Abstract
In order to assess the potential of the stem bark of Kigelia africana (Lam.) Benth as source of new anti-malarial leads, n-hexane and ethyl acetate (EtOAc) extracts and four compounds isolated from the stem bark were screened in vitro against the chloroquine-resistant W-2 and two field isolates of Plasmodium falciparum using lactate dehydrogenase assay. The products were also tested for their cytotoxicity on LLC/MK2 monkey kidney cells. The EtOAc extract exhibited a significant antiplasmodial activity (IC50 = 11.15 μg/mL on W-2; 3.91 and 4.74 μg/mL on field CAM10 and SHF4 isolates, respectively), whereas the n-hexane fraction showed a weak activity (IC50 = 73.78 μg/mL on W-2 and 21.85 μg/mL on SHF4). Three out of the four compounds showed good activity against all the three different parasite strains (IC50 < 5 μM). Specicoside exhibited the highest activity on W-2 (IC50 = 1.54 μM) followed by 2β, 3β, 19α-trihydroxy-urs-12-en-28-oic acid (IC50 = 1.60 μM) and atranorin (IC50 = 4.41 μM), while p-hydroxycinnamic acid was the least active (IC50 = 53.84 μM). The EtOAc extract and its isolated compounds (specicoside and p-hydroxycinnamic acid) were non-cytotoxic (CC50 > 30 μg/mL), whereas the n-hexane extract and two of its products, atranorin and 2β, 3β, 19α-trihydroxy-urs-12-en-28-oic acid showed cytotoxicity at high concentrations, with the last one being the most toxic (CC50 = 9.37 μg/mL). These findings justify the use of K. africana stem bark as antimalaria by traditional healers of Western Cameroon, and could constitute a good basis for further studies towards development of new leads or natural drugs for malaria.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The emergence and spread of resistance to almost all the anti-malarial drugs including artemisinin-based combinations is a clear indication that there is an urgent need to discover new anti-malarials. In the absence of an effective vaccine, it is obvious that the fight against malaria will continue to rely on the use of drugs for treatment and vector control (Ridley 2002).
Despite the recent successes in rational drug design and synthetic chemistry techniques by pharmaceutical companies, natural products and particularly medicinal plants remain an important source of new drugs (Geysen et al. 2003; Lombardino and Lowe 2004). The potential for finding new compounds is enormous as only about 1% of the estimated 250,000 flowering tropical plant species have been studied for their pharmaceutical potential so far (Jachak and Arvind 2007). According to Newman et al. in (2003) 61% of the 877 small molecules introduced as drugs worldwide between 1981 and 2002 were inspired by natural products. These include natural products and derivatives, and synthetic compounds designed from natural products or with natural product pharmacophores (Newman et al. 2003). Several products of plant origin have recently been introduced to the market, like artemether and artesunate derived from artemisinin isolated from Artemisia annua. Many others like Gossypol and derivatives from cotton seeds are currently involved in late-phase clinical trials (Razakantoanina et al. 2000). Natural products formulation approaches are currently highly encouraged as they are more likely to procure local and affordable remedies to the diseases in poor and vulnerable communities of tropical regions (Titanji et al. 2008).
The plant family Bignoniaceae has been shown to be one of the most promising sources of anti-malarial compounds. For example, lapachol, a hydroxynaphthoquinone with anti-malarial, antifungal, antibacterial and anticancer activity, which is present in many members of the Bignoniaceae, was used as template for the synthetic anti-malarial atovaquone (Schwikkard and Van Heerden 2002). Kigelia africana (Lam.) Benth. syn. Kalanchoe pinnata (Jacq.) DC a member of this family is widely used across the African continent to treat malaria. The species is widely distributed in South, Central and West Africa, and it is known as cucumber or sausage tree because of its huge fruits (average 0.6 m in length and 4 kg in weight). It spreads abundantly across wet savannah and riverside areas. This plant has a long history of use by rural and African countries particularly for medicinal properties. Several parts of the plant are employed in folk medicine to treat a wide range of skin ailments. It also has internal application including the treatment of dysentery, ringworm, tapeworm, postpartum haemorrhage and malaria (Jachak and Saklani 2009). Previous investigations by Clarkson et al. in (2004) revealed a moderate activity of methylene chloride crude extract of leaves (with an IC50 of 51 μg/mL) on the chloroquine-sensitive strain (D10) of Plasmodium falciparum. In the Western region of Cameroon, traditional healers use the stem bark of this plant, either boiled in water or powdered and mixed with palm oil for ingestion as treatment of malaria and other fevers. The use of the stem bark has also been reported that in South Western Nigeria, where an infusion is prepared mixed with stem bark of Anarcardium occidentalis, Mangifera indica and Terminalia catappa (Oladele and Adewunmi 2008). However, to the best of our knowledge, K. africana stem bark especially the Cameroonian species, is yet to be investigated for its claimed anti-malarial potential. This prompted us to investigate the antiplasmodial properties of extracts and pure compounds of the stem bark of K. africana.
Material and methods
Collection of plant materials
Stem bark of K. Africana was collected in Bandjoun (Kong Khi division, Western Region, Cameroon) in December 2008. The sample identification was confirmed by Mr. Tadjouteu, botanist at the Cameroon National Herbarium in Yaounde, where a voucher specimen was deposited (Voucher No 20527/SRF/Cam).
Preparation of crude extracts
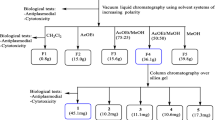
The air-dried and powdered plant material (5 kg) was macerated for 3 days at room temperature in 15 L of a methylene chloride/methanol (1:1) mixture, filtered with Whatman paper and the solvent evaporated using a Rotavapor system (BÜCHI Labortechnik AG, Switzerland). The crude extract obtained (750 g) was defatted with n-hexane followed by EtOAc.The n-hexane and EtOAc soluble portions obtained were then respectively concentrated to dryness affording 33 and 72 g of viscous residues further stored at 4°C.
Fractionation, purification and characterization of pure compounds
Thirty grammes of the n-hexane extract (KAEh) were chromatographed in a 70–230 mesh silica gel (400 g) column with stepwise gradient elution by n-hexane/EtOAc mixtures. The 52 fractions collected were combined, according to their TLC profiles on pre-coated Kiesegel 60 F254 plates developed with n-hexane/EtOAc mixtures, to give four major fractions: (a) 8 g, n-hexane/EtOAc 9:1, (b) 2 g, n-hexane/EtOAc 4:1 and 7:3, (c) 2.8 g, n-hexane/EtOAc 3:2 and 1:1 and (d) 6 g, EtOAc. These major fractions were purified through silica gel column chromatography yielding after re-crystallisation of generated sub-fractions three pure products codified KAE1 (from a), KAE3 (from d) and KAE5 (from c). 70 g of EtOAc extract were similarly fractionated through silica gel (1 kg) column chromatography eluting with n-hexane/EtOAc mixtures followed by EtOAc/MeOH mixtures of increasing polarity. Fractions eluted with AcOEt/MeOH 9:1 and 17:3 afforded after filtration KAE7. Those eluted with EtOAc gave KAE10 after re-crystallisation in methylene chloride. The structures of isolated products were elucidated using spectroscopic analysis as previously described Tane et al. (2005).
Parasite strains
The W-2 (MRA-157) strain was ordered from MR4 (Manassas, VA, USA), and maintained in continuous culture. The field isolates (named CAM10 and SHF4) were collected from Bolifamba, a small town in Fako division, South West Region of Cameroon, stored in liquid nitrogen and used as required.
P. falciparum culture and maintenance
Field isolates and laboratory strains of P. falciparum were grown and maintained in culture using the method of Trager and Jensen with some modification (Nkhoma et al. 2007). Cultures consisted of a 4% hematocrit suspension of O + human erythrocytes in RPMI 1640 medium (Sigma Aldrich Inc.) supplemented with gentamicin solution at 0.01 mg/mL, 25 mM HEPES buffer, 25 mM NaHCO3 and 1% Albumax II (Gibco; Invitrogen, USA). Cultures were fed with a gas mixture containing 5% CO2 and incubated at 37°C.
The estimation of the parasitaemia, as well as parasite visualisation before incubation, was done using both fluorescence (acridine orange) and light (Giemsa stain) microscopes.
Determination of in vitro antiplasmodial activity
Drug sensitivity assay was carried out in 96-well microtitration plates as described by Ngemenya et al. (2006) with some modifications. Drugs were dissolved in dimethyl sulfoxide (200 μL) and pre-diluted with culture medium to make a final concentration of 1,000 μg/mL for crude extracts and 50 μg/mL for pure compounds. All stock solutions were sterilized by passing them through a 0.2-μm filter and stored at −20°C until required. Similarly, chloroquine stock was prepared from liquid chloroquine phosphate (Greenfield Pharmaceutical LTD, Jiang Su, China).
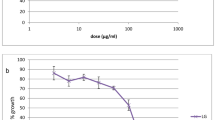
Dose–response assay was carried out to obtain the 50% inhibitory concentration (IC50) of the individual drugs. Ring stage-infected erythrocytes (100 μL per well with 2% hematocrit and 1% parasitaemia) were incubated in triplicate with twofold serial dilution of each drug for 48 h. Each experiment was performed in triplicate separate experiment. Parasitaemia was measured using the parasite lactate dehydrogenase (pLDH) assay.
Lactate dehydrogenase (pLDH) assay
While incubation of parasites with products is going on, the two reagents for detecting and measuring the LDH enzyme were prepared (Nkhoma et al. 2007 ). The first of these is the Malstat reagent, which was made by dissolving 400 μL of Triton X-100 in 80 mL of deionised water, adding L-lactate (4.00 g), Tris buffer (1.32 g), and 0.022 g of 3- acetylpyridine adenine dinucleotide, adjusting the pH to 9 with hydrochloric acid, and bringing the volume up to 200 mL with deionised water. The second reagent, NBT/PES solution, was prepared by dissolving nitro blue tetrazolium salt (0.160 g) and phenazine ethosulfate (0.008 g) in 100 mL of deionized water. The solution was stored in a foil-covered container and kept in the refrigerator until required. All reagents for preparing the Malstat reagent and NBT/PES solution were purchased from Sigma-Aldrich Inc (Germany). When incubation was complete, plates were harvested and subjected to three 20-min freeze–thaw cycles to release the cell contain and re-suspend the culture. Thereafter, 100 μL of Malstat reagent and 25 μL of NBT/PES solution were added to each well of a new, triplicate flat-bottomed 96-well microtiter plate. The culture in each well was mixed and 20 μL of the culture taken from each well and added to the corresponding well of the Malstat plate, 25 μL of NTB/PES were then added to each well, thereby initiating the LDH reaction. Colour development of the LDH plate was monitored colorimetrically at 650 nm with the aid of a plate reader (Emax-Molecular Devices Corporation, CA, USA) after an hour of incubation in the dark.
Analysis of test results from the LDH assay
The LDH assay generates optical density (OD) values at various concentrations of the drug as raw data. OD values from control wells represent the maximum amount of LDH that is produced by parasites and OD values from blank wells represent background LDH activity. A 100% growth value, which corresponds to maximum LDH activity, was obtained by subtracting the mean OD value of blank wells from that of control wells. Likewise, the growth value at each concentration of the drug was obtained by adjusting OD values from drug-treated wells for background LDH activity (parasite-free red blood cells). These values were then expressed as a percentage of the 100% growth value and plotted against corresponding concentrations of the drug using GraphPad Software (San Diego CA, USA, www.graphpad.com) to generate log dose–response curves from which IC50 values were obtained. Each extract or pure products were tested in triplicate. IC50 values were log-transformed and expressed as geometric mean IC50 and 95% confidence intervals for the geometric mean calculated for the replicates.
Cytotoxicity study of active compounds and extracts
The cytotoxicities of the extract and pure compounds were estimated against LLC-MK2 monkey kidney epithelial cells according to the procedure previously described (Malebo et al. 2009) with some modifications. Cells were cultured in the same conditions as P. falciparum. For the determination of extracts and pure compounds toxicity, cells were distributed in 96-well plates at 20,000 cells per well in 100 μL culture medium. Cells were allowed to attach for 24 h, the medium removed completely the next day, and 100 μL of fresh medium added to all the wells. Then, 100 μL of crude extract (1,000 μg/mL) or pure compounds (200 μg/mL) were added in triplicate in raw H and two-fold serial dilution made upward ending at raw B, and 100 μL of medium added to all the wells to have concentration range of 250–3.90 (crude extract) or 50–0.78 (pure compounds), cells at raw A serving as control without drug. The plates were incubated for 72 h at 37°C in 5% gas mixture. Cell concentration and viability in the presence of extract or pure compounds were compared with that of control cultures without extracts. The definition of the cytotoxicity used (Malebo et al. 2009) was CC50 < 1.0 μg/ml (high cytotoxicity); CC50 1.0–10.0 μg/ml (moderate); CC50 10.0–30.0 μg/ml (mild); and CC50 > 30 μg/ml (nontoxic). The selectivity index defined as SI = CC50/IC50 was also considered.
Results
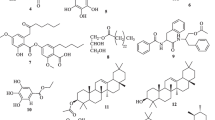
Chemical characterization of the compounds isolated
Table 1 below summarizes the chemical characterization of the five compounds isolated from K. africana stem bark.
Antiplasmodial activity of isolated products
Using the pLDH assay, the n-hexane (KAEh) and ethyl acetate (KAEae) extracts and four of their products (KAE1, KAE3, KAE7 and KAE10) were assayed against W-2, chloroquine-resistant laboratory strain and two field isolates (CAM10 and SHF4). The IC50 values of the different products tested and that of chloroquine phosphate (CQ) are shown in Tables 2 and 3 below.
The ethyl acetate extract was moderately active against the W-2 strain and more considerably active against the two field isolates, while the n-hexane extract presented a weak activity against W-2 and a low activity against the SHF4 field isolate. Three of the four pure compounds showed good antiplasmodial activity with IC50 < 5 nM on the three parasite strains. The ability of chloroquine to inhibit parasite growth was shown to be more potent that the one of the pure products extracted from the K. africana stem bark by 5–15, 8–28 and 14–42-folds on W-2, CAM10 and SHF4, respectively. Chloroquine was used to assess the sensitivity of the field isolates. The IC50s obtained on both isolates are significantly higher than 0.1 μM indicating that CAM10 and SHF4 used in this study were chloroquine-resistant.
Cytotoxicity study of isolated products
Cytotoxicity test of all the products assayed for anti-malarial activity was carried out on LLC/MK2 monkey kidney epithelial cells; the results are summarized in Table 4. The two crude extracts presented the same level of toxicity (SI value of 11.15 for W-2). But considering CC50s, KAEh appeared to be more moderately toxic, whereas KAEae had mild cytotoxicity. All the two products isolated from the hexane extract were toxic against the mammalian cells, with KAE3 having the lowest selectivity index, below 8 for SHF4.
Discussion
We isolated five products from the stem bark of K. Africana and tested four of them against the chloroquine-resistant W-2 laboratory strain and two field isolates (CAM10 and SHF4).
Characteristics of the compounds isolated
All the four compounds are well known and had been isolated from other plant species, but none of them was known before as occurring in K. africana. It was also the first time that their anti-malarial activities were investigated.
From its spectral data, KAE1 was identified to be atranorin previously isolated from Hopea sangal (Dipterocarpaceae) by Nasser et al. (2009). Likewise, 2β, 3β, 19α-trihydroxy-urs-12-en-28-oic acid, isolated and codified as KAE3, is a well-known molecule, from Paradrymonia macrophylla (Gesneriaceae) (Masao et al. 1988). The 2β-hydroxyoleanolic acid (KAE5) was also previously isolated from a different plant, the Phytolaccaceae Phytolacca dodecandra (Parkurst et al. 1990). KAE5 was structurally very close to KAE3 from which it differs with two methyl groups at positions 19 and 20. The structure of KAE7 was described by Tetsuo et al. (1990) who attributed the name of specicoside to the molecule isolated from the plant Radermachia sinica of the Bignoniaceae family. The structure of KAE10 was found to perfectly similar to a portion of KAE7. One could easily think that this product just occurred as a result of a cleavage of KAE7, but considering the quantity of KAE10 (about 2/3 of that of KAE7), this hypothesis is hardly valuable. Furthermore, literature search revealed KAE10 as a well-known compound previously isolated from the Rhizome of Agropyron repens (Koetter et al. 1994). In general, KAE7 happened to be the most abundant compound from ethyl acetate extract of K. africana, followed by KAE10.
Antiplasmodial activity
The activities obtained for each product vary widely from one parasite strain to another indicating the genetic differences among the strains used. The results obtained indicate that n-hexane extract showed a weak activity against the W-2 laboratory strain and considerable activity on the SHF4 field isolate. The ethyl acetate extract for its own was considerably active (IC50 < 50 μg/mL) on W-2 and very active (IC50 < 5 μg/mL) against the both field isolates.
The in vitro antiplasmodial properties of K. africana has previously been reported for the leaves, roots and wood, but we did not find any report on the activity of the stem bark. Clarkson et al. in (2004) investigated the in vitro antiplasmodial activity of the leaves of the plant from South Africa using different solvent systems and observed a wide range of activity with methylene chloride extract having 51 μg/mL against the chloroquine-sensitive D10 strain of P. falciparum. IC50 range obtained with stem bark (21–73 μg/mL) using the same solvent are therefore comparable to these previous finding, and confirm the antiplasmodial properties of K. africana. Studies carried out on the leaves collected from Kenya showed highest activity, with ethyl acetate extract presenting the lowest IC50 compared to methylene chloride extract, 29.01 and 13.50 μg/mL on D6 strain (Akeng'a Ayuko et al. 2009). From the present study, the activity of the ethyl acetate extract was also higher than the one of the non-polar hexane extract (11.15 and 73.98 μg/mL, respectively on chloroquine-resistant W-2). These findings, corroborating with the previous results, may indicate that the most active ingredients of K. africana are polar compounds. Four naphtoquinones isolated from the plant were also tested for their antiplasmodial properties and exhibited a good antiplasmodial activity with 2-(1-hydroxyethyl) naphtha [2, 3-b] furan-a, 9-dione being the most active with an IC50 of 627nM for the K1 and 718 nM for T9-96 strains (Carvalho et al. 1988).
In the western region of Cameroon, the stem bark of the plant prepared as infusion, water decoction or mixed with palm oil is commonly used to treat malaria and inflammatory ailments. The presence of active ingredients in both n-hexane and ethyl acetate extracts seems to justify the use of so diversified solvent systems in the preparation of traditional remedies from K. africana.
Four out of the five isolated compounds were submitted to antiplasmodial testing against P. falciparum malaria parasite following the testing of the crude extracts, among which: KAE1, KAE3, KAE7 and KAE10. Three exhibited significant activity against all the three different parasite strains (IC50 < 5 μg/mL). KAE7 showed the highest activity followed by KAE3 and KAE1, while KAE10 was the least active. The very low activity of KAE10 compared to KAE7 (about 35-fold for W-2 and 3-fold for the both field isolates) may indicate that the glycosidic or irridoic portions of KAE7 are crucial for antiplasmodial activity. KAE3 was shown to be a 2, 19-dihydroxylated form of tomentosolic acid, a compound isolated from Spathodea campanulata and showed considerable in vivo anti-malarial activity against Plasmodium berghei (Amusan et al. 1990). Several terpenoids have been isolated from different medicinal plants with anti-malarial activity. For example, the epi-oleanolic acid, a triterpenoid isolated from petroleum extract of Viola verecunda was shown to display high antiplasmodial activity with an IC50 0.18 μg/ml on the chloroquine-resistant FcB1 strain of P. falciparum (Moon et al. 2006). The anti-malarial properties of terpenoids have also been demonstrated in mice by (2008). Ineupatorolides A, a sesquiterpene isolated from Carpesium rosulatum (Compositae) was found to have a significant blood schizontocidal activity in 4-day early infection by P. berghei in mice. One of the most interesting cases of anti-malarial terpenoids is gedunin isolated for the first time from Azadiracta indica by Bray et al. in (1990) and which was about three times more active than chloroquine. Comparison of activities of gedurin and dihydrogerudin suggested that the reduction of the double bond in αβ-unsaturated keto function lead to a decrease of anti-malarial activity and increase in toxicity (Saxena et al. 2003). It was already established that αβ-unsaturated keto function is an important feature for anti-malarial activity in quassinoids (Adoum 2008). Oxidizing the hydroxyl groups of KAE3 may therefore increase its anti-malarial activity while decreasing the toxicity.
Cytotoxicity
The crude extracts and pure products isolated from K. africana were screened for cytotoxicity against the LLC/MK2 monkey kidney epithelial cells. The results indicate that hexane extract was cytotoxic at high concentration (CC50 = 125 μg/mL). The two products isolated from this extract also presented mild (KAE1; CC50 = 27.78 μg/mL) to moderate (KAE3; CC50 = 9.37 μg/mL) cytotoxicity, confirming the result obtained with their mother crude extract. Based on their selectivity index, the hexane extract were toxic (SI = 1.69 on W-2; 5.72 on SHF4). However, none of the products could be considered toxic, except KAE3 which had an SI less than 8 for the SHF4 field isolate. The ethyl acetate extract was nontoxic (CC50 < 30 μg/mL) and this observation was confirmed by the derived products (KAE7 and KAE10). Brine shrimp lethality bioassay was carried out by Adoum in (2008) using Artemia salina (Leach) eggs also showed the polar extract of stem bark of K. africana to be safe.
In conclusion, the results obtained from the present study show that ethyl acetate extract of K. africana stem bark has a higher anti-malarial activity and least cytotoxicity than the hexane extract, indicating that polar solvents are more appropriate in the preparation of traditional medicine from this plant. Both extracts contain ingredients with significant antiplasmodial activity with 2β, 3β, 19α-trihydroxy-urs-12-en-28-oic acid, atranorin and specicoside being the most active. These findings justify the use of stem bark of K. africana by traditional healers of the West region of Cameroon for the treatment of malaria. Therefore, there is a need to advance the work on the products isolated through further in vivo testing in animal models of malaria followed by sub-acute and chronic toxicity tests. This is likely to reveal suitable candidate molecules which may serve as leads and which can be optimized followed by development into new anti-malarials.
References
Adoum OA (2008) Determination of toxicity effects of some savannah plants using brine shrimp test (BST). Int J P App Sci 2(3):1–5
Akeng'a Ayuko T, Njihia Njau R, Cornelius W, Leah N, Ndiege IO (2009) In vitro antiplasmodial activity and toxicity assessment of plant extracts used in traditional malaria therapy in the Lake Victoria Region. Mem Inst Oswaldo Cruz 104(5):689–694
Amusan OOG, Adesogan EK, Makinde JM (1990) Antimalarial active principles of Spathoda companulata stem bark. Phytother Res 10(8):692–693
Bray DH, Warhurst DC, Connolly JD, O'Neil MJ, Phillipson (1990) Plants as source of antimalarial drug. Pt.7 activity of some species of Meliaceae plants and their constituent limonoids. Phytother Res 4(1):29–35
Carvalho LH, Rocha EMM, Raslan DS, Oliveira AB, Krettli AU (1988) In vitro activity of natural and synthetic naphthoquinones against erythrocytic stages of Plasmodium falciparum. Braz J Med Biol Res 21(3):485–487
Clarkson C, Maharaj VJ, Crouch NR, Grace OM, Matsabisa PPMG, Bhagwandin N et al (2004) In vitro antiplasmodial activity of medicinal plants native to or naturalised in South Africa. J Ethnopharmacol 92(2–3):177–191
Geysen HM, Schoenen F, Wagner D, Wagner R (2003) Combinatorial compound libraries for drug discovery: an ongoing challenge. Nat Rev Drug Discov 2(3):222–230
Jachak M, Arvind Saklani (2007) Challenges and opportunities in drug discovery from plants. Sanjay Current Sci 92(9):1252–1257
Jachak SM, Saklani A (2009) Kigelia africana (Lam) Beth—an overview. Nat Prod Rad 8(2):190–197
Koetter U, Kaloga M, Schilcher H (1994) Isolation and structure elucidation of p-Hydroxycinnamic acid esters from the rhizom of Agropyron repens, Part II. Planta Med 60(5):488–489
Lombardino JG, Lowe JA III (2004) The role of the medicinal chemist in drug discovery—then and now. Nat Rev Drug Discov 3(10):853–862
Malebo HM, Tanja W, Cal M, Swaleh SAM, Omolo MO, Hassanali A et al (2009) Antiplasmodial, anti-trypanosomal, anti-leishmanial and cytotoxicity activity of selected Tanzanian medicinal plants. Tanzania J Health Res 11(4):226–234
Masao H, Kue-Ping K, Yue-Zhong S, Yasuhiro T, Tohru K, Tsuneo N (1988) A triterpene from the fruits of Rubus chingii. Phytochemistry 27(12):3975–3976
Nasser JA, Yaacob WA, Din LB, Yamin BM, Latip J (2009) Isolation of atranorin, bergenin and goniothalamin from Hopea sangal. ARPN J Eng Appl Sci 4(1):92–95
Newman DJ, Cragg GM, Snader KM (2003) Natural products as sources of new drugs over the period 1981–2002. J Nat Prod 66(7):1022–1037
Ngemenya MN, Akam TM, Yong JN, Tane P, Fanso-Free SN, Berzins K, Titanji VPK (2006) Antiplasmodial activities of some products from Turreanthus africanus (Meliaceae). Afr J Health Sci 13(1–2):33–39
Nkhoma S, Molyneux M, Ward S (2007) In vitro antimalarial susceptibility profile and Prcrt/Pfmdr-1 genotypes of Plasmodium falciparum field isolates from Malawi. Am J Trop Med Hyg 76(6):1107–1112
Oladele AT, Adewunmi CO (2008) Medicinal plants used in the management of malaria among the Traditional Medicine Practitioners (TMPs) in South Western Nigeria. Afr J Infect Dis 2(1):51–59
Parkurst MR, Thomas AW, Adams TR, Makhubu LP, Mthupha BM, Wolde YL et al (1990) Triterpene aglycones from various Phytolacca dodecandra populations. Phytochemistry 29(8):1171–1174
Razakantoanina V, Nguyen Kim PP, Jaureguiberry G (2000) Antimalarial activity of new gossypol derivatives. Parasitol Res 86(8):665–668
Ridley RG (2002) Medical need, scientific opportunity and the drive for antimalarial drugs. Nature 415(7):686–693
Saxena S, Pant N, Jain DC, Bhakuni RS (2003) Antimalarial agents from plant sources. Current Sci 85(9):1314–1329
Schwikkard S, Van Heerden RF (2002) Antimalarial activity of plant metabolites. Nat Prod Rep 19(6):675–692
Tane P, Wabo HK, Connolly JD (2005) A new benzophenanthridine alkaloid from Zanthoxylum buesgenii. Fitoterapia 6(7–8):656–660
Tetsuo I, Hiroaki A, Tsunao H, Shizuo S, Ronghui S, Nobuyuki H, Mujo K (1990) Monoterpenoids from Rabermachia sinica. Phytochemistry 29(6):1913–1916
Titanji VPK, Zofou D, Ngemenya MN (2008) The anti-malarial potential of medicinal plants used for the treatment of malaria in Cameroonian folk medicine. Afr J Trad CAM 5(3):302–321
Moon H, Jung J, Lee J (2006) Antiplasmodial activity of triterpenoid isolated from whole plants of Viola genus from South Korea. Parasitol Res 100(3):641–644
Chung I, Kim M, Moon H (2008) Antiplasmodial activity of sesquiterpene lactone from Carpesium rosulatum in mice. Parasitol Res 103(2):341–344
Acknowledgements
This work received financial support in the form of research grants awarded to Professor Vincent P.K. Titanji by the International Programme in the Chemical Sciences (IPICS, CAM:01) and Microsoft Corporation, and a research grant from the International Foundation for Science (IFS) awarded to Dr. Mathieu Tene (RGA No. F/4238-1).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zofou, D., Kengne, A.B.O., Tene, M. et al. In vitro antiplasmodial activity and cytotoxicity of crude extracts and compounds from the stem bark of Kigelia africana (Lam.) Benth (Bignoniaceae). Parasitol Res 108, 1383–1390 (2011). https://doi.org/10.1007/s00436-011-2363-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00436-011-2363-y