Abstract
Blastocystis sp. has been described as the most common intestinal parasite in humans and has an increased impact in public health. To improve our understanding of the molecular epidemiology of this human-emerging parasite, we determined the Blastocystis subtypes (STs) and their relative frequency in Egyptian patients living in or in the vicinity of Cairo and presenting gastrointestinal symptoms. We obtained a total of 20 stool samples identified as positive for Blastocystis by microscopic examination of smears. Genotyping using partial small subunit ribosomal RNA gene analysis identified a total of 21 Blastocystis isolates corresponding to 19 single infections and one mixed infection (ST1 and ST3). Three STs were identified: ST3 was the most common ST in the present Egyptian population (61.90%) followed by ST1 (19.05%) and ST2 (19.05%). Together with previous studies carried out in different areas in Egypt, a total of five STs (ST1, ST2, ST3, ST4, and ST6) have been found in symptomatic patients. These data were compared to those available in the literature, and we underlined variations observed in the number and relative proportions of STs between and within countries. On the whole, it seemed that Blastocystis infection is likely not associated with specific STs even if some STs are predominant in the epidemiologic studies, but rather with a conjunction of factors in the course of infection including environmental risk and parasite and host factors.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Blastocystis sp. is a single-celled anaerobic and enteric parasite that inhabits the lower gastrointestinal tract of humans and many animals, such as a large variety of vertebrates (for reviews, see Stenzel and Boreham 1996; Abe et al. 2002; Tan et al. 2002; Tan 2004, 2008; Stensvold et al. 2009a). This emerging parasite with a worldwide distribution is often identified as the most common eukaryotic organism reported in human fecal samples, and its prevalence has shown a dramatic increase in recent years (for review see Tan 2008). Moreover, its prevalence is higher in developing countries, and this has been linked to poor hygiene practices, exposure to animals, and consumption of contaminated food or water (Li et al. 2007b; Leelayoova et al. 2008) since the fecal–oral route is considered to be the main mode of transmission of this parasite. Indeed, a higher risk of infection has been found in humans with close animal contact (food and animal handlers) reinforcing the zoonotic nature of this parasite (Salim et al. 1999; Yan et al. 2007; Yoshikawa et al. 2009).
At the morphological level, Blastocystis is a highly polymorphic organism with various forms (Stenzel and Boreham 1996; Tan et al. 2002; Zhang et al. 2007; Suresh et al. 2009). The water-resistant infective cyst possibly represents the transmissible stage of the parasite (Moe et al. 1996; Suresh et al. 2005), whereas the large amoeboid form has been suggested as being associated with pathogenicity (Tan and Suresh 2006; Katsarou-Katsari et al. 2008). However, we have recently shown that the presence of the amoeboid form may be poorly correlated with patient status (Souppart et al. 2009). Although its role in human diseases was widely debated in the literature during the two last decades, numerous recent in vivo and in vitro studies strongly suggest that Blastocystis is a pathogen (for reviews, see Stark et al. 2007; Boorom et al. 2008; Tan 2008; Stensvold et al. 2009a). Case reports have linked Blastocystis infection with various gastrointestinal and extraintestinal symptoms including diarrhea, abdominal pain, depression, fatigue, vomiting, constipation, anorexia, urticaria, skin rash, headaches, and flatulence, but this parasite may also play a significant role in several chronic gastrointestinal illnesses such as irritable bowel syndrome and inflammatory bowel disease (Giacometti et al. 1999; Yakoob et al. 2004; Boorom et al. 2008; Dogruman-Al et al. 2009; Jones et al. 2009; Stensvold et al. 2009c). Moreover, Blastocystis infection is frequent in cancer and HIV/AIDS patients with gastrointestinal symptoms (Tan et al. 2009).
Blastocystis isolates from humans and other animals have been reported to be morphologically similar. However, extensive genetic variation among numerous isolates has been identified using molecular methods including, in most cases, polymerase chain reaction (PCR)–restriction fragment length polymorphism, random amplified polymorphic DNA, and PCR using sequence-tagged site primers (for reviews see Stensvold et al. 2007b; Tan 2008). This large diversity was subsequently re-emphasized by molecular phylogenies inferred from small subunit (SSU) ribosomal RNA (rRNA) gene sequences (Noël et al. 2003, 2005; Scicluna et al. 2006; Li et al. 2007a; Rivera 2008; Stensvold et al. 2009b). Recently, a consensus approach recommended assigning Blastocystis isolates from humans, mammals, and birds to one of nine subtypes (ST1 to ST9), each of them exhibiting sufficient genetic diversity to be classified as separate species (Stensvold et al. 2007b). Interestingly, all nine of the STs described in mammals and birds can be found in humans as well, confirming the low host specificity and zoonotic potential of Blastocystis. This also suggests animals may serve as a large potential reservoir for infections in humans. Lately, a novel subtype (ST10) was shown to be hosted by primates and artiodactyls (Stensvold et al. 2009b).
Epidemiological surveys have reported the frequency of subtypes from symptomatic and asymptomatic patients in several countries (for reviews see Boorom et al. 2008; Tan 2008; Souppart et al. 2009). These studies have provided new insights into the taxonomy of this parasite, host range, transmission route, and contamination sources, and data concerning a hypothetical link between genotype and pathogenic potential of this parasite. In the present study, in the aim at enhancing our understanding of the influence of ST differences in pathogenicity, genotypes of several Blastocystis clinical isolates were identified from symptomatic patients living in or in the vicinity of Cairo in Egypt. This study is of crucial interest regarding the high prevalence (33.3%) of this parasite in this country (Rayan et al. 2007).
Materials and methods
Patient selection
Samples were collected at the Laboratory of Parasitology of the Cairo University, Egypt during parasitological examinations of stool samples from symptomatic patients living in or in the vicinity of Cairo. Blastocystis sp. was identified in a total of 20 stool samples through direct light microscopy of smears. Patients were classified as “symptomatic” (= with digestive symptoms) based on responses to a standardized questionnaire. Stool samples were examined for the presence of other parasitic infections. No information was available on potential viral or bacterial infections.
DNA extraction
DNA was extracted from fecal samples cultured in Jones' medium (Jones 1946), supplemented with 10% horse serum (Invitrogen, Groningen, The Netherlands), 100 UI/ml penicillin, and 100 µg/ml streptomycin (Sigma-Aldrich, St. Louis, MO, USA) at 37°C for 2–3 days. The cultures were screened for Blastocystis cells by standard light microscopy and subcultured for an additional 2–3 days in fresh medium. Approximately 106 Blastocystis cells were isolated by centrifugation at 500×g for 10 min. The pellet was washed two times with phosphate-buffered saline (pH 7.4) before centrifugation. The supernatant was discarded, and genomic DNA was extracted from the pellet with the High Pure PCR Template Preparation Kit (Roche Applied Science, Mannheim, Germany) according to the manufacturer's protocol.
PCR amplification, cloning, and sequencing
For each sample, genomic DNA was submitted to a nested PCR using two Blastocystis-specific pairs of primers targeting the SSU rRNA gene. The primers used in this study were previously designed by Souppart et al. (2009). It allowed the amplification of the same genomic region than that described by Scicluna et al. (2006). This amplified domain of the SSU rDNA coding region has been shown to provide sufficient information for accurate subtyping. Successive PCR amplifications were performed as previously described (Souppart et al. 2009). The products of the second PCR were separated by agarose gel electrophoresis, and bands of the expected size (approximately 600 bp) were purified using the Wizard SV Gel and PCR clean-up system (Promega, Madison, WI, USA). Purified PCR products were cloned in the T-vector, pCR 2.1-TOPO (Invitrogen) and amplified in Escherichia coli One Shot TOP10 competent cells. Minipreparations of plasmid DNA were done using the QIAprep Spin Miniprep kit (Qiagen). Two clones containing inserts of approximately the expected size were arbitrarily selected for each sample and sequenced on both strands. The SSU rRNA gene sequences obtained in this study have been deposited in GenBank under accession numbers GU130218 to GU130247. These new sequences were aligned with the use of the BioEdit version 7.0.1 package (http://www.mbio.ncsu.edu:BioEdit/bioedit.html) then compared with all the Blastocystis SSU rRNA gene sequences available from the National Centre for Biotechnology Information database (http://www.ncbi.nlm.nih.gov) using the basic local alignment search tool (BLAST) program (Altschul et al. 1997). Subtypes were identified by determining the exact match or closest similarity against all known Blastocystis subtypes according to the last classification by Stensvold et al. (2007b).
Results and discussion
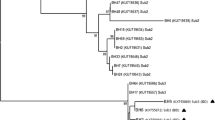
In this study, stool specimens were collected from a total of 20 gastrointestinal symptomatic patients, nine males and 11 females, ranging in age from 8 to 45 years (Table 1). These patients presented various symptoms including diarrhea, abdominal pain, flatulence, and constipation. Stool samples were cultured in Jones’ medium, and DNA was extracted for each of the isolates. All 20 isolates were successfully amplified by nested PCR, and two clones were completely sequenced for each of the 20 samples. Each of the SSU rDNA gene sequences obtained in this study showed very high similarity (from 98% to 100%) to homologous sequences of the other Blastocystis isolates reported so far. The comparison with representative sequences of all known Blastocystis STs using the BLAST software allowed the direct genotyping of the new isolates (Table 1) according to the consensus terminology by Stensvold et al. (2007b). For ten of the 20 samples, the two sequenced clones were identical (Table 1). Clones showed one nucleotide difference for five of the samples. For the samples AC11 (ST3), AC17 (ST1), AC18 (ST3), and AC19 (ST2), the number of nucleotide differences between the two clones was of 6, 11, 4, and 3, respectively. Despite the differences, the two different clones for each of these four latter samples belonged to the same Blastocystis subtype according to BLAST search. The differences observed between the two clones of a same sample are unlikely to be due to PCR artifacts, as the study used high fidelity Taq DNA polymerase. Therefore, as suggested in previous studies (Scicluna et al. 2006; Souppart et al. 2009), the cause may be coinfection with two variants within the same subtype or sequence variations between SSU rDNA gene copies within the same isolate. In the present analysis, we have considered that two different clones of the same subtype identified in the same patient derived from the same strain of Blastocystis. In contrast, for one of the 20 samples (AC6, Table 1), we identified mixed infection containing two subtypes of Blastocystis (ST1 and ST3). The prevalence of mixed infections observed in the present study (5%) was roughly similar to that described in other countries such as Germany (5%; Böhm-Gloning et al. 1997), Turkey (4.3%; Dogruman-Al et al. 2008), France (7.5%; Souppart et al. 2009), and different Chinese regions (2.6% to 14.3%; Yan et al. 2006; Li et al. 2007a, b). In these latter studies, most are coinfections with ST1 and ST3.
The majority of the samples included in this study (19/20) represented single infections. With the addition of one mixed infection, we analyzed a total of 21 isolates. As summarized in Table 1, three STs were identified, and ST3 was the most common in our Egyptian population (61.90%) followed by ST1 (19.05%) and ST2 (19.05%). Globally, the subtype distribution in the present study showed similarities with that found in other countries (for review, see Souppart et al. 2009). Indeed, all these studies reported that a large majority of human Blastocystis infections (an average of more than 60%) were attributable to ST3 isolates whereas the proportions of other subtypes differ between locations. Although the most dominant genotype was ST3, the ST distribution reported here significantly differed to that described in a recent epidemiologic survey performed in another Egyptian area (Hussein et al. 2008). Indeed, in this latter study including 16 symptomatic and 28 asymptomatic patients, four Blastocystis STs (ST1, ST3, ST6, and ST7) were detected (18.2%, 54.5%, 18.2%, and 9.1%, respectively). In addition, only three STs (ST1, ST3, and ST6) but not ST2 were identified (28.6%, 57.1%, and 14.3%, respectively) in the symptomatic group. On the other hand, ST6 was not detected in our Egyptian population. However, the ST distribution observed in the present study was similar to that described in a third Egyptian area (only 14 isolates collected) in which four STs were identified (ST1 to ST4; Elwakil and Talaat 2009) according to the new classification by Stensvold et al. (2007b). Recently, the genetic diversity of Blastocystis was studied in different parts of China (Li et al. 2007a). Prevalence of Blastocystis was shown to be different in the four epidemiological settings analyzed. However, unlike Egypt, the number of subtypes identified and their relative frequency were roughly similar from a Chinese province to the other one. On the other hand, in Japan, two epidemiological studies were performed in the Osaka (Yoshikawa et al. 2004) and Tokyo (Kaneda et al. 2001) area and like Egypt, showed differences in the number of subtypes identified and their relative frequency. As previously suggested (Li et al. 2007a; Stensvold et al. 2009a), such variations in the number and relative proportions of STs between countries as within the same country could reflect true differences between communities and might indicate divergent epidemiological contexts including reservoirs and ways of transmission. These differences are likely due to prevailing local living conditions and customs, rather than differences in the susceptibility to infection. In China (Li et al. 2007b), it has been shown that the consumption of raw water plants was a risk factor for ST1 infections while drinking unboiled water was significantly associated with infections involving ST3. The dominant ST3 was suggested to be the only subtype of human origin, while the remaining subtypes would be zoonotic (Böhm-Gloning et al. 1997; Kaneda et al. 2001; Yoshikawa et al. 2004; Noël et al. 2005; Tan 2008). In the present study, the predominance of ST3 might be explained by large-scale human-to-human transmission in Egypt as in other countries. Regarding ST1 and ST2, their presence in our Egyptian population might be linked to zoonotic transmission from farm animals and monkeys (Noël et al. 2005; Tan 2008). The finding that these latter STs were common in our Egyptian population suggested that contamination from mammals and birds might be a significant source of transmission. To clarify this point, future attempts to identify specific risk factors for infection with different Blastocystis STs have to be performed in other areas of Egypt in the aim at exploring the potential association of STs with specific environmental compartments and reservoirs.
In the recent literature, it is still matter of debate whether disease is ST related. Clark (1997) was the first to suggest that Blastocystis ST correlates with the pathogenic potential of this parasite. In that way, a study by Kaneda et al. (2001) suggested that ST1, ST2, and ST4 were associated with gastrointestinal symptoms while the most commonly isolated ST3 was not responsible for symptoms. More recently, Yan et al. (2006) overrepresented only ST1 in a group of symptomatic patients, while ST3 was isolated predominantly from asymptomatic individuals. In parallel, Hussein et al. (2008) confirmed this hypothesis by concluding that ST1 was the most virulent, while ST3 and ST6 consisted of pathogenic and nonpathogenic strains, and ST7 was irrelevant to the pathogenicity of Blastocystis. In contrast, several studies showed that none of the STs was significantly correlated with intestinal disease (Böhm-Gloning et al. 1997; Yoshikawa et al. 2004; Özyurt et al. 2008; Souppart et al. 2009). To evaluate the pathogenic potential of the different Blastocystis STs in our Egyptian population, we examined the phylogenetic distribution of isolates from symptomatic individuals. Two symptomatic patients (AC11 and AC15) coinfected with Entamoeba sp. or Giardia were excluded in this analysis because these might have caused similar symptoms. The symptomatic patient (AC6) with mixed infection was also removed from the present analysis, as the preferential growth of a particular isolate could potentially impact analysis (Parkar et al. 2007; Yan et al. 2007). In other patients of this study, Blastocystis was the sole pathogen found. Therefore, in the final analysis, 17 isolates from symptomatic patients were considered. The most dominant genotype was ST3 (64.70%), followed by ST1 (17.65%) and ST2 (17.65%). Interestingly, the three ST2 isolates included in our analysis were associated with symptoms. However, it has first been suggested that this subtype was a nonpathogenic genotype of Blastocystis sp., (Dogruman-Al et al. 2008) although in a more recent study, the same authors identified ST2 to be associated with chronic infection in symptomatic individuals (Dogruman-Al et al. 2009). Besides, ST2 represented one of the predominant genotype in Danish (Stensvold et al. 2007a, 2009c) and Turkish (Özyurt et al. 2008) symptomatic individuals. To the author's knowledge, it is the first time that ST2 strains are isolated from Egyptian symptomatic patients. We also confirmed that ST1 isolates could be associated with pathogenicity as suggested in previous studies carried out in Egypt (Hussein et al. 2008; Elwakil and Talaat 2009) and others countries (Yan et al. 2006; Dominguez-Marquez et al. 2009; Souppart et al. 2009; Stensvold et al. 2007a, 2009c). Moreover, ST3 was the most common in our Egyptian symptomatic group as described in other countries (Stensvold et al. 2007a, 2009c; Wong et al. 2008; Eroglu et al. 2009; Jones et al. 2009; Souppart et al. 2009; Tan et al. 2009). In addition, ST4 was not found in our symptomatic group as well as in that of the previous Egyptian study by Hussein et al. (2008). In the opposite, ST4 was identified in only four Egyptian symptomatic patients by Elwakil and Talaat (2009). However, ST4 was recently identified as the most frequent genotype in a large symptomatic group of patients in Spain (Dominguez-Marquez et al. 2009), which included 94.1% of the isolates.
Almost all the data available from epidemiological surveys of symptomatic groups of patients including this study confirmed that several Blastocystis STs would be involved in human infections associated with gastrointestinal symptoms, and that the implicated STs and their relative frequency would broadly differ between countries as within the same country. In Turkey, striking differences were observed between communities since four STs (ST1 to ST4) were detected by Özyurt et al. (2008), while ST1 was the only ST detected in 20 symptomatic patients (Eroglu et al. 2009). In Egypt, together with the studies by Hussein et al. (2008) and Elwakil and Talaat (2009), a total of five STs (ST1, ST2, ST3, ST4, and ST6) have been identified in symptomatic patients. In recent studies, at least four different STs (ST1 to ST4) were also found in Danish (Stensvold et al. 2009c), French (Souppart et al. 2009), Turkish (Özyurt et al. 2008), and Malaysian (Tan et al. 2009) individuals with gastrointestinal symptoms. In numerous studies, the existence of pathogenic and nonpathogenic variants in different STs including ST3 was also suggested (Hussein et al. 2008; Özyurt et al. 2008; Dogruman-Al et al. 2009; Souppart et al. 2009). Therefore, according to all these observations, it may be suggested that Blastocystis infection is likely not due to a particular ST even if some STs are frequently dominant in epidemiologic studies but rather to a conjunction of factors in the course of infection including environmental risk (transmission routes and contamination sources) and parasite (pathogenic and zoonotic potential) and host factors (genotype, immunity, and age). The apparent lack of correlation between symptoms and subtypes as the differences noticed in the frequency of the STs between and within countries could undoubtedly be explained by taking into account these parameters in further studies.
References
Abe N, Nagoshi M, Takami K, Sawano Y, Yoshikawa H (2002) A survey of Blastocystis sp. in livestock, pets, and zoo animals in Japan. Vet Parasitol 106:203–212
Altschul SF, Madden TL, Schäffer AA, Zhang J, Zhang Z, Miller W, Lipman DJ (1997) Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res 25:3389–3402
Böhm-Gloning B, Knobloch J, Walderich B (1997) Five subgroups of Blastocystis hominis isolates from symptomatic and asymptomatic patients revealed by restriction site analysis of PCR-amplified 16 S-like rDNA. Trop Med Int Health 2:117–118
Boorom KF, Smith H, Nimri L, Viscogliosi E, Spanakos G, Parkar U, Li LH, Zhou XN, Ok UZ, Leelayoova S, Jones MS (2008) Oh my aching gut: irritable bowel syndrome, Blastocystis, and asymptomatic infection. BMC Parasit Vectors 1:40
Clark CG (1997) Extensive genetic diversity in Blastocystis hominis. Mol Biochem Parasitol 87:79–86
Dogruman-Al F, Dragci H, Yoshikawa H, Kurt Ö, Demirel M (2008) A possible link between subtype 2 and asymptomatic infections of Blastocystis hominis. Parasitol Res 103:685–689
Dogruman-Al F, Kustimur S, Yoshikawa H, Tuncer C, Simsek Z, Tanyuksel M, Araz E, Boorom K (2009) Blastocystis subtypes in irritable bowel syndrome and inflammatory bowel disease in Ankara, Turkey. Mem Inst Oswaldo Cruz 104:724–727
Dominguez-Marquez MV, Guna R, Munoz C, Gomez-Munoz MT, Borras R (2009) High prevalence of subtype 4 among isolates of Blastocystis hominis from symptomatic patients of a health district of Valencia (Spain). Parasitol Res 105:949–955
Elwakil HS, Talaat RM (2009) Genetic analysis of Blastocystis hominis isolated from symptomatic and asymptomatic human hosts in Egypt. J Egypt Soc Parasitol 39:99–109
Eroglu F, Genc A, Elgun G, Koltas IS (2009) Identification of Blastocystis hominis isolates from asymptomatic and symptomatic patients by PCR. Parasitol Res 105:1589–1592
Giacometti A, Cirioni O, Fiorentini A, Fortuna M, Scalise G (1999) Irritable bowel syndrome in patients with Blastocystis hominis infection. Eur J Clin Microbiol Infect Dis 18:436–439
Hussein EM, Hussein AM, Eida MM, Atwa MM (2008) Pathophysiological variability of different genotypes of human Blastocystis hominis egyptian isolates in experimentally infected rats. Parasitol Res 102:853–860
Jones WR (1946) The experimental infection of rats with Entamoeba histolytica with a method for evaluating the anti-amoeboic properties of new compounds. Ann Trop Med Parasitol 40:130–140
Jones MS, Whipps CM, Ganac RD, Hudson NR, Boroom K (2009) Association of Blastocystis subtype 3 and 1 with patients from an Oregon community presenting with chronic gastrointestinal illness. Parasitol Res 104:341–345
Kaneda Y, Horiki N, Cheng XJ, Fujita Y, Maruyama M, Tachibana H (2001) Ribodemes of Blastocystis hominis isolated in Japan. Am J Trop Med Hyg 65:393–396
Katsarou-Katsari A, Vassalos CM, Tzanetou K, Spanakos G, Papadopoulou C, Vakalis N (2008) Acute urticaria associated with amoeboid forms of Blastocystis sp. subtype 3. Acta Derm Venereol 88:80–81
Leelayoova S, Siripattanapipong S, Thathaisong U, Naaglor T, Taamasri P, Piyaraj P, Mungthin M (2008) Drinking water: a possible source of Blastocystis spp. subtype 1 infection in school children of a rural community in central Thailand. Am J Trop Med Hyg 79:401–406
Li LH, Zhang XP, Shan L, Yoshikawa H, Zhiliang W, Steinmann P, Utzinger J, Tong XM, Chen SH, Zhou XN (2007a) Cross-sectional surveys and subtype classification of human Blastocystis isolates from four epidemiological settings in China. Parasitol Res 102:83–90
Li LH, Zhou XN, Du ZW, Wang XZ, Wang LB, Jiang JY, Yoshikawa H, Steinmann P, Utzinger J, Wu Z, Chen JX, Chen SH, Zhang L (2007b) Molecular epidemiology of human Blastocystis in a village in Yunnan province, China. Parasitol Int 56:281–286
Moe KT, Singh M, Howe J, Ho LC, Tan SW, Ng GC, Chen XQ, Yap EH (1996) Observations on the ultrastructure and viability of the cystic stage of Blastocystis hominis from human feces. Parasitol Res 82:439–444
Noël C, Peyronnet C, Gerbod D, Edgcomb VP, Delgado-Viscogliosi P, Sogin ML, Capron M, Viscogliosi E, Zenner L (2003) Phylogenetic analysis of Blastocystis isolates from different hosts based on the comparison of small-subunit rRNA gene sequences. Mol Biochem Parasitol 126:119–123
Noël C, Dufernez F, Gerbod D, Edgcomb VP, Delgado-Viscogliosi P, Ho LC, Singh M, Wintjens R, Sogin ML, Capron M, Pierce R, Zenner L, Viscogliosi E (2005) Molecular phylogenies of Blastocystis isolates from different hosts: implications for genetic diversity, identification of species, and zoonosis. J Clin Microbiol 43:348–355
Özyurt M, Kurt O, Molbak K, Nielsen HV, Haznedaroglu T, Stensvold CR (2008) Molecular epidemiology of Blastocystis infections in Turkey. Parasitol Int 57:300–306
Parkar U, Traub RJ, Kumar S, Mungthin M, Vitali S, Leelayoova S, Morris K, Thompson RCA (2007) Direct characterization of Blastocystis from faeces by PCR and evidence of zoonotic potential. Parasitology 134:359–367
Rayan HZ, Ismail OA, El Gayar EK (2007) Prevalence and clinical features of Dientamoeba fragilis infections in patients suspected to have intestinal parasite infection. J Egypt Soc Parasitol 37:599–608
Rivera WL (2008) Phylogenetic analysis of Blastocystis isolates from animal and human hosts in the Philippines. Vet Parasitol 156:178–182
Salim HR, Kumar GS, Vellayan S, Mak JW, Anuar AK, Init I, Vennila GD, Saminathan R, Ramakrishnan K (1999) Blastocystis in animal handlers. Parasitol Res 85:1032–1033
Scicluna SM, Tawari B, Clark CG (2006) DNA Barcoding of Blastocystis. Protist 157:77–85
Souppart L, Sanciu G, Cian A, Wawrzyniak I, Delbac F, Capron M, Dei-Cas E, Boorom K, Delhaes L, Viscogliosi E (2009) Molecular epidemiology of human Blastocystis isolates in France. Parasitol Res 105:413–421
Stark D, van Hal S, Marriott D, Elis J, Harkness J (2007) Irritable bowel syndrome: a review on the role of intestinal protozoa and the importance of their detection and diagnosis. Int J Parasitol 37:11–20
Stensvold CR, Arendrup MC, Jespersgaard C, Molbak K, Nielsen HV (2007a) Detecting Blastocystis using parasitologic and DNA-based methods: a comparative study. Diag Microbiol Infect Dis 59:303–307
Stensvold CR, Suresh GK, Tan KSW, Thompson RCA, Traub RJ, Viscogliosi E, Yoshikawa H, Clark CG (2007b) Terminology for Blastocystis subtypes—a consensus. Trends Parasitol 23:93–96
Stensvold CR, Nielsen HV, Mølbak K, Smith HV (2009a) Pursuing the clinical significance of Blastocystis—diagnostic limitations. Trends Parasitol 25:23–29
Stensvold CR, Alfellani MA, Nørskov-Lauritsen S, Prip K, Victory EL, Maddox C, Nielsen HV, Clark CG (2009b) Subtype distribution of Blastocystis isolates from synanthropic and zoo animals and identification of a new subtype. Int J Parasitol 39:473–479
Stensvold CR, Lewis HC, Hammerum AM, Porsbo LJ, Nielsen SS, Olsen KEP, Arendrup MC, Nielsen HV, Molbak K (2009c) Blastocystis: unravelling potential risk factors and clinical significance of a common but neglected parasite. Epidemiol Infect 137:1655–1663
Stenzel DJ, Boreham PFL (1996) Blastocystis hominis revisited. Clin Microbiol Rev 9:563–584
Suresh K, Smith HV, Tan TC (2005) Viable Blastocystis cysts in Scottish and Malaysian sewage samples. Appl Environ Microbiol 71:5619–5620
Suresh K, Venilla GD, Tan TC, Roheia M (2009) In vivo encystation of Blastocystis hominis. Parasitol Res 104:1373–1380
Tan KSW (2004) Blastocystis in humans and animals: new insights using modern methodologies. Vet Parasitol 126:121–144
Tan KSW (2008) New insights on classification, identification, and clinical relevance of Blastocystis spp. Clin Microbiol Rev 21:639–665
Tan TC, Suresh KG (2006) Predominance of amoeboid forms of Blastocystis hominis in isolates from symptomatic patients. Parasitol Res 98:189–193
Tan KSW, Singh M, Yap EH (2002) Recent advances in Blastocystis hominis research: hot spots in terra incognita. Int J Parasitol 32:789–804
Tan TC, Ong SC, Suresh KG (2009) Genetic variability of Blastocystis sp. isolates obtained from cancer and HIV/AIDS patients. Parasitol Res 105:1283–1286
Wong KHS, Ng GC, Lin RTP, Yoshikawa H, Taylor MB, Tan KSW (2008) Predominance of subtype 3 among Blastocystis isolates from a major hospital in Singapore. Parasitol Res 102:663–670
Yakoob J, Jafri W, Jafir N, Khan R, Islam M, AsimBeg M, Zaman V (2004) Irritable bowel syndrome: in search of an etiology: role of Blastocystis hominis. Am J Trop Med Hyg 70:383–385
Yan Y, Su S, Lai R, Liao H, Ye J, Li X, Luo X, Chen G (2006) Genetic variability of Blastocystis isolates in China. Parasitol Res 99:597–601
Yan Y, Su S, Ye J, Lai X, Lai R, Liao H, Chen G, Zhang R, Hou Z, Luo X (2007) Blastocystis sp. subtype 5: a possibly zoonotic genotype. Parasitol Res 101:1527–1532
Yoshikawa H, Wu Z, Kimata I, Iseki M, Ali IKMD, Hossain MB, Zaman V, Haque R, Takahashi Y (2004) Polymerase chain reaction-based genotype classification among human Blastocystis hominis populations isolated from different countries. Parasitol Res 92:22–29
Yoshikawa H, Wu Z, Pandey K, Pandey BD, Sherchand JB, Yanagi T, Kanbara H (2009) Molecular characterization of Blastocystis isolates from children and rhesus monkeys in Kathmandu, Nepal. Vet Parasitol 160:295–300
Zhang X, Qiao JY, Zhou XJ, Yao FR, Wei ZC (2007) Morphology and reproductive mode of Blastocystis hominis in diarrhea and in vitro. Parasitol Res 101:43–51
Acknowledgments
This work was developed in the framework of EA3609 scientific program (French Ministry of Research) and supported by the Institut Pasteur de Lille, the Institut National de la Santé et de la Recherche Médicale, the Centre National de la Recherche Scientifique, and the Université Lille Nord de France.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Souppart, L., Moussa, H., Cian, A. et al. Subtype analysis of Blastocystis isolates from symptomatic patients in Egypt. Parasitol Res 106, 505–511 (2010). https://doi.org/10.1007/s00436-009-1693-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00436-009-1693-5