Abstract
Malaria is an overwhelming impact in the poorest countries in the world due to their prevalence, virulence and drug resistance ability. Currently, there is inadequate armoury of drugs for the treatment of malaria. This underscores the continuing need for the discovery and development of new effective and safe antimalarial drugs. To evaluate the in vitro and in vivo antimalarial activity of the leaf ethyl acetate extract of Murraya koenigii, bioassay-guided chromatographic fractionation was employed for the isolation and purification of antimalarial compounds. The in vitro antimalarial activity was assayed by the erythrocytic stages of chloroquine-sensitive strain of Plasmodium falciparum (3D7) in culture using the fluorescence-based SYBR Green I assay. The in vivo assay was done by administering mice infected with Plasmodium berghei (NK65) four consecutive daily doses of the extracts through oral route following Peter’s 4-day curative standard test. The percentage suppression of parasitaemia was calculated for each dose level by comparing the parasitaemia in untreated control with those of treated mice. Cytotoxicity was determined against HeLa cells using MTT assay. Histopathology was studied in kidney, liver and spleen of isolated compound-treated Swiss albino mice. The leaf crude ethyl acetate extract of M. koenigii showed good in vitro antiplasmodial activity against P. falciparum. The in vivo test of the leaf crude ethyl acetate extract (600 mg/kg) showed reduced malaria parasitaemia by 86.6 % against P. berghei in mice. Bioassay-guided fractionation of the leaf ethyl acetate extract of M. koenigii led to the isolation of two purified fractions C3B2 (2.84 g) and C3B4 (1.97 g). The purified fractions C3B2 and C3B4 were found to be active with IC50 values of 10.5 ± 0.8 and 8.25 ± 0.2 μg/mL against P. falciparum, and in vivo activity significantly reduced parasitaemia by 82.6 and 88.2 % at 100 mg/kg/body weight on day 4 against P. berghei, respectively. The isolated fractions C3B2 and C3B4 were monitored by thin-layer chromatography until a single spot was obtained with R f values of 0.36 and 0.52, respectively. The pure compounds obtained in the present investigation were subjected to UV–visible spectroscopy, Fourier transformer infrared spectroscopy, 1D and 2D 1H-Nuclear magnetic resonance (NMR), 13C NMR, DEPT, COSY and Mass spectral analysis. Based on the spectral analysis, it is concluded that the isolated compounds were myristic acid (C3B2) and β-caryophyllene (C3B4). The cytotoxic effect of myristic acid and β-caryophyllene showed the TC50 values of >100 and 80.5 μg/mL, respectively against HeLa cell line. The histopathology study showed that protection against nephrotoxicity of kidney, hepatic damage of liver and splenocytes protection in spleen was achieved with the highest dose tested at 100 mg/kg/body weight. The present study provides evidence of antiplasmodial compounds from M. koenigii and is reported for the first time.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Malaria caused by protozoan parasite of the genus Plasmodium is transmitted to man through the bites of female Anopheles mosquitoes. Plasmodium falciparum, the most deadly species, predominantly occurs in Africa and is known to be responsible for 90 % of all world malaria deaths (WHO 2012). In spite of considerable control efforts in many countries, malaria remains a major cause of global morbidity and mortality with substantial global public health costs and with most of the burden in sub-Saharan Africa (Greenwood et al. 2005; Muller 2011). Resistance to the majority of available antimalarial drugs has been reported in a growing number of countries worldwide, and such resistance threatens future progress in malaria control (WHO 2012; Zofou et al. 2012). WHO recommended treating uncomplicated Plasmodium falciparum malaria with artemisinin-based combination therapy (ACT) in order to reduce the risk of resistance. However, resistance to artemisinin derivatives has been recently described in Southeast Asia (Noedl et al. 2008; Carrara et al. 2009; Dondorp et al. 2010; Phyo et al. 2012).
The use of plants for malaria treatment extends over at least three continents, including several countries in Africa, Americas and Asia (Gurib-Fakim 2006). The most widely used compounds to treat malaria, quinine from Cinchona bark and artemisinin from Artemisia annua (Li and Rieckmann 1992), are based on traditional medicine and derived from plant extracts (Willcox et al. 2004). Hence, the identification of natural product leads from diverse natural sources that critically augment the search in anti-parasitic drug discovery (Bero and Quetin-Leclercq 2011; Li and Vederas 2009; Newman and Cragg 2012). Murraya koenigii (Rutaceae) is native to India and distributed in most of Southern Asia. The leaves of this plant known as curry leaves are widely used in conventional Indian food preparations since they add characteristic flavour and aroma (Sathaye et al. 2011).
Previous pharmacological study revealed that the methanol extracts of M. koenigii could decrease insulin secretion when they were subjected to both in vivo and in vitro antidiabetic tests (Adebajo et al. 2004). Khan et al. (1995) reported that the curry leaf has no adverse effects, as evident from the unchanged blood parameters/constituents and normal histopathology of hepatic tissue rats. The leaf petroleum ether extract of M. koenigii has been shown to possess antioxidant potential and protection against oxidative stress induced in diabetes (Arulselvan and Subramanian 2007), and the aqueous leaf extract showed the lipid peroxidation reduction and decrease cellular damage, thereby protecting liver from ethanol-induced toxicity (Sathaye et al. 2011). The leaf chloroform extract of M. koenigii exhibited profound and exclusive lactate dehydrogenase inhibitory activity against chloroquine (CQ)-sensitive (NE) and CQ-resistant (MRC-2) strains of Plasmodium falciparum and Plasmodium vivax (Keluskar and Ingle 2012). Simonsen et al. (2001) reported that the stem ethanol extract showed moderate activity against CQ-susceptible strain (3D7) of Plasmodium falciparum. Orwa et al. (2013) have reported that the fruit ethyl acetate extract of Toddalia asiatica (Rutaceae) showed high activity against CQ-resistant (W2) and CQ-sensitive (D6) strains of Plasmodium falciparum as well as against Plasmodium berghei (ANKA). The leaf ethyl acetate and methanol extracts of Aegle marmelos showed promising antiplasmodial activity against CQ-sensitive (3D7) and CQ-resistant (INDO) strains of Plasmodium falciparum (Kamaraj et al. 2012a).
The carbazole alkaloids isolated from M. koenigii had significantly antioxidant and radical-scavenging biological activities (Rao et al. 2007). The major constituents responsible for the aroma and flavour have been reported as pinenes, carene, sabinene, caryophyllene, cadinol and cadinene (Chowdhury et al. 2008; Malwal and Sarin 2010; Onayade and Adebajo 2000; Rana et al. 2004; Walde et al. 2006). Raina et al. (2002) confirmed the occurrence of four distinct chemotypes (β-pinene, α-phellandrene-β-caryophyllene, α-pinene, β-caryophyllene) of M. koenigii. The compound myristic acid exhibited significant mosquito attractancy (P < 0.05) against unfed adult female Aedes aegypti (Mathew et al. 2013), and it was also shown the potential antagonistic effects against root-knot nematodes, Meloidogyne incognita (Zhang et al. 2012). Liu and Huang (2012) have reported that the curcumin-loaded myristic acid microemulsions inhibited the bacterial growth against Staphylococcus epidermidis and the myristic acid also exhibited significant anti-HIV activity (Agarwal et al. 2013). Sivakumar et al. (2011) who have observed the tetradecanoic acid (myristic acid) exhibited effective larvicidal activity against Culex quinquefasciatus and Aedes aegypti. Several biological activities were attributed to β-caryophyllene, such as anticancer activity against amelanotic melanoma C32 and human breast adenocarcinoma MCF-7 cell lines (Tundis et al. 2009), cytotoxic and antioxidative activities against several solid tumour cell lines (Kubo et al. 1996), weak larvicidal potency against Aedes aegypti (Doria et al. 2010), effective antimicrobial activity against Bacillus subtilis and Escherichia coli (Macleod and Rasmussen 1999), anti-microbial (Alma et al. 2003; Lourens et al. 2004), anti-oxidant (Singh et al. 2006) and also possessed skin penetration enhancing properties (Cornwell and Barry 1994).
Ndjonka et al. (2012) reported the isolated compounds, polyphenols, geraniin from the leaves of Phyllanthus muellerianus and ellagic, gentisic and gallic acids isolated from the bark of Anogeissus leiocarpus, which showed significant inhibitory activity against Plasmodium falciparum (3D7). Specicoside, 2β, 3β, 19α-trihydroxy-urs-12-en-28-oic acid and atranorin isolated from the stem bark ethyl acetate extract of Kigelia africana exhibited significant antiplasmodial activity against CQ-resistant (W-2) strain of Plasmodium falciparum (Zofou et al. 2011). The bioassay-guided isolation of two antiplasmodial principles, 6-(8′Z-pentadecenyl)-salicylic acid (1) and 6-(8′Z, 11′Z, 14′Z-heptadecatrienyl)-salicylic acid (2), have been isolated from the whole plant petroleum ether extract of Viola websteri and showed significant activity against CQ-sensitive strain of Plasmodium falciparum (Lee et al. 2009). Morais et al. (2012) have reported that the bioactive compound jacaranone [methyl (1-hydroxy-4-oxo-2,5-cyclohexandienyl) acetate] was isolated from the leaf methanol extract of Pentacalia desiderabilis which showed antimalarial activity against CQ-resistant strain (K1) of Plasmodium falciparum. The flavonoids, lupinifolin (1), citflavanone (2), erythrisenegalone (3), lonchocarpol A (4), liquiritigenin (5) and 8-prenyldaidzein (6), were isolated from the stem bark ethyl acetate extract of Erythrina fusca showing notable activity against the multi-drug-resistant strain (K1) of Plasmodium falciparum (Khaomek et al. 2008). Shuaibu et al. (2008) have reported that the leaf, root or stem ethyl acetate and butanolic fractions of Anogeissus leiocarpus and Terminalia avicennioides exhibited better antiplasmodial activity against CQ-sensitive (3D7) and CQ-resistant (K1) strains of Plasmodium falciparum. The stilbene glycosides, piceid (1), piceid-(1 → 6)-beta-d-glucopyranoside (2), resveratrol (3), longistylin A (4) and longistylin C (5), were isolated from the leaf methanol extract of Parthenocissus tricuspidata exhibiting potential inhibition against CQ-sensitive (D10) strain of Plasmodium falciparum (Son et al. 2007).
Inspired by the use of M. koenigii in the treatment of different diseases, in the present study the bioassay-guided fractionation of M. koenigii extract was carried out in order to evaluate their pharmacological potentials. The objective of the present study was to identify its bioactive antimalarial components by using column chromatographic fractionation, and structural elucidation was carried out for isolated compounds, myristic acid and β-caryophyllene.
Materials and methods
Plant material
The leaves of M. koenigii were selected in the present study based upon their medicinal uses and biological activities. The material was collected from Malaiyur Hills, Dharmapuri district (11° 53′ 28″ N, 078° 30′ 26″ E, altitude 959 m), Tamil Nadu, South India in May 2012. The taxonomic identification was made by Dr. C. Hema, Department of Botany, Arignar Anna Government Arts College for Women, Walajapet, Vellore, India. The voucher specimens were numbered and kept in our research laboratory for further reference.
Preparation of plant extracts
The leaves (1.5 kg) were air-dried for 4–7 days in the shade at environmental temperatures (27–37 °C daytime) and powdered mechanically using a commercial electrical stainless steel blender and extracted with ethyl acetate (4,600 mL, Qualigens) in a Soxhlet apparatus (boiling point range 60–80 °C) for 8 h. The extract was concentrated under a reduced pressure of 22–26 mmHg at 45 °C, and the residue obtained was stored at 4 °C.
In vitro cultivation of Plasmodium falciparum
CQ-sensitive (3D7) strain of Plasmodium falciparum was used in in vitro blood stage culture to test the antimalarial efficacy of fractions and isolated compounds. The culture was maintained at the Unit of Nanotechnology and Bioactive Natural Products, Post Graduate and Research Department of Zoology, C. Abdul Hakeem College, Melvisharam, Vellore District, Tamil Nadu, India. Plasmodium falciparum culture was maintained according to the method described by Trager and Jensen (1976), with minor modifications. Plasmodium falciparum (3D7) culture was maintained in fresh O+ve human erythrocytes suspended at 4 % hematocrit in Roswell Park Memorial Institute (RPMI) 1640 (Sigma) containing 0.2 % sodium bicarbonate, 0.5 % albumax, 45 μg/L hypoxanthine and 50 μg/L gentamycin and incubated at 37 °C under a gas mixture of 5 % O2, 5 % CO2 and 90 % N2. Every day, infected erythrocytes were transferred into a fresh complete medium to propagate the culture (Kamaraj et al. 2012a, b).
Drug dilutions
Stock solutions of CQ were prepared in water (milli-Q grade). The test compounds were prepared in dimethyl sulfoxide (DMSO; Qualigens). All stocks were then diluted with culture medium to achieve the required concentrations (in all cases except CQ, the final solution contained 0.4 % DMSO, which was found to be non-toxic to the parasite). Drugs and test compounds were then placed in 96-well flat bottom tissue culture grade plates (Dorin et al. 2001; Kumari et al. 2012).
Assay for antiplasmodial activity
The fractions and isolated compounds were dissolved in 0.5 mL of sterile distilled water with 0.4 % DMSO to obtain a stock concentration of 5 mg/mL. CQ stock concentration of 0.5 mg/mL was used as positive control, while 0.4 % DMSO was used as the negative control. From these stock solutions, a tenfold dilution was made with RPMI 1640 medium (without bicarbonate). One hundred-microlitre aliquots of diluted extract dispensed into 96-well plates obtained 6.3, 12.5, 25, 50 and 100 μg/mL as final concentrations against Plasmodium falciparum (3D7) as per the procedure followed by Smilkstein et al. (2004). Fifty microlitres of RPMI 1640 medium plus sodium bicarbonate and serum A+ as complete serum was aliquoted into all the wells. Twenty-five microlitres of infected red cells of each isolate was separately added. The starting parasitaemia was between 2.5 and 5.0 %. However, the negative controls were without the extract. After this, the plates were covered, shaken gently and incubated in desiccators at 37 °C for 24–48 h. After incubation, the contents of both the controls and tested wells were harvested, and the deposited red cells were transferred to a slide to form thick film. The film was dried and stained with Giemsa, and the parasites were assessed for growth. The numbers of schizonts with three or more nuclei out of a total of 200 asexual parasites were noted. A graph of percent inhibition of parasite growth against the concentration of the extract, compounds and the standard agents was also plotted, and the IC50 values were determined. CQ (Sigma-Aldrich) was used as standard; infected and uninfected erythrocytes were added as positive and negative controls, respectively (Bhat and Surolia 2001; Bagavan et al. 2011a, b).
In vivo bioassay test
Parasite strain
A chloroquine-sensitive Plasmodium berghei (NK 65) obtained from the National Institute of Malaria Research (NIMR), Delhi, India was used to assess the in vivo intrinsic antimalarial activity.
Experimental animals
Male and female mice (Swiss albino mice) weighing 20–30 g, 6 weeks old, obtained from the Institute of Veterinary Preventive Medicine, Ranipet, Tamil Nadu, India were used for this study. The mice were grouped and housed in polyacrylic cages (38 × 23 × 10 cm) with three animals per cage and maintained under standard laboratory condition (temperature at 27 ± 2 °C with dark/light cycle 12:12 h). They were allowed standard pellet diet (Hindustan Lever Limited, Mumbai, India) and clean drinking water ad libitum (Shakya 2012). The study was conducted in accordance with the permission and approval of Government of India, Ministry of Environment and Forest, New Delhi, India (committee for the purpose of control and supervision of experiments on animals; Reg. No. 1011/c/CPCSEA).
Parasite inoculation
Parasitized erythrocytes were obtained from a donor-infected mouse by cardiac puncture in heparin and diluted with sterile blood from similar age group mice. Animals were inoculated intraperitoneally with infected blood suspension (0.2 mL) containing 106 parasitized erythrocytes lethal inoculum on day 0. Infected mice with parasitaemia of 5–7 % were allocated to four groups of three mice each (David et al. 2004).
In vivo antiplasmodial bioassay
Experiments were performed using Peter’s 4-day curative standard test (Peters et al. 1975; David et al. 2004; Peter and Anatoli 1998) and employing the chloroquine-sensitive Plasmodium berghei (NK 65). For bioassay test, three mice were used for isolated compound (C3B2 and C3B4) test group; chloroquine (standard drug, Sigma) and untreated control groups of mice were tested separately. Tween 80 (Qualigens) was used as an emulsifier at the concentration of 0.3 % in the final test solution. The plant extracts were orally administered to the test groups at a different dose level (150–600 mg/kg body weight) from 0 to 4 days to infected mice of Plasmodium berghei. Chloroquine (Sigma) was used as a standard drug with normal saline (0.9 %) at 1.25–5 mg/kg. Saline (0.9 %) and Tween 80 (0.3 %) with distilled water were used as control for four experimental days. Treatments were performed daily for four consecutive days starting 24 h after infection receiving a total of four oral doses.
Estimation of parasitaemia
The parasitaemia was monitored in all the groups starting from 0 to 4 days using thick and thin smears of blood films made from the tail vein of the mice (Ene et al. 2008). The smears were stained with 10 % Giemsa at pH 7.2 for 15 min and examined under the microscope at ×100 to assess the level of parasitaemia. The percentage parasitaemia was calculated according to the method outlined by Iwalewa et al. (1997).
Cytotoxic activity on HeLa cells using MTT assay
The cytotoxic effects of isolated compounds (C3B2 and C3B4) on host cells were assessed by functional assay as described by Mosmann (1983) using HeLa cells cultured in RPMI containing 10 % foetal bovine serum, 0.21 % sodium bicarbonate (Sigma) and 50 μg/mL gentamicin (complete medium). Briefly, cells (104 cells/200 μL/well) were seeded into 96-well flat bottom tissue culture plates in complete medium. Drug solutions were added after 24 h of seeding and incubated for 48 h in a humidified atmosphere at 37 °C and 5 % CO2. DMSO (as positive inhibitor) was added at 10 %. Twenty microlitres of a stock solution of MTT (5 mg/mL in 1× phosphate-buffered saline) was added to each well, gently mixed and incubated for another 4 h. After spinning the plate at 1,500 rpm for 5 min, supernatant was removed and 100 μL of DMSO (stop agent) was added. Formation of formazon was read on a microtiter plate reader (Versa max tunable multi-well plate reader) at 570 nm. Fifty percent cytotoxic concentration (TC50) of drug was determined by the analysis of dose–response curves.
Preliminary screening of crude extract
In our earlier study, in preliminary screening, the leaf ethyl acetate extract of M. koenigii exhibited good antimalarial activity against CQ-sensitive (3D7) and CQ-resistant (INDO) strains of Plasmodium falciparum (Kaushik et al. 2013). It showed low cytotoxicity against human HeLa cell lines with therapeutic index. The leaf ethyl acetate extract of M. koenigii was selected for bioassay-guided isolation, purification and identification of bioactive compounds using column chromatography (Bagavan et al. 2008).
Bioassay-guided fractionation of leaf ethyl acetate extract of M. koenigii
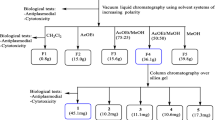
The residue (yield, 27.63 g, 2.21 %) obtained from the leaf ethyl acetate extract of M. koenigii was subjected to column chromatography (gravity), and each fraction obtained was tested in vitro against Plasmodium falciparum (3D7) at the concentrations of 6.25, 12.5, 25, 50 and 100 μg/mL. Those fractions showing IC50 values of <15 μg/mL were considered to be highly active and selected for further separation by column chromatography. The active crude leaf ethyl acetate extract of M. koenigii was subjected to column chromatography (50 × 5 cm, gravity, 1:2 charcoal/Si gel, 60–120 mesh, 220 g) to obtain five fractions A, B, C, D and E by increasing polarity of elution stepwise with a linear gradient of hexane and ethyl acetate 100:0 (5 × 200 mL), 75:25 (19 × 200 mL), 50:50 (16 × 200 mL), 25:75 (5 × 200 mL) and 0:100 (2 × 200 mL), respectively (Bagavan et al. 2008). Further elution of the column with different proportions of chloroform and methanol yielded three more fractions, namely F, G and H with the elutions of 100:0 (5 × 200 mL), 50:50 (12 × 200 mL) and 0:100 (3 × 200 mL), respectively. Each fraction (A–H) obtained was tested in vitro against CQ-sensitive strain (3D7) of Plasmodium falciparum, and those fractions showing 50 % parasite inhibition in 48 h alone were selected for further column chromatographic separation. Fractions A, B, C, D, E, F, G and H showed the IC50 values of 17.0, 26.5, 6.5, 14.25, 30.2, 38.5, 22.5 and 16.2 μg/mL at the concentrations of 6.25, 12.5, 25, 50 and 100 μg/mL, respectively. Among the fractions tested, fraction C showed promising activity with IC50 value of 6.5 μg/mL. Fraction C was selected for further separation by column chromatography.
Fractions C (16.32 g), C3 (10.43 g), C3B (8.12 g), C3B2 (2.84 g) and C3B4 (1.97 g) were subjected to a subsequent repeated column chromatography (gravity) separately using different Si gel meshes (70–320 mesh 240 g and 230–400 mesh 180 g) with varying proportions of hexane and ethyl acetate as eluents to collect different sub-fractions. Bioassay-guided fractionation was carried out, and the pure compounds C3B2 (2.84 g) and C3B4 (1.97 g) were obtained from the fourth column with the elution of 95:5 (22 × 50 mL) and 75:25 (8 × 50 mL). The fractions collected were combined based on Thin-layer chromatography (TLC) results. The obtained fractions were tested in vitro against CQ-sensitive strain (3D7) of Plasmodium falciparum, and those fractions showing 50 % parasite inhibition in 48 h alone were selected. All fractions were monitored by TLC (pre-coated plate, 0.02 mm thick, E. Merck, Germany 60 F254) until a single spot was obtained. The plates were air-dried and exposed in iodine vaporized chamber to locate the spots. Retardation factor (R f) values of isolated fractions C3B2 and C3B4 showed single band (TLC result) with R f values of 0.36 and 0.52, respectively. The identification and characterization of the purified compounds were done by analysing different spectroscopic data.
Spectral analysis
The pure compounds obtained in the present investigation were subjected to UV–visible spectroscopy (UV), Fourier transformer infrared spectroscopy (FTIR), 1D and 2D 1H-Nuclear magnetic resonance (NMR), 13C NMR, DEPT, COSY and Mass spectral analysis. UV spectra were recorded on Shimadzu 160A, and IR spectra were recorded on Thermo Electron (Madison, WI, USA) instrument. The 1H and 13C NMR spectra were recorded on a Bruker DRX 500 NMR instrument operating at 500 MHz for 1H and 125 MHz for 13C at room temperature. Mass spectrum was obtained using Q-TOF Waters Ultima instrument (Q-TOF GAA 082, Waters, Manchester, UK).
Histopathological studies
In the present study, liver, kidney and spleen were removed from the experimental mice through the dissection, washed in 0.9 % sodium chloride solution and placed in 10 % formalin for fixation. The organs were dehydrated by increasing concentrations of alcohol (0–100 %) and embedded in paraffin blocks which were sectioned in 4 μm thickness using Leica rotary microtome. The sections were stained in haematoxylin and eosin for parasite visualization and evaluation of tissue morphology using light microscopy with a camera (×40) (Labomed, India). Examination of liver, kidney and spleen sections was undertaken to determine morphological changes. Organs were harvested from myristic acid, β-caryophyllene, chloroquine-treated, untreated (control infected) and non-infected (control normal) mice (Moore et al. 2008).
Statistical analysis
The parasitaemia was determined, and the doses were performed in triplicate by using three sets of mice for each group. The results were analysed statistically using one-way and two-way ANOVA methods to identify the differences between treated group and control. The data were considered significant at P < 0.05. The mean % reduction of parasitaemia for each group was recorded, and the effective dose (ED50) of the extract was determined from log (dose)/% reduction via an Excel graph (Traore et al. 2008).
Results
In vitro antiplasmodial activity of purified fraction
Bioassay-guided fractionation of the leaf ethyl acetate extract of M. koenigii led to the isolation of two purified fractions C3B2 (2.84 g) and C3B4 (1.97 g). The fractions were bioassayed for in vitro antimalarial activity against CQ-sensitive (3D7) strain of Plasmodium falciparum. The purified fractions C3B2 and C3B4 were found to be active with IC50 values of 10.5 ± 0.8 and 8.25 ± 0.2 μg/mL, respectively (Table 1).
In vivo antiplasmodial of purified fraction
After the chromatographic separations, the sub-fractions C3B2 and C3B4 showed promising in vitro antimalarial activity against 3D7 strain of Plasmodium falciparum with IC50 values of 10.5 and 8.25 μg/mL, respectively, and showed single band in TLC result. The in vitro active purified fractions C3B2 and C3B4 were further evaluated for in vivo activity against Plasmodium berghei-infected mouse model. The purified fractions C3B2 and C3B4 significantly reduced parasitaemia 82.6 ± 2.04 and 88.2 ± 0.69 %, respectively, at 100 mg/kg on day 4 after parasite inoculation. Chloroquine was tested in parallel which inhibited 96.7 ± 0.86 % at 5 mg/kg. The effective dose (ED50) values were determined for purified fractions which had significant antimalarial activities as compared to chloroquine. ED50 values were observed for C3B2, C3B4 and chloroquine as 144.23 ± 2.42, 112.68 ± 2.00 and 48.60 ± 2.03 mg/kg, respectively (Table 2). Tween 80 (Qualigens) was used as an emulsifier at the concentration of 0.3 % in the final test solution. Chloroquine (standard drug) was prepared with normal saline (0.9 %) and Tween 80 (0.3 %) with distilled water used as control for experiments.
Cytotoxic effect of isolated fractions
The cytotoxicity was assessed against HeLa cell line. The percentage of cell viability was observed in purified fractions C3B2 and C3B4 at 54.86 and 42.14 % for the highest concentration 500 μg/mL. The TC50 values of purified fractions C3B2 and C3B4 were observed to be >100 and 80.5 μg/mL, respectively, and exhibited promising therapeutic index 9.52 and 9.76 μg/mL, respectively (Table 3), against HeLa cells.
R f values of different fractions calculated using TLC
The isolated fractions were monitored by TLC until a single spot was obtained. The plates were air-dried and exposed to iodine chamber to locate the spots. Fraction C showed six bands with R f values of 0.24, 0.26, 0.32, 0.49, 0.55 and 0.62; fraction C3 showed four bands with R f values 0.28, 0.62, 0.68 and 0.76; fraction C3B showed three bands with R f values of 0.32, 0.46 and 0.64 and fractions C3B2 and C3B4 showed single band with R f values of 0.36 and 0.52, respectively. The identification and characterization of the purified compounds were elucidated by the analysis of spectroscopic data.
Spectral analysis of compound 1 (C3B2)
The compound 1 of ethyl acetate extract is a colourless solid (10 mg) with melting point of 53 °C and molecular formula: C14H28O2. The UV (Me OH) showed a band at 243 nm. The FTIR spectra for compound 1 (Fig. 1a) and the absorbance frequencies were obtained at 3,416.19 cm−1 (–OH), 2,920.98 cm−1 (–CH) and 1,712.42 cm−1 (–C=O).
a FTIR spectrum of the compound 1 (C3B2) from the leaf ethyl acetate extract of M. koenigii. b 1H NMR spectrum of the compound 1 (C3B2) from the leaf ethyl acetate extract of M. koenigii. c 13C spectrum of the compound 1 (C3B2) from the leaf ethyl acetate extract of M. koenigii. d GC–MS of the compound 1 (C3B2) from the leaf ethyl acetate extract of M. koenigii. e HRMS of the compound 1 (C3B2) from the leaf ethyl acetate extract of M. koenigii. f Structure of myristic acid isolated from the leaf ethyl acetate extract of M. koenigii (molecular formula: C14H28O2)
The 1H NMR spectrum (DMSO-d6, 500 MHz) showed a sharp singlet at δ 1.256–1.315 for nine CH2 in the compound. The triplet at δ 0.861–0.895 corresponds to the terminal CH3. The peak at δ 1.300–1.315 corresponds to methyl group. A broad multiplet found in the range of δ 1.554–1.666 corresponds to CH2. A sharp triplet in the range of 2.325–2.363 corresponds to CH2 (Fig. 1b).
The 13C NMR (DMSO-d6, 125 MHz) showed a signal at δ 14.11 for methyl carbon (C-14). The signals at δ 22.693 could be attributed for methylene carbon C-13 adjacent to methyl carbon. The signal at δ 24.67, 29.06, 29.35, 29.43, 29.59, 29.64 and 29.67 corresponds to the methylene protons in the structure. The remaining methylene carbon adjacent to methyl carbon and acid functionality carbon resonates at δ 31.92 and δ 34.10, respectively. The –C=O in the acid functionality resonates at δ 180.46, thus accounting for 14 carbons in the compound (Fig. 1c). The DEPT-135, HSQC and COSY techniques further confirmed the correlation of carbons and hydrogen in the molecule. The Gas chromatography–mass spectrometry (GC–MS) and High-resolution mass spectrometry (HRMS) of the compound support the molecular weight m/z 228.30 (Cal 228.37) (Fig. 1d, e). Based on the above spectral analysis, it is concluded that the isolated compound is to be the myristic acid (n-tetradecanoic acid) (Fig. 1f).
Spectral analysis of compound 2 (C3B4)
Compound 2 of ethyl acetate extract is a colourless liquid (1 mL). The UV (MeOH) showed a band at 243 nm. The FTIR spectrum for compound 2 is shown in Fig. 2a. The absorbance frequencies were obtained at 2,949, 2,926 and 2,856 cm−1 (–CH, –CH2, –CH3). The 1H NMR spectrum (DMSO-d6, 500 MHz) showed a broad peak between δ1and 2.5 due to the presence of more number of aliphatic hydrogens in the structure (Fig. 2b).
a FTIR spectrum of the compound 1 (C3B2) from the leaf ethyl acetate extract of M. koenigii. b 1H NMR spectrum of the compound 1 (C3B2) from the leaf ethyl acetate extract of M. koenigii. c 13C spectrum of the compound 1 (C3B2) from the leaf ethyl acetate extract of M. koenigii. d GC–MS of the compound 1 (C3B2) from the leaf ethyl acetate extract of M. koenigii. e HRMS of the compound 1 (C3B2) from the leaf ethyl acetate extract of M. koenigii. f Structure of myristic acid isolated from the leaf ethyl acetate extract of M. koenigii (molecular formula: C14H28O2)
13C NMR (DMSO-d6, 125 MHz) showed that a signal at δ 29.84, 29.36 and 17.91 corresponds to four methyl carbons. The signals at δ 110.80, 40.35, 34.85, 34.78 and 30.06 correspond to five methylene carbons. The signal at δ 53.56, 48.48, 124.86 and 135.39 corresponds to –CH carbons in the structure, and the remaining –C– carbon resonates at δ 154.59 (Fig. 2c). The DEPT-135, HSQC and COSY techniques further confirm the correlation of carbons and hydrogen in the molecule. The HRMS of the compound supports the molecular weight m/z 204.39 (Cal 204.09) and had molecular formula C15H24 (Fig. 2d). Based on the above spectral analysis, it is concluded that the isolated compound is to be the β-caryophyllene ((trans-(1R, 9S)-4, 11, 11-trimethyl-8-methylenebicyclo [7.2.0] undec-4-ene) [87-44-5]) (Fig. 2e).
Histopathological studies
The histopathology of kidney section showed normal (non-infected) architecture of cells in control mice. No significant lesions were observed in the kidney cells treated with myristic acid and β-caryophyllene at 100 mg/kg/day once daily for 4 days (Fig. 3a, b, respectively). Figure 3c shows that the kidney sections of untreated (infected) mice were observed for malarial pigments in cells and intracellular gaps. Chloroquine treated mice at 5 mg/kg/day once daily for 4 days, where no significant lesion was found compared with untreated control mice. The liver sections of Swiss albino mice were treated with purified compounds myristic acid, β-caryophyllene and chloroquine once daily for 4 days. The normal hepatocytes showed devoid of intracellular gaps in the liver section of normal control mice. The myristic acid and β-caryophyllene treated mice at 100 mg/kg/day for 4 days, and no morphological changes of hepatocytes and also normal intracellular gaps were observed in the liver (Fig. 3d, e, respectively). In the liver section of untreated mice, infiltration of hepatocytes and deposition of malarial pigment were observed (Fig. 3f). Chloroquine treated mice at 5 mg/kg/day for 4 days, and no significant lesions in the control mice were observed.
a, b Myristic acid- and β-caryophyllene-treated mice at 100 mg/kg once daily for 4 days. There is no significant lesion observed in the treated mice kidney cells. c Deposition of malarial pigments in cells and intracellular gaps in the kidney section of untreated (infected) mice. d, e Purified compounds myristic acid- and β-caryophyllene-treated mice. No lesion of lymphocytes and also normal intracellular gaps were observed in the liver. f Infiltration of lymphocytes in the liver section of untreated mice. g, h Isolated compounds myristic acid- and β-caryophyllene-treated mice at 100 mg/kg/day once daily for 4 days, exhibited normal red pulp and no lesion observed in splenocytes. i Expanded red pulp in the spleen section of untreated mice
Histopathology photomicrographs of spleen sections of albino mice treated with myristic acid, β-caryophyllene and chloroquine once daily for 4 days were shown. Normal texture of splenocytes showed in control mice. The isolated compounds myristic acid and β-caryophyllene (Fig. 3g, h) treated mice at 100 mg/kg/day for 4 days, and it was reported that no lesion was found in splenocytes and normal red pulp in spleen sections. The spleen sections of untreated mice exhibited expanded red pulp along with the deposition of malarial pigments present in the macrophages (Fig. 3i). Chloroquine treated mice at 5 mg/kg/day for 4 days; no significant lesions were observed in the control mice.
Discussion
Natural products have been the basis of vast majority of current antimalarial medicines. Molecules such as quinine, lapachol and artemisinin have been originally isolated from herbal medicinal products (Ginsburg and Deharo 2011). The success of these drugs has broadened the search for natural plant products as a source of novel drugs for malaria. In the present study, ethyl acetate extract was subjected to bioassay-guided fractionation using silica gel column chromatography, and each fraction was tested for in vitro antiplasmodial activity against 3D7 strain of Plasmodium falciparum. Among the tested fractions, C3B2 (myristic acid) and C3B4 (β-caryophyllene) were found to be most active with IC50 values of 10.5 and 8.25 μg/mL, respectively against Plasmodium falciparum (3D7). The in vivo antimalarial activity of isolated fractions showed significantly reduced parasitaemia of 82.6 ± 2.04 and 88.2 ± 0.69 %, respectively, at 100 mg/kg/day on day 4 against Plasmodium berghei (NK65)-infected mice. Ondo et al. (2012) have proven that the leaf and stem bark ethyl acetate fraction (EA1) of Vitex madiensis exhibited high in vitro antiplasmodial activity against CQ-resistant (FCB) strain of Plasmodium falciparum with IC50 values ranging from 0.53 to 4.87 μg/mL. Zofou et al. (2011) have isolated four compounds from the stem bark ethyl acetate fraction of K. africana (Bignoniaceae), and the compound specicoside (1) exhibited the highest activity (IC50 = 1.54 μM), followed by 2β, 3β, 19α-trihydroxy-urs-12-en-28-oic acid (2) (IC50 = 1.60 μM) and atranorin (3) (IC50 = 4.41 μM), while p-hydroxycinnamic acid (4) was reported to possess low activity (IC50 = 53.84 μM) against CQ-resistant (W-2) strain of Plasmodium falciparum. The new bithienyl compound 2-hydroxymethyl-non-3-ynoic acid 2-[2,2′]-bithiophenyl-5-ethyl ester isolated from the roots ethyl acetate fraction of Tagetes erecta exhibited significant schizonticidal activity against CQ-sensitive and CQ-resistant strains of Plasmodium falciparum with IC50 values of 0.01 and 0.02 mg/mL, respectively (Gupta and Vasudeva 2010). The present results revealed that the hexane/ethyl acetate fraction (C3B4) also showed good activity compared with previous authors reporting against 3D7 strain of Plasmodium falciparum with the IC50 value of 8.25 μg/mL.
Severino et al. (2009) have reported that the isolated methyl 5,7-dimethoxy-2,2-dimethyl-2H-1-benzopyran-6-propanoate from the leaves of Hortia oreadica showed promising antimalarial activity with IC50 value of 23.6 μM against CQ-sensitive (3D7) strain of Plasmodium falciparum. Similarly, Ramalhete et al. (2010) have also reported that the isolated balsaminoside A and karavilagenin E from Momordica balsamina showed effective antimalarial activity against 3D7 strain of Plasmodium falciparum with IC50 values of 4.6 and 7.4 μM, respectively. The bioactive compounds geraniin isolated from Phyllanthus muellerianus and ellagic acid isolated from Anogeissus leiocarpus exhibited potent activity against Plasmodium falciparum (3D7) with IC50 values of 11.74 and 2.88 μM, respectively (Ndjonka et al. 2012). The new azafluorenone alkaloid 5,8-dihydroxy-6-methoxyonychine (1) and known natural product 5-hydroxy-6-methoxyonychine (2) isolated from the roots of Mitrephora diversifolia (Annonaceae) displayed good antimalarial activity against Plasmodium falciparum (3D7) with IC50 values of 9.9 and 11.4 μM, respectively (Mueller et al. 2009). Similar study has been conducted by Shuaibu et al. (2008) who have reported different purified compounds castalagin, ellagic acid, flavogallonic acid, punicalagin and terchebulin isolated from the butanolic fraction of Anogeissus leiocarpus and Terminalia avicennioides (Combretaceae) which showed relatively better activity with IC50 values ranging between 8–21 and 8–40 μg/mL against CQ-sensitive (3D7) and CQ-resistant (K1) strains of Plasmodium falciparum, respectively. Lee et al. (2009) have isolated two main antiplasmodial principles, 6-(8′Z-pentadecenyl)-salicylic acid (1) and 6-(8′Z, 11′Z, 14′Z-heptadecatrienyl)-salicylic acid (2), from the whole plant petroleum ether extract of Viola websteri and showed significant activity against CQ-sensitive (3D7) strain of Plasmodium falciparum with IC50 values of 10.1 ± 3.2 and 13.3 ± 6.7 μM, respectively. The compound stilbene glycoside, piceid-(1 → 6)-beta-d-glucopyranoside (2), was isolated from the leaf methanol extract of Parthenocissus tricuspidata exhibiting most potential inhibition against CQ-sensitive (D10) strain of Plasmodium falciparum with IC50 value of 5.3 μM (Son et al. 2007). In the present observation, the purified compounds myristic acid and β-caryophyllene also showed promising antimalarial activity compared with earlier authors’ report against 3D7 strain of Plasmodium falciparum with IC50 values of 10.5 and 8.25 μg/mL, respectively.
In the present observation, myristic acid and β-caryophyllene compounds showed potential in vivo antiplasmodial activity with reducing parasitaemia 88.2 ± 0.69 and 82.6 ± 2.04 % against Plasmodium berghei (NK65), respectively. Similarly, Silva et al. (2011) have also reported the isolated compound 4‐nerolidylcatechol from the root chloroform/ethanol (1:1) extracts of Piper peltatum which exhibited significant in vivo antiplasmodial activity evidenced by suppression of growth up to 63 % against CQ-sensitive strain of Plasmodium berghei (NK65) at 600 mg/kg/day. Rukunga et al. (2007) reported the purified five known spermine alkaloids: budmunchiamine K (1), 6-hydroxybudmunchiamine K (2), 5-normethyl-budmunchiamine K (3), 6-hydroxy-5-normethylbudmunchiamine K (4) and 9-normethyl-budmunchiamine K (5) from the alkaloidal fraction of Albizia gummifera. These alkaloids showed percentage chemosuppression of parasitaemia in mice ranging from 43 to 72 % against Plasmodium berghei (ANKA). This is in accordance with the report of earlier authors that the present results revealed good in vivo antimalarial activity against Plasmodium berghei-infected mice.
In the present observation, the histological slide showed histopathology of myristic acid- and β-caryophyllene-treated kidney section in normal mice kidney cells. Histopathology of myristic acid- and β-caryophyllene-treated liver section did not show significant pathological changes in kidney and hepatic cells compared with control mice on day 4 at 100 mg/kg/day. This is in accordance with the report of George et al. (2011) who have proven that the hepatoprotective effects of aqueous extract of Aframomum sceptrum (350 mg/kg/day) moderately brought to the central vein, hepatic cell with preserved cytoplasm and prominent nucleus in Plasmodium berghei-infected mice were observed. Adewoye et al. (2010) observed that the bark methanol extract of Chrysophyllum albidum (750–1,500 mg/kg/day) exhibited significant (P < 0.05) schizontocidal activities against Plasmodium berghei berghei (NK 65)-infected mice, and the organ and tissue pathology of hearts, brains, lungs, kidneys, livers, spleens and stomachs of infected and uninfected mice treated with the various doses of methanolic bark extract did not show any appreciable gross and histological changes. Results from this investigation suggest that the isolated compound β-caryophyllene exhibited promising antiplasmodial activities and showed non-toxic to kidney, liver and spleen of Plasmodium berghei (NK65)-infected mice administered at 100 mg/kg/day. It was observed that the artesunate and artelinate administered at 30–240 mg/kg were safer and exhibited less toxic effects in Plasmodium berghei-infected rats whereas it was reported that in the uninfected rats, both drugs caused irreversible vascular irritation, reversible nephrotoxicity and no neurotoxicity at high doses (Li et al. 2007).
In conclusion, myristic acid (1) and β-caryophyllene (2) which are active against in vitro and in vivo antimalarial activity against CQ-sensitive (3D7) strain of Plasmodium falciparum and Plasmodium berghei (NK65), have low cytotoxic effect against HeLa cells and have histopathology of liver, kidney and spleen microscopic examination clearly indicated no pathological changes in the control and purified fraction and combined fraction in treated mice. The compounds myristic acid (1) and β-caryophyllene (2) have been isolated and identified for the first time to study from the leaves of M. koenigii. The structures have been elucidated by systematic analysis of various spectral data obtained for each compound. The isolated compound structures were elucidated by the bioassay-guided fractionation of crude natural plant extract. The present study results revealed the development of natural drug formulations against malarial disease and revealed that the formulation may be safer and environmental friendly and might replace the existing drugs.
References
Adebajo AC, Olayiwola G, Verspohl JE, Iwalewd EO, Omisore NOA, Bergenthal D (2004) Evaluation of the ethnomedical claims of Murraya koenigii. Pharm Biol 42:610–620
Adewoye EO, Salami AT, Taiwo VO (2010) Anti-plasmodial and toxicological effects of methanolic bark extract of Chrysophyllum albidum in albino mice. J Physiol Pathophysiol 1(1):1–9
Agarwal HK, Chhikara BS, Bhavaraju S, Mandal D, Doncel GF, Parang K (2013) Emtricitabine prodrugs with improved anti-HIV activity and cellular uptake. Mol Pharm 10(2):467–476
Alma MH, Mavi A, Yildirim A, Digrak M, Hirata T (2003) Screening chemical composition and in vitro antioxidant and antimicrobial activities of the essential oils from Origanum syriacum L. growing in Turkey. Biol Pharm Bull 26:1725–1729
Arulselvan P, Subramanian SP (2007) Beneficial effects of Murraya koenigii leaves on antioxidant defense system and ultrastructural changes of pancreatic b-cell in experimental diabetes. Chem Biol Interact 165:155–164
Bagavan A, Rahuman AA, Kamaraj C, Geetha K (2008) Larvicidal activity of saponin from Achyranthes aspera against Aedes aegypti and Culex quinquefasciatus (Diptera: Culicidae). Parasitol Res 103(1):223–229
Bagavan A, Rahuman AA, Kamaraj C, Kaushik NK, Mohanakrishnan D, Sahal D (2011a) Antiplasmodial activity of botanical extracts against Plasmodium falciparum. Parasitol Res 108:1099–1109
Bagavan A, Rahuman AA, Kaushik NK, Sahal D (2011b) In vitro antimalarial activity of medicinal plant extracts against Plasmodium falciparum. Parasitol Res 108:15–22
Bero J, Quetin-Leclercq J (2011) Natural products and tropical diseases. Planta Med 77:571
Bhat GP, Surolia N (2001) In vitro antimalarial activity of three plants used in the traditional medicine of India. Am J Trop Med Hyg 65(4):304–308
Carrara IV, Zwang J, Asley AE, Price RA, Stepniewka K, Barends M, Brock-man A, Anderson T, McGready R, Phaiphun L, Proux S, Vugt M, van Hutagalung R, Lwin MK, PyaePhyo A, Preechapornkul P, Imwong M, Pukrittayakamee S, Singhasivanon P, White NJ, Nosten F (2009) Changes in the treatment response to artesunate–mefloquine on the north western border of Thailand during 13 years of continuous deployment. PLoS ONE 4:2
Chowdhury JU, Bhuiyan MNI, Yusuf M (2008) Chemical composition of the essential oils of Murraya koenigii (L.) Spreng and Murraya paniculata (L.) Jack. Bangladesh J Pharmacol 3:59–63
Cornwell PA, Barry BW (1994) Sesquiterpene components of volatile oils as skin penetration enhancers for the hydrophilic permeant 5-fluorouracil. J Pharm Pharmacol 46:261–269
David AF, Philip JR, Simon RC, Reto B, Solomon N (2004) Antimalarial drug discovery: efficiency models for compound screening. Nat Rev 3:509–520
Dondorp AM, Yeung S, Ngguonn C, Day NP, Socheat D, VonSeidlein L (2010) Artemisinin resistance: current status and scenarios for containment. Nat Rev Microbiol 8:272–280
Doria GA, Silva WJ, Carvalho GA, Alves PB, Cavalcanti SC (2010) A study of the larvicidal activity of two Croton species from northeastern Brazil against Aedes aegypti. Pharm Biol 48(6):615–620
Dorin D, Le Roch K, Sallicandro P, Alano P, Parzy D, Poullet P, Meijer L, Doerig C (2001) Pfnek-1, a NIMA-related kinase from the human malaria parasite Plasmodium falciparum. Biochemical properties and possible involvement in MAPK regulation. Eur J Biochem 268:2600–2608
Ene AC, Atawodi SE, Ameh DA, Kwanshie HO, Agomo PU (2008) Experimental induction of chloroquine resistance in Plasmodium berghei NK 65. Trends Med Res 3(1):16–23
George BO, Osioma E, Okpoghono J, Aina OO (2011) Changes in liver and serum transaminases and alkaline phosphatase enzyme activities in Plasmodium berghei infected mice treated with aqueous extract of Aframomum sceptrum. Afr J Biochem Res 5(9):277–281
Ginsburg H, Deharo E (2011) A call for using natural compounds in the development of new antimalarial treatment an introduction. Malar J 10(Suppl 1):S1
Greenwood BM, Bojang K, Whitty CJM, Targett GAT (2005) Malaria. Lancet 365:1487–1498
Gupta P, Vasudeva N (2010) In vitro antiplasmodial and antimicrobial potential of Tagetes erecta roots. Pharm Biol 48(11):1218–1223
Gurib-Fakim A (2006) Medicinal plants: tradition of yesterday and drugs of tomorrow. Mol Asp Med 27:1–93
Iwalewa EO, Oguntoye L, Rai PP, Iyaniwura TT (1997) In vivo and in vitro antimalarial activity of two crude extracts of Cassia occidentalis leaf. Niger J Pharm Sci 5:23–28
Kamaraj C, Kaushik NK, Mohanakrishnan D, Elango G, Bagavan A, Zahir AA, Rahuman AA, Sahal D (2012a) Antiplasmodial potential of medicinal plant extracts from Malaiyur and Javadhu hills of South India. Parasitol Res 111(2):703–715
Kamaraj C, Kaushik NK, Rahuman AA, Mohanakrishnan D, Bagavan A, Elango G, Zahir AA, Santhoshkumar T, Marimuthu S, Jayaseelan C, Kirthi AV, Rajakumar G, Velayutham K, Sahal D (2012b) Antimalarial activities of medicinal plants traditionally used in the villages of Dharmapuri regions of South India. J Ethnopharmacol 141(3):796–802
Kaushik NK, Bagavan A, Rahuman AA, Mohanakrishnan D, Kamaraj C, Elango G, Zahir AA, Sahal D (2013) Antiplasmodial potential of selected medicinal plants from eastern Ghats of South India. Exp Parasitol 134(1):26–32
Keluskar P, Ingle S (2012) Ethnopharmacology guided screening of traditional Indian herbs for selective inhibition of Plasmodium specific lactate dehydrogenase. J Ethnopharmacol 144:201–207
Khan BA, Abraham A, Leelamma S (1995) Haematological and histological studies after curry leaf (Murraya koenigii) & mustard (Brassica juncea) feeding in rats. Indian J Med Res 102:184–186
Khaomek P, Ichino C, Ishiyama A, Sekiguchi H, Namatame M, Ruangrungsi N, Saifah E, Kiyohara H, Otoguro K, Omura S, Yamada H (2008) In vitro antimalarial activity of prenylated flavonoids from Erythrina fusca. J Nat Med 62(2):217–220
Kubo I, Chaudhuri SK, Kubo Y, Sanchez Y, Ogura T, Saito T, Ishikawa H, Haraguchi H (1996) Cytotoxic and antioxidative sesquiterpenoids from Heterotheca inuloides. Planta Med 62:427–430
Kumari P, Sahal D, Jain SK, Chauhan VS (2012) Bioactivity guided fractionation of leaves extract of Nyctanthes arbor tristis (Harshringar) against P. falciparum. PLoS One 7(12):e51714
Lee SJ, Park WH, Moon HI (2009) Bioassay-guided isolation of antiplasmodial anacardic acids derivatives from the whole plants of Viola websteri Hemsl. Parasitol Res 104(2):463–466
Li X, Rieckmann K (1992) A bioassay for derivatives of qinghaosu (artemisinin). Trop Med Parasitol 43:195–196
Li JWH, Vederas JC (2009) Drug discovery and natural products: end of an era or an endless frontier? Science 325:161–165
Li Q, Xie LH, Johnson TO, Si Y, Haeberle AS, Weina PJ (2007) Toxicity evaluation of artesunate and artelinate in Plasmodium berghei-infected and uninfected rats. Trans R Soc Trop Med Hyg 101(2):104–112
Liu CH, Huang HY (2012) Antimicrobial activity of curcumin-loaded myristic acid microemulsions against Staphylococcus epidermidis. Chem Pharm Bull (Tokyo) 60(9):1118–1124
Lourens AC, Reddy D, Baser KH, Viljoen AM, Van Vuuren SF (2004) In vitro biological activity and essential oil composition of four indigenous South African Helichrysum species. J Ethnopharmacol 95:253–258
Macleod JK, Rasmussen HB (1999) A hydroxy-β-caryophyllene from Pterocaulon serrulatum. Phytochem 50:105–108
Malwal M, Sarin R (2010) Chemical characterization and antimicrobial screening of volatile components of Murraya koenigii (L.) Spreng—an Indian aromatic tree. J Pharm Res 3(8):1782–1784
Mathew N, Ayyanar E, Shanmugavelu S, Muthuswamy K (2013) Mosquito attractant blends to trap host seeking Aedes aegypti. Parasitol Res 112(3):1305–1312
Moore BR, Jago JD, Batty KT (2008) Plasmodium berghei: parasite clearance after treatment with dihydroartemisinin in an asplenic murine malaria model. Exp Parasitol 118:458–467
Morais TR, Romoff P, Fávero OA, Reimão JQ, Lourenço WC, Tempone AG, Hristov AD, Di Santi SM, Lago JH, Sartorelli P, Ferreira MJ (2012) Anti-malarial, anti-trypanosomal, and anti-leishmanial activities of jacaranone isolated from Pentacalia desiderabilis (Vell.) Cuatrec. (Asteraceae). Parasitol Res 110(1):95–101
Mosmann T (1983) Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods 65:55–63
Mueller D, Davis RA, Duffy S, Avery VM, Camp D, Quinn RJ (2009) Antimalarial activity of azafluorenone alkaloids from the Australian tree Mitrephora diversifolia. J Nat Prod 72(8):1538–1540
Muller O (2011) Challenges for control and elimination in the 21st century. In: Razum O (ed) Malaria in Africa, vol 60. Peter Lang, Frankfurt, p 193
Ndjonka D, Bergmann B, Agyare C, Zimbres FM, Luersen K, Hensel A, Wrenger C, Liebau E (2012) In vitro activity of extracts and isolated polyphenols from West African medicinal plants against Plasmodium falciparum. Parasitol Res 111(2):827–834
Newman DJ, Cragg GM (2012) Natural products as sources of new drugs over the 30 years from 1981 to 2010. J Nat Prod 75:311–335
Noedl H, Se Y, Socheat D, Fukuda M (2008) Evidence of artemisinin-resistant malaria in western Cambodia. N Engl J Med 359:2619–2620
Onayade OA, Adebajo AC (2000) Composition of the leaf volatile oil of Murraya koenigii growing in Nigeria. J Herbs Spices Med Plants 7(4):59–66
Ondo JP, Lekana-Douki JB, Bongui JB, Zang Edou ES, Zatra R, Toure-Ndouo FS, Elomri A, Lebibi J, Seguin E (2012) In vitro antiplasmodial activity and cytotoxicity of extracts and fractions of Vitex madiensis, medicinal plant of Gabon. Trop Med Int Health 17(3):316–321
Orwa JA, Ngeny L, Mwikwabe NM, Ondicho J, Jondiko IJ (2013) Antimalarial and safety evaluation of extracts from Toddalia asiatica (L) Lam. (Rutaceae). J Ethnopharmacol 145(2):587–590
Peter LT, Anatoli VK (1998) The current global malaria situation. Malaria: parasite biology, pathogenesis and protection. ASM Press, Washington, DC, pp 11–22
Peters W, Portus JH, Robinson BL (1975) The chemotherapy of rodent malaria. XXII. The value of drug-resistant strains of Plasmodium berghei in screening for blood schizontocidal activity. Ann Trop Med Parasitol 69:155–171
Phyo AP, Nkhoma S, Stepniewska K, Ashley EA, Nair S, McGready R, Ler Moo C, Al-Saai S, Dondorp AM, Lwin KM, Singhasivanon P, Day NPJ, White NJ, Anderson TJ C, Nosten F (2012) Emergence of artemisinin-resistant malaria on the western border of Thailand: a longitudinal study. Lancet 379:1960–1966
Raina VK, Lal RK, Tripathi S, Khan M, Syamasundar KV, Srivastava SK (2002) Essential oil composition of genetically diverse stocks of Murraya koenigii from India. Flav Fragr J 17:144–146
Ramalhete C, Lopes D, Mulhovo S, Molnar J, Rosario VE, Ferreira MU (2010) New antimalarials with a triterpenic scaffold from Momordica balsamina. Bioorganic Med Chem 18:5254–5260
Rana VS, Juyal JP, Rashmi Blazquez MA (2004) Chemical constituents of the volatile oil of Murraya koenigii leaves. Int J Aromather 14:23–25
Rao LJM, Ramalakshmi K, Borse BB, Raghavan B (2007) Antioxidant and radical scavenging carbazole alkaloids from the oleoresin of curry leaf (Murraya koenigii Spreng). Food Chem 100:742–747
Rukunga GM, Muregi FW, Tolo FM, Omar SA, Mwitari P, Muthaura CN, Omlin F, Lwande W, Hassanali A, Githure J, Iraqi FW, Mungai GM, Kraus W, Kofi-Tsekpo WM (2007) The antiplasmodial activity of spermine alkaloids isolated from Albizia gummifera. Fitoterapia 78(7–8):455–459
Sathaye S, Bagul Y, Gupta S, Kaur H, Redkar R (2011) Hepatoprotective effects of aqueous leaf extract and crude isolates of Murraya koenigii against in vitro ethanol induced hepatotoxicity model. Exp Toxicol Pathol 63:587–591
Severino VG, Cazal Cde M, Forim MR, da Silva MF, Rodrigues-Filho E, Fernandes JB, Vieira PC (2009) Isolation of secondary metabolites from Hortia oreadica (Rutaceae) leaves through high-speed counter-current chromatography. J Chromatogr A 1216(19):4275–4281
Shakya A (2012) Antimalarial activity of Acacia nilotica plant on Plasmodium berghei in mice. IJGHC 1(2):145–150
Shuaibu MN, Wuyep PA, Yanagi T, Hirayama K, Tanaka T, Kouno I (2008) The use of microfluorometric method for activity-guided isolation of antiplasmodial compound from plant extracts. Parasitol Res 102(6):1119–1127
Silva LFRE, Pinto ACS, Pohlit AM, Quignard EL, Vieira PP, Tadei WP, Chaves FC, Samonek JF, Lima CA, Costa MR, Alecrim M, Andrade-Neto VF (2011) In vivo and in vitro antimalarial activity of 4-nerolidylcatechol. Phytother Res 25(8):1181–1188
Simonsen HT, Nordskjold JB, Smitt UW, Nyman U, Palpu P, Joshi P, Varughese G (2001) In vitro screening of Indian medicinal plants for antiplasmodial activity. J Ethnopharmacol 74(2):195–204
Singh G, Marimuthu P, de Heluani CS, Catalan CA (2006) Antioxidant and biocidal activities of Carum nigrum (seed) essential oil, oleoresin, and their selected components. J Agric Food Chem 54:174–181
Sivakumar R, Jebanesan A, Govindarajan M, Rajasekar P (2011) Larvicidal and repellent activity of tetradecanoic acid against Aedes aegypti (Linn.) and Culex quinquefasciatus (Say.) (Diptera: Culicidae). Asian Pac J Trop Med 4(9):706–710
Smilkstein M, Sriwilaijaroen N, Kelly JX, Wilairat P, Riscoe M (2004) Simple and inexpensive fluorescence-based technique for high throughput antimalarial drug screening. Antimicrob Agents Chemother 48:1803–1806
Son IH, Chung IM, Lee SJ, Moon HI (2007) Antiplasmodial activity of novel stilbene derivatives isolated from Parthenocissus tricuspidata from South Korea. Parasitol Res 101(1):237–241
Trager W, Jensen JB (1976) Human malaria parasites in continuous culture. Science 193:673–675
Traore M, Diallo A, Nikiema JB, Tinto H, Dakuyo ZP, Ouedraogo JB, Guissou IP, Guiguemde TR (2008) In vitro and in vivo antiplasmodial activity of ‘saye’, an herbal remedy used in Burkina Faso traditional medicine. Phytother Res 22(4):550–551
Tundis R, Loizzo MR, Bonesi M, Menichini F, Dodaro D, Passalacqua NG, Statti G, Menichini F (2009) In vitro cytotoxic effects of Senecio stabianus Lacaita (Asteraceae) on human cancer cell lines. Nat Prod Res 23(18):1707–1718
Walde SG, Jyothirmayi T, Rao PGP, Srinivas P (2006) Flavour volatiles of flowers and stalks of Murraya koenigii L. Flav Fragr J 21:581–584
WHO (World Health Organization) (2012) World malaria report. World Global Malaria Program. http://www.who.int/malaria/publications/world_malaria_report_2012/wmr2012_full_report.pdf
Willcox M, Bodeker R, Rasoanaivo P (2004) Traditional herbal medicines for modern times. Traditional medicinal plants and malaria. CRC, Boca Raton, p 431
Zhang WP, Ruan WB, Deng YY, Gao YB (2012) Potential antagonistic effects of nine natural fatty acids against Meloidogyne incognita. J Agric Food Chem 60(46):11631–11637
Zofou D, Kengne AB, Tene M, Ngemenya MN, Tane P, Titanji VP (2011) In vitro antiplasmodial activity and cytotoxicity of crude extracts and compounds from the stem bark of Kigelia africana (Lam.) Benth (Bignoniaceae). Parasitol Res 108(6):1383–1390
Zofou D, Tene M, Tane P, Titanji VP (2012) Antimalarial drug interactions of compounds isolated from Kigelia africana (Bignoniaceae) and their synergism with artemether, against the multidrug-resistant W2mef Plasmodium falciparum strain. Parasitol Res 110(2):539–544
Acknowledgments
The authors are grateful to C. Abdul Hakeem of the College Management and Dr. S. Y. Anver Sheriff, Principal and Dr. Hameed Abdul Razack, HOD of Zoology Department for providing the facilities to carry out this work. We thank the Management of VIT University, Vellore for providing necessary spectral analysis facilities to carry out this study. We are thankful to Dr. C.R. Pillai, Emeritus Medical Scientist, National Institute of Malaria Research, Delhi, India for providing the Plasmodium berghei (NK65) strain. Chinnaperumal Kamaraj gratefully thanks CSIR, New Delhi for Senior Research Fellowship (CSIR Sc. No. 8/524 (0005)/2011EMR-1).
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Kamaraj, C., Rahuman, A.A., Roopan, S.M. et al. Bioassay-guided isolation and characterization of active antiplasmodial compounds from Murraya koenigii extracts against Plasmodium falciparum and Plasmodium berghei . Parasitol Res 113, 1657–1672 (2014). https://doi.org/10.1007/s00436-014-3810-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00436-014-3810-3