Abstract
Irritable bowel syndrome (IBS) is a chronic functional gastrointestinal disease presenting clinically by abdominal pain with alteration of bowel habits. Although IBS has uncertain etiology, chronic gut inflammation due to persistent exposure to an infectious agent including Blastocystis sp. was proposed. The aim of this study was to detect the prevalence of Blastocystis sp. subtype (ST) isolated from stool of IBS patients and to assess Blastocystis sp. and H. pylori co-infection in IBS patients from Beni-Suef Governorate, Egypt. Stool samples were collected from 115 IBS patients, following Rome III criteria. All stool samples were microscopically examined by wet mount and permanent trichrome stain, cultured on Jones’ medium with further sequencing of positive Blastocystis isolates and screened for detection of H. pylori coproantigen. Blastocystis sp. was the predominant parasite in IBS patients; it had statistical significant association with both rural residence (OR = 10) and flatulence (OR = 8.2). There was a predominance of Blastocystis sp. ST3 (81%) followed by ST1 (19%). Blastocystis culture results (19.1%) were superior than microscopy (16.5%). The majority of Blastocystis-positive IBS patients (72.7%) were co-infected with H. pylori with statistical significance; however, H. pylori was higher in Blastocystis-negative IBS patients (47/64) than in Blastocystis-positive IBS patients (17/64). Interestingly, IBS is usually associated with gut dysbiosis, while the most prevalent parasite in our IBS patients was Blastocystis sp., which is frequently found in asymptomatic individuals. Whether Blastocystis sp. is a cause or a consequence of IBS still needs further investigation, with a particular focus on correlation of IBS with different Blastocystis sp. subtypes and gut microbiomes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Irritable bowel syndrome (IBS) is one of the most common functional gastrointestinal illnesses representing 25–50% of all gastroenterology consultations (Longstreth et al. 2006). According to the “Rome Committee for the Classification of Functional Gastrointestinal Disorders”, IBS is defined by the presence of continuous or repeated abdominal and bowel manifestations for a minimum of 3 months in the absence of physical, laboratory, or radiological abnormalities (Dorn et al. 2009).
Although the pathophysiology of IBS is still obscure, several interconnected factors including altered gut reactivity of psychosomatic origin or gut hypersensitivity with increased pain perception may play a role (Mertz 2003; Stark et al. 2007; Surangsrirat et al. 2010). Hereditary and environmental factors, particularly dietary, were also suggested as predisposing factors (Levy et al. 2001; Brandt et al. 2002).
There is increasing evidence that persistent exposure to an infectious agent could induce a chronic state of gut inflammation with subsequent change in gastrointestinal motility and visceral sensitivity (Nouh et al. 2008). Several intestinal protozoa have IBS-like symptoms and have been investigated as causative agents of IBS with contradictory results, including Blastocystis sp., Giardia lamblia (G. lamblia), Entamoeba spp., Dientamoeba fragilis, Cryptosporidium spp., Cystoisospora belli, and Cyclospora cayetanensis (Stark et al. 2007).
By far, Blastocystis sp. is the most commonly found protozoa in symptomatic patients in the USA, 28.5 times more than G. lamblia (Amin 2002). Human infection is likely acquired via fecal-oral route, most commonly human-human; animal-human transmission is now thought to be uncommon (Stensvold and Clark 2016). Currently, more than 13 Blastocystis subtypes (STs) have been identified in humans and animals. Nine of these STs (ST1–9) have been detected in humans, with ST1–4 being the most frequent, representing around 90% of all human surveyed. Other STs (5–9), which were sporadically detected in humans, were most frequently found in non-human hosts and may be of zoonotic transmission: ST5 in livestock, ST6 and ST7 in birds, and ST8 in non-human primates. ST9 has not been found in non-human hosts (Clark et al. 2013; Stensvold and Clark 2016).
Infection with Blastocystis sp. may be asymptomatic or commonly associated with symptoms such as abdominal discomfort, diarrhea or constipation, anorexia, nausea, vomiting, bloating, dehydration, weight loss, and pruritis (Boorom et al. 2008). Numerous studies have linked Blastocystis sp. infection to IBS which may be subtype-dependent (Tan et al. 2010) or IBS-induced intestinal changes that facilitates Blastocystis growth (Roberts et al. 2014).
Some bacteria including Helicobacter pylori (H. pylori) were also incriminated in the pathogenesis of IBS (Ali 2012; Abdelrazak et al. 2015). Recent studies have confirmed that IBS is associated with low-grade inflammation and the distribution of vacuolating cytotoxin A alleles and H. pylori cytotoxin-associated gene in IBS (IBS-D) patients (Yakoob et al. 2012). H. pylori is a gram-negative bacillus that colonizes the gastric mucosa causing chronic gastritis, gastric and duodenal ulcers, and stomach cancer (Blaser 2006). Although IBS was significantly linked to H. pylori infection, it is not well established whether H. pylori is a primary or secondary cause to IBS (Ali and Mohamed 2014).
Few studies have investigated co-infection between Blastocystis sp. and H. pylori in IBS patients. Hence, this study aim was to detect the prevalence of Blastocystis sp., its predominant subtypes, and frequency of co-infection with H. pylori in IBS patients from Beni-Suef Governorate, Egypt.
Material and methods
Study population
This cross-sectional study included 115 patients attending the Outpatient Clinic of Tropical Medicine, Beni-Suef University Hospital, for a year (January 2015–January 2016).
Inclusion criteria were the following: patients with symptoms suggestive of IBS according to the “Rome (III) Criteria”: repeated abdominal pain for at least 3 days monthly in the last 3 months, accompanied by a minimum two of the following: improvement with defecation, change in frequency of defecation (> 3 daily or < 3 weekly), or change in stool form (Longstreth et al. 2006; David and Lola 2007).
Exclusion criteria were the following: (1) alarming signs such as age > 60 years, progressive severe symptoms, nocturnal symptoms awaking the patient, bleeding, anemia, fever, marked weight loss, family history of celiac disease, colon cancer, inflammatory bowel disease, or lactose intolerance: (2) chronic diseases such as diabetes mellitus, hepatic, and renal failure; and (3) history of drug intake in the last 10 days especially NSAID, proton pump inhibitors, antibiotics, or other drugs that stimulate the gut irritability such as sedatives or hypnotics.
Relative demographic and clinical data were collected from all studied cases using standardized questionnaire sheet. Also, these cases were subjected to full routine clinical examination, abdominal ultrasonography, upper GIT endoscopy with duodenal biopsy and colonoscopy with segmental biopsies, and laboratory investigations to exclude chronic diseases or other causes of IBS.
Collection and processing of samples
A fresh stool sample was collected from each IBS patient in a clean covered labeled container. Each stool sample was further subdivided into three portions according to the following techniques:
-
1-
Direct microscopy and permanent staining of fecal smears:
A small portion of each stool sample was microscopically examined immediately after collection using saline and iodine wet preparations. Also, a part of PVA-preserved fecal specimens were further stained with trichrome stain following the manufacturer’s instruction to exclude the presence of other parasites and to identify cases of Blastocystis infection (Garcia 2003).
-
2-
In vitro cultivation on modified Jones’ medium (Jones 1946):
The second portion (about 50 mg) of each fresh stool sample was inoculated into sterile screw-capped tubes containing 5 ml Jones’ medium enhanced with 10% horse serum. Culture tubes were incubated at 37 °C, and the sediment was examined by the × 40 objective after 24, 48, 72, and 96 h using a sterile pipette. The cultures were considered negative if no Blastocystis sp. was seen up to 96 h later, while positive samples were further subcultured in fresh medium (Zaman and Khan 1994). After one or two subcultures, Blastocystis suspensions were centrifuged at 12,000×g for 1 min and the final pellet was kept at − 20 °C for molecular assay and genotyping (Yoshikawa et al. 2004; Uobeed et al. 2015).
-
3-
Detection of H. pylori coproantigen
The final portion (about 50 mg) of each stool sample was frozen at − 20 °C for detecting H. Pylori coproantigen using a one-step OnSite H. pylori Ag Rapid test-Cassette (CTK Biotech, Inc., San Diego, CA, USA). The samples were completely thawed out and brought at room temperature before testing, and the results were analyzed following the manufacturer’s instruction.
-
4-
Molecular identification of Blastocystis STs:
-
4.1-
DNA extraction and PCR
Parasite genomic DNA was extracted from Blastocystis-positive subculture stool samples utilizing FavorPrep Stool DNA Mini Kit (Favorgen Biotech corporation ping-Tung 908, Taiwan) following the manufacturer’s instructions. Blastocystis sp. DNA was amplified using PCR. DNA concentrations of the extracted DNA were measured, were adjusted to 5 ng/ul, and were stored at − 20 °C until processed.
A forward primer, RD5 (ATCTGGTTGATCCTGCCAGT) (Clark 1997), and reverse primer, BhRDr (GAGCTTTTTAACTGCAACAACG) (Scicluna et al. 2006), were used. The primers amplified a 550–585 bp fragment of the Blastocystis SSU rDNA sequence according to Blastocystis ST. The Blastocystis DNA amplification was performed with a thermocycler following the PCR cycling and reaction conditions described previously (Scicluna et al. 2006). Amplified DNA products were detected with 1.5% agarose gel electrophoresis using ultraviolet trans-illumination after ethidium bromide staining.
-
4.2-
Sequencing and phylogenetic analysis of Blastocystis isolates
PCR products were purified using genome DNA purification Kit and then sequenced using the primer pair (RD5 and BhRDr) with Big-Dye® Terminator v3.1, Ready Reaction Cycle Sequencing Kit (Applied Biosystems, Foster City, CA, USA) following the manufacturer’s instructions of the ABI Prism 310 genetic analyzer (Applied Biosystems, Foster City, CA, USA). Sequences of Blastocystis isolates were matched with reference sequences deposited in the GenBank database utilizing the online BLAST program available at the National Center for Biotechnology Information (NCBI) (http://www.ncbi.nlm.nih.gov/BLAST). The ClustalW program of the BioEdit software was used to align all sequences (Hall 1999). The phylogenetic tree for the sequences was done using the method of neighbor joining (Saitou and Nei 1987) utilizing the Molecular and Evolution Genetic Analysis v7 (MEGA7) software (Kumar et al. 2016). Bootstrapping (1000 replicates) was used to evaluate the reliability of the phylogenetic tree. The Maximum–Likelihood algorithm with Tamura-3 parameter substitution model was used to compute the evolutionary distances using MEGA7.
Statistical analysis
Analysis of results was done using the SPSS-23 (IBM, Somers, NY, USA) software. Numerical data were presented as mean ± SD while categorical data were expressed as number and percentage. The association between any two qualitative variables was studied by chi-square. P value was statistically significant at ≤ 0.05. Variables which were significantly associated with Blastocystis prevalence were analyzed as estimated risks using univariate logistic regression.
The diagnostic yield of microscopically examined trichrome-stained smears, including sensitivity, specificity, negative predictive value (NPV), positive predictive value (PPV), and diagnostic accuracy, was evaluated in comparison with Blastocystis culture, the “gold standard” method.
Ethical consideration
Ethical approval was attained from the Committee of Research, Publications and Ethics of the Faculty of Medicine, Beni-Suef University.
Results
Out of 115 IBS patients, 54 (47%) were males while 61 (53%) were females, with an age range between 13 and 60 years and a mean of 34.9 (± 9.2) years. Thirty-one (27%) patients were from urban areas while 84 (73%) were from rural areas. The bowel habits were regarded as diarrhea in 103 patients (89.6%) and constipation in only 12 patients (10.4%). The predominant manifestations in IBS patients were abdominal pain (92.2%) followed by flatulence (61.7%), dyspepsia (55.7%), nausea (33%), vomiting (12.2%), and finally anorexia (2.6%).
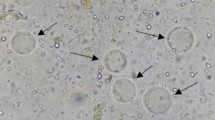
Microscopic examination of all stool samples revealed the following parasites in descending order: Blastocystis sp. in 19 patients (16.5%), E. coli in 13 (11.3%), G. lamblia in 3 patients (2.6%), Capillaria phlippinensis in 3 patients (2.6%), Entamoeba complex in 2 patients (1.5%), and Cystoisospora oocyst in 1 patient (0.9%). There was no co-parasitism. Different forms of Blastocystis sp. were detected in wet mount, iodine, and trichrome-stained smears, with the vacuolated form being the most common (Fig. 1 ).
Stool cultured on modified Jones’ medium revealed Blastocystis sp. in 22 out of 115 samples (19.1%), a higher percentage than those detected by microscopic examination. None of the Blastocystis-positive stool culture showed co-infection with other parasites. Different forms of Blastocystis were detected as shown in Fig. 2. Among the demographic and clinical data studied, only residence in rural areas and flatulence (P value < 0.05) showed statistical significant association with Blastocystis sp. infection in IBS patients (Table 1). Univariate analysis of these variables, using logistic regression, showed an estimated increase in the risk of Blastocystis infection in rural areas 10 times greater than that in urban areas, and IBS patients with flatulence were 8.2 times more likely to have Blastocystis than IBS patients without flatulence (Table 2).
Light microscopy of different forms of Blastocystis sp. with varied sizes in culture, each containing 1–4 nuclei and mitochondrion-like organelles at the periphery. a Vacuolar form: round and has a large central vacuole occupying 90% of the whole cell. b Granular form: contains granules in the central vacuole. c Amoeboid form: amoeba-like and possesses one or two large pseudopods. d Cells undergoing binary fission with thin cytoplasmic rims
Considering the culture method as the “gold standard” method for microscopic detection of Blastocystis, microscopic examination of trichrome-stained smears had 86.4% sensitivity, 100% specificity, 100% PPV, 96.8% NPV, and an overall accuracy of 97.4% (Table 3).
H. pylori was revealed in 64 patients (55.7%) using coproantigen detection, of which 23 were male while 41 were females (Fig. 3). Co-infection between Blastocystis sp. and H. pylori was detected in 17 out of 22 (77.3%) Blastocystis-positive cases with statistical significance (Table 4).
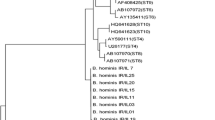
Two Blastocystis STs (ST1 and ST3) were identified by the phylogenetic analyses of the SSU rDNA sequences of the clinical samples from IBS patients, with a predominance of ST3 [18 (81.8%) samples], while only 4 (18.2%) samples were ST1. The studied Blastocystis STs were in the same cluster as there was no genetic variability within studied Blastocystis STs matched with the database of NCBI (Fig. 4 ). All the obtained sequence data of the 22 Blastocystis isolates were deposited in GenBank with accession numbers MF417460.1–MF417466.1 as follow: ST3 (MF417463.1 [samples 4–10], MF417464.1 [samples 11–14], MF417465.1 [samples 15–18], and MF417466.1 [samples 19–22]), and ST1 (MF417460.1 [sample 1], MF417461.1 [samples 2–3], and MF417462.1 [sample 4]).
Phylogenetic tree of SSU rDNA sequences of Blastocystis STs from IBS patients. Neighbor-joining tree showing the evolutionary history of Blastocystis isolates, inferred by distance-based analysis of Blastocystis SSU rDNA sequence. Bootstrap value is 100 with the sum of the branch length = 0.1. The monophyletic clades of ST1 (samples 1–4) and ST3 (samples 5–22) were supported by high bootstrap values
Discussion
IBS is one of a wider group of disorders known as functional gastrointestinal disorders presenting with abdominal pain and diarrhea or constipation in the absence of an identified organic cause (Boorom et al. 2008). In some developing countries, the prevalence of IBS ranges from 35 to 43% (Quigley et al. 2006; Schmulson et al. 2006) with 2:1 predominance in females (Stark et al. 2007). But, in our study, there was almost an equal percentage of females (53%) and males (47%) having IBS.
Due to an absence of alarm indicators, IBS is usually diagnosed by exclusion depending upon symptoms alone. Constipation or diarrhea may alternate, or predominate, yet in the present study, most cases presented with diarrhea (89.6%) and had constipation (10.4%).
Blastocystis sp. was described in many studies as having a possible role in the etiology of IBS. Its detection is usually dependent upon microscopic examination of stool wet mount or trichrome-stained smears (Surcsh and Smith 2004). Also, Jones’ medium has been successfully selected in many studies as the medium of choice for culturing Blastocystis sp. (Parkar et al. 2007).
In the present study, culturing stool on Jones’ medium yielded higher results (22 cases) in detection of Blastocystis than microscopic examination of stool using wet mount and trichrome-stained smears (19 cases). Three cases were missed by microscopy even after trichrome staining which may be explained by the polymorphism of Blastocystis spp. and tiny size of some isolates (6–8 Mm) which makes microscopical detection difficult even after staining, especially when present in small numbers. Also, Blastocystis may be missed in stool due to its irregular shedding (Vennila et al. 1999). Furthermore, some forms may be mistaken for leukocytes, fat globules, or other artifacts found in the stool.
This result agrees with several similar studies that showed greater sensitivity of short-term in vitro culture for detecting Blastocystis sp. than iodine or trichrome-stained smears (Termmathurapoj et al. 2004; Leelayoova et al. 2004; Thamrongwittawatpong and Surangsrirat 2006; Stensvold et al. 2007; Eida and Eida, 2008; Roberts et al. 2011). This is opposite to Kukoschke et al. (1990) who found no advantage in culturing Blastocystis sp. over microscopy.
Although short-term in vitro culture of Blastocystis sp. on modified Jones’ medium is time consuming (last 2–4 days), it was chosen in the present study for detecting Blastocystis sp. owing to its higher sensitivity and specificity as suggested by Surangsrirat et al. (2010) and Popruk et al. (2013).
In our study, Blastocystis sp. was the predominant parasitic infection in IBS patients with a percentage (19.1%) similar to that of Tungtrongchitr et al. (2004) and Surangsrirat et al. (2010) (13.6 and 16.7%, respectively). Conversely, it was lower than results reported for IBS patients by others where Blastocystis sp. percentage was 44.6, 46,41, 49, 71, and 76% (Eida and Eida 2008; Yakoob et al. 2003; Yakoob et al. 2010a; Yakoob et al. 2010b; Dogruman et al. 2010).
Blastocystis sp. was linked to IBS due to similarity of symptoms between Blastocystis infection and IBS; IBS-induced changes in the intestinal environment may facilitate Blastocystis growth and persistent antigenic exposure may lead to low-grade inflammation (Stark et al. 2007).
Intestinal environmental changes induced by IBS may affect virulent Blastocystis by reducing its generation time and genetic variability. Polymorphisms in genes which encode inflammatory cytokines might increase relative risk of IBS in Blastocystis carriers and may play a role in the IBS pathophysiology (Olivo-Diaz et al. 2012).
In this study, the estimated increase in the risk of Blastocystis infection was 10 times greater in rural areas than in urban areas. Similarly, Fujita et al. (1993) and Nimri and Meqdam (2004) reported a higher prevalence rate of Blastocystis sp. infection in lower socioeconomic communities or those with lower standards of personal hygiene. Ramirez-Miranda et al. (2010) observed that bloating was the only gastrointestinal symptom that showed statistical differences in both control and IBS patients. This is to some extent in accordance with our results, where flatulence was higher in Blastocystis-positive patients (90.9%) than in Blastocystis- negative patients (9.1%) (P value = 0.01, OR = 8.2).
In our study, H. pylori was detected in 64 IBS patients (55.7%) using coproantigen detection (Table 4). Likewise, Ali and Mohamed (2014) and Abdelrazak et al. (2015) found H. pylori infection in 41.7 and 42.7% of IBS cases, respectively. H. pylori infection was previously confirmed as a risk factor for the presence of dyspepsia in IBS patients by Su et al. (2000) and as triggering visceral hypersensitivity inducing typical abdominal discomfort by Gerards et al. (2001). Meanwhile, another study done by Nouh et al. (2008) detected no direct correlation between H. pylori infection and IBS except that there was an intense inflammatory reaction with of H. pylori.
Few studies have reported an association between Blastocystis sp. and H. pylori in IBS patients. In the present study, the majority of Blastocystis-positive IBS patients (77.2%) were co-infected with H. pylori with statistical significance (P value = 0.01); however, H. pylori was higher in Blastocystis-negative IBS patients (47/64) than in Blastocystis-positive IBS patients (17/64). This agrees with another study in which H. pylori was detected in gastric biopsies, more frequently in the positive Blastocystis sp. cases [19/26 (73.1%)] than in the controls [15/38 (39.5%)] (Chen et al. 2003). This relationship can be explained by the fact that both organisms are transmitted feco-orally. The presence of accompanying bacteria in cultures of Blastocystis sp. isolates allows for faster growth which may also occur in vivo (Tan et al. 2002); further investigations are needed to study the interaction of Blastocystis and/or H.pylori with gut microbiota, their role in gut dysbiosis, and the development of gut dysfunctions.
Nine Blastocystis sp. STs have been isolated from humans; some of them were reported to be zoonotic; furthermore, there are controversial explanations for the pathogenesis of Blastocystis, which may be explained by ST variations in virulence (Clark et al. 2013; Stensvold and Clark 2016). Consequently, we were interested in identifying the predominant Blastocystis STs in IBS patients, as subtyping would help us to detect routes of transmission and potential sources of STs in Beni-Suef Governorate, Egypt.
PCR testing of extracted DNA from stools or cultures is the only method for Blastocystis STs differentiation. In the present study, DNA was extracted directly from subculture to avoid PCR inhibitors in stool samples that hinder the detection process.
Studies showed differences in virulence between Blastocystis STs; some reported that STs 1 and 3 are found in symptomatic cases (Stensvold et al. 2007; Jones et al. 2009), while ST2 in asymptomatic carriages (Dogruman et al. 2008). Others reported that the pathogenicity is mostly related to STs 1, 4, and 7 while STs 2 and 3 are nonpathogenic (Tan et al. 2010).
There is a lack of consensus on the existence of an association between Blastocystis STs and IBS. This study showed predominance of ST3 of Blastocystis sp. [18 (81.8%) samples], followed by ST1 in only 4 (18.2%) samples within IBS patients. Our results show some matching with Dogruman et al. (2009) who revealed that ST3 followed by ST2 are the most predominant STs in IBS. In Egypt, Souppart et al. (2010) found a predominance of ST3 (61.9%) isolated from 20 stool samples, while Hussein et al. (2008) found ST3 to be the most prevalent STs (54.5%) from 44 stool samples. Meanwhile, he detected ST1, ST4, and ST2 in 18.2, 18.2, and 9.1% of the samples, respectively. In contrast, another study in Egypt reported that ST1 was the most detected STs in IBS patients, while ST1 was not present in the control group (Fouad et al. 2011).
Yakoob et al. (2010b) detected ST1 in the IBS group in a higher incidence compared to that in the control group but ST3 from both groups was equal in number. In Colombia, ST3 was identified in 100% of IBS patients harboring Blastocystis (Ramirez et al. 2013).
ST3 is the most common ST identified in humans with a worldwide distribution, detected in Egypt, Japan, Germany, USA, Singapore, and Turkey (Jantermtor et al. 2013), findings which agree with our results.
The route of transmission of ST3 is suggested to be zoonotic as it was previously isolated from other mammals such as dogs, cattle, pigs, and rodents. However, Stensvold et al. (2012) reported that most of ST3 Blastocystis found in human reside in a particular clade and the ST3 Blastocystis detected in animals are found in a different clade, supporting the idea that ST3 Blastocystis is due to human-human transmission.
Some studies reported that ST1 has a zoonotic transmission from different animals such as birds, cattle, monkeys, pigs, and rodents (Yoshikawa et al. 2004; Tan 2008). However, water-borne transmission and person-to-person transmission has been recorded for ST1 (Leelayoova et al. 2008; Thathaisong et al. 2013).
While Blastocystis colonization is usually associated with gut eubiosis (a healthy gut microbiota) (Scanlan et al. 2014), our work highlighted that Blastocystis sp. was the most prevalent parasites in IBS patients, whereas it is usually associated with gut dysbiosis (abnormal gut microbiota) (Lyra and Lahtinen 2012). Whether Blastocystis sp. is a cause or a consequence of IBS still needs further investigation, with a particular focus on correlation of IBS with different Blastocystis sp. subtypes and gut microbiomes.
Conclusion
Our study highlighted Blastocystis sp. as the most prevalent parasites in IBS patients, with a majority of Blastocystis-positive IBS patients showing co-infection between Blastocystis sp. and H. pylori. Since the precise pathogenesis of IBS is still elusive, it is recommended for all IBS patients to exclude intestinal protozoa and H. pylori as they may cause IBS-similar symptoms or cause persistent low-grade inflammation causing IBS manifestations. In IBS patients, the role of co-infection of Blastocystis and H.pylori in gut microbiota disturbance and the development of gut dysfunctions needs further studies.
The variations of Blastocystis STs in IBS patients in different studies highlights the necessity for further research to explore the relation between IBS and Blastocystis STs, their clinical significance, virulence and zoonotic potential.
References
Abdelrazak MA, Walid FE, Abdelrahman M, Mahmoud MA (2015) Interrelation between helicobacter pylori infection, infantile colic, and irritable bowel syndrome in pediatric patients. J Med Bio Sci Res 1(7):85–91
Ali AMM (2012) Helicobacter pylori and infantile colic. Arch Pediatr Adolesc Med 166(7):648–650. https://doi.org/10.1001/archpediatrics.2011.1241
Ali AMM, Mohamed MA (2014) Irritable bowel syndrome in children; is there an association with infantile colic in infants? Am Assoc Sci Technol 1(2):56–61
Amin OM (2002) Seasonal prevalence of intestinal parasites in the United States during 2000. Am J Trop Med Hyg 66(6):799–803. https://doi.org/10.4269/ajtmh.2002.66.799
Blaser MJ (2006) Who are we? Indigenous microbes and the ecology of human diseases. EMBO Rep 7(10):956–960. https://doi.org/10.1038/sj.embor.7400812
Boorom KF, Smith H, Nimri L, Viscogliosi E, Spanakos G, Parkar U, Li LH, Zhou XN, Ok UZ, Leelayoova S, Jones MS (2008) Oh my aching gut: irritable bowel syndrome, Blastocystis and asymptomatic infection. Parasit Vectors 1(1):40. https://doi.org/10.1186/1756-3305-1-40
Brandt LJ, Bjorkman D, Fennerty MB, Locke GR, Olden K, Peterson W, Quigley E, Schoenfeld P, Schuster M, Talley N (2002) Systematic review on the management of irritable bowel syndrome in North America. Am J Gastroenterol 97(11 Suppl):S7–26
Chen TL, Chan CC, Chen HP, Fung CP, Lin CP, Chan WL, Liu CY (2003) Clinical characteristics and endoscopic findings associated with Blastocystis hominis in healthy adults. Am J Trop Med Hyg 69(2):213–216
Clark CG (1997) Extensive genetic diversity in Blastocystis hominis. Mol Biochem Parasitol 87(1):79–83. https://doi.org/10.1016/S0166-6851(97)00046-7
Clark CG, van der Giezen M, Alfellani MA, Stensvold CR (2013) Recent developments in Blastocystis research. Adv Parasitol 82:1–32
David QS, Lola YK (2007) All roads lead to Rome: update on Rome III criteria and new treatment options. Gastroenterol Rep 1(2):56–65
Dogruman AF, Dagci H, Yoshikawa H, Kurt O, Demirel M (2008) A possible link between ST2 and asymptomatic infections of Blastocystis hominis. Parasitol Res 103(3):685–689. https://doi.org/10.1007/s00436-008-1031-3
Dogruman AF, Kustimur S, Yoshikawa H, Tuncer C, Simsek Z, Tanyuksel M et al (2009) Blastocystis subtypes in irritable bowel syndrome and inflammatory bowel disease in Ankara, Turkey. Mem Inst Oswaldo Cruz 104(5):724–727. https://doi.org/10.1590/S0074-02762009000500011
Dogruman AF, Simsek Z, Boorom K, Ekici E, Sahin M, Tuncer C, Kustimur S, Altinbas A (2010) Comparison of Methods for Detection of Blastocystis Infectionin Routinely Submitted Stool Samples, and also in IBS/IBD Patients in Ankara, Turkey. PLoS One 5(11):e15484. https://doi.org/10.1371/journal.pone.0015484
Dorn SD, Morris CB, Hu Y, Toner BB, Diamant N, Whitehead WE, Bangdiwala SI, Drossman DA (2009) Irritable bowel syndrome subtypes defined by Rome II and Rome III criteria are similar. J Clin Gastroenterol 43(3):214–220. https://doi.org/10.1097/MCG.0b013e31815bd749
Eida AM, Eida MM (2008) Identification of Blastocystis hominis in patients with irritable bowel syndrome using microscopy and culture compared to PCR. PUJ 1(2):87–92
Fouad SA, Basyoni MM, Fahmy RA, Kobaisi MH (2011) The pathogenic role of different Blastocystis hominis genotypes isolated from patients with irritable bowel syndrome. Arab J Gastroenterol 12(4):194–200. https://doi.org/10.1016/j.ajg.2011.11.005
Fujita O, Ohnishi M, Diaz L, Martinez L, Kamiya M (1993) Epidemiological investigation for intestinal parasitic infection in children in rural communities in Paraguay. Jpn J Parasitol 42:409–414
Garcia LS (2003) Selection and use of laboratory procedures for diagnosis of parasitic infections of the gastrointestinal tract, Cumitech 30A, ASM Press., Washington, D.C
Gerards C, Leodolter A, Glasbrenner B, Malfertheiner P (2001) H. pylori infection and visceral hypersensitivity in patients with irritable bowel syndrome. Dig Dis 19(2):170–173. https://doi.org/10.1159/000050673
Hall TA (1999) BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucl. Acids Symp Ser 41:95–98
Hussein EM, Hussein AM, Eida MM, Atwa MM (2008) Pathophysiological variability of different genotypes of human Blastocystis hominis Egyptian isolates in experimentally infected rats. Parasitol Res 102(5):853–860
Jantermtor S, Pinlaor P, Sawadpanich K, Pinlaor S, Sangka A, Wilailuckana C, Wongsena W, Yoshikawa H (2013) Subtype identification of Blastocystis spp. isolated from patients in a major hospital in northeastern Thailand. Parasitol Res 112(4):1781–1786. https://doi.org/10.1007/s00436-012-3218-x
Jones MS, Whipps CM, Ganac RD, Hudson NR, Boroom K (2009) Association of Blastocytis subtype 3 and 1 with patients from an Oregon community presenting with chronic gastrointestinal illness. Parasitol Res 104(2):341–345. https://doi.org/10.1007/s00436-008-1198-7
Jones WR (1946) The experimental infection of rats with Entamoeba histolytica. Ann Trop Med Parasitol 40(2):130–140. https://doi.org/10.1080/00034983.1946.11685270
Kukoschke KG, Necker A, Muller HE (1990) Detection of Blastocystis hominis by direct microscopy and culture. Eur J Clin Microbiol lnfc 9(4):305–307. https://doi.org/10.1007/BF01968071
Kumar S, Stecher G, Tamura K (2016) MEGA7: Molecular Evolutionary Genetics Analysis version 7.0 for bigger datasets. Mol Biol Evol 33(7):1870–1874. https://doi.org/10.1093/molbev/msw054
Leelayoova S, Siripattanapipong S, Thathaisong U, Naaglor T, Taamasri P, Piyaraj P et al (2008) Drinking water: a possible source of Blastocystis spp. subtype 1 infection in schoolchildren of a rural community in central Thailand. Am J 79(3):401–406
Leelayoova S, Rangsin R, Taamasri P, Naaglor T, Thathaisong U, Mungthin M (2004) Evidence of waterborne transmission of Blastocystis hominis. Am J Trop Med Hyg 70(6):658–662
Levy RL, Jones KR, Whithead WE, Feld SI, Talley NJ, Corey LA (2001) Irritable bowel syndrome in twins: heredity and social learning both contribute to etiology. Gastroenterology 121(4):799–804. https://doi.org/10.1053/gast.2001.27995
Longstreth GF, Thompson WG, Chey WD, Houghton LA, Mearin F, Spiller RC (2006) Functional bowel disorders. Gastroenterology 130(5):1480–1491. https://doi.org/10.1053/j.gastro.2005.11.061
Lyra A, Lahtinen S (2012) Dysbiosis of the intestinal microbiota in IBS, in Current concepts in colonic disorders (ed. Dr Lule, G.) 261–276, In Tech, DOI: https://doi.org/10.5772/25759
Mertz HR (2003) Irritable bowel syndrome. N Engl J Med 349(22):2136–2146. https://doi.org/10.1056/NEJMra035579
Nimri LF, Meqdam M (2004) Enteropathogens associated with cases of gastroenteritis in a rural population in Jordan. Clin Microbial Infect 10(7):634–639. https://doi.org/10.1111/j.1469-0691.2004.00891.x
Nouh MA, Abd El-Wahed MM, Anis SEM, Nassar MRA (2008) Study of the relationship between helicobacter pylori infection and irritable bowel syndrome. MMMJ 21(1):5–18
Olivo-Diaz A, Romero-Valdovinos M, Gudino-Ramirez A, Reyes-Gordillo J, Jimenez-Gonzalez DE, Ramirez-Miranda ME, Martinez-Flores WA, Martinez- Hernandez F, Flisser A, Maravilla P (2012) Findings related to IL-8 and IL-10 gene polymorphisms in a Mexican patient population with irritable bowel syndrome infected with Blastocystis. Parasitol Res 111(1):487–491
Parkar U, Traub RJ, Kumar S, Mungthin M, Vitali S, Leelayoova S, Morris K, Thompson RC (2007) Direct characterization of Blastocystis from faeces by PCR and evidence of zoonotic potential. Parasitology 134(Pt 3):359–367. https://doi.org/10.1017/S0031182006001582
Popruk S, Pintong A, Radomyos P (2013) Diversity of Blastocystis subtypes in humans. J Trop Med Parasitol 36:88–97
Quigley EM, Locke GR, Mueller-Lissner S, Paulo LG, Tytgat GN, Helfrich I, Schaefer E (2006) Prevalence and management of abdominal cramping and pain: a multinational survey. Aliment Pharmacol Ther 24(2):411–419. https://doi.org/10.1111/j.1365-2036.2006.02989.x
Ramirez JD, Sanchez LV, Bautista DC, Corredor AF, Florez AC, Stensvold CR (2013) Blastocystis subtypes detected in humans and animals from Colombia. Infect Genet Evol 22:223–228. https://doi.org/10.1016/j.meegid.2013.07.020
Ramirez-Miranda ME, Hernandez-Castellanos R, Lopez-Escamilla E, Moncada D, Rodriguez-Magallan A, Pagaza-Melero C, Gonzalez-Angulo A, Flisser A, Kawa-Karasik S, Maravilla P (2010) Parasites in Mexican patients with irritable bowel syndrome: a case-control study. Parasit Vectors 3(1):96. https://doi.org/10.1186/1756-3305-3-96
Roberts T, Barratt J, Harkness J, Ellis J, Stark D (2011) Comparison of microscopy, culture, and conventional polymerase chain reaction for detection of Blastocystis sp. in clinical stool samples. Am J Trop Med Hyg 84(2):308–312. https://doi.org/10.4269/ajtmh.2011.10-0447
Roberts T, Stark D, Harkness J, Ellis J (2014) Update on the pathogenic potential and treatment options for Blastocystis sp. Gut Pathogens 6(1):17. https://doi.org/10.1186/1757-4749-6-17
Saitou N, Nei M (1987) The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol Biol Evol 4(4):406–425
Scanlan PD, Stensvold CR, Rajilić-Stojanović M, Heilig HG, De Vos WM, O'Toole PW, Cotter PD (2014) The microbial eukaryote Blastocystis is a prevalent and diverse member of the healthy human gut microbiota. FEMS Microbiol Ecol 90(1):326–330. https://doi.org/10.1111/1574-6941.12396
Schmulson M, Ortiz O, Santiago-Lomeli M, Gutierrez-Reyes G, Gutierrez-Ruiz MC, Robles-Diaz G, Morgan D (2006) Frequency of functional bowel disorders among healthy volunteers in Mexico City. Dig Dis 24(3–4):342–347. https://doi.org/10.1159/000092887
Scicluna SM, Tawari B, Clark CG (2006) DNA barcoding of Blastocystis. Protist 157(1):77–85. https://doi.org/10.1016/j.protis.2005.12.001
Souppart L, Moussa H, Cian A, Sanciu G, Poirier P, EI Alaoui H et al (2010) Subtype analysis of Blastocystis isolates from symptomatic patients in Egypt. Parasitol Res 106(2):505–511. https://doi.org/10.1007/s00436-009-1693-5
Stark D, van Hal S, Marriott D, Ellis J, Harkness J (2007) Irritable bowel syndrome: a review on the role of intestinal protozoa and the importance of their detection and diagnosis. Int J Parasitol 37(1):11–20. https://doi.org/10.1016/j.ijpara.2006.09.009
Stensvold CR, Clark CG (2016) Current status of Blastocystis: a personal view. Parasitol Int 65(6):763–771. https://doi.org/10.1016/j.parint.2016.05.015
Stensvold CR, Alfellani M, Clark CG (2012) Levels of genetic diversity vary dramatically between Blastocystis subtypes. Infect Genet Evol 12(2):263–273. https://doi.org/10.1016/j.meegid.2011.11.002
Stensvold CR, Arendrup MC, Jespersgaard C, Molbak K, Nielsen HV (2007) Detecting Blastocystis using parasitologic and DNA-based methods: a comparative study. Diagn Microbiol Infect Dis 59(3):303–307. https://doi.org/10.1016/j.diagmicrobio.2007.06.003
Su YC, Wang WM, Wang SY, Lu SN, Chen LT, Wu DC, Chen CY, Jan CM, Horowitz M (2000) The association between helicobacter pylori infection and functional dyspepsia in patients with irritable bowel syndrome. Am J Gastroenterol 95(8):1900–1905
Surangsrirat S, Thamrongwittawatpong L, Piyaniran W, Naaglor T, Khoprasert C, Taamasri P, Mungthin M, Leelayoova S (2010) Assessment of the association between Blastocystis infection and irritable bowel syndrome. J Med Assoc Thail 93(Suppl. 6):S119–S124
Surcsh K, Smith H (2004) Comparisons of methods for detecting: Blastocystis hominis. Europ J Clin Microbiol Infec Dis 23(6):509–511. https://doi.org/10.1007/s10096-004-1123-7
Tan KS, Mirza H, Teo JD, Wu B, Macary PA (2010) Current views on the clinical relevance of Blastocystis spp. Curr Infect Dis Rep 12(1):28–35. https://doi.org/10.1007/s11908-009-0073-8
Tan KS, Singh M, Yap EH (2002) Recent advances in Blastocystis hominis research: hot spots in terra incognita. Int J Parasitol 32(7):789–804. https://doi.org/10.1016/S0020-7519(02)00005-X
Tan KS (2008) New insights on classification, identification, and clinical relevance of Blastocystis spp. Clin Microbiol Rev 21(4):639–665. https://doi.org/10.1128/CMR.00022-08
Termmathurapoj S, Leelayoova S, Aimpun P, Thathaisong U, Nimmanon T, Taamasri P, Mungthin M (2004) The usefulness of short-term in vitro cultivation for the detection and molecular study of Blastocystis hominis in stool specimens. Parasitol Res 93(6):445–447. https://doi.org/10.1007/s00436-004-1157-x
Thamrongwittawatpong L, Surangsrirat S (2006) Assessment of the association between Blastocystis hominis infection and irritable bowel syndrome in Phramongkutklao Hospital. Thai J Gastroenterol 7(2):88–92
Thathaisong U, Siripattanapipong S, Mungthin M, Pipatsatitpong D, Tan-ariya P, Naaglor T et al (2013) Identification of Blastocystis subtype 1 variants in the home for girls, Bangkok, Thailand. Am J Trop Med Hyg 88(2):352–358. https://doi.org/10.4269/ajtmh.2012.12-0237
Tungtrongchitr A, Manatsathit S, Kositchaiwat C, Ongrotchanakun J, Munkong N, Chinabut P, Leelakusolvong S, Chaicumpa W (2004) Blastocystis hominis infection in irritable bowel syndrome patients. Southeast Asian J Trop Med Public Health 35(3):705–710
Uobeed AH, Ali GB, Mohammed SK (2015) Isolation and identification of Blastocystis hominis isolated from irritable bowel syndrome patients using phenotypic and genotypic methods. Int J Scientific Engineering Res 6(11):1183–1190
Vennila G, Suresh Kumar G, Khairul Anuar A, Rajah S, Saminathan R, Sivanandan S, Ramakrishnan K (1999) Irregular shedding of Blastocystis hominis. Parasitol Res 85(2):162–164. https://doi.org/10.1007/s004360050528
Yakoob J, Jafri W, Beg MA, Abbas Z, Naz S, Islam M, Khan R (2010a) Blastocystis hominis and Dientamoeba fragilis in patients fulfilling irritable bowel syndrome criteria. Parasitol Res 107(3):679–684. https://doi.org/10.1007/s00436-010-1918-7
Yakoob J, Jafri W, Beg MA, Abbas Z, Naz S, Islam M, Khan R (2010b) Irritable bowel syndrome: is it associated with genotypes of Blastocystis hominis. Parasitol Res 106(5):1033–1038. https://doi.org/10.1007/s00436-010-1761-x
Yakoob J, Abbas Z, Islam M, Jafri W (2012) Virulence markers of Helicobacter pylori in patients with diarrhoea-dominant irritable bowel syndrome. Br J Biomed Sci 69(1):6–10. https://doi.org/10.1080/09674845.2012.11669914
Yakoob J, Jafri W, Jafri N, Khan R, Baig MA, Zaman V (2003) Irritable bowel syndrome: search of an etiology: role of Blastocystis hominis. Am J Gastroenterol 98(9, Supplement 1):S264–S265
Yoshikawa H, Wu Z, Kimata I, Iseki M, Ali IK, Hossain MB, Zaman V, Haque R, Takahashi Y (2004) Polymerase chain reaction-based genotype classification among human Blastocystis hominis populations isolated from different countries. Parasitol Res 92(1):22–29. https://doi.org/10.1007/s00436-003-0995-2
Zaman V, Khan K (1994) A comparison of direct microscopy with culture for the diagnosis of Blastocystis hominis. Southeast Asian J Trop ed Hyg Public Health 25:792–793
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical approval was obtained from the Committee of Research, Publications and Ethics of the Faculty of Medicine, Beni-Suef University.
Rights and permissions
About this article
Cite this article
El-Badry, A.A., Abd El Wahab, W.M., Hamdy, D.A. et al. Blastocystis subtypes isolated from irritable bowel syndrome patients and co-infection with Helicobacter pylori . Parasitol Res 117, 127–137 (2018). https://doi.org/10.1007/s00436-017-5679-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00436-017-5679-4