Abstract
This study deals with two range-extending brown algae from Tahitian coral reefs, Sargassum mangarevense and Turbinaria ornata; their alginate properties, mannitol and phenolic contents, antioxidant and antimicrobial activities were determined. Turbinaria ornata showed the richest alginate content with the highest extraction yield (19.2 ± 1.3% dw). Their alginates also exhibited the highest viscosity (50 ± 18 mPa.s), but the M:G ratios (mannuronic acid to glucuronic acid) of alginates (1.25–1.42) were similar in both species. Alginate yield displayed spatial variations, but no significant seasonal changes. The highest mannitol content was found in S. mangarevense (12.2 ± 2.1% dw) during the austral winter. With respect to other tropical Fucales, both algae exhibited also a high phenolic content (2.45–2.85% dw) with significant spatio-temporal variations. Furthermore, high antioxidant activity and activity against Staphylococcus aureus were also detected in extracts. According to these preliminary results, these two range-extending algae are of key interest in numerous industrial areas.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Over the past few decades, French Polynesian reefs have experienced a large algal bloom by two members of the Fucales, Sargassum mangarevense (Grunow) Setchell and Turbinaria ornata (Turner) J. Agardh, accompanied with reef degradation (Stiger and Payri 1999a, b). This proliferation is a threat to the equilibrium of the coral reef ecosystem while leading to a spatial extension of the populations in the Tuamotu Archipelago via a long-range dispersal strategy (Payri and Stiger 2001). In this context, it would be worthwhile to make use of this biological material for industrial applications in order to sustain the control of these two species while contributing to the conservation of the reef ecosystem. Within the Tahitian coral reef ecosystem, the mass of attached algae was estimated by a remote-sensing tool (IKONOS satellite imagery) at 739.269 ± 337.462 tons of dry matter (Arue, Punaauia and Paea) (Andréfouët et al. 2004). Moreover, biochemical analyses have confirmed these algae to be of suitable quality for industrial uses (Zubia et al. 2003) with respect to their mineral salts content (notably potassium, nitrogen, calcium and iron), soluble fibres and proteins contents, and their numerous polyunsaturated fatty acids.
In order to collect additional data to further supplement the prospective study about possible industrial uses of these algae, it seemed relevant to conduct a detailed analysis of some compounds of specific interest. Indeed, amongst the remarkable diversity of natural compounds of brown algae, polysaccharides (e.g., alginate and fucoidan), mannitol and phlorotannins have proved to have great properties for industrial uses (Kornprobst 2005). Alginate is the major structural component of the brown algal cell wall, and mainly consists of β-D-mannuronic acid and α-L-guluronic acid units. In a wide range of industrial applications, alginates are essential compounds as thickening, gelling or stabilizing agents (McHugh 1987; Perez et al. 1992). In temperate areas, they are mainly extracted from the brown seaweeds Macrocystis pyrifera, Ascophyllum nodosum and Laminaria spp., whereas in tropical regions (China, Philippines, India, and Vietnam), Sargassum, Turbinaria, and Padina are the major sources (Critchley and Ohno 1998). Mannitol is a sugar alcohol produced by photosynthesis and is universally found in brown algae and can account for 20–30% dw in some Laminaria species (Kornprobst 2005). Mannitol exhibits hydrating and antioxidant properties used in numerous cosmetic and pharmaceutical applications (Iwamoto and Shiraiwa 2005). Though the mannitol produced by chemical synthesis is cheaper than the natural one extracted from seaweeds, it is worth using the latter because of the preference exhibited by consumers for natural cosmetic products. Other bioactive molecules are phenolic compounds: these secondary metabolites are found, mainly as phlorotannins, at high levels in Fucales (20–30% dw) (Ragan and Glombitza 1986). These molecules are assumed to function as chemical defenses against grazers, pathogens and epiphytes (see Amsler and Fairhead 2006 for review), and are also involved in mechanisms of photoprotection against solar radiation, especially UV radiation (Pavia et al. 1997). They have a wide range of biological activities (antimicrobial, antioxidant, antitumoral, antiviral; Lacaille-Dubois and Wagner 1996) of high interest for applications in pharmaceutical and cosmetic processes. These considerations explain why the screening of the bioactivity of seaweed extracts is paramount. The genera Sargassum and Turbinaria are well-known for their biological activities (see Zubia 2003 for review), and chemical defenses are supposed to increase in species from coral reef ecosystems where biodiversity, grazing and competition for space are enhanced (Hay 1996). Moreover, tropical macroalgae exhibit high radical scavenging activities leading to an effective antioxidant defense system (Zubia et al. 2007). Thus, S. mangarevense and T. ornata from Tahiti (French Polynesia) could be a valuable source of secondary metabolites. However, previous biochemical studies on these seaweeds have dealt only with phenolic contents (Stiger et al. 2004) and antitumor activities (Deslandes et al. 2000).
Together, these considerations led us to pursue these investigations by focusing the present study on an assessment of alginate properties, mannitol and phenolic contents, and biological activities (antioxidant and antimicrobial). Spatio-temporal fluctuations of some parameters were also explored in order to optimise the harvest of these species for future industrial applications.
Materials and methods
Sargassum mangarevense and Turbinaria ornata were collected by snorkeling at two sites, Arue (149°32′W, 17°30′S) and Punaauia (149°37′W, 17°35′S), both located on the northwest inner barrier reef of Tahiti (French Polynesia). These sites were selected because of their different environmental conditions. In order to detect eventual seasonal changes, sampling was conducted during the hot and wet season in February 2000 and during the cool and dry season in July 2000. For each season, and for each site, 30 attached individuals of each species were randomly sampled from well-developed Sargassum and Turbinaria patches. Once back in the laboratory, the algae were sorted out to remove epiphytes and epifauna and washed with demineralized water to remove sand and other detritus. They were then mixed and blended according to site, season and species. For each batch, three or four subsamples were selected randomly for each analysis. The subsamples were sun-dried for alginates, oven-dried at 60°C for 48 h for mannitol, and frozen at −20°C prior to extraction for phenolic content and antioxidant activity analysis. Additional material was collected for antimicrobial activity in January 2002, from the reef front of Faa’a barrier reef (149°36′W, 17°33′S). Twenty fixed individuals for each species were sampled, washed with demineralized water and immediately extracted.
Alginates
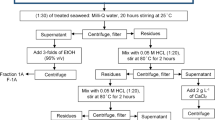
The extraction protocol of Perez et al. (1992) was followed; 10 g of sun-dried alga was stirred for 12 h in 500 mL of 1% formaldehyde, then washed with deionized water prior to acidification (H2SO4 0.2 N, 4 h, 25°C). The algal sample was then washed a second time with deionised water prior to alginates extraction by stirring in 500 mL of 1% Na2CO3 for 12 h at 25°C. After filtration, the alginates were precipitated in ethanol 95% (1:2 v/v) as sodium salt. The precipitate was washed first with absolute ethanol then with acetone, dried for 24 h at 40°C and milled before storage. Alginate yield was expressed as a percentage of dry weight (% dw).
For viscosity analysis, 3 g of sodium alginate were dissolved in 397 ml of distilled water prior to the addition of 0.72 g of sodium carbonate, and stirred to obtain 1% sodium alginate in a uniform gel structure. The viscosity of the solution was measured in triplicate at 20°C with a Brookfield Model LVF apparatus and expressed in mPas.s.
Alginates are composed of two uronic acids: the mannuronic acid (M) and the guluronic acid (G) present as blocks of homopolymeric (M-blocks or G-blocks) and heteropolymeric (MG-blocks) sequences. The mannuronic acid-to-guluronic acid ratio (M:G ratio) gives information about the formed gel (Perez et al. 1992). The uronic acids from alginates were determined by 1H- and 13C-NMR spectrometry (Brucker AM400 spectrometer) at 25.05 MHz according to the method by Tako et al. (2001). Ten mg of alginates were dissolved in 4 mL of D2O, and data were recorded at 80°C. Chemical shifts were expressed in parts per million (ppm) relative to tetramethylsilane as internal reference.
Mannitol
Mannitol was measured according to Cameron et al. (1948) on each subsamples from the Arue site only. The complete oxidation of mannitol was by an excess of 0.1 N periodic acid. The amount of periodic acid used was then determined against a blank by titration with 0.1 N sodium thiosulfate after addition of potassium iodate (2 g) and 4 N sulfuric acid, and expressed as a percentage of dry weight (% dw).
Phenolic content and antioxidant activity
For each subsample, one extraction was performed from 10 g of fresh algae mixed with methanol/demineralized water (50/50, v/v) at 40°C for 3 h in the dark with stirring. The extracts were then filtered, concentrated to a final volume of 10 ml by evaporation under vacuum at 40°C and stored at −20°C.
The Folin-Ciocalteu method described in Zubia (2003) was used to determine, by spectrophotometry at 700 nm, the total content of phenolic compounds in algal extracts. Each extract was measured in triplicate against a phloroglucinol standard curve, and phenolic content expressed as % dw.
Radical scavenging/antioxidant activities of the different extracts were assessed using the DPPH (2,2-diphenyl-1-picrylhydrasyl) free-radical method (Blois 1958). Aliquots of 300 μL of extracts were added to 3 ml of DPPH solution [0.0141 g in 100 mL methanol/water mixture (90/10)]. After standing for 60 min at room temperature, absorbance was read at 517 nm against demineralised water as blank. Each extract was measured in triplicate and absorbance was then transformed into a percentage of inhibition.
Antimicrobial activity
Extracts were prepared by stirring 100 g of fresh algae with 300 mL solvent (demineralized water or ethanol 50%) for 24 h at 40°C in the dark. After filtration, the alcoholic extracts were concentrated under reduced pressure at 40°C, re-suspended in 25 mL of distilled water and lyophilized for storage. The aqueous extracts were directly lyophilized after filtration.
Antimicrobial activity testing of the extracts was performed in agar-plated Petri dishes by the disc diffusion technique (Micromer, Brest, France). Each extract was tested for five common pathogenic microorganisms: one Gram-positive bacterium (Staphylococcus aureus), two Gram-negative bacteria (Escherischia coli and Pseudomonas aeruginosa), one yeast (Candida albicans) and one mould (Aspergillus niger). The Petri dishes were incubated at 30°C for 72 h for bacteria and 25°C for 96 h for fungi. The activity was then estimated by measuring the diameter (in mm) of the inhibition zones around the discs. There were three replicates for each assay and for the control (no extracts).
Statistical analysis
All statistical analyses were performed using Statistica 5.1. The data were tested for normality (Shapiro–Wilk test), and the homogeneity of variances groups was verified (Bartlett or Levene tests) at the 0.05 significance level. To satisfy the criteria of normality and homoscedasticity for parametric tests, some data were arcsine transformed (Underwood 1999). The differences between 2 independent groups were assessed with the Student t-test. Moreover, different sources of variations, i.e. species, site and season, were tested on different variables (alginate yield, phenolic content and antioxidant activity) with three-way analysis of variance (ANOVA) with fixed and crossed factors. Mannitol content was tested with two-way factorial ANOVA (factors: season and specie). Post-hoc tests (Tukey HSD or SNK) were performed when data showed significant differences (p < 0.05).
Results
Alginate
Table 1 gives the mean of alginate properties (yield, viscosity and M:G ratios) of Sargassum mangarevense and Turbinaria ornata. Yield and viscosity are statistically higher in T. ornata than in S. mangarevense (p < 0.0000 and p = 0.0103 respectively). M:G ratios are alike in both species (p = 0.3052). The high standard deviations for yield and viscosity data are indicative of the great variability of alginate properties. Alginate yield and viscosity vary in time and space (Fig. 1) depending on species. For both parameters, higher values were observed in T. ornata from the Arue samples (Table 2; p = 0.0472). However, no significant seasonal variation of alginate yield was observed (Table 2; p = 0.0592) and the significant interaction observed between season and species factors (Table 2, p = 0.0010) indicated that seasonal variations are species-dependent. Though the reduced number of analysed samples prevented us from statistically testing viscosity values, they tended to be more elevated during the austral winter and at Arue for every sample tested.
Alginate yield (% dw, histogram) and viscosity (mPa.s, curve) of Turbinaria ornata and Sargassum mangarevense collected at Arue and Punaauia in austral summer and winter. Bars represent the standard deviation. The means of alginate yield and number of analysed subsamples (in brackets). Viscosity data correspond to the analysis in triplicate of a single subsample of alginate
Mannitol
Mannitol contents and seasonal variations are given respectively in Table 3 and Fig. 2. Mannitol levels were statistically higher in S. mangarevense than in T. ornata (12.2 ± 2.1% dw compared to 5.9 ± 1.5% dw, p < 0.0000) and exhibited significant seasonal variations (p = 0.0006) with elevated concentrations in the austral winter for both species, but with a more marked variation in T. ornata (interaction season x species, p = 0.0252).
Phenolic content
The phenolic contents are quite similar (Table 2; p = 0.1175) in both species S. mangarevense and T. ornata (2.85 ± 1.12% dw and 2.45 ± 0.61% dw, respectively), but show spatio-temporal variations (Fig. 3). They are significantly higher over the austral winter than in the austral summer (p < 0.0000) and at Arue site (p = 0.0084) (Table 2). The significant interaction between the three factors (season x site x species) (p = 0.0214) likely results from the high intra-variability of phenolic content (Fig. 3).
Antioxidant activity
The antioxidant activities of S. mangarevense (84 ± 2% dw) and T. ornata (83 ± 4% dw) are similar (Table 2; p = 0.5870). Moreover, the samples exhibited great intra-sample-variability despite the lack of significant spatio-temporal variation (Fig. 3).
Antimicrobial activity
The results of the antimicrobial screening of the extracts are shown in Table 4. Sargassum mangarevense and T. ornata both displayed an antimicrobial activity only against the Gram-positive bacterium, Staphylococcus aureus, and the aqueous extract of S. mangarevense showed the smallest inhibition.
Discussion
Alginates
In our study, alginate yields in Polynesian seaweeds were in the range of 6.0 to 21.1% dw, with the highest values shown in Turbinaria ornata. These ratios are comparatively lower than those from other alginophytes used in the industry (13–38% dw; Perez et al. 1992). Sargassum mangarevense showed a lower alginate yield than T. ornata (6.0–12.4% dw against 16.8–21.1% dw). Despite the great intra-species variability in part regulated by abiotic and biotic factors, i.e., age, parts of the thalli, physiological stage, season, tide, depth (Aponte de Otaola et al. 1983), and by the diversity among extraction procedures (Chou and Chiang 1977; Davis et al. 2004), it is worth noting that S. mangarevense values are usually below the levels reported in the literature for the genus Sargassum, while those of T. ornata agree with the available data (Table 1). Seasonal variations in alginate yields are correlated with the physiological stage of seaweeds. Indeed, in most Sargassum species (Umamaheswara Rao 1969; Chennubhotla et al. 1982; Aponte de Otaola et al. 1983; Thomas and Subbaramaiah 1991; Ragaza and Hurtado 1999; Saraswathi et al. 2003) and Turbinaria species (Umamaheswara Rao 1969; Kaliaperumal and Kalimuthu 1976) the highest and lowest yields have been recorded during the growth period and over the reproduction stage, respectively. In the present study, no statistical evidence of seasonal variations was observed, in contrast to the spatial ones. Polynesian seaweeds displayed the highest alginate yield in Arue probably due to specific environmental parameters (i.e., temperature, hydrodynamism). In fact, alginate yields in Sargassum and T. ornata are known to be affected by environmental factors (Aponte de Otaola et al. 1983; Kaliaperumal et al. 1989; Ragaza and Hurtado 1999) in relation with the biological functions of alginic acid (e.g., prevention of dessication, flexibility) (Aponte de Otaola et al. 1983). It would be worthwhile to conduct monthly measurements of alginate yield in Arue reef to gain more insight into alginate variations in Polynesian seaweeds in order to optimize their harvest.
The development of any alginophyte industry goes through an assessment of alginate quality. For commercial and scientific purposes, the key property of alginates is their ability to form viscous solutions in water. According to the criteria used in the industry (Perez et al. 1992), the viscosities of Polynesian seaweed alginates are low (9–78 mPa.s), which is in agreement with the frequent observations of low viscosity in alginates obtained from tropical or subtropical species compared to cold water species (McHugh 1987). In this study, T. ornata exhibited a higher viscosity than S. mangarevense with a mean value agreeing with literature data (Table 1). Such a comparison is difficult for the genus Sargassum because of the great variability among the numerous alginate viscosity values available in the literature (Table 1), which are dependent upon various factors (e.g., parts of the thalli, physiologic stage, season, storage) (Tewari et al. 1983; Thomas and Subbaramaiah 1991; Ragaza and Hurtado 1999; Saraswathi et al. 2003; Jothisaraswathi et al. 2006) and also upon extraction protocols (Chou and Chiang 1977). In Tahiti, austral winter and the Arue site could be the best season and location for harvesting large brown algae as their alginates are of the highest quality.
Whereas viscosity determines the quality of alginates, their M:G ratio gives insight into the nature of a gel formed with divalent cations. In both species studied, M:G ratios were quite high (1.25–1.42) in comparison with the literature data (Table 1). However, those from common alginophytes are frequently higher, i.e. 2–3.6 (Minghou et al. 1984; McHugh 1987; Perez et al. 1992). These observations favor the use of Polynesian seaweed alginates for the manufacture of strong gels as reported previously for Sargassum and Turbinaria (Minghou et al. 1984; McHugh 1987; Larsen et al. 2003; Davis et al. 2004; Jothisaraswathi et al. 2006). However, further analyses are required to confirm these preliminary results, especially as M:G ratios are known to depend strongly on factors such as extraction procedures (Davis et al. 2004), age (Omar et al. 1988; Honya et al. 1993) and seasonality (Minghou et al. 1984; Jothisaraswathi et al. 2006).
Mannitol
Mannitol content ranged from 3.90 to 14.42% dw, with S. mangarevense displaying a higher mannitol content than T. ornata, and that of other Sargassum species recorded in the literature (Table 3) with the exception of S. pteropleuron (Prince and Daly 1981). Values for T. ornata are in agreement with the highest values reported in the literature for that genus (Table 3). However, a great variability of mannitol level was observed within the same genus (1.4–8.7% dw for Turbinaria and 1–34% dw for Sargassum; Table 3) depending on season, site and species (Umamaheswara Rao 1969; Umamaheswara Rao and Kalimuthu 1972; Joshi and Gowda 1975; Kaliaperumal and Kalimuthu 1976; Mehta and Parekh 1978; Kalimuthu 1980; Prince and Daly 1981; Kaliaperumal et al.1989; Thomas and Subbaramaiah 1991), and also the thallus parts examined (Thomas and Subbaramaiah 1991). A close examination of the seasonal variations recorded in our study indicated that, in both species, the growth stage (during austral winter) corresponds to the highest mannitol concentration. This observation agrees with previous reports on Sargassum and Turbinaria (Umamaheswara Rao and Kalimuthu 1972; Kaliaperumal and Kalimuthu 1976; Prince and Daly 1981; Thomas and Subbaramaiah 1991). It is worth recalling that in brown seaweeds, where mannitol is the primary product from photosynthesis, changes in mannitol concentrations are indicative of variations in photosynthetic activities (Prince and Daly 1981; Thomas and Subbaramaiah 1991).
Phenolic content
In our study, the content of phenolic compounds was higher contents than previously reported in tropical and subtropical species of Sargassum and Turbinaria (Steinberg 1986; Zubia 2003; Stiger et al. 2004), and agrees with the results of Targett et al. (1992). The results conflict with the previous assumption that temperate brown Fucales would generally contain more phenolic compounds (>2% dw) than tropical representatives (see: Steinberg 1986; Van Alstyne and Paul 1990; Pereira and Yoneshigue-Valentin 1999; for the most cited). The higher phenolic contents in S. mangarevense with respect to T. ornata were also reported by Steinberg (1986) working on Tahitian Fucales. He explained interspecific variations by assuming that the very tough nature of species such as Turbinaria puts them less at risk of attacks by herbivores and could explain the reduced production of chemical defenses. Conversely, Stiger et al. (2004), working on the same species, have shown a lower phenolic content in S. mangarevense which suggests that further studies are needed for a better understanding of the significace of the variation in polyphenolic contents.
The great variability observed in the phenolic contents oberved could originate in external environmental factors such as herbivory, light, depth, salinity, nutrients, seasonality as well as intrinsic ones such as age, length, type of the tissues. All these factors could act on the spatio-temporal regulation of the phenolic metabolic expression inducing marked qualitative and quantitative variations among individuals at a very small scale, together with intra-individual variations (see Amsler and Fairhead 2006 for review). Despite the numerous studies dedicated to seasonal variation of phenolic content in brown algae, no consensus has been found because of the lack of a clear trend. The complexity of seasonal variations suggests a stronger correlation between phenolic contents and local environmental factors, e.g., grazing intensity in different areas of the coral reef, than with larger scale factors, i.e., months, seasons, and latitudinal gradients.
Antioxidant activity
In accord with previous studies on the genera Sargassum (Matsukawa et al. 1997; Yan et al. 1998; Kim et al. 2005; Nakai et al. 2006; Zhang et al. 2007; Zubia et al. 2007) and Turbinaria (Matsukawa et al. 1997; Santoso et al. 2004; Zubia et al. 2007), extracts from Polynesian S. mangarevense and T. ornata displayed a great antioxidant activity whatever the site and the season. Positive correlations between phenolic contents and antioxidant activities have often been reported in Sargassum species (see: Kang et al. 2003; Kim et al. 2005; Connan et al. 2006; Nakai et al. 2006; Zhang et al. 2007; for the most cited). Phenolic compounds are assumed to protect algal thalli from photodestruction by UV radiation (Pavia et al. 1997) and to exhibit free-radical scavenging properties (Nakai et al. 2006). However, macroalgae are known to contain many other antioxidant compounds such as tocopherols, ascorbic acid, carotenoids, phospholipids, chlorophyll-related compounds, mycosporines-like amino acids or polysaccharides. Among those isolated from Sargassum species, it is worth mentioning meroterpenoids from S. siliquastrum (Jang et al. 2005), plastoquinones from S. micracanthum (Iwashima et al. 2005) and some aromatic compounds from S. thunbergii (Seo et al. 2004). It is likely that the poor sensitivity of the method in use in this study prevented us from detecting spatio-temporal variations. However, antioxidant activities of seaweeds are likely subject to a huge variability, including at very small scales as in the study of Connan et al. (2006), in which intra-thallus variations in antioxidant activity were described for Ascophyllum nodosum. Abiotic stresses could induce changes in the antioxidant defenses of macroalgae since the antioxidative capacity of the cell made up the major part of the general stress tolerance (Collen and Davison 1999).
Antimicrobial activity
Aqueous and ethanolic extracts of S. mangarevense and T. ornata showed antibacterial activity against the Gram-positive Staphylococcus aureus as expected from previous studies on Sargassum and Turbinaria species (Table 5), in which seaweed extracts are preferentially active against S. aureus. The screening of seaweeds for antimicrobial activity is an issue frequently addressed in the literature. Extracts have usually been found to be more effective against Gram-positive bacteria than against Gram-negative ones known to have a more complex cell wall structure (Reichelt and Borowitzka 1984; Ballantine et al. 1987; Sreenivasa Rao et al. 1988; Kubo et al. 1992). However, according to other studies, Sargassum extracts have shown antimicrobial activities against E. coli, P. aeruginosa, C. albicans and A. niger, and T. ornata extracts against E. coli (Table 5). The low antibacterial and antifungal activities observed in this study may result from the low concentration in specific metabolites of the crude extracts studied. Indeed, whenever studies have shown some activity against Gram-negative bacteria, the experiments had been mainly carried out on fractionated extracts of Sargassum and Turbinaria (Sreenivasa Rao et al. 1988; Sreenivasa Rao 1989, 1990; Sastry and Rao 1994, 1995; Anggadiredja et al. 1996). In crude extracts, inhibitory or antagonist compounds could mask other antimicrobial activities (Sreenivasa Rao et al. 1988; Robles-Centeno et al. 1996; Kumar and Rengasamy 2000). Several antimicrobial compounds of different chemical natures have so far been isolated from Sargassum and Turbinaria: dioctyl phthalate from S. wightii (Sastry and Rao 1995), triterpenes from S. crassifolium and T. ornata (Anggediredja et al. 1996), crinitol from S. tortile (Kubo et al. 1992) and sulphoglycerolipid from S. wightii (Arunkumar et al. 2005). Extraction by water and ethanol in our study favors polar and apolar antibacterial compounds. The antimicrobial activity of compounds extracted from seaweeds is subject to a great variability in relation to season, physiological stage and environmental conditions (Ballantine et al. 1987; Robles-Centeno et al. 1996; Padmakumar and Ayyakkannu 1997; Kumar and Rengasamy 2000). Hence, the antimicrobial activity could be restricted to one or two months in the year as Robles-Centeno et al. (1996) have demonstrated for some Rhodophyta species. This consideration emphasizes the importance for further screening programmes to perform a stronger sampling effort.
Conclusions
Our study indicates that T. ornata would be a good source of raw materials for the preparation of G-rich alginates while S. mangarevense would be the target species for use in cosmetic products because of its high mannitol and phenolic contents. Moreover, the harvest of Polynesian seaweeds should take place preferably during the austral winter and at the Arue site. Furthermore, the high phenolic levels, coupled with antioxidant and antibacterial activities, leads us to consider both species as valuable sources of bioactive compounds. Future research effort should be targeted at the isolation of new bioactive natural compounds useful as natural antioxidant, pharmaceutical agents or dietary supplements.
References
Amsler CD, Fairhead VA (2006) Defensive and sensory chemical ecology of brown algae. Adv Bot Res 43:1–91
Andréfouët S, Zubia M, Payri CE (2004) Mapping and biomass estimation of the invasive brown algae Turbinaria ornata (Turner) J. Agardh and Sargassum mangarevense (Grunow) Setchell on heterogeneous Tahitian coral reefs using 4-meter resolution Ikonos satellite data. Coral Reefs 23:26–38
Anggadiredja J, Hasanudin Sadiq SA, Pratomo S, Rudyansyah A (1996) Screening of marine algae from Warambadi seashore of Sumba Island of Indonesia for antibacterial activity. Phytomed 3 (Suppl. 1):37
Aponte de Otaola NE, Diaz-Piferrer M, Graham HD (1983) Seasonal variations and anatomical distribution of alginic acid in Sargassum spp. found along the coasts of Puerto Rico. J Agric Puerto Rico 67:464–475
Arunkumar K, Selvapalam N, Rengasamy R (2005) The antibacterial compound sulphoglycerolipid 1-0 palmitoyl-3-0(6′-sulpho-alpha-quinovopyranosyl)-glycerol from Sargassum wightii Greville (Phaeophyceae). Bot Mar 48:441–445
Ballantine DL, Gerwick WH, Velez SM, Alexander E, Guevara P (1987) Antibiotic activity of lipid-soluble extracts from Caribbean marine algae. Hydrobiologia 151/152:463–469
Behairy AKA, El-Sayed MM (1983) Biochemical composition of some marine brown algae from Jeddah Coast, Saudi Arabia. Ind J Mar Sci 12:200–201
Blois MS (1958) Antioxidant determinations by the use of a stable free radical. Nature 181:1199–1200
Cameron MC, Ross AG, Percival EGV (1948) Methods for the routine estimation of mannitol, alginic acid and combined fucose in seaweeds. J Soc Chem Ind 67:161–164
Chennubhotla VSK, Kaliaperumal N, Kalimuthu S, Selvaraj M, Ramalingam JR, Najmuddin M (1982) Seasonal changes in growth and alginic acid and mannitol contents in Sargassum ilicifolium (Turner) J. Agardh and S. myriocystum J. Agardh. Ind J Mar Sci 11:195–196
Chou HN, Chiang YM (1976) Studies on algin from brown algae of Taiwan. I. Estimation of yield and quality of algin. Acta Oceanogr Taiwanica 6:135–139
Chou HN, Chiang YM (1977) Studies on algin from brown algae of Taiwan. II. Conditions for the extraction of algin from Sargassum cristaefolium C. Agardh. Acta Oceanogr Taiwanica 7:193–199
Collen J, Davison IR (1999) Reactive oxygen metabolism in intertidal Fucus spp. (Phaeophyceae). J Phycol 35:54–61
Connan S, Delisle F, Deslandes E, Ar Gall E (2006) Intra-thallus phlorotannin content and antioxidant activity in Phaeophyceae of temperate waters. Bot Mar 49:34–46
Critchley AT, Ohno M (eds) (1998) Seaweed resources of the world. Japan International Agency, Yokosuka
Davis TA, Ramirez M, Mucci A, Larsen B (2004) Extraction, isolation and cadmium binding of alginate from Sargassum spp. J Appl Phycol 16:275–284
Deslandes E, Pondaven P, Auperin T, Roussakis C, Guézennec J, Stiger V, Payri C (2000) Preliminary study of the in vitro antiproliferative effect of a hydroethanolic extract from the subtropical seaweed Turbinaria ornata (Turner J. Agardh) on a human non-small-cell bronchopulmonary carcinoma line (NSCLC-N6). J Appl Phycol 12:257–262
Febles CI, Arias A, Hardisson A, Sierra Lopez A (1995) Antimicrobial activity of extracts from some Canary species of Phaeophyta and Chlorophyta. Phyto Res 9:385–387
Hay ME (1996) Marine chemical ecology: what’s known and what’s next? J Exp Mar Biol Ecol 200:103–134
Honya M, Kinoshita T, Ishikawa M, Nori H, Nisizawa K (1993) Monthly determination of alginate, M/G ratio, mannitol and minerals in cultivated Laminaria japonica. Bull Jap Soc Sci Fish 59:295–299
Itoh K, Hori K (1989) Seaweed: chemical composition and potential food uses. Food Rev Int 5:101–144
Iwamoto K, Shiraiwa Y (2005) Salt-regulated mannitol metabolism in algae. Mar Biotech 7:407–415
Iwashima M, Mori J, Ting X, Matsunaga T, Hayashi K, Shinoda D, Saito H, Sankawa U, Hayashi T (2005) Antioxidant and antiviral activities of plastoquinones from the brown alga Sargassum micracanthum, and a new chromene derivative converted from the plastoquinones. Biol Pharm Bull 28:374–377
Jang KH, Lee BH, Choi BW, Lee HS, Shin J (2005) Chromenes from the brown alga Sargassum siliquastrum. J Nat Prod 68:716–723
Jing M, Wei T (1984) Screening for antimicrobial activities in marine algae from the Qingdao coast, China. Hydrobiologia 116/117:517–520
Joshi GV, Gowda CA (1975) Seasonal variations in chemical composition of Sargassum ilicifolium Grun. and seawater. Ind J Mar Sci 4:165–168
Jothisaraswathi S, Babu B, Rengasamy R (2006) Seasonal studies on alginate and its composition II: Turbinaria conoides (J.Ag.) Kütz. (Fucales, Phaeophyceae). J Appl Phycol 18:161–166
Kaliaperumal N, Kalimuthu S (1976) Changes in growth, alginic acid and mannitol contents of Turbinaria decurrens Bory. Bot Mar 19:157–159
Kaliaperumal N, Kalimuthu S, Ramalingam JR (1989) Agar, algin and mannitol from seaweeds of Lakshadweep. J Mar Biol Assoc Ind 31:303–305
Kalimuthu S (1980) Variations in growth and mannitol and alginic acid contents of Sargassum myriocystum J. Agardh. Ind J Fish 27:265–266
Kang K, Park Y, Hwang HJ, Kim SH, Lee JG, Shin HC (2003) Antioxidative properties of brown algae polyphenolics and their perspectives as chemopreventive agents against vascular risk factors. Arch Pharm Res 26:286–293
Kim SJ, Woo S, Yun H, Yum S, Choi E, Do JR, Jo JH, Kim D, Lee S, Lee TK (2005) Total phenolic contents and biological activities of Korean seaweed extracts. Food Sci Biotechnol 14:798–802
Kornprobst JM (2005) Substances naturelles d’origine marine. Chimiodiversité-Pharmacodiversité-Biotechnologie. 1. Généralités-Micro-organismes-Algues. Lavoisier, Paris
Kubo I, Himejima M, Tsujimoto K, Murci H (1992) Antibacterial activity of crinitol and its potentiation. J Nat Prod 55:780–785
Kumar KA, Rengasamy R (2000) Antibacterial activities of seaweed extracts/fractions obtained through a TLC profile against the phytopathogenic bacterium Xanthomonas oryzae pv. oryzae. Bot Mar 43:417–421
Lacaille-Dubois MA, Wagner H (1996) Importance pharmacologique des dérivés polyphénoliques. Acta Bot Gallic 143:555–562
Larsen B, Salem DMSA, Sallam MAE, Mishrikey MM, Beltagy AI (2003) Characterization of the alginates from algae harvested at the Egyptian Red Sea coast. Carbohydr Res 338:2325–2336
Martinez-Lozano SJ, Garcia S, Heredia N, Villareal-Rivera L, Garcia-Pailla CA (2000) Antifungal activity of extract of Sargassum filipendula. Phyton 66:179–182
Matsukawa R, Dubinsky Z, Kishimoto E, Masaki K, Masuda Y, Takeuchi T, Chihara M, Yamamoto Y, Niki E, Karube I (1997) A comparison of screening methods for antioxidant activity in seaweeds. J Appl Phycol 9:29–35
McHugh DJ (1987) Production, properties and uses of alginates. FAO Fish Tech Pap 288:58–115
Mehta BR, Parekh RG (1978) Mannitol content in brown algae of the coast of Saurashtra. Bot Mar 21:251–252
Minghou J, Yujun W, Zuhong X, Yucai G (1984) Studies on the M:G ratios in alginate. Hydrobiologia 116/117:554–556
Nakai M, Kageyama N, Nakahara K, Miki W (2006) Phlorotannins as radical scavengers from the extract of Sargassum ringgoldianum. Mar Biotech 8:409–414
Omar S, Ahmad N, Ahmad F (1988) Composition of alginates from brown seaweeds, Sargassum and Padina spp. Pertanika 11(1):79–85
Padmakumar K, Ayyakkannu K (1997) Seasonal variation of antibacterial and antifungal activities of the extracts of marine algae from Southern coasts of India. Bot Mar 40:507–515
Pavia H, Cervin G, Lindgren A, Åberg P (1997) Effects of UV-B radiation and simulated herbivory on phlorotannins in the brown alga Ascophyllum nodosum. Mar Ecol Prog Ser 157:139–146
Payri CE, Stiger V (2001) Macroalgal community changes on French Polynesian reefs, 1980–2000. Phycologia 40:111
Pereira RC, Yoneshigue-Valentin Y (1999) The role of polyphenols from tropical brown alga Sargassum furcatum on the feeding by amphipod herbivores. Bot Mar 42:441–448
Perez R, Kaas R, Campello F, Arbault S, Barbaroux O (1992) La culture des algues marines dans le monde. IFREMER, France
Prince JS, Daly EL (1981) The ecology of Sargassum pteropleuron Grunow (Phaeophyceae, Fucales) in the waters off South Florida. IV. Seasonal variation in mannitol, protein, ash and laminaran. Phycologia 20:232–241
Ragan MA, Glombitza KW (1986) Phlorotannins, brown algal polyphenols. In: Round FE, Chapman DJ (eds) Progress in phycological research, vol. 4. Biopress, Bristol, pp 129–241
Ragaza AR, Hurtado AQ (1999) Sargassum studies in Currimao, Ilocos Norte, Northern Philippines. II. Seasonal variations in alginate yield and viscosity of Sargassum carpophyllum J. Agardh, Sargassum ilicifolium (Turner) C. Agardh and Sargassum siliquosum J. Agardh (Phaeophyta, Sargassaceae). Bot Mar 42:327–331
Reichelt JL, Borowitzka MA (1984) Antimicrobial activity from marine algae: results of a large scale screening programme. Hydrobiologia 116/117:158–168
Robles-Centeno PO, Ballantine DL, Gerwick WH (1996) Dynamics of antibacterial activity in three species of Caribbean marine algae as a function of habitat and life history. Hydrobiologia 326/327:457–462
Santoso J, Yoshie-Stark Y, Suzuki T (2004) Anti-oxidant activity of methanol extracts from Indonesian seaweeds in an oil emulsion model. Fish Sci 70:183–188
Saraswathi SJ, Babu B, Rengasamy R (2003) Seasonal studies on the alginate and its biochemical composition I: Sargassum polycystum (Fucales) Phaeophyceae. Phycol Res 51:240–243
Sastry VMVS, Rao GRK (1994) Antibacterial substances from marine algae: successive extraction using benzene, chloroform and methanol. Bot Mar 37:357–360
Sastry VMVS, Rao GRK (1995) Dioctyl phthalate and antibacterial compound from the marine brown alga Sargassum wightii. J Appl Phycol 7:185–186
Seo Y, Lee HJ, Park KE, Kim YA, Ahn JW, Yoo JS, Lee BJ (2004) Peroxynitrite-scavenging constituents from the brown alga Sargassum thunbergii. Biotech Bioprocess Eng 9:212–216
Sreenivasa Rao PP (1989) Biological investigation of Indian Phaeophyceae. 7. Efficiency of different parts of Sargassum johnstonii Setchell and Gardner in the evaluation of antibacterial substances. Ind Bot Reptr 8(1):28–30
Sreenivasa Rao PP (1990) Biological investigations of Indian phaephyceae. 11. Screening of different parts of Sargassum wightii Greville for their antibacterial activity. Ind Bot Reptr 9(2):52–55
Sreenivasa Rao PP, Sreenivasa Rao P, Karmarkar SM (1986) Antibacterial substances from brown algae. II. Efficiency of solvents in the evaluation of antibacterial substances from Sargassum johnstonii Setchell and Gardner. Bot Mar 29:503–507
Sreenivasa Rao PP, Sreenivasa Rao P, Karmarkar SM (1988) Antibacterial activity from Indian Species of Sargassum. Bot Mar 31:295–298
Steinberg PD (1986) Chemical defenses and the susceptibility of tropical marine brown algae to herbivores. Oecologia 69:628–630
Stiger V, Payri CE (1999a) Spatial and seasonal variations in the biological characteristics of two invasive brown algae, Turbinaria ornata (Turner) J. Agardh and Sargassum mangarevense (Grunow) Setchell (Sargassaceae, Fucales) spreading on the reefs of Tahiti (French Polynesia). Bot Mar 42:295–306
Stiger V, Payri CE (1999b) Spatial and temporal patterns of settlement of the brown macroalgae Turbinaria ornata and Sargassum mangarevense in a coral reef on Tahiti. Mar Ecol Prog Ser 191:91–100
Stiger V, Deslandes E, Payri CE (2004) Phenolic contents of two brown algae, Turbinaria ornata and Sargassum mangarevense on Tahiti (French Polynesia): interspecific, ontogenic and spatio-temporal variations. Bot Mar 47:402–409
Tako M, Kiyuna S, Uechi S, Hongo F (2001) Isolation and characterization of alginic acid from commercially cultured Nemacystus decipiens (Itomozuku). Biosci Biotechnol Biochem 65:654–657
Targett NM, Coen LD, Boettcher AA, Tanner CE (1992) Biogeographic comparisons of marine algal polyphenolics: evidence against a latitudinal trend. Oecologia 89:464–470
Tewari A, Joshi HV,Ramavat BK (1983) Studies on preservation of Sargassum. III. Effect of storage on quality and quantity of alginic acid in Sargassum tenerrimum. Phykos 22:113–119
Thomas PC, Subbaramaiah K (1991) Seasonal variations in growth, reproduction, alginic acid, mannitol, iodine and ash contents of brown alga Sargassum wightii. Ind J Mar Sci 20:169–175
Umamaheswara Rao M (1969) Seasonal variations in growth, alginic acid and mannitol contents of Sargassum wightii and Turbinaria conoïdes from the gulf of Manna, India. Proc Int Seaweed Symp 6:579–584
Umamaheswara Rao M, Kalimuthu S (1972) Changes in mannitol and alginic acid contents of Turbinaria ornata (Turner) J. Agardh in relation to growth and fruiting. Bot Mar 15:57–59
Underwood AJ (1999) Experiments in ecology: their logical design and interpretation using analysis of variance. Cambridge University Press, Cambridge
Van Alstyne KL, Paul VJ (1990) The biogeography of polyphenolic compounds in marine macroalgae : temperate brown algal defenses deter feeding by tropical herbivorous fishes. Oecologia 84:158–163
Vlachos V, Critchley AT, Von Holy A (1996) Establishment of a protocole for testing antimicrobial activity in Southern African macroalgae. Microbios 88:115–123
Yan X, Nagata T, Fan X (1998) Antioxidative activities in some seaweeds. Plant Foods Hum Nutr 52:253–262
Zhang WW, Duan XJ, Huang HL, Zhang Y, Wang BG (2007) Evaluation of 28 marine algae from the Qingdao coast for antioxidative capacity and determination of antioxidant efficiency and total phenolic content of fractions and subfractions derived from Symphyocladia latiuscula (Rhodomelaceae). J Appl Phycol 19:97–108
Zubia M (2003) The industrial valorisation of the invasive brown algae (Fucales) of French Polynesia-Prospective study to fight against their growth and contribute to a lasting management of the coral reef environment. PhD Dissertation, University of French Polynesia
Zubia M, Payri C, Deslandes E, Guezennec J (2003) Chemical composition of attached and drifted brown algae, Sargassum mangarevense and Turbinaria ornata, from Tahiti (French Polynesia). Bot Mar 46:562–571
Zubia M, Robledo D, Freile-Pelegrin Y (2007) Antioxidant activities in marine macroalgae from the coasts of Quintana Roo and Yucatan, Mexico. J Appl Phycol 19:449–458
Aknowledgments
This work was supported by a grant from ANRT and the CAIRAP. It is a contribution to the Invasive Macroalgae Programme of the laboratory “Terre-Océan”. We thank Marie-Paule Friocourt and Antoine D.R. N’Yeurt for their help with correction of the English of this paper.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zubia, M., Payri, C. & Deslandes, E. Alginate, mannitol, phenolic compounds and biological activities of two range-extending brown algae, Sargassum mangarevense and Turbinaria ornata (Phaeophyta: Fucales), from Tahiti (French Polynesia). J Appl Phycol 20, 1033–1043 (2008). https://doi.org/10.1007/s10811-007-9303-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10811-007-9303-3