curtis similarity based on untransformed parasite abundance data for each of the 179 individual R. brasiliensis across six parasite species in three zones of the Argentine sea. Results of a hierarchical agglomerative clustering performed for parasite species is overlaid on the MDS plot with similarity levels represented by a gray scale. b Idem after down-weighting the importance of very abundant species
SIMPER were similar and relatively low within localities showing that samples were little homogeneous in terms of parasite assemblages composition (Table 5). The main contributor to similarity in samples from MDP was Nybelinia sp., whereas C. australe was the most important contributor in MDPjun and SJG, but was also relevant in similarity among infracommunities from MDP; finally, C. genypteri contributed substantially to similarity within both Bonaerense samples. All these species contributed consistently to similarity in these inter-comparisons within localities, as shown by the rate between their contributions and standard deviations. On the other hand, a high average dissimilarity was observed among all samples (Table 5), the highest being that among MDPjun and SJG and the lowest between both Bonaerense samples. C. australe, despite being typical of all samples, was important and consistent discriminator among them; the other important discriminator species were C. genypteri among all samples and Nybelinia sp. between MDP and SJG.
Analyses after down-weighting the importance of dominant species showed similar patterns. Fish from all sampling localities differed significantly in their parasite species composition (Global R ANOSIM: 0.77, P < 0.01) with all pair-wise comparisons showing significant differences (all P < 0.01) and MDS showed a clear separation between samples, although in this case the stress values was somewhat higher (0.15; Fig. 4b). Ordination of parasite species showed a similar pattern to that of untransformed data, also with a low stress level (0.0) and a higher similarity between C. australe and species typical from the Bonaerense zone (Fig. 5b). Similarity percentages increased within localities (MDPjun: 66.24; MDP: 64.36; SJG: 61.47) but dissimilarity between zones decreased (MDPjun-MDP: 48.87; MDPjun-SJG: 67.59; MDP-SJG: 69.02), however the same subset of typical and discriminator species was observed within and between samples, respectively, although G. carvajalregorum and H. aduncum were also discriminator species in those comparisons involving fish from MDPjun.
Discussion
The total sample of R. brasiliensis harbored 11 metazoan parasites species, all of them constituting new host records. Nonetheless, all these parasite species have been previously recorded in other host species inhabiting the Argentine Sea.
The raneya showed low values of both species richness and abundance in relation to other fish species from the same region, but similar to those recorded in other small-bodied fishes that also occupy a low level in the regional trophic web (Lanfranchi et al. 2009; Timi and Lanfranchi 2009a). Host features are important determinants of parasite species richness (Poulin 2004; Poulin and Morand 2004); some studies have shown that the deciding factors for explaining parasite species richness in fishes are the host size and its ecology (Guégan et al. 2005; Luque et al. 2004). Host body size has proved to be positively correlated with parasite diversity in several studies, although this tendency is far from being universal (Poulin 2000). In larger hosts, the available niches for parasites colonization are more diverse, which in turn allows hosts to harbor greater species richness (Poulin 2004). On the other hand, trophic level of a fish host in the food web may be a good indicator that fishes at higher levels should be exposed to more infective helminth larvae from a broader range of parasite taxa via their diet than those at lower trophic levels over evolutionary time (Luque and Poulin 2008). Thus, the paucity of taxa found in the raneya can be explained by its small size and low level in the food chain.
Most parasites found in R. brasiliensis were at larval stage, the exception being C. genypteri, which was found as larvae as well as immature adults free in the intestines or encapsulated in the intestine wall and liver. Thus, by the nature of their different life cycles, parasites in a host population provide information about the role of the host in the food webs (Campbell et al. 1980; Marcogliese 2002; Marcogliese 2003; Marcogliese 2001; Marcogliese and Cone 1997). Many helminths use fish either as their second intermediate or as a paratenic host (Marcogliese 1995; Poulin and Valtonen 2002). The use of organisms as paratenic hosts serve to promote transmission and maintain parasites in the environment even at low densities (Marcogliese 1995). Given that larval helminths in fish hosts are transmitted to their definitive hosts by predation, clearly the more adequate fish species to act as intermediate hosts would be small-bodied enough to serve as prey, and they should be near the bottom or middle of the food chain (George-Nascimento 1987; Marcogliese 2002). All these features are present in the raneya, and could explain the larval composition of their parasite communities.
Larval helminths complete their life cycle when the raneya is ingested by their definitive host: marine mammals, marine birds and fishes. However, due to the parasites of raneya are widely distributed among fish species in the region, its importance in the parasite’s life cycles depends on the relevance that this fish has in the diet of the definitive hosts. Trophically transmitted parasites provide natural biological indicators of trophic links between organisms within ecosystems (Lafferty et al. 2008; Marcogliese 2003; Marcogliese and Cone 1997). Indeed, the raneya is one of the most important prey items in Patagonian waters for both the South American sea lion Otaria flavescens (Koen-Alonso et al. 2000) and the South American fur seal Arctocephalus australis, this fish could have some relevance serving as paratenic host and source of the acanthocephalan C. australe for these definitive hosts in this zone. In fact, and contrarily to the findings of the present paper, C. australe shows low levels of parasitism in other fish species from Patagonian waters in relation to northern zones (Timi 2003, 2007).
C. genypteri is only known at adult stage as a parasite of two ophidiid species in the Southwest Atlantic, the kingclips Genypterus blacodes and G. brasiliensis (Sardella et al. 1997). Although knowledge about the life cycle of cucullanids is still scarce, there is some evidence that this group is primitively heteroxenous and uses vertebrates as intermediate hosts, but in some species the intermediate host has been replaced by a histotropic phase in the definitive host (Anderson 2000). This nematode probably attain an adult stage in the raneya because of the phylogenetic affinities between this fish and Genypterus spp.; however, the histotropic nature of these adults, as well as the fact that no gravid females were found in the gut, indicate that the raneya is an unsuitable definitive host for this species. In any case, this fish could serve as a paratenic host for C. genypteri, with the advantage of a developmental precocity of this parasite when is consumed by the definitive hosts.
Several authors have claimed that the lack of replication is the rule in studies on parasite community structure and that their short-scale spatial and temporal variations have usually been neglected (Díaz and George-Nascimento 2002; Vidal-Martínez and Poulin 2003). In fact, whereas steady patterns of parasite community structure have been found in some studies where more than one sample have been analyzed in a single place or in localities situated close each other (Chávez et al. 2007; Díaz and George-Nascimento 2002; Flores and George-Nascimento 2009), the opposite trend has been observed in others (Ferrer-Castelló et al. 2007; George-Nascimento et al. 2009). This lack of repeatability has been claimed as a flaw in comparative studies, such as those designed to discriminate fish populations, carried out with single samples in each place or by pooling samples from the same locality, and suffering therefore from pseudoreplication problems (Ferrer-Castelló et al. 2007).
In the present study however, and in spite of the differences found in fish size between samples caught in Mar del Plata in October and November, no differences were observed in comparisons of their parasite populations, as well as of infracommunity descriptors, showing clearly that these hosts from close localities (distant about 37 km each other) belong to a single population. This repeatability was also demonstrated by similarity analyses, validating the assumption that infection levels are more similar between samples from the same locality than between samples from different localities (Chávez et al. 2007), and making reliable the inferences about stock structure in the other two identified populations of raneya based on single samples from each locality.
Despite the low number of individuals in the sample from MDPjun, which could affect the results of the comparisons of population and community indices, the differences observed between samples were strong enough to consider them as the result of genuine biological processes. These differences cannot be attributed to the variability in the size of hosts between regions, since the abundance of most species did not vary with fish length. Furthermore, and as an example C. australe, G. carvajalregorum, C. genypteri, and S. polymorphus, which displayed a cumulative effect with host size, were more prevalent and abundant in MDPjun than in SJG, where fish were larger.
The most conspicuous result of the analyses is the difference in parasite community structure between fishes from the Bonaerense and Patagonian regions. At the parasite population level, significant differences in population descriptors, especially the prevalence, as well as in infracommunity indices, between both regions for all parasite species demonstrate their potential for discriminating discrete stocks of raneya, each having their own indicator species. Evidence supporting the existence of different stocks was also obtained by multivariate analyses. The parasite species that contributed most to the separation of the samples in the MDS analysis agreed with those identified as potential biological markers in the analyses at population level, being C. australe and C. genypteri identified as the best indicator species in similarity analysis. The dominance and high prevalence of C. australe in the parasite assemblages from both zones was responsible of the similarity within component communities, however the differential abundance of this species, even after down-weighting its importance was the main determinant of the observed geographical variations.
Differences found in the structure of parasite communities between zones are a consequence of the presence of latitudinal gradients in the oceanographic conditions in the study area, mainly related to water temperature; which in turn influences the latitudinal distribution of parasites (Timi 2007). In fact, C. australe, Grillotia sp., Nybelinia sp. and S. polymorphus have been always recorded at higher abundance and prevalence in Bonaerense waters in relation to Patagonian ones, in all fish species analyzed comparatively between these zones, whereas A. simplex and H. aduncum showed the opposite pattern (Timi 2003; Sardella and Timi 2004; Timi et al. 2008; Timi and Lanfranchi 2009b). These differences between zones can be due to the influence of environmental conditions on the fitness of parasite species, as well as on the biota in general, and consequently, on the distribution of other hosts for these species. In fact, samples from both zones can be assigned to different faunistic provinces in the Atlantic environment of South America: those from the Bonaerense region belong to the Argentine Province (Bonaerense region), and those from Patagonia to the Magellanic Province (Bogazzi et al. 2005; Floeter and Gasparini 2000).
Despite samples were obtained in different faunistic provinces, variations in composition of parasite assemblages between both zones were less important than differences in abundance and/or prevalence, as well as in infracommunity descriptors. Parasite communities in the same host species form interconnected networks of component communities or metacommunities which are linked by migrations of individual hosts between host populations or via other dispersal routes, such as migrations of other hosts involved in the life cycle of the parasites (Poulin 2007). No migratory behavior is known to occur in R. brasiliensis, therefore the similarity in species composition of parasite communities between both zones is expected to be the outcome of migration of definitive hosts for the parasites harbored by the raneya, such as marine mammals.
Focusing in the comparisons among Bonaerense samples, an in spite of being more similar each other than in relation to those from SJG, they showed differences of abundance for C. australe, Nybelinia sp. and Prosorhynchus sp., with the latter two species also differing in prevalence. These differences, especially the abundance of C. australe, were responsible for both the higher total abundance and dominance values in MDPjun and the separation of this species from the other indicator species of the Bonaerense region in the MDS analyses. Therefore, the raneya from MDPjun should be considered as members of a different population than those of MDP.
As the coastal fleet operating at the port of Mar del Plata changes its fishing stations depending on fishing success, vessels can move northwards to the vicinity of Villa Gesell (37° 15′S, 57° 23′W), a zone greatly influenced by the Brazil current (tropical warm and saline waters), and also affected by the discharge of the Rio de la Plata (Bakun and Parrish 1991; Guerrero and Piola 1997).
In this zone, fish assemblages show temporal persistence in species composition and geographical location (Jaureguízar et al. 2006). In fact, discrete populations, discriminated by their parasites, have been recorded for other host species in the Bonaerense zone (Timi and Lanfranchi 2009b; Timi et al. 2009). Furthermore, C. australe displays higher values of abundance in several host species in the northern boundaries of this region (Timi 2003; Braicovich and Timi 2008; Timi and Lanfranchi 2009b; Timi et al. 2009, 2010). The notably higher levels of parasitism by C. australe could indicate that these samples come from this zone. This is also supported by the dissimilarity values among samples, which are much higher between distant localities (between regions) than between close ones (between localities within the same region), being the maximum value observed for the comparisons between MDPjun and SJG. This is a result of distance separating samples, a common phenomenon that usually emerges as the best predictor of similarity in analyses of parasite communities in relation to the characteristics of the habitat (Poulin and Morand 1999; Poulin 2003). Patterns of distance decay in similarity among communities of other fish species have been found in the southwestern Atlantic (Timi et al. 2010), and as the lowest the similarity the highest the distance, the geographical origin of the samples from June should be located at the north of Mar del Plata. Further analyses of samples from the northern limit of the Argentine sea are necessary to test this hypothesis.
The dominance of larval and little specific parasite species seems, therefore, to be a recurrent pattern in the Bonaerense region, and as it was observed in other species of small benthic fishes (Lanfranchi et al. 2009; Timi and Lanfranchi 2009a). In this region the composition and structure of parasite assemblages of R. brasiliensis are mainly determined by the composition of the compound community. The same reasoning applies to raneyas from Patagonian waters, although also depending from parasite species typical from this zone, namely H. aduncum and A. simplex s.l. However, in this case this pattern differs from other fish species for which the guild of adult endoparasites is the most important component (Timi et al. 2010).
Whereas the occurrence of recurrent patterns appear to be the rule in parasite communities of several fish species in Bonaerense waters; no repeatability in the composition of such assemblages was found in Patagonia; further studies including different host species are needed to shed some light on the apparently contingent patterns in this region.
References
Acha EM, Mianzan HW, Guerrero RA, Favero M, Bava J (2004) Marine fronts at the continental shelves of austral South America: physical and ecological processes. J Marine Syst 44:83–105
Anderson RC (2000) Nematode parasites of vertebrates: their development and transmission, 2nd edn. CABI, Wallingford
Bakun A, Parrish RH (1991) Comparative studies of coastal pelagic fish reproductive habitats: the anchovy (Engraulis anchoita) of the southwestern Atlantic. ICES J Mar Sci 48:343–361
Begg GA, Waldman JR (1999) An holistic approach to fish stock identification. Fish Res 43:35–44
Bogazzi E, Baldoni A, Rivas A, Martos P, Reta R, Oresanz JM, Lasta M, Dell’Arciprete P, Werner F (2005) Spatial correspondence between areas of concentration of Patagonian scallop (Zygochlamys patagonica) and frontal systems in the southwestern Atlantic. Fish Oceanogr 14:359–376
Braicovich PE, Timi JT (2008) Parasites as biological tags for stock discrimination of the Brazilian flathead Percophis brasiliensis in the south-west Atlantic. J Fish Biol 73:557–571
Bush A, Aho J, Kennedy C (1990) Ecological versus phylogenetic determinants of helminth parasite community richness. Evol Ecol 4:1–20
Bush AO, Lafferty KD, Lotz JM, Shostak AW (1997) Parasitology meets ecology on its own terms: Margolis et al. revisited. J Parasitol 83:575–583
Campbell RA, Haedrich RL, Munroe TA (1980) Parasitism and ecological relationships among deep-sea benthic fishes. Mar Biol 57:301–313
Carvalho-Filho A (1999) Peixes: costa brasileira, 3rd edn. Metro, São Paulo
Clarke KR (1993) Non-parametric multivariate analyses of changes in community structure. Austral Ecol 18:117–143. doi:10.1111/j.1442-9993.1993.tb00438.x
Clarke KR, Warwick RM (2001) Change in marine communities: an approach to statistical analysis and interpretation, 2nd edn. PRIMER-E, Plymouth
Clarke KR, Gorley RN (2006) PRIMER V6: user manual/tutorial. PRIMER-E, Plymouth
Cremonte F, Sardella NH (1997) The parasite fauna of Scomber japonicus Houttuyn, 1782 (Pisces: Scombridae) in two zones of the Argentine Sea. Fish Res 31:1–9
Chambers CB, Cribb TH, Jones MK (2000) Tetraphyllidean metacestodes of teleosts of the Great Barrier Reef, and the use of in vitro cultivation to identify them. Folia Parasit 47:285–292
Chávez RA, Valdivia IM, Oliva ME (2007) Local variability in metazoan parasites of the pelagic fish species, Engraulis ringens: implications for fish stock assessment using parasites as biological tags. J Helminthol 81:113–116
Díaz F, George-Nascimento M (2002) Estabilidad temporal de las infracomunidades de parásitos en la borrachilla Scartichthys viridis (Valenciennes, 1836) (Pisces: Blenniidae) en la costa central de Chile. Rev Chil Hist Nat 75:641–649
Esch GW, Fernández JC (1993) A functional biology of parasitism: ecological and evolutionary implications. Chapman & Hall, London
Ferrer-Castelló E, Raga JA, Aznar FJ (2007) Parasites as fish population tags and pseudoreplication problems: the case of striped red mullet Mullus surmuletus in the Spanish Mediterranean. J Helminthol 81:169–178
Floeter SR, Gasparini JL (2000) The southwestern Atlantic reef fish fauna: composition and zoogeographic patterns. J Fish Biol 56:1099–1114
Flores K, George-Nascimento M (2009) Las infracomunidades de parásitos de dos especies de Scartichthys (Pisces: Blenniidae) en localidades cercanas del norte de Chile. Rev Chil Hist Nat 82:63–71
George-Nascimento M (1987) Ecological helminthology of wildlife animals hosts from South America: a literature review and search for patterns in marine food webs. Rev Chil Hist Nat 60:181–202
George-Nascimento M, Mellado A, Saavedra S, Carvajal J (2009) Variabilidad de las comunidades de parásitos metazoos del róbalo Eleginops maclovinus (Cuvier & Valenciennes, 1830) (Pisces: Eleginopidae) en Chile. Rev Chil Hist Nat 82:199–207
González MT, Poulin R (2005) Spatial and temporal predictability of the parasite community structure of a benthic marine fish along its distributional range. Int J Parasitol 35:1369–1377
Gosztonyi AE, Kuba L, Mansur LE (2007) Estimation of body size using morphometric relationships of head bones, pectoral fin bones and bony precaudal distance in Raneya brasiliensis (Kaup, 1856) (Pisces, Ophidiiformes, Ophidiidae) in Patagonian waters. Rev Biol Mar Oceanogr 42:1–5
Graça-Lopes R, Tomás ARG, Tutui SL, Severino-Rodrigues E, Puzzi A (2002) Fauna acompanhante da pesca camaroeira no litoral do estado de São Paulo, Brasil. Bol Inst Pesca São Paulo 28:173–188
Guégan JF, Morand S, Poulin R (2005) Are there general laws in parasite community ecology? The emergence of spatial parasitology and epidemiology. In: Thomas F, Renaud F, Guégan JF (eds) Parasitism and ecosystems. Oxford University Press, Oxford, pp 22–42
Guerrero RA, Piola AR (1997) Masas de agua en la plataforma continental. In: Boschi E (ed) El Mar Argentino y sus Recursos Pesqueros. Antecedentes históricos de las exploraciones en el mar y las características ambientales, vol. 1. INIDEP, Mar del Plata, pp 107–118
Jaureguízar A, Menni R, Lasta C, Guerrero R (2006) Fish assemblages of the northern Argentine coastal system: spatial patterns and their temporal variations. Fish Oceanogr 15:326–344
Koen-Alonso M, Crespo EA, Pedraza SN, García NA, Coscarella MA (2000) Food habits of the South American sea lion, Otaria flavescens, off Patagonia, Argentina. Fish Bull-NOAA 98:250–263
Lafferty KD, Allesina S, Arim M, Briggs CJ, Leo GD, Dobson AP, Dunne JA, Johnson PTJ, Kuris AM, Marcogliese DJ, Martínez ND, Memmott J, Marquet PA, McLaughlin JP, Mordecai EA, Pascual M, Poulin R, Thieltges DW (2008) Parasites in food webs: the ultimate missing links. Ecol Lett 11:533–546
Lanfranchi AL, Rossin MA, Timi JT (2009) Parasite infracommunities of a specialized marine fish species in a compound community dominated by generalist parasites. J Helminthol 83:373–378
Luque JL, Poulin R (2004) Use of fish as intermediate hosts by helminth parasites: a comparative analysis. Acta Parasitol 49:353–361
Luque JL, Poulin R (2008) Linking ecology with parasite diversity in Neotropical fishes. J Fish Biol 72:189–204
Luque JL, Mouillot D, Poulin R (2004) Parasite biodiversity and its determinants in coastal marine teleost fishes of Brazil. Parasitology 128:671–682
MacKenzie K (2002) Parasites as biological tags in population studies of marine organisms: an update. Parasitology 124:153–163
MacKenzie K, Abaunza P (1998) Parasites as biological tags for stock discrimination of marine fish: a guide to procedures and methods. Fish Res 38:45–56
Magurran AE (1988) Ecological diversity and its measurement. Princeton University Press, Princeton
Marcogliese DJ (1995) The role of zooplankton in the transmission of helminth parasites to fish. Rev Fish Biol Fish 5:336–371
Marcogliese DJ (2001) Pursuing parasites up the food chain: implications of food web structure and function on parasite communities in aquatic systems. Acta Parasitol 46:82–93
Marcogliese DJ (2002) Food webs and the transmission of parasites to marine fish. Parasitology 124:83–99
Marcogliese DJ (2003) Food webs and biodiversity: are parasites the missing link? J Parasitol 82:389–399
Marcogliese DJ, Cone DK (1997) Food webs: a plea for parasites. Trends Ecol Evol 12:320–325
Menni R, Lopez HL (1974) Presencia en la Argentina de Raneya fluminensis (Miranda Ribeiro, 1903) Robins, 1961 (Teleostomi, Ophidiidae). Neotróp Notas Zool Amer 20:1–6
Nekola JC, White PS (1999) The distance decay of similarity in biogeography and ecology. J Biogeogr 26:867–878
Nielsen JG, Cohen DM, Markle DF, Robins CR (1999) FAO species catalogue, vol 18. Ophidiiform fishes of the world (Order Ophidiiformes). An annotated and illustrated catalogue of pearlfishes, cusk-eels, brotulas and other ophidiiform fishes known to date. FAO Fisheries Synopsis, Rome
Poulin R (2000) Variation in the intraspecific relationship between fish length and intensity of parasitic infection: biological and statistical causes. J Fish Biol 56:123–137
Poulin R (2003) The decay of similarity with geographical distance in parasite communities of vertebrate hosts. J Biogeogr 30:1609–1615
Poulin R (2004) Macroecological patterns of species richness in parasite assemblages. Basic Appl Ecol 5:423–434
Poulin R (2007) Are there general laws in parasite ecology? Parasitology 134:763–776
Poulin R, Morand S (1999) Geographical distances and the similarity among parasite communities of conspecific host populations. Parasitology 119:369–374
Poulin R, Morand S (2004) Parasite biodiversity. Smithsonian Institution Press, Washington DC
Poulin R, Valtonen ET (2002) The predictability of helminth community structure in space: a comparison of fish populations from adjacent lakes. Int J Parasitol 32:1235–1243
Power AM, Balbuena JA, Raga JA (2005) Parasite infracommunities as predictors of harvest location of bogue (Boops boops L.): a pilot study using statistical classifiers. Fish Res 72:229–239
Sardella NH, Timi JT (1996) Parasite communities of Merluccius hubbsi from the Argentinian-Uruguayan common fishing zone. Fish Res 27:81–88
Sardella NH, Timi JT (2004) Parasites of Argentine hake in the Argentine Sea: population and infracommunity structure as evidence for host stock discrimination. J Fish Biol 65:1472–1488
Sardella NH, Navone GT, Timi JT (1997) A new species of Cucullanus (Nematoda: Cucullanidae) parasite of Genypterus blacodes and G. brasiliensis (Pisces: Ophidiidae) in the South West Atlantic. Parasite 4:41–47
Soininen J, McDonald R, Hillebrand H (2007) The distance decay of similarity in ecological communities. Ecography 30:3–12
Timi JT (2003) Parasites of Argentine anchovy in the south-west Atlantic: latitudinal patterns and their use for discrimination of host populations. J Fish Biol 63:90–107
Timi JT (2007) Parasites as biological tags for stock discrimination in marine fish from South American Atlantic waters. J Helminthol 81:107–111
Timi JT, Lanfranchi AL (2009a) The importance of the compound community on the parasite infracommunity structure in a small benthic fish. Parasitol Res 104:295–302
Timi JT, Lanfranchi AL (2009b) The metazoan parasite communities of the Argentinean sandperch Pseudopercis semifasciata (Pisces: Perciformes) and their use to elucidate the stock structure of the host. Parasitology 136:1209–1219. doi:10.1017/S0031182009990503, ISSN 0031-1820. 136:1209-1219
Timi JT, Poulin R (2003) Parasite community structure within and across host populations of a marine pelagic fish: how repeatable is it? Int J Parasitol 33:1353–1362
Timi JT, Luque JL, Sardella NH (2005) Parasites of Cynoscion guatucupa along South American Atlantic coasts: evidence for stock discrimination. J Fish Biol 67:1603–1618
Timi JT, Lanfranchi AL, Etchegoin JA, Cremonte F (2008) Parasites of the Brazilian sandperch Pinguipes brasilianus Cuvier: a tool for stock discrimination in the Argentine Sea. J Fish Biol 72:1332–1342. doi:10.1111/j.1095-8649.2008.01800.x, ISSN 0022-1112, 72: 1332-1342
Timi JT, Lanfranchi AL, Etchegoin JA (2009) Seasonal stability and spatial variability of parasites in Brazilian sandperch Pinguipes brasilianus from the Northern Argentine Sea: evidence for stock discrimination. J Fish Biol 74:1206–1225. doi:10.1111/j.1095-8649.2009.02190.x, ISSN 0022-1112, 74: 1206-1225
Timi JT, Lanfranchi AL, Luque JL (2010) Similarity in parasite communities of the teleost fish Pinguipes brasilianus in the southwestern Atlantic: infracommunities as a tool to detect geographical patterns. Int J Parasitol 40:243–254. doi:10.1016/j.ijpara.2009.07.006, ISSN 0020-7519, 40:243-254
Vera GR, Soares LSH (2008) Variabilidade alimentar de Raneya brasiliensis na plataforma continental de Ubatuba e Cabo Frio, Brasil. Braz J Oceanogr 1:303–315
Vidal-Martínez VM, Poulin R (2003) Spatial and temporal repeatability in parasite community structure of tropical fish hosts. Parasitology 127:387–398
Zar JH (1996) Biostatistical analysis. Prentice-Hall, New Jersey
Acknowledgments
The authors wish to thank to Lic. M.S. Leonardi (CENPAT-CONICET) for her valuable suggestions on an earlier version of the manuscript. Thanks are also given to the fishing companies Alpesca S.A. and Harengus S.A. for the collection of fishes for this study. This work was supported by CONICET (J.T.T., PIP # 112-200801-00024); ANPCYT (J.T.T., PICT # 02199), (E.A.C., PICT 01-04025 A); Fundación BBVA (E.A.C., BIOCON 04) and United Nations Development Program (E.A.C. ARG-02/018).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Vales, D.G., García, N.A., Crespo, E.A. et al. Parasites of a marine benthic fish in the Southwestern Atlantic: searching for geographical recurrent patterns of community structure. Parasitol Res 108, 261–272 (2011). https://doi.org/10.1007/s00436-010-2052-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00436-010-2052-2