Abstract
Seaweeds are one of the most widely studied natural resources for their biological activities. Novel seaweed compounds with unique chemical structures have been reported for their pharmacological properties. The urge to search for novel insecticidal compound with a new mode of action for development of botanical insecticides supports the relevant scientific research on discovering the bioactive compounds in seaweeds. The mosquitocidal potential of seaweed extracts and their isolated compounds are documented in this review paper, along with the discussion on bioactivities of the major components of seaweeds such as polysaccharides, phenolics, proteins, terpenes, lipids, and halogenated compounds. The effects of seaweed extracts and compounds toward different life stages of mosquito (egg, larva, pupa, and adult), its growth, development, and reproduction are elaborated. The structure-activity relationships of mosquitocidal compounds are discussed to extrapolate the possible chemical characteristics of seaweed compounds responsible for insecticidal properties. Furthermore, the possible target sites and mode of actions of the mosquitocidal seaweed compounds are included in this paper. The potential synergistic effects between seaweeds and commercial insecticides as well as the toxic effects of seaweed extracts and compounds toward other insects and non-target organisms in the same habitat are also described. On top of that, various factors that influence the mosquitocidal potential of seaweeds, such as abiotic and biotic variables, sample preparation, test procedures, and considerations for a precise experimental design are discussed. The potential of active seaweed extracts and compounds in the development of effective bioinsecticide are also discussed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Seaweeds are photosynthetic, non-vascular, and eukaryotic organisms. They are marine macroalgae that occupy littoral zone. The form of seaweeds is very diverse. They can be filamentous that are only a few millimeters in height or in huge fronds up to 60 m long (Coppejans et al. 2009). Seaweeds are classified into three higher taxa, which include brown seaweed (Phaeophyceae), green seaweed (Chlorophyceae), and red seaweed (Rhodophyceae) according to their pigmentation. Pigmentation determines the color of seaweed. The pigments in green seaweeds are α-, β-, and γ-carotene, chlorophylls a and b, lutein, siphonoxanthin, and siphonein, while the pigments in brown seaweeds are chlorophylls a, c1, and c2, β-carotene, and fucoxanthin. Chlorophyll a, r-phycocyanin, allophycocyanin, c-phycoerythrin, α- and β-carotene, on the other hand, are pigments commonly found in red seaweeds (Sharma 2011).
Seaweeds, which live in the harsh marine environment, have different bioactive compounds from those organisms found in the terrestrial environment. These seaweed compounds are with varied chemical structures and used for food (FAO 2004), medical (Smit 2004), and industrial (Cardozo et al. 2007) products. Early reports have envisaged the insecticidal activity of seaweeds against mosquito larvae (Thangam and Kathiresan 1991b). As the necessity of finding a novel insecticidal agent in mosquito control is evident, there is growing interest in looking for natural alternatives sourced from seaweeds. However, it is vital to study the bioactive components of seaweeds and determine their mosquitocidal activity. Understanding of the seaweed chemical constituents provides information on the structure-activity relationships of active compounds that are responsible for the killing action (Dias and Moreas 2014).
Mosquitoes are one of the targeted disease vectors as declared by World Health Organization (WHO 1996). These insects are midge-like flies belonging to the family of Culicidae. These insects go through four life stages—egg, larva, pupa, and adult. The duration of each life stage and life span varies from species to species. There are approximately 3,100 mosquito species, and the important human-blood-sucking species belongs to the genera of Aedes, Anopheles, Culex, Haemagogus, Mansonia, Sabethes, and Psorophora (Service 1980). These bloodsuckers are not only nuisance to human but also vectors of harmful infectious diseases. For instance, Aedes species is of primary medical importance as it is the vector of viral diseases such as yellow fever, dengue fever, and chikungunya (Christophers 1960). WHO (2013) estimated around 50 to 100 million cases of dengue infection worldwide every year. In 2010, the dengue fever cases reported across the Americas, Southeast Asia, and Western Pacific exceeded 2.3 million (WHO 2013). The parasitic disease, malaria, is transmitted by the Anopheles species while lymphatic filariasis can be spread by a wide variety of mosquito species (Service 1980). Drug treatment is expensive yet not effective for some of the vector-borne diseases. Therefore, the efficient way to prevent the transmission of mosquito borne diseases is to combat the disease-carrying mosquitoes (Pitasawat et al. 2007).
Insecticides are broadly applied in the control measures during outbreaks of vector-borne diseases. Synthetic organic insecticides such as organochlorine, organophosphate, and carbamate have been discovered and developed since 1940s, gaining wide acceptance due to their economical price and rapid effectiveness. However, uncontrolled usage of synthetic insecticides elicits insecticide resistance in mosquito populations and poses detrimental effects on the environment and other organisms (Paeporn et al. 2003). Researchers are looking for alternative solutions with limited adverse effects in eradicating the pests. Therefore, research on natural products which aims at discovering the active constituents from natural insecticide agents that are safe and target-specific has gained attention. Successful stories have been reported on the quest for insecticides of natural origin. Some examples that signify huge advancement in the insecticide research include the discovery of pyrethrins from chrysanthemum flowers (Casida 1980), insecticidal products derived from neem plant (Schmutterer 1990), and endotoxins originating from bacteria Bacillus thuringiensis var. israelensis (Schnepf et al. 1998).
Plant, as a natural source of food and drug, is also well known for its insecticidal (Roark 1947) and repellent properties (Amer and Mehlhorn 2006a). However, there is limited review articles published on the usage of phytochemicals for mosquito control. One of the earliest articles by Roark (1947) discussed some insecticidal plants from the genera of Derris and Lonchocarpus. Sukumar et al. (1991) described the mosquitocidal activity of 344 species which included trees, herbs, grasses, aquatic plants, fungi, microalgae, and five seaweeds. In their review, the toxicity effects, growth and reproductive inhibition effects, repellence and ovipositional deterrence effects of botanical chemicals were discussed. However, that review discussed only the plant parts used in mosquitocidal activity, not the active insecticidal compounds. Shaalan et al. (2005) described the larvicidal activity of 69 plant species and the effects of 48 plant species on the growth, development, reproduction, hatch rates, and fertility of mosquitoes. Other than the toxic effects of botanical phytochemicals, their review also explained the screening methodology of the mosquitocidal phytochemicals, mechanism and site of action of mosquitocidal compounds, field evaluation aspects of potential bioinsecticides, and resistance development in mosquito populations. They also included the larvicidal activity of two seaweeds in their review. However, Shaalan et al. (2005) shared limited information on modes and sites of action of larvicidal phytochemicals at molecular level and did not describe the intoxication of larvae such as the behavioral changes and morphological aberrations after their exposure to the plant. In the review by Ghosh et al. (2012), application of phytochemicals as mosquito larvicide as part of the integrated mosquito management was described. The review listed the botanical extracts that posses the ability to reduce or control the population of mosquitoes. In some of the other studies, the potential of terrestrial plant essential oils and active constituents in controlling the disease vector has also been discussed (Jantan et al. 2003, 2005; Amer and Mehlhorn 2006b; Zoubiri and Baaliouamer 2011; Dias and Moreas 2014).
A data search using the major databases (ScienceDirect, Springerlink, PubMed, etc.) with the keywords (“seaweeds,” “macroalgae,” “mosquitocidal activity,” “larvicidal activity,” and “larvicide”) has been done to identify the relevant original articles for this review. The results of the data searching showed that there is a lack of reviews on mosquitocidal potential of seaweeds and their compounds; thus, there is a need to acknowledge and further investigate the importance and potential of seaweeds as a mosquitocidal agent. This review will serve as an important piece of data for future work. In this review, the major components of seaweeds are elaborated to give a brief account of the bioactive seaweed constituents. Besides, the current knowledge on mosquitocidal seaweed species, mosquitocidal seaweed compounds, growth and reproduction inhibiting seaweed species, mode and site of action of mosquitocidal compounds, synergistic effects of seaweeds and insecticide mixtures, toxic effects of mosquitocidal seaweed species on non-target organisms, and potential of seaweeds in the development of novel insecticides are discussed in this paper.
Major components of seaweeds
Extensive research on chemical constituents of seaweeds has been carried out. The bioactive components of seaweeds include polysaccharides, phenolics, phlorotannins, proteins, peptides, amino acids, terpenes, terpenoids, lipids, and halogenated compounds. However, the content of seaweeds varies with species, season, locality, and environmental factors (Black 1954; FAO 2004). The bioactivity of the major components of seaweeds is discussed as follows.
Polysaccharides
Polysaccharides are polymers of monosaccharides that are linked together. The total content of polysaccharides in seaweeds is up to 76 % (dry weight). Different chemical structures of polysaccharides are related to taxonomic classification and cell structure of the seaweed. Agar, carrageenan, xylan, floridean starch, water-soluble sulfated galactan, and porphyran are the common polysaccharides in red seaweeds. Green seaweeds contain polysaccharides such as sulfuric acid polysaccharide, sulfated galactan, and xylan, while brown seaweeds contain alginic acid, fucoidan, laminarin, and sargassan (Chandini et al. 2008). Some polysaccharides are only present in seaweeds and not in the terrestrial plants (Ferriera et al. 2012). For example, galactan, fucoidan, laminarin, and alginate are the important polysaccharides only found in seaweeds (Ferriera et al. 2012). There is a great demand for seaweed polysaccharides in industries that produce products such as stabilizer, emulsifier, food, and beverages (Cardozo et al. 2007).
Alginate and carrageenan are hydrocolloids extracted from various red and brown seaweeds. Alginate or alginic acid is a polysaccharide that contains 1,4-linked β-d-mannuronic acid and α-l-guluronic acid residues. Most of the alginate production depends on the extraction of wild brown seaweeds (FAO 2004). Extracted alginates are available in acid and salt forms. Sodium alginate is impregnated into gauze dressing, cotton, and swabs for external use and internal application onto bleeding points during abdominal operations (Khotimchenko et al. 2001). The polysaccharide base of these products stimulates reparative processes, displays protective properties, and shields mucous membranes and damaged skin against irritation from unfavorable environments (Glyantsev et al. 1993).
Carrageenans are linear polysaccharide chains with sulfate half-esters attached to the sugar unit (Rasmussen and Morrissey 2007). Carrageenan production depends on the extraction of cultivated red seaweeds such as Kappaphycus alvarezii and Eucheuma denticulatum (FAO 2004). Besides their application as stabilizers in food industry, carrageenans are also used in a wide range of medical applications because of their antitumor, antiviral, anticoagulant, and immunomodulation properties (Sen et al. 1994; Schaeffer and Krylov 2000; Zhou et al. 2005). For example, Carraguard is a carrageenan-based vaginal microbiocide that entered the clinical phase III trial conducted by Population Council Centre in South Africa and Botswana in 2003 (Spieler 2002).
Fucoidans are polysaccharide commonly found in brown seaweeds. They account for 10 to 20 % of dry weight of the seaweed and consist of l-fucose and sulfate ester groups (Li et al. 2008). Fucoidans of both Ecklonia kurome and Laminaria angustata var. longissima exhibit high activity of anticoagulant action (Nishino and Nagumo 1987) and high antithrombin activity (Kitamura et al. 1991), respectively. Fucoidans also show antiviral activity against Herpes simplex virus (Hemmingson et al. 2006; Hayashi et al. 2008).
Laminarins are major polysaccharides found in brown seaweeds especially in Laminaria species. The content of laminarin in seaweeds varies seasonally, and it contributes to 10 to 32 % of dry weight (Holdt and Kraan 2011). They have been reported for their medical and pharmaceutical uses as prebiotic, anticoagulant, and antioxidant (Chattopadhyay et al. 2010; Holdt and Kraan 2011).
Phenolics and phlorotannins
Phenolic compounds are characterized structurally by an aromatic ring with one or more hydroxyl substituents. The polyphenols of seaweeds are derived from polymerized phloroglucinol units (1,3,5-trihydroxybenzene). Green and red seaweeds have lower concentration of phenol as compared to brown seaweeds. The phenol content in seaweeds ranges from 1 to 4 % of dry weight (Holdt and Kraan 2011). Phenolic compounds are known for their bioactivity. The phenylethanol and phenylethanol sulfate bromophenols isolated from red seaweed Rhodomela confervoides show moderate cytotoxicity against several cell lines, namely, human colon cancer (HCT-8), hepatoma (Bel7402), stomach cancer (BGC-823), lung adenocarcinoma (A549), and human ovarian cancer (A2780) (Ma et al. 2006).
Phlorotannins are polymers of phloroglucinol with a molecular skeleton consisting of eight phenol rings. They are produced by secondary metabolism in brown seaweeds. Phlorotannins with a wide range of molecular sizes (400 to 400,000 Da) have been reported for their various bioactivities. Ahn et al. (2007) reported good radical scavenging activity and inhibition effects of three phlorotannins, namely, phloroglucinol, eckol, and dieckol (purified from brown seaweed Ecklonia cava) on H2O2-mediated DNA damage. Bioactivities of phlorotannins such as eckol, phlorofucofuroeckol A, and dieckol derived from Ecklonia species have also been reported, with antimicrobial activity (Nagayama et al. 2002) and inhibitory activity against angiotensin-converting enzyme (ACE) (Jung et al. 2006).
Proteins, peptides, and amino acids
The protein content of seaweeds varies by species, location, biotic interactions, and spatial and temporal changes in the environmental parameters (Stengel et al. 2011). Seaweeds are a rich source of amino acids, namely, aspartic acid, glutamic acid, and leucine. Threonine, lysine, tryptophan, sulfur amino acids, and histidine are also found in seaweed, though in nominal amount (Dawczynski et al. 2007). Stengel et al. (2011) revealed that the protein content of 34 seaweeds they tested was consistently rich in amino acids such as threonine, valine, leucine, lysine, glycine, and alanine. Generally, red seaweeds contain higher concentration of taurine as compared to brown seaweeds (Dawczynski et al. 2007).
Domoic acid (1), isolated from red seaweed Chondria armata, is a potent excitatory neurotransmitter and also a nitrogen atom containing heterocyclic compound. Domoic acid (1) was the cause of the mass food poisoning which happened in 1987, caused by domoic-acid-contaminated blue mussel from Prince Edward Island, Canada (Iverson et al. 1989). Besides domoic acid (1), isodomoic acid A (2), isodomoic acid B (3), and isodomoic acid C (4), isolated from C. armata, are also lethal to cockroach (Fig. 1) (Maeda et al. 1984, 1986).
α-Kainic acid (5), an amino acid isolated from red seaweed Digenea simplex, shows a potent neurophysiology activity in mammals (Ferkany and Coyle 1983). The toxicity properties of compound 5 are directly related to neurochemical and histopathological changes in the rat’s brain (Sperk et al. 1983). On the other hand, the compound exhibits antihelmintic activity against roundworm Ascaris lumbricoides (Rim et al. 1974). α-Kainic acid (5) also exhibits killing effects toward American cockroach Periplaneta americana (Maeda et al. 1984).
Kahalalides are sequences of amino and hydroxy carboxylic acid residues. For example, kahalalides A and F are polypeptides isolated from sacoglossan mollusk Elysia rufescens and its diet—green seaweed Bryopsis species (Hamann et al. 1996). These kahalalides have in vitro antituberculosis activity which inhibits the growth of Mycobacterium tuberculosis (El Sayed et al. 2000; Bourel-Bonnet et al. 2005). Kahalalide F can interfere with lysosome function, and it has been introduced into clinical phase I trial by Pharma Mar S. A. as an anticancer agent against prostate cancer (Bonnard et al. 2003; Rademaker-Lakhai et al. 2005).
Terpenes
Terpenes are secondary metabolites made up of isoprene units. Monoterpenes are terpenes that consist of two isoprene units. The halogenated monoterpenes isolated from red seaweed Plocamium cartilagineum, such as violacene (6), mertensene (7), dibromomertensene (8), dihydromertensene (9), and 1,4,6-trichloro-3-(2′-chlorovinyl)-1,3-dimethylcycohexane (10) exhibit insecticidal potential against various insects (San-Martin et al. 1991; Argandoña et al. 2000).
Sesquiterpenes consisting of three isoprene units are known for their bioactivity. Laurepinnacin (11) and Isolaurepinnacin (12), which are acetylinic sesquiterpene ethers isolated from red seaweed Laurencia pinnata, are potent toward Azuki bean beetle Callosobruchus chinensis (Fukuzawa and Masamune 1981). Elatol (13), a halogenated sesquiterpene isolated from red seaweed Laurencia dendroidea, exhibits potent larvicidal effects against mosquito Aedes aegypti (Bianco et al. 2013). The compound also has antileishmanial, antitumor, acaricidal, and repellent activities (Dos Santos et al. 2010; Born et al. 2012; Campos et al. 2012). Another sesquiterpene isolated from red seaweed Laurencia nipponica, deoxyprepacifenol (14), exhibits larvicidal activity against mosquito Culex pipiens (Watanabe et al. 1989b).
Diterpenes with four isoprene units can be isolated from various seaweeds. Seven brominated diterpenes of the parguerene and isoparguerene series derived from red seaweed Jania rubens exhibit marked antitumor activities, cytotoxicity activities, and antihelmintic effects against earthworm Allolobophora caliginosa (Awad 2004). The cytotoxicity activity of these diterpenes depends on the number of acetoxy groups present. Neoirietetraol, a brominated diterpene isolated from red seaweed Laurencia yonaguniensi is toxic to brine shrimp, and it demonstrates antibacterial activity against Alcaligenes aquamarines and Escherichia coli (Takahashi et al. 2002).
Sargaquinoic acid, a meroterpenoid isolated from brown seaweed Sargassum species has antimalarial activity against chloroquine-sensitive strain (D10) of Plasmodium falciparum (Afolayan et al. 2008). Meroterpenoids from brown seaweed Cystoseira crinita collected from south coast of Sardinia, Italy, have been tested for their antioxidative properties in the 1,1-diphenyl-2-picrylhydrazyl radical (DPPH) and thiobarbituric acid reactive substances (TBARS) assays. Among the meroterpenoids tested, six tetraprenyltoluquinols and two triprenyltoluquinols from C. crinita have exhibited radical-scavenging effect (Fisch et al. 2003).
Carotenoids are colored terpenes that occur as common natural pigments in fruits, vegetables, seaweeds, etc. (Rao and Rao 2007). Carotenoids like α- and β-carotene, lutein, zeaxanthin, fucoxanthin, chlorophyll a, and phaeophytin a are identified in seaweeds (Terasaki et al. 2009). Fucoxanthin isolated from brown seaweed Sargassum species has strong antioxidant properties and cytotoxicity activity against breast cancer (MCF-7) cells (Ayyad et al. 2011). The pigment also has antiobesity and antidiabetic properties (Maeda et al. 2009).
Lipids
There is a great variation in lipid content of seaweeds. A study of 27 tropical Indian seaweeds has revealed that although the lipid content of seaweeds is low, the polyunsaturated fatty acid (PUFA) content is the same with or higher than the terrestrial plants (Kumari et al. 2009). Seaweeds are rich in PUFAs containing C18 and C20 (Chandini et al. 2008). Kumari et al. (2009) reported that brown and red seaweeds were rich in arachadonic acid and eicosapentaenoic acid. Saturated fatty acids such as capric acid (15), lauric acid (16), and myristic acid (17), and monounsaturated fatty acid like palmitoleic acid (18) isolated from green seaweed Cladophora glomerata have been tested against mosquito larva Aedes triseriatus, and lethal concentration 50 (LC50) values ranging from 3 to 14 ppm have been obtained (LaLonde et al. 1979).
The major sterol in Rhodophyceae is cholesta-5,24(25)-dien-3β-ol while the methylation product of 24-methylenecholesterol is the main sterol in Phaeophyceae and Chlorophyceae. Fucosterol is a commonly found sterol in brown seaweeds such as Fucus species (Kapetanovic et al. 2005). This sterol demonstrates antidiabetic effects and decreases the serum glucose concentration in rats (Lee et al. 2004).
Halogenated compounds
Marine environment is rich in halogens, and marine plants use these chemical entities to produce halogenated compounds (Sharma 2011). The possession of halogenated compounds is a unique chemical characteristic for seaweeds as compared to terrestrial plants. Halogenated compounds are mainly isolated from red and brown seaweeds. These compounds are found in different classes of primary and secondary metabolites and are important for inducing biological activities (Smit 2004). Antibacterial properties of bromophenols derived from red seaweed Rhodomela confervoides have been reported. In a study done by Xu et al. (2003), bis(2,3-dibromo-4,5-dihydroxybenzyl) ether was the most active compound among all the bromophenols tested against five strains of bacteria with the minimum inhibition concentration (MIC) of less than 70 μg/ml. Halomon, a polyhalogenated monoterpene isolated from red seaweed Portieria hornemannii, has been selected as an in vitro antitumor agent for the development of preclinical drug (Fuller et al. 1992, 1994). The compound is a moderate inhibitor of DNA methyl transferase-1 (Andrianasolo et al. 2006). Z-Laureatin (19) and Z-isolaureatin (20) are brominated oxygen heterocyclics isolated from red seaweed L. nipponica that have larvicidal effect against mosquito Cx. pipiens with LC50 values of 2.86 and 6.14 ppm, respectively (Watanabe et al. 1989b). Bromophycolide A, isolated from red seaweed Callophycus serratus displays cytotoxic activity against several human tumor cell lines. Besides, the compound also exhibits moderate antibacterial (against methicillin-resistant Staphylococcus aureus and vancomycin-resistant Enterococcus faecium), antifungal (against amphotericin-B-resistant Candida albicans), and antivirus (against two human immunodeficiency virus strains) activities (Kubanek et al. 2005).
Mosquitocidal properties of seaweeds
Primary and secondary seaweed metabolites have important economical and medicinal values (Smit 2004). Furthermore, many reports have revealed that seaweeds have pronounced insecticidal properties (Table 1) that are comparable to other bioinsecticides derived from land plants (Oliveira et al. 2010; Ghosh et al. 2012), fungi (Matasyoh et al. 2011; Mnyone et al. 2011), microalgae (Ahmad et al. 2004), other marine organisms (Samidurai and Saravanakumar 2011), etc. Subramonia Thangam and Kathiresan (1996) screened seaweeds, seagrasses, and mangroves for their larvicidal, skin repellent and smoke repellent activities. They classified larvicide with larvicidal activity less than LC50 100 mg/L as effective larvicide, LC50 between 100 and 200 mg/L as less effective larvicide, and LC50 more than 200 mg/L as ineffective larvicide. Among 15 seaweed species tested by them, six seaweeds showed active larvicidal activity (LC50 lower than 100 mg/L). Besides seaweed extracts, seaweed compounds isolated from red (Maeda et al. 1984; Watanabe et al. 1989a; San-Martin et al. 1991) and green seaweeds (LaLonde et al. 1979; Alarif et al. 2010) also have strong insecticidal activity (Table 2).
In the process of screening the insecticidal activity of seaweed extracts, the active seaweed extract is fractioned and tested repeatedly to identify the compound that is responsible for the killing action. Bianco et al. (2013) reported that at 10 ppm, the hexane extract of red seaweed L. dendroidea exhibited the strongest larvicidal effect (100 % mortality) against the mosquito larva Ae. aegypti as compared to ethyl acetate (37 % mortality), dichloromethane (70 % mortality), and methanol (15 % mortality) extracts. Sequential fractionation of the hexane extract of L. dendroidea yielded elatol (13) that exhibited potent larvicidal activity against Ae. aegypti (LC50 of 10.7 ppm) (Bianco et al. 2013). However, some pure compounds have been reported to have a decrement in their efficiency when separated from the fraction/extract. This may be due to the synergistic effects of several compounds that are responsible for the bioactivity.
In this review, 40 % of the seaweeds reported for their mosquito larvicidal potential are brown seaweeds, followed by green seaweeds (35 %) and red seaweeds (25 %). The main active mosquitocidal compounds are derived from the family of Rhodomelaceae and Plocamiaceae of red seaweeds which exhibit LC50 ranging from 0.1 to 36.9 ppm against several mosquito larva species (Table 2). It is interesting to note that majority of the active larvicidal compounds are halogenated aromatic compounds (Fig. 2).
The mosquito larvae treated with insecticidal agent manifest intoxicated symptoms through several ways, such as aberrations of morphological and histological features, change of swimming behavior, abnormal growth and development, decrement of longevity, reduced fecundity, and reproductive ability. These toxic effects of seaweed are discussed in the following section.
Effects on mortality
The susceptibility of different mosquito species toward seaweed treatment varies (Ali et al. 2013). Manilal et al. (2011) reported that mosquito larvae of Ae. aegypti were more susceptible (with a lower LC50 values) toward the treatment of seaweed extracts (Lobophora variegata, Spatoglossum asperum, Stoechospermum marginatum, Sargassum wightii, Acrosiphonia orientalis, Centroceras clavulatum, and Padina tetrastromatica) as compared to Culex quinquefasciatus. Similar observations were obtained by Govindarajan et al. (2011) who reported that the Anopheles stephensi had the lowest LC50 value followed by Ae. aegypti, and Cx. quinquefasciatus had the highest LC50 value after treatment with benzene extract of evergreen shrub Ervatamia coronaria. However, a different trend was observed by Shaalan et al. (2005), who reported that Aedes larvae were less susceptible (with a higher LC50 values) to insecticides and botanical extracts (land plants) compared to Culex larvae. For example, ginger Zingiber officinalis is a more effective larvicide against Cx. quinquefasciatus (LC50 value of 154 ppm) compared to Ae. aegypti (LC50 value of 197 ppm) (Khandagle et al. 2011).
Another factor that affects the effectiveness of seaweed extract is the age of mosquito tested. There is a higher mortality rate for younger larvae compared to older larvae under the same concentration of treatment. The tolerance of larvae toward treatment increases with the larval age (Alarif et al. 2010; Abou-Elnaga et al. 2011). Furthermore, field and resistance-strain mosquitoes may not be as susceptible as the lab-strain mosquitoes to bioinsecticides.
Research on the adulticidal effects of seaweed against mosquito is scarce. However, the repellent activity of brown seaweed S. wightii toward adult mosquito Anopheles sundaicus has been reported. The percentage of repellency was 89 % when 10 mg/L of S. wightii methanol extract was applied in the study done by Kumar et al. (2012). On the other hand, essential oils from terrestrial plants have been widely reported for their adulticidal and repellent properties (de Lima et al. 2013; Gkinis et al. 2014).
Seaweed extracts and compounds also exhibit their lethality effects toward various life stages of other insects (Table 3). Brown seaweed Padina pavonica and green seaweeds Ulva fasciata and Ulva lactuca exhibit nymphicidal and ovicidal activities against red cotton bug Dysdercus cingulatus (Sahayaraj and Kalidas 2011; Asha et al. 2012).
Effects on growth, development, and reproduction
Exposure to insecticidal agents causes acute mortality and sublethal effects to the mosquito larvae. The sublethal effects of toxin do not cause immediate death to the exposed larvae, but alter the morphogenesis process and induce changes to the larval external structure (Arias and Mulla 1975). Besides, the toxic effects of insecticidal agents also affect the growth, development, fecundity, fertility, and adult longevity of mosquitoes (Kumar et al. 2011; Asha et al. 2012).
The developmental aberrations of the mosquito larvae treated by juvenile hormone were described by Ratanatham et al. (1994, 1996). These observations were categorized into eight groups: (1) normal larva, (2) deformed larva, (3) prepupa that has not completely come out of the larval exoskeleton, (4) white pupa, (5) deformed pupa, (6) dead normal brown pupa, (7) adult attached to the pupal case, and (8) normal adult. Similar observations have also been reported for mosquito larvae treated with neem kernel extract (Sharma et al. 2006).
Elbanna and Hegazi (2011) observed a longer larval duration for mosquito Cx. pipiens compared to the control larvae after the treatment of dried ground seaweeds (Caulerpa prolifera, Caulerpa serrulata, Jania rubens, Nitophyllum punctatum, Cystoseira myrica, and P. pavonica). The authors also reported that some of the tested green (C. serrulata) and red (N. punctatum and J. rubens) seaweeds exhibited inhibition effects toward the growth regulatory activity and resulted in prolongation in metamorphosis of mosquito larvae.
Toxic effects of seaweed extracts and compounds toward the metabolism of other insects have been reported (Table 4). In the study of Asha et al. (2012), two green seaweeds, U. fasciata and U. lactuca caused reduction of body wet weight, adult longevity, female fecundity, and hatchability percentage of red cotton bug D. cingulatus. Significant reduction in total body protein and DNA content were observed in D. cingulatus nymphs treated with benzene extract of brown seaweed P. pavonica in the study of Sahayaraj and Kalidas (2011). In another study, two monoterpene derivatives—dibromomertensene (8) and dihydromertensene (9) derived from red seaweed Plocamium cartilagineum—have reduced 64 and 54 % of the reproduction index of cereal aphid Schizaphis graminum, respectively after 72 h of treatment (Argandoña et al. 2000).
Structure-activity relationships of seaweed larvicidal compounds
The structure-activity relationships of plant origin inseciticidal compounds and derivatives have been assessed in the previous studies. Such investigations aim at identifying the structural characteristic that contributes to the killing action (Barbosa et al. 2012; Dias and Moreas 2014).
A number of reports have demonstrated that larvicidal activity is dependent on the lipophilic profile of the compound involved (Barbosa et al. 2012; Dias and Moreas 2014). Generally, the lipophilic compounds are more potent compared to non-lipophilic compounds (Dias and Moreas 2014). It is evident in a study on fatty acids isolated from green seaweed Cladophora glomerata, which display different degrees of larvicidal activity against the mosquito larvae of Ae. triseriatus. Capric acid (15) with a 10-carbon hydrocarbon chain (LC50 of 14 ppm), is twice less potent than lauric acid (16) with a 12-carbon hydrocarbon chain (LC50 of 7 ppm), and 3.5 times less potent than myristic acid (17) with a 14-carbon hydrocarbon chain (LC50 of 4 ppm) (LaLonde et al. 1979). Among the four fatty acids tested against the mosquito larvae of Ae. triseriatus in the study conducted by LaLonde et al. (1979), palmitoleic acid (18) was the most potent compound (LC50 of 3 ppm) that has the longest hydrocarbon chain (16 carbons) and a double bond. The activity comparison of these fatty acids showed that longer hydrocarbon chain exhibited stronger lipophilic profile and at the same time resulted in stronger larvicidal action. Furthermore, the presence of double bond in the chemical structure of compound was suggested to induce an overall increment in larvicidal potency level. This is supported by the report of Laurens et al. (1997) where the reduction of double bond of cardonol, cardol, and anacardic acids isolated from the shell of cashew nut Anacardium occidentale caused a decrement in the larvicidal activity of A. occidentale against the mosquito larvae of Aedes atropalpus.
Caulerpin (21) and caulerpinic acid (22), isolated from green seaweed Caulerpa racemosa are derivatives that exhibit different levels of larvicidal activity. Compound 21 has higher larvicidal activity (LC50 = 1.42, 1.81, and 1.99 ppm) than compound 22 (LC50 = 3.04, 3.90, and 4.89 ppm) against the second-, third-, and fourth-instar larvae of Cx. pipiens (Alarif et al. 2010). Compound 21 has a stronger lipophilic profile than compound 22, as the ester group of compound 21 is less polar than the carboxylic acid group of compound 22. Thus, Caulerpin (21) exhibits a more potent killing action against Cx. pipiens. This is another example that demonstrates that a compound with a stronger lipophilic character exhibits a stronger larvicidal potential (Barbosa et al. 2012).
Mode and target site of action of seaweed larvicidal compounds
To date, seaweed extracts and compounds have been investigated only for their mosquitocidal properties; their mode of action and target site at molecular level have not been fully elucidated compared to the insecticidal compounds derived from terrestrial plants. Rattan (2010) explained the action mechanism of insecticidal secondary metabolites originating from terrestrial plants. The author described that cholinergic, gamma-aminobutyric acid (GABA), mitochondrial, and octopaminergic systems were the major targets of the insecticides derived from terrestrial plants. For instance, plant compounds inhibit acetylcholinesterase (AChE) enzymes (Senthil-Nathan et al. 2008) and also affect insect cholinergic system by acting as a receptor agonist or antagonist (Kukel and Jennings 1994). Terrestrial plant compounds inhibit mitochondrial activity by disrupting sodium and potassium ion exchange (Casida 1980), affecting calcium channels (Copping and Menn 2000) and interrupting nerve cell membrane action (Bloomquist 1996). Phytochemical compounds are able to affect the function of receptors. For instance, thymol isolated from thyme essential oil is suggested to potentiate GABA(A) receptors (Priestley et al. 2003). Previous studies have also revealed that the essential oil compound—eugenol decreases cAMP level in HEK-293 cells (which express octopamine receptors from American cockroach Periplaneta americana) (Enan 2005).
Seaweed extracts and compounds also exhibit significant inhibition effects on cholinergic system. Ryu et al. (2003) reported that the farnesylacetone derivatives and plastoquinones isolated from brown seaweed Sargassum sagamianum showed inhibition effects toward acetylcholinesterase enzymes isolated from bovine erythrocytes. On the other hand, the methanol extracts of brown seaweed Sargassum species, red seaweed Gracilaria gracilis, and green seaweed Cladophora fascicularis showed inhibition effects against cholinesterase enzymes isolated from freshwater Nile tilapia Oreochromis niloticus (Natarajan et al. 2009).
Besides, aplysia terpenoid A (23) and telfairine (24) isolated from red seaweed Plocamium telfairiae show strong larvicidal activity against mosquito Anopheles gambiae. Their mode of action is similar to cyclodiene (Watanabe et al. 1990). Cyclodiene is an organochloride insecticide that blocks GABA-gated chloride channel, resulting in reduction of neuronal inhibition, hyper-excitation of the central nervous system, convulsion, and death (Bloomquist 1993).
Morphological aberrations of insect organ and cuticular sculpturing are the toxic effects of insecticide. Since anal papillae are important for regulation of electrolyte balance (Christophers 1960), structural deformation of anal papillae leads to dysfunction and is probably associated with the death of mosquito larvae (Chaithong et al. 2006). Bianco et al. (2013) reported darkening of anal papillae of the red seaweed L. dendroidea treated mosquito larvae. The findings correspond to the work by Chaithong et al. (2006) which revealed that pepper-treated mosquito larvae had deformed and shrunkened anal gills. Warikoo and Kumar (2013) suggested that cytotoxic effects of flowering thistle Argemone mexicana extracts led to the discharge of electrolytes from the anal region resulting in violent anal biting of larvae.
Besides outer morphology, the inner structure of larvae is also susceptible to the deleterious effect of treatment. The midgut region of the Culicidae larvae is one of the common target sites of insecticidal phytochemicals. The toxin causes detachment and vacuolization of the epithelial midgut cells, damage of microvilli, modified peritrophic matrix structure, and finally cell death. Severe damage of the midgut region disrupts the midgut epithelium integrity and function (David et al. 2000; Al-Mehmadi and Al-Khalaf 2010). Furthermore, phytochemicals also affect the gastric caeca and Malpighian tubules in mosquito larvae (David et al. 2000).
Toxin-treated mosquitoes show signs of being poisoned in their behavior as one of their manners of intoxication (Manilal et al. 2011; Kovendan et al. 2013). Previous reports have shown that the brown seaweed Lobophora variegata treated mosquito larvae of Cx. quinquefasciatus and Ae. aegypti showed abnormal behavior before death (Manilal et al. 2011). In the study, the larvae exhibited primary phase of behavioral changes such as being excited, restless, sinking down, and floating up frequently, after being exposed to 200 μg/ml of L. variegata methanol extract for an hour. After 6 h, the secondary phase began and the larvae showed sluggishness. During the tertiary phase (12 to 24 h), the larvae lost response and paralyzed, followed by sinking to the bottom of the holding container and died (Manilal et al. 2011). Similar symptomatological observations have been reported for the sand ginger Kaempferia galanga-treated mosquito larvae of Cx. quinquefasciatus (Insun et al. 1999) and pepper-plant-treated mosquito larvae of Ae. aegypti (Chaithong et al. 2006) except for the time and duration of exhibiting the toxic symptoms. In addition to the symptoms mentioned earlier, the flowering thistle Argemone mexicana extract treated Ae. aegypti larvae show behavior of self biting of anal papillae prior to paralysis (Warikoo and Kumar 2013). These intoxication symptoms which are similar to those caused by synthetic nerve poisons suggest that the insecticidal extracts may act as cytolysin that affects the neuromuscular coordination in chemical synapses (Warikoo and Kumar 2013).
Synergism between seaweed extract and insecticide
Studies on the synergistic effect of insecticide and phytochemical mixture have been conducted in mosquito control investigation (Table 5). These works have investigated the potential of binary mixture of the synthetic insecticide/bioinsecticide and phytochemical. The bioefficacy of the combination of phytochemical and insecticide is described as synergistic factor (SF). A SF value of the binary mixture that is less than 1 indicates the presence of synergism between two insecticidal agents. Smaller SF value indicates greater synergistic activity (Kalyanasundaram and Babu 1982; Kalyanasundaram and Das 1985). Kalyanasundaram and Das (1985) calculated SF by using the formula below.
Combination of brown seaweed S. wightii and bacteria Bacillus thuringiensis var. israelensis shows high synergistic larvicidal activity toward the second-instar larvae of An. sundaicus (Kumar et al. 2012). Besides, a combination of two bioinsecticides (mixture of seaweed and synthetic insecticide) has also been tested for its synergistic effects. Among different combinations of three synthetic insecticides and three marine plants used in the study of Thangam and Kathiresan (1991a), green seaweed Caulerpa scalpelliformis and synthetic insecticide benzene hexachloride exhibited the highest synergistic larvicidal effects (SF = 0.71) against the mosquito larvae of Ae. aegypti (Table 5). The synergistic interaction between the seaweed extract and insecticide increased the larvicidal effects against the mosquito larvae. This could be due to the presence of active compounds in the seaweed extract that inhibits the activity of insect-detoxifying enzymes which act against the synthetic insecticides (Thangam and Kathiresan 1991a). The consumption of synthetic insecticide and the risk of chemical pollution can be reduced by combining two insecticidal agents.
Effect on non-target organisms
Although conventional synthetic insecticides are effective, application of chemicals elicits resistance in mosquito populations and results in harmful effects to human and other organisms (Paeporn et al. 2003). Therefore, bioinsecticides which have been proven effective should be assessed for their impact on the beneficial and non-target organisms. A safe and effective mosquitocidal agent should be target-specific, posing no or little harm to the other organisms that share the same habitat. It is crucial when bioinsecticide is used in the integrated pest management of mosquito whereby chemical and biological control agents are used. Non-target fauna such as fingerlings, frog tadpoles, aquatic insects, and other invertebrates could be the test subjects to assess the relative toxicity of mosquitocidal agents (Manilal et al. 2009; Patil et al. 2011). Table 6 shows the toxic effects of seaweed extracts and compounds on non-target organisms. Furthermore, field trial should be carried out to evaluate the efficacy of mosquitocidal agents in reducing mosquito populations at different breeding habitats (Kovendan et al. 2013).
Brine shrimp nauplius, a simple zoologic organism, has been used in a number of bioassays in natural product research (Meyer et al. 1982). The lethality test that uses brine shrimp nauplii is relatively simple, inexpensive, and rapid compared to the other in vivo tests. Furthermore, McLaughlin et al. (1998) observed a positive correlation between the brine shrimp and 9 KB (human nasopharyngeal carcinoma) cytotoxicity tests. The ED50 value of the cytotoxicity test was about one tenth the LC50 value obtained from the brine shrimp test. It is considered a prescreen procedure for antitumor and pesticidal natural products (McLaughlin et al. 1998). Assessment of the active mosquitocidal extract/compound in the brine shrimp lethality test offers a simple toxicity prediction method that can be used on mammals.
Major variables affecting mosquitocidal activity
The bioactive properties of seaweed extracts and fractions are highly dependent on the content of the extracts/fraction which is associated with natural variability and sample preparation. Furthermore, the standardized procedures recommended by the authorities eases the comparison of bioactivity between such extracts from different sources/laboratories in the evaluation of mosquitocidal potential.
Abiotic and biotic variables
Abiotic and biotic factors influence the chemical composition of seaweeds. Abiotic factors from the environment such as nutrients, light, temperature, pH, and water movement induce changes in biomass production (Wong and Phang 2004) and chemical composition of seaweeds (Stengel et al. 2011). Differences in chemical composition caused by environmental influences can be directly linked to different levels of bioactivity. Seasonal variations in lipid composition were reported for the seaweeds harvested in the sea of Japan by Kostetsky et al. (2004). The authors observed a lower content of phospholipids in seaweeds in summer than in spring. Other than natural factors from the environment, contaminants such as heavy metals also influence the chemical constituents of seaweeds. Copper has been reported to reduce total phenolic contents and alter phenolic composition of brown seaweeds (Ascophyllum nodosum and Fucus vesiculosus) (Connan and Stengel 2011).
The influences of biotic factors on chemical compositions of seaweeds such as grazing by herbivores have been studied (Cronin and Hay 1996). Grazing effects induce elevation of defensive secondary metabolite concentration in brown seaweeds (Cronin and Hay 1996). Antigrazing metabolites in seaweeds such as phlorotannins, fatty-acid-derived compounds, terpenoids, and halogenated compounds showed significant bioactivities (Stengel et al. 2011). In addition, the chemical composition of macroalgae also varies with growth cycle; for example, the content of mannitol, laminaran, and cellulose along the blades of brown seaweeds varies to age and developmental stages (Zubia et al. 2008).
Sample preparation
Every sample preparation step is crucial for maintaining the performance of the sample. Drying the raw material is one of the important steps to preserve the relevant chemical composition in the seaweeds. Some metabolites are more heat- and light-sensitive than others. Therefore, appropriate measures must be taken to ensure the potential constituents are not lost or destroyed during sample preparation. For example, phenolic compounds can be degraded in oven-drying (Wong and Cheung 2001) while the content of polyunsaturated fatty acids decreased after freeze-drying process (Chan et al. 1997).
The bioefficacy of seaweed extract/compound depends on sample preparation. It is crucial to choose a suitable solvent for the extraction process. Solvents of different polarities extract different chemical constituents (Karmegam et al. 1997), and the chemical composition obtained affects the bioactivity of the crude extract. Basically, a solvent with high polarity index extracts more polar molecules, while a solvent with low polarity index extracts more non-polar molecules.
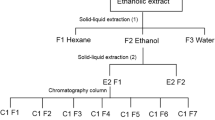
Fractionation of crude extract by sequential use of solvent from high to low polarity is usually being carried out prior to bioassay testing. This allows better determination of fraction(s) that exhibit active insecticidal properties and assist in identification of active compounds(s). Khanavi et al. (2011) reported that among the solvent fractions derived from brown seaweed Sargassum swartzii which were tested against the mosquito larvae of An. stephensi, ethyl acetate fraction induced the highest mortality rate (96.1 %) compared to chloroform (0 %) and the methanol-water (6.1 %) fractions. This indicated the presence of active insecticidal compound(s) or synergist effects of the compounds in the ethyl acetate fraction. This further suggested that chloroform and the methanol-water fractions of S. swartzii contributed to little or no active constituents that promote the killing activity (Khanavi et al. 2011).
Test procedure
Bioactivity screening of natural products requires standard protocol. It is important to harmonize the testing procedures carried out in different laboratories and institutions for generation of valid data for bioactivity comparison. Guidelines for laboratory and field testing of mosquito larvicides (WHO 2005) and test procedures for insecticide resistance monitoring in malaria vectors, bioefficacy, and persistence of insecticides on treated surfaces (WHO 1998) provide standardized procedures and guidelines for conducting mosquito bioassays. Besides, WHO also provides generic models for risk assessment of mosquito larvicides and adulticides toward human, environment, and non-target organisms (WHO 2011a, b).
Guidelines from WHO serve as “golden” rules for the researchers in conducting mosquitocidal assay. However, there are some primary requirements/recommendations that help researchers define a sound mosquitocidal potential in seaweeds. Inclusion of one or more reference insecticides as a positive control to ascertain the test results and proper interpretation of the efficacy of a tested sample are necessary. Commercially available insecticides sourced from reliable chemical suppliers or WHO is preferred. Additional appropriate controls such as blank and negative control can be considered in the testing. Besides, each test should contain a number of replicates and be repeated for a few times. In larvicidal and adulticidal assays, the efficacy of a tested sample is expressed by values such as mortality (%), lethal concentration 50 (LC50), or lethal concentration 90 (LC90). Dose-response curves are established by using the data range of at least three concentrations. Following stringent endpoint criteria, only plant extracts that exhibit LC50 values of less than 100 μg/ml against mosquito larvae are considered as effective larvicides. Test mosquitoes are preferably lab strain that has been cultured for several generations. Resistant strain may be used only if the resistance level of the mosquito has been characterized.
Conclusion and perspectives
The extracts and compounds derived from seaweeds have proven their mosquitocidal potential in the research of mosquito control. Some seaweed extracts or compounds act as general toxicants which cause acute mortality to various life stages of mosquito (Abou-Elnaga et al. 2011). However, some extracts can potentially be used as growth, feeding, and reproduction inhibitors (Elbanna and Hegazi 2011; Asha et al. 2012). The bioactivity of a seaweed compound is related to the compound’s chemical structure and chemical reactions (Barbosa et al. 2012; Dias and Moreas 2014). The studies of structure-activity relationships and killing action of the insecticidal compounds are crucial and useful in the selection of proprietary compounds in the development of insecticides. These approaches predict the biological potential of the compounds. By understanding the bioactivity and chemistry properties of the existing insecticidal compounds, researchers can design a chemically synthesized derivative, which is safe, environmentally friendly, and can possess multiple modes of actions (Rattan 2010).
It is noteworthy that many studies have envisaged the pronounced toxicity of seaweed extracts and compounds against medically important insects and agricultural pests. These extracts or compounds may have the same significant lethality against mosquitoes since most of the insects share similar physiological aspects. For instance, (12E)-cis maneonene-E (25), an acetogenin isolated from red seaweed Laurencia papillosa, is lethal to both mosquito and beetle larvae (Abou-Elnaga et al. 2011). Thus, the reported insecticidal seaweed extracts and compounds can potentially be used for the screening and development of active mosquitocidal agents.
Seaweed-derived mosquitocidal compounds have been proven effective and highly biodegradable, making them a potentially safe and environmentally sound control agent. Several issues are yet to be focused and investigated to improve the effectiveness of seaweed-based mosquitocidal agents such as standardization of the testing protocols, degradation rate, persistence rate and quality assessment of the agents, residual effects of the agents toward the non-target organisms, and potency of resistance development in the mosquito populations (Shaalan et al. 2005). The current knowledge on the mode of actions of insecticides is direct and linear. In reality, the mechanisms, reactions, and interactions between the insecticides and target sites are more complex. Therefore, effective field trial application rates should be established.
References
Abou-Elnaga ZS, Alarif WM, Al-lihaibi SS (2011) New larvicidal acetogenin from the red alga Laurencia papillosa. CLEAN-Soil Air Water 39(8):787–794. doi:10.1002/clen.201000597
Afolayan AF, Bolton JJ, Lategan CA, Smith PJ, Beukes DR (2008) Fucoxanthin, tetraprenylated toluquinone and toluhydroquinone metabolites from Sargassum heterophyllum inhibit the in vitro growth of the malaria parasite Plasmodium falciparum. Z Naturforsch C 63:848–852
Ahmad R, Chu WL, Ismail Z, Lee HL, Phang SM (2004) Effect of ten chlorophytes on larval survival, development and adult body size of the mosquito Aedes aegypti. Southeast Asian J Trop Med Public Health 35(1):79–87
Ahn GN, Kim KN, Cha SH, Song CB, Lee J, Heo MS, Yeo IK, Lee NH, Jee YH, Kim JS, Heu MS, Jeon YJ (2007) Antioxidant activities of phlorotannins purified from Ecklonia cava on free radical scavenging using ESR and H2O2-mediated DNA damage. Eur Food Res Technol 226:71–79
Alarif WM, Abou-Elnaga ZS, Ayyad SE, Al-lihaibi SS (2010) Insecticidal metabolites from the green alga Caulerpa racemosa. CLEAN-Soil Air Water 38(5–6):548–557. doi:10.1002/clen.201000033
Ali MS, Ravikumar S, Beula JM (2013) Mosquito larvicidal activity of seaweeds extracts against Anopheles stephensi, Aedes aegypti and Culex quinquefasciatus. Asian Pac J Trop Dis 3(3):196–201. doi:10.1016/S2222-1808(13)60040-7
Al-Mehmadi RM, Al-Khalaf AA (2010) Larvicidal and histological effects of Melia azedarach extract on Culex quinquefasciatus Say larvae (Diptera: Culicidae). J King Saud Univ (Science) 22:77–85
Amer A, Mehlhorn H (2006a) Repellency effect of forty-one essential oils against Aedes, Anopheles, and Culex mosquitoes. Parasitol Res 99(4):478–490. doi:10.1007/s00436-006-0184-1
Amer A, Mehlhorn H (2006b) Larvicidal effects of various essential oils against Aedes, Anopheles, and Culex larvae (Diptera, Culicidae). Parasitol Res 99(4):466–472. doi:10.1007/s00436-006-0182-3
Andrianasolo EH, France D, Cornell-Kennon S, Gerwick WH (2006) DNA methyl transferase inhibiting halogenated monoterpenes from the Madagascar red marine alga Portieria hornemannii. J Nat Prod 69:576–579
Argandoña V, Pozo Del T, San-Martín A, Rovirosa J (2000) Insecticidal activity of Plocamium cartilagineum monoterpenes. Bol Soc Chil Quím. doi:10.4067/S0366-16442000000300006
Arias JR, Mulla MS (1975) Morphogenetic aberrations induced by a juvenile hormone analogue in the mosquito Culex tarsalis (Diptera: Culicidae). J Med Entomol 12(3):309–318
Asha A, Martin Rathi J, Patric Raja D, Sahayaraj K (2012) Biocidal activity of two marine green algal extracts against third instar nymph of Dysdercus cingulatus (Fab.) (Hemiptera: Pyrrhocoridae). J Biopest 5(Supplementary):129–134
Awad NE (2004) Bioactive brominated diterpenes from the marine red alga Jania rubens (L.) Lamx. Phytother Res 18:275–279
Ayesha H, Sultana V, Ara J, Ehteshamul-Haque S (2010) In vitro cytotoxicity of seaweeds from Karachi Coast on brine shrimp. Pak J Bot 42(5):3555–3560, http://www.pakbsorg/pjbot/tcontents/tcontent42(5)61–70.html. Accessed 15 June 2014
Ayyad SEN, Ezmirly ST, Basaif SA, Alarif WM, Badria AF, Badria FA (2011) Antioxidant, cytotoxic, antitumor, and protective DNA damage metabolites from the red sea brown alga Sargassum sp. Pharmacognosy Res 3:160–165. doi:10.4103/0974-8490.85000
Bantoto V, Dy D (2013) The larvicidal activity of brown algae Padina minor (Yamada 1925) and Dictyota linearis (Greville 1830) against the dengue vector, Aedes aegypti (Linn 1762) (Diptera: Culicidae). J Vect Borne Dis 50:68–70
Barbosa JDF, Silva VB, Alves PB, Gumina G, Santos RLC, Sousa DP, Cavalcanti SCH (2012) Structure–activity relationships of eugenol derivatives against Aedes aegypti (Diptera: Culicidae) larvae. Pest Manag Sci 68:1478–1483. doi:10.1002/ps.3331
Bianco EM, Pires L, Santos GKN, Dutra KA, Reis TNV, Vasconcelos ERTPP, Cocentino ALM, Navarro DMAF (2013) Larvicidal activity of seaweeds from northeastern Brazil and of a halogenated sesquiterpene against the dengue mosquito (Aedes aegypti). Ind Crop Prod 43:270–275. doi:10.1016/j.indcrop.2012.07.032
Black WAP (1954) Concentration gradients and their significance in Laminaria saccharina (L.) Lamour. J Mar Biol Assoc UK 33(1):49–60
Bloomquist JR (1993) Toxicology, mode of action and target site-mediated resistance to insecticides acting on chloride channels. Comp Biochem Physiol C 106(2):301–314
Bloomquist JR (1996) Ion channels as targets for insecticides. Ann Rev Entomol 41:163–190
Bonnard I, Manzanares I, Rinehart KL (2003) Stereochemistry of Kahalalide F. J Nat Prod 66(11):1466–1470
Born FS, Bianco ÉM, Da Camara CAG (2012) Acaricidal and repellent activity of terpenoids from seaweeds collected in Pernambuco, Brazil. Nat Prod Commun 7:463–466
Bourel-Bonnet L, Rao KV, Hamann MT, Ganesan A (2005) Solid-phase total synthesis of Kahalalide A and related analogues. J Med Chem 48(5):1330–1335
Campos A, Souza CB, Lhullier C, Falkenberg M, Schenkel EP, Ribeiro-do-Valle RM, Siqueira JM (2012) Anti-tumour effects of elatol, a marine derivative compound obtained from red algae Laurencia microcladia. J Pharm Pharmacol 64(8):1146–1154. doi:10.1111/j.2042-7158.2012.01493
Cardozo KH, Guaratini T, Barros MP, Falcão VR, Tonon AP, Lopes NP, Campos S, Torres MA, Souza AO, Colepicolo P, Pinto E (2007) Metabolites from algae with economical impact. Comp Biochem Physiol C Toxicol Pharmacol 146(1–2):60–78. doi:10.1016/j.cbpc.2006.05.007
Casida JE (1980) Pyrethrum flowers and pyrethroid insecticides. Environ Health Perspect 34:189–202
Chaithong U, Choochote W, Kamsuk K, Jitpakdi A, Tippawangkosol P, Chaiyasit D, Champakaew D, Tuetun B, Pitasawat B (2006) Larvicidal effect of pepper plants on Aedes aegypti (L.) (Diptera: Culicidae). J Vector Contr 31(1):138–144
Chan JCC, Cheung PCK, Ang PO (1997) Comparative studies on the effect of three drying methods on the nutritional composition of seaweed Sargassum hemiphyllum (Turn.) C. Ag. J Agric Food Chem 45(8):3056–3059. doi:10.1021/jf9701749
Chandini S, Kumar GP, Suresh PV, Bhaskar N (2008) Seaweeds as source of nutritionally beneficial compounds—a review. J Food Sci Technol 45(1):1–13, http://irc.ftri.com/1608/. Accessed 11 May 2014
Chattopadhyay N, Ghosh T, Sinha S, Chattopadhyay K, Karmakar P, Ray B (2010) Polysaccharides from Turbinaria conoides: structural features and antioxidant capacity. Food Chem 118:823–829. doi:10.1016/j.foodchem.2009.05.069
Christophers SR (1960) Aëdes aegypti (L.) the yellow fever mosquito: Its life history, bionomics, and strucure. Cambridge University Press, New York
Connan S, Stengel DB (2011) Impacts of ambient salinity and copper on brown algae: 2. Interactive effects on phenolic pool and assessment of metal binding capacity of phlorotannin. Aquat Toxicol 104(1–2):1–13
Coppejans E, Leliaert F, Dargent O, Gunasekara R, De Clerck O (2009) Sri Lankan seaweeds—methodologies and field guide to the dominant species. Abc Taxa 6(i-viii):265
Copping LG, Menn JJ (2000) Biopesticides: a review of their action, applications and efficacy. Pest Manag Sci 56:651–676
Crews P, Mayers BL, Naylor S, Clason EL, Jacobs RS, Staal GB (1984) Bio-active monoterpenes from red seaweeds. Phytochemistry 23(7):1449–1451
Cronin G, Hay ME (1996) Induction of seaweed chemical defenses by amphipod grazing. Ecology 77(8):2287–2301
David J, Rey D, Pautou M, Meyran J (2000) Differential toxicity of leaf litter to dipteran larvae of mosquito developmental sites. J Invertebr Pathol 75:9–18
Dawczynski C, Schubert R, Jahreis G (2007) Amino acids, fatty acids, and dietary fibre in edible seaweed products. Food Chem 103:891–899. doi:10.1016/j.foodchem.2006.09.041
de Lima GP, de Souza TM, de Paula FG, Farias DF, Cunha AP, Ricardo NM, de Morais SM, Carvalho AF (2013) Further insecticidal activities of essential oils from Lippia sidoides and Croton species against Aedes aegypti L. Parasitol Res 112(5):1953–1958. doi:10.1007/s00436-013-3351-1
Devi P, Solimabi W, D’Souza L, Kamat SY (1997) Toxic effects of coastal and marine plant extracts on mosquito larvae. Bot Mar 40:533–535
Devi P, Solimabi W, D’Souza L, Kamat SY (1998) Larvicidal activity of some marine macrophytes against Artemia salina. Ad Bios 17(11):75–84, http://drs.nio.org/drs/handle/2264/1922. Accessed 11 May 2014
Dias CN, Moreas DFC (2014) Essential oils and their compounds as Aedes aegypti L. (Diptera: Culicidae) larvicides: review. Parasitol Res 113(2):565–592. doi:10.1007/s00436-013-3687-6
Dos Santos AO, Veiga-Santos P, Ueda-Nakamura T, Dias-Filho BP, Sudatti DB, Bianco EM, Pereira RC, Nakamura CV (2010) Effect of elatol, isolated from red seaweed Laurencia dendroidea, on Leishmania amazonensis. Mar Drugs 8:2733–2743. doi:10.3390/md8112733
El Sayed K, Bartyzel P, Shen X, Perry TL, Zjawiony JK, Hamann MT (2000) Marine natural products as antituberculosis agents. Tetrahedron 56:949–953
Elbanna SM, Hegazi MM (2011) Screening of some seaweeds species from South Sinai. Red Sea as potential bioinsecticides against mosquito larvae; Culex pipiens. Egypt Acad J Biol Sci 4(2):21–30, http://entomology.eajbs.eg.net/vol4-no2.html. Accessed 15 June 2014
Enan EE (2005) Molecular and pharmacological analysis of an octopamine receptor from American cockroach and fruit fly in response to plant essential oils. Arch Insect Biochem 59(3):161–171
FAO (Food and Agriculture Organization of the United Nations) (2004) The state of world fisheries and aquaculture. Part 3. Highlights of special FAO studies. http://www.fao.org/docrep/007/y5600e/y5600e00.htm. Accessed 24 Jan 2014
Ferkany JW, Coyle JT (1983) Kainic acid selectively stimulates the release of endogenous excitatory acidic amino acids. J Pharmacol Exp Therapeut 225:399–406
Ferriera LG, Noseda MN, Gonҫalves AG, Ducatti DRB, Fujii MT, Duarte MER (2012) Chemical strucute of the complex pyruvylated and sulfated agaran from the red seaweed Palisada flagellifera (Cermiales, Rhodophyta). Carbohydr Res 347:83–94. doi:10.1016/j.carres.2011.10.007
Fisch K, Böhm V, Wright AD, König GM (2003) Antioxidative meroterpenoids from the brown alga Cystoseira crinita. J Nat Prod 66(7):968–975
Fukuzawa A, Masamune T (1981) Laurepinnacin and isolaurepinnacin, new acetylenic cyclic ethers from the marine red alga Laurencia pinnata Yamada. Tetrahedron Lett 22(41):4081–4084
Fuller RW, Cardellina JH, Kato Y, Brinen LS, Clardy J, Sander KM, Boyad MR (1992) A pentahalogenated monoterepene from the red alga Portieria hornemannii produced a novel cytotoxicity profile against a diverse panel of human tumor cell lines. J Med Chem 35:3007–3011
Fuller RW, Cardellina JH, Jurek J, Scheuer PJ, Alvarado-Linder B, McGuier M, Gray GN, Steiner IR, Clardy I, Menez E, Shoemaker RH, Newman DI, Sander KM, Boyad MR (1994) Isolation and structure/activity features of halomon-related antitumor monoterpenes from the red alga Portieria hornemannii. J Med Chem 37:4407–4411
Gerwick W, Fenical W (1981) Ichthytoxic and cytotoxic metabolite of the tropical brown alga Stypopodium zonale (Lamouroux) Papenfuss. J Org Chem 46:22–27
Ghosh A, Chowdhury N, Chandra G (2012) Plant extracts as potential mosquito larvicides. Indian J Med Res 135:581–598
Gkinis G, Michaelakis A, Koliopoulos G, Ioannou E, Tzakou O, Roussis V (2014) Evaluation of the repellent effects of Nepeta parnassica extract, essential oil, and its major nepetalactone metabolite against mosquitoes. Parasitol Res 113(3):1127–1134. doi:10.1007/s00436-013-3750-3
Glyantsev S, Annaev A, Savvina TV (1993) Morphological basis for selecting composition and structure of biologically active compounds based on sodium alginate for wound treatment. Byull Eksp Biol Med 115:65–67
Govindarajan M, Mathivanan T, Elumalai K, Krishnappa K, Anandan A (2011) Mosquito larvicidal, ovicidal, and repellent properties of botanical extracts against Anopheles stephensi, Aedes aegypti, and Culex quinquefasciatus (Diptera: Culicidae). Parasitol Res 109(2):353–367. doi:10.1007/s00436-011-2263-1
Hamann MT, Otto CS, Scheuer PJ, Dunbar DC (1996) Kahalides: bioactive peptides from a marine mollusk Elysia rufescens and its algal diet Bryopsis sp. J Org Chem 61:6594–6600
Hayashi K, Nakano T, Hashimoto M, Kanekiyo K, Hayashi T (2008) Defensive effects of a fucoidan from brown alga Undaria pinnatifida against Herpes simplex virus infection. Int Immunopharmacol 8:109–116
Hemmingson JA, Falshaw R, Furneaux RH, Thompson K (2006) Structure and antiviral activity of the galactofucan sulfates extracted from Undaria pinnatifida (Phaeophyta). J Appl Phycol 18:185–193. doi:10.1007/s10811-006-9096-9
Holdt SL, Kraan S (2011) Bioactive compounds in seaweeds: functional food applications and legislation. J Appl Phycol 23:543–597
Insun D, Choochote W, Jitpakdi A, Chaithong U, Tippawangkosol P, Pitasawat B (1999) Possible site of action of Kaempferia galanga in killing Culex quinquefasciatus larvae. Southeast Asian J Trop Med Public Health 30(1):195–199
Iverson F, Truelove J, Nera E, Tryphonas L, Campbell J, Lok J (1989) Domoic acid poisoning and mussel-associated intoxication: Preliminary investigations into the response of mice and rats to toxic mussel extract. Food Chem Toxicol 27(6):377–384
Jantan I, Ping WO, Visuvalingam SD, Ahmad NW (2003) Larvicidal activity of essential oils and methanol extracts of Malaysian plants on Aedes aegypti. Pharm Biol 41:234–236
Jantan I, Yalvema MF, Ahmad NW, Jamal JA (2005) Insecticidal activities of the leaf oils of eight Cinnamomum species against Aedes aegypti and Aedes albopictus. Pharm Biol. doi:10.1080/13880200500220771
Jung HA, Hyun SK, Kim HR, Choi JS (2006) Angiotensin-converting enzyme I inhibitory activity of phlorotannins from Ecklonia stolonifera. Fish Sci 72:1292–1299
Kalyanasundaram M, Babu CJ (1982) Biological active plant extracts as mosquito larvicides. Indian J Med Res 76:102–106
Kalyanasundaram M, Das PK (1985) Larvicidal and synergistic activity of plant extracts for mosquito control. Indian J Med Res 82:19–23
Kapetanovic R, Sladic D, Popov S, Zlatovic M, Kljajic Z, Gasic MJ (2005) Sterol composition of the Adriatic sea algae Ulva lactuca, Codium dichotomum, Cystoseira adriatica and Fucus virsoides. J Serb Chem Soc 70:1395–1400
Karmegam N, Sakthivadivel M, Anuradha V, Daniel T (1997) Indigenous plant extracts as larvicidal agents against Culex quinquefasciatus Say. Bioresour Technol 59:137–140
Khanavi M, Toulabi PB, Abai MR, Sadati N, Hadjiakhoondi F, Hadjiakhoondi A, Vatandoost H (2011) Larvicidal activity of marine algae, Sargassum swartzii and Chondria dasyphylla, against malaria vector Anopheles stephensi. J Vector Borne Dis 48(4):241–244
Khandagle AJ, Tare VS, Raut KD, Morey RA (2011) Bioactivity of essential oils of Zingiber officinalis and Achyranthes aspera against mosquitoes. Parasitol Res 109(2):339–343. doi:10.1007/s00436-011-2261-3
Khotimchenko YS, Kovalev VV, Savchenko OV, Ziganshina OA (2001) Physical–chemical properties, physiological activity, and usage of alginates, the polysaccharides of brown algae. Russ J Mar Biol 27:53–64
Kitamura K, Matsuo M, Yasui T (1991) Fucoidan from brown seaweed Laminaria angustata var. longissima. Agric Biol Chem 55(2):615–616
Kostetsky EY, Goncharova SN, Sanina NM, Shnyrov VL (2004) Season influence on lipid composition of marine macrophytes. Bot Mar 47:134–139
Kovendan K, Murugan K, Kumar PM, Thiyagarajan P, William SJ (2013) Ovicidal, repellent, adulticidal and field evaluations of plant extract against dengue, malaria and filarial vectors. Parasitol Res 112(3):1205–1219. doi:10.1007/s00436-012-3252-8
Kubanek I, Prusak AC, Snell TW, Giese RA, Hardcastle KI, Fairchild CR, Aalbersberg W, Raventos-Suarez C, Hay ME (2005) Antineoplastic diterpene-benzoate macrolides from the Fijian red alga Callophycus serratus. Org Lett 7:261–264
Kukel CF, Jennings KR (1994) Delphinium alkaloids as inhibitors of alpha-bungarotoxin binding to rat and insect neural membranes. Can J Physiol Pharmacol 72:104–107
Kumar S, Singh AP, Nair G, Batra S, Seth A, Wahab N, Warikoo R (2011) Impact of Parthenium hysterophorus leaf extracts on the fecundity, fertility and behavioural response of Aedes aegypti L. Parasitol Res 108(4):853–859. doi:10.1007/s00436-010-2126-1
Kumar KP, Murugan K, Kovendan K, Kumar AN, Hwang JS, Barnard DR (2012) Combined effect of seaweed (Sargassum wightii) and Bacillus thuringiensis var. israelensis on the coastal mosquito, Anopheles sundaicus, in Tamil Nadu, India. ScienceAsia 38:141–146
Kumari P, Kumar M, Gupta V, Reddy CRK, Jha B (2009) Tropical marine macroalgae as potential sources of nutritionally important PUFAs. Food Chem 120:749–757. doi:10.1016/j.foodchem.2009.11.006
LaLonde RT, Morris CD, Wong CF, Gardener LC, Eckert DJ, King DR, Zimmerman RH (1979) Response of Aedes triseriatus larvae to fatty acids of Cladophora. J Chem Ecol 5(3):371–381
Laurens A, Fourneau C, Hocquemiller R, Cave A, Bories C, Loiseau PM (1997) Antivectorial activities of cashew nut shell extracts from Anacardium occidentale L. Phytother Res 11:145–146
Lee YS, Shin KH, Kim BK, Lee S (2004) Anti-diabetic activities of fucosterol from Pelvetia siliquosa. Arch Pharmacol Res 27(11):1120–1122
Li B, Lu F, Wei X, Zhao Z (2008) Fucoidan: structure and bioactivity. Molecules 13:1671–1695
Ma M, Zhao J, Wang S, Li S, Yang Y, Shi J, Fan X, He L (2006) Bromophenols coupled with methyl gamma-ureidobutyrate, bromophenol sulfates from the red alga Rhodomela confervoides. J Nat Prod 69:206–210
Maeda M, Kodama T, Tanaka T, Ohfune Y, Nomoto K, Nishimura K, Fujita T (1984) Insecticidal and neuromuscular activities of domoic acid and its related compounds. J Pestic Sci 9(1):27–32
Maeda M, Kodama T, Tanaka T, Yoshizumi H, Takemoto T, Nomoto K, Fujita T (1986) Structures of isodomoic acids A, B and C, novel insecticidal amino acids from the red alga Chondria armata. Chem Pharm Bull 34:4892–4895
Maeda H, Hosokawa M, Sashima T, Murakami-Funayama K, Miyashita K (2009) Anti-obesity and anti-diabetic effects of fucoxanthin on diet-induced obesity conditions in a murine model. Mol Med Rep 2:897–902
Manilal A, Sujith S, Kiran GS, Selvin J, Shakir C, Gandhimathi R, Panikkar MVN (2009) Biopotentials of seaweeds collected from southwest coast of India. J Mar Sci Technol 17(1):67–73, http://jmst.ntou.edu.tw/marine/search2.php?keyin=17(1). Accessed 15 June 2014
Manilal A, Thajuddin N, Selvin J, Idhayadhulla A, Kumar RS, Sujith S (2011) In vitro mosquito larvicidal activity of marine algae against the human vectors, Culex quinquefasciatus (Say) and Aedes aegypti (Linnaeus) (Diptera: Culicidae). Int J of Zool Res 7(3):272–278
Margaret Beula J, Ravikumar S, Syed Ali M (2011) Mosquito larvicidal efficacy of seaweed extracts against dengue vector of Aedes aegypti. Asian Pac J Trop Biomed. 1(2)Supplement:S143–S146. doi:10.1016/S2221-1691(11)60143-3
Matasyoh JC, Dittrich B, Schueffler A, Laatsch H (2011) Larvicidal activity of metabolites from the endophytic Podospora sp. against the malaria vector Anopheles gambiae. Parasitol Res 108(3):561–566. doi:10.1007/s00436-010-2098-1
McLaughlin JL, Rogers LL, Anderson JE (1998) The use of biological assays to evaluate botanicals. Drug Inf J 32:513–524. doi:10.1177/009286159803200223. http://dij.sagepub.com/content/32/2.toc. Acessed 18 July 2014
Meyer BN, Ferrigni NR, Putnam JE, Jacobsen LB, Nichols DE, McLaughlin JL (1982) Brine shrimp: a convenient general bioassay for active plant constituents. Planta Med 45:31–34
Mnyone LL, Kirby MJ, Mpingwa MW, Lwetoijera DW, Knols BGJ, Takken W, Koenraadt CJM, Russell TL (2011) Infection of Anopheles gambiae mosquitoes with entomopathogenic fungi: effect of host age and blood-feeding status. Parasitol Res 108(2):317–322. doi:10.1007/s00436-010-2064-y
Nagayama K, Iwamura Y, Shibata T, Hirayama I, Nakamura T (2002) Bacterial activity of phlorotannins from the brown alga Ecklonia kurome. J Antimicrob Chemoth 50:889–893
Natarajan S, Pandian K, Pandima S, Kasi D (2009) Cholinesterase inhibitors from Sargassum and Gracilaria gracillis: seaweeds inhabiting south Indian coastal areas (Hare Island, Gulf of Mannar). J Nat Prod Res 23(4):355–369
Nishino T, Nagumo T (1987) Sugar constituents and blood-anticoagulant activities of fucose-containing sulfated polysaccharides in nine brown seaweed species. Nippon Nogeikagaku Kaishi 61:361–363
Oliveira PV, Ferreira JC Jr, Moura FS, Lima GS, de Oliveira FM, Oliveira PE, Conserva LM, Giulietti AM, Lemos RP (2010) Larvicidal activity of 94 extracts from ten plant species of northeastern of Brazil against Aedes aegypti L. (Diptera: Culicidae). Parasitol Res 107:403-407. doi:10.1007/s00436-010-1880-4
Paeporn P, Komalamisra N, Deesin V, Rongsriyam Y, Eshita Y, Thongrungkiat S (2003) Temephos resistance in two forms of Aedes aegypti and its significance for the resistance mechanism. Southeast Asian J Trop Med Public Health 34(4):786–792
Patil CD, Patil SV, Salunke BK, Salunkhe RB (2011) Bioefficacy of Plumbago zeylanica (Plumbaginaceae) and Cestrum nocturnum (Solanaceae) plant extracts against Aedes aegypti (Diptera: Culicide) and nontarget fish Poecilia reticulata. Parasitol Res 108(5):1253–1263. doi:10.1007/s00436-010-2174-6
Pitasawat B, Champakaew D, Choochote W, Jitpakdi A, Chaithong U (2007) Aromatic plant-derived essential oil: An alternative larvicide for mosquito control. Fitoterapia 78:205–210
Priestley CM, Williamson EM, Wafford KA, Sattelle DB (2003) Thymol, a constituent of thyme essential oil, is a positive allosteric modulator of human GABA(A) receptors and a homo-oligomeric GABA receptor from Drosophila melanogaster. Br J Pharmacol 140(8):1363–1372
Rademaker-Lakhai JM, Horenblas S, Meinhardt W, Stokvis E, Reijke TM, Jimeno JM, Lopez-Lazaro L, Lopez Martin JA, Beijnen JH, Schellens JHM (2005) Phase I clinical and pharmacokinetic study of Kahalalide F in patients with advanced androgen refractory prostate. Cancer Clin Cancer Res 11:1854–1862
Rao AV, Rao JG (2007) Carotenoids and human health. Pharmacol Res 55(3):207–216. doi:10.1016/j.phrs.2007.01.012
Rasmussen RS, Morrissey MT (2007) Marine biotechnology for production of food ingredients. Adv Food Nutr Res 52:237–292
Ratanatham S, Rojanasunan W, Upatham ES (1994) Morphological aberrations induced by methoprene, a juvenile hormone analogue, in Anopheles dirus s.s. and An. Sawadwongporni (Diptera: Culicidae). J Sci Soc Thailand 20:171–182
Ratanatham S, Dinh PX, Upatham ES, Sukhapanth N, Chitramvong Y (1996) Effects of diflubenzuron against the larval stages of Anopheles (cellia) dirus and Anopheles (cellia) maculatus (Diptera: Anophelinae). J Sci Soc Thailand 22:189–200
Rattan RS (2010) Mechanism of action of insecticidal secondary metabolites of plant origin. Crop Prot 29:913–920. doi:10.1016/j.cropro.2010.05.008
Rim HJ, Ha JH, Lee JS, Hyun I, Uh KB (1974) Pyrantel embonate in mass treatment of ascariasis and comparison with piperazine adipate and santonin-kainic acid complex. Korean J Parasitol 12(2):141–146
Roark RC (1947) Some promising insecticidal plants. Econ Bot 1:437–445
Ryu G, Hee S, Sook E, Wook B, Ryu S, Ho B (2003) Cholinesterase inhibitory activity of two farnesylacetone derivatives from the brown alga Sargassum sagamianum. J Arch Pharm Res 26(10):796–799
Sahayaraj K, Kalidas S (2011) Evaluation of nymphicidal and ovicidal effect of a seaweed, Padina pavonica (Linn.) (Phaeophyceae) on cotton pest, Dysdercus cingulatus (Fab.). Indian J Mar Sci 40(1):125–129, http://www.niscair.res.in/sciencecommunication/researchjournals/rejour/ijms/ijms2k11/ijms_feb11.asp. Accessed 15 June 2014
Samidurai K, Saravanakumar A (2011) Mosquitocidal properties of nereistoxin against Anopheles stephensi, Aedes aegypti and Culex quinquefasciatus (Diptera: Culicidae). Parasitol Res 109(4):1107–1112
San-Martin A, Negrete R, Rovirosa J (1991) Insecticide and acaricide activities of polyhalogenated monoterpenes from Chilean Plocamium cartilagineum. Phytochemistry 30(7):2165–2169
Schaeffer DJ, Krylov VS (2000) Anti-HIV activity of extracts and compounds from algae and cyano bacteria. Ecotoxicol Environ Saf 45:208–227
Schmutterer H (1990) Properties and potential of natural pesticides from the neem tree, Azadirachta indica. Annu Rev Entomol 35:197–271
Schnepf E, Crickmore N, Van Rie J, Lereclus D, Baum J, Feitelson J, Zeigler DR, Dean DH (1998) Bacillus thuringiensis and its pesticidal crystal proteins. Microbiol Mol Biol Rev 62(3):775–806
Selvin J, Lipton AP (2004) Biopotentials of Ulva fasciata and Hypnea musciformis collected from the peninsular coast of India. J Mar Sci Technol 12(1):1–6, http://jmst.ntou.edu.tw/marine/search2.php?keyin=12(1). Accessed 15 June 2014
Sen AK, Das AK, Banerji N, Siddhanta AK, Mody KH, Ramavat BK, Chauhan VD, Vedasiromoni JR, Ganguly DK (1994) A new sulfated polysaccharides with potent blood anti-coagulant activity from the new seaweed Grateloupia indica. Int J Biol Macromol 16:279–280
Senthil-Nathan S, Choi MY, Paik CH, Seo HY, Kalaivani K, Kim JD (2008) Effect of azadirachtin on acetylcholinesterase (AChE) activity and histology of the brown planthopper Nilaparvata lugens (Stål). Ecotoxicol Environ Saf 70:244–250
Service MW (1980) A guide to medical entomology Macmillan tropical and sub-tropical medical texts. Macmillan, London
Shaalan EAS, Canyon D, Younes MWF, Abdel-Wahab H, Mansour AH (2005) A review of botanical phytochemicals with mosquitocidal potential. Environ Int 31:1149–1166
Sharma OP (2011) Series on diversity of microbes and cryptogams Algae. McGraw Hill, New Delhi
Sharma P, Mohan L, Srivastava CN (2006) Impact analysis of neem kernel extracts on the development profile of Anopheles stephensi. J Asia-Pac Entomol 9(1):11–17. doi:10.1016/S1226-8615(08)60270-8
Smit AJ (2004) Medicinal and pharmaceutical uses of seaweed natural products: a review. J Appl Phycol 16(4):245–262. doi:10.1023/B:JAPH.0000047783.36600.ef
Sperk G, Lassmann H, Baran H, Kish SJ, Seitelberger F, Hornykiewicz O (1983) Kainic acid induced seizures: neurochemical and histopathological changes. Neurosci 10(4):1301–1315
Spieler R (2002) Seaweed compound’s anti-HIV efficacy will be tested in southern Africa. Lancet 359(9318):1675. doi:10.1016/S0140-6736(02)08605-1
Stengel DB, Connan S, Popper ZA (2011) Algal chemodiversity and bioactivity: sources of natural variability and implications for commercial application. Biotechnol Adv 29(5):483–501. doi:10.1016/j.biotechadv.2011.05.016
Subramonia Thangam T, Kathiresan K (1996) Marine plants for mosquito control. In: Wildey KB (ed) Proceedings of the second international conference on urban pests. Edinburgh, Scotland
Sukumar K, Perich MJ, Boobar LR (1991) Botanical derivatives in mosquito control: a review. J Am Mosq Control Assoc 7(2):210–217
Takahashi Y, Daitoh M, Suzuki M, Abe T, Masuda M (2002) Halogenated metabolites from the new Okinawan red alga Laurencia yonaguniensis. J Nat Prod 65:395–398
Terasaki M, Hirose A, Narayan B, Baba B, Kawagoe B, Yasui H, Saga H, Hosokawa M, Miyashita K (2009) Evaluation of recoverable functional lipid components of several brown seaweeds (Phaeophyta) from Japan with special reference to fucoxanthin and fucosterol contents. J Phycol 45(4):974–980. doi:10.1111/j.1529-8817.2009.00706
Thangam TS, Kathiresan K (1991a) Mosquito larvicidal activity of marine plant extracts with synthetic insecticides. Bot Mar 34:537–539
Thangam TS, Kathiresan K (1991b) Mosquito larvicidal effect of seaweed extracts. Bot Mar 34:433–435
Warikoo R, Kumar S (2013) Impact of Argemone mexicana extracts on the cidal, morphological, and behavioral response of dengue vector, Aedes aegypti L. (Diptera: Culicidae). Parasitol Res 112(10):3477–3484. doi:10.1007/s00436-013-3528-7
Watanabe K, Miyakado M, Ohno N, Okada A, Yanagi K, Moriguchi K (1989a) A polyhalogenated insecticidal monoterpene from the red alga, Plocamium telfairiae. Phytochemistry 28(1):77–78
Watanabe K, Umeda K, Miyakado M (1989b) Isolation and identification of three insecticidal principles from the red alga, Laurencia nipponica. Agric Biol Chem 53:2513–2515
Watanabe K, Umeda K, Kurita Y, Takayama C, Miyakado M (1990) Two insecticidal monoterpenes, telfairine and aplysiaterpenoid A, from the red alga Plocamium telfairiae: Structure elucidation, biological activity, and molecular topographical consideration by a semiempirical molecular orbital study. Pestic Biochem Physiol 37(3):275–286
Wong K, Cheung PC (2001) Influence of drying treatment on three Sargassum species. J Appli Phyco 13(1):43–50
Wong CL, Phang SM (2004) Biomass production of two Sargassum species at Cape Rachado. Hydrobiologia 512:79–88
World Health Organization (1996) Evaluation on the testing of insecticides—report of the WHO informal consultation, 7–11 October 1996, Geneva. Ref: CTD/WHOPES/IC/96.1. http://www.who.int/whopes/resources/resources_1996/en/. Accessed 11 May 2014
World Health Organization (1998) Test procedures for insecticide resistance monitoring in malaria vectors, bioefficacy and persistence of insecticides on treated surfaces. Ref: WHO/CDS/CPC/MAL/98.12. http://www.who.int/whopes/resistance/en/. Accessed 18 July 2014
World Health Organization (2005) Guidelines for laboratory and field testing of mosquito larvicides. Ref: WHO/CDS/WHOPES/GCDPP/2005.13. http://www.who.int/whopes/guidelines/en/. Accessed 14 Jun 2014
World Health Organization (2011a) Generic risk assessment model for insecticides used for larviciding. Ref: WHO/HTM/NTD/WHOPES/2010.4.Rev1. http://www.who.int/whopes/guidelines/en/. Accessed 14 Jun 2014
World Health Organization (2011b) Generic risk assessment model for indoor residual spraying of insecticides. Ref: WHO/HTM/NTD/WHOPES/2010.5.Rev1. http://www.who.int/whopes/guidelines/en/. Accessed 14 Jun 2014
World Health Organization (2013) Dengue and severe dengue. http://www.who.int/mediacentre/factsheets/fs117/en/. Accessed 2 February 2014
Xu N, Fan X, Yan X, Li X, Niu R, Tseng CK (2003) Antibacterial bromophenols from the marine red alga Rhodomela confervoides. Phytochemistry 62(8):1221–1224
Zhou GF, Sun Y, Xin H, Zhang Y, Li Z, Xu Z (2005) In vivo antitumor and immunomodulation activities of different molecular weight lambda-carrageenans from Chondrus ocellatus. Pharmacol Res 50:47–53
Zoubiri S, Baaliouamer A (2011) Potentiality of plants as source of insecticide principles. J Saudi Chem Soc. doi:10.1016/j.jscs.2011.11.015
Zubia M, Payri C, Deslandes E (2008) Alginate, mannitol, phenolic compounds and biological activities of two range-extending brown algae, Sargassum mangarevense and Turbinaria ornata (Phaeophyta: Fucales), from Tahiti (French Polynesia). J Appl Phycol 20(6):1033–1043
Acknowledgments
Financial assistance from Universiti Kebangsaan Malaysia postgraduate fund is gratefully acknowledged. The authors thank Prof. Simon Gibbons from University College London for his input to the manuscript and Miss Chiong Kai Shing from Universiti Malaya for proofreading the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yu, KX., Jantan, I., Ahmad, R. et al. The major bioactive components of seaweeds and their mosquitocidal potential. Parasitol Res 113, 3121–3141 (2014). https://doi.org/10.1007/s00436-014-4068-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00436-014-4068-5