Abstract
The emergence and spread of Plasmodium falciparum with resistance to chloroquine (CQ), the safest and cheapest anti-malarial drug, coupled with the increasing cost of alternative drugs especially in developing countries have necessitated the urgent need to tap the potential of plants for novel anti-malarials. The present study investigates the anti-malarial activity of the methanolic extracts of 13 medicinal plants from the Malaiyur and Javadhu hills of South India against blood stage CQ-sensitive (3D7) and CQ-resistant (INDO) strains of P. falciparum in culture using the fluorescence-based SYBR Green I assay. Sorbitol-synchronized parasites were incubated under normal culture conditions at 2% hematocrit and 1% parasitemia in the absence or presence of increasing concentrations of plant extracts. CQ and artemisinin were used as positive controls, while 0.4% DMSO was used as the negative control. The cytotoxic effects of extracts on host cells were assessed by functional assay using HeLa cells cultured in RPMI containing 10% fetal bovine serum, 0.21% sodium bicarbonate and 50 μg/mL gentamycin (complete medium). Plant extracts (bark methanol extracts of Annona squamosa (IC50, 30 μg/mL), leaf extracts of Ocimum gratissimum (IC50, 32 μg/mL), Ocimum tenuiflorum (IC50, 31 μg/mL), Solanum torvum (IC50, 31 μg/mL) and Justicia procumbens (IC50, 63 μg/mL), showed moderate activity. The leaf extracts of Aristolochia indica (IC50, 10 μg/mL), Cassia auriculata (IC50, 14 μg/mL), Chrysanthemum indicum (IC50, 20 μg/mL) and Dolichos biflorus (IC50, 20 μg/mL) showed promising activity and low activity was observed in the flower methanol extracts of A. indica , leaf methanol extract of Catharanthus roseus, and Gymnema sylvestre (IC50, >100 μg/mL). These four extracts exhibited promising IC50 (μg/mL) of 17, 24, 19 and 24 respectively also against the CQ resistant INDO strain of P. falciparum. The high TC50 in mammalian cell cytotoxicity assay and the low IC50 in anti-malarial P. falciparum assay indicates selectivity and good resistance indices in the range of 0.9–1.7 for leaf extracts of A. indica, C. auriculata, C. indicum and D. biflorus suggests that these may serve as anti-malarial agents even in their crude form. These results indicate a possible explanation of the traditional use of some of these medicinal plants against malaria or malaria-like conditions.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Malaria is still a major public health problem, especially in tropical and sub-tropical regions. It is estimated that, in 2006, 3.3 billion people were at risk of contracting malaria, which causes nearly one million deaths each year, mostly of African children aged below 5 years who are susceptible to this disease (WHO 2008). As reported recently, 406 million Indians were at risk of stable Plasmodium falciparum transmission in 2007 with an uncertainty point estimate of 101.5 million clinical cases (95% CI 31.0–187.0 million cases; Hay et al. 2010). Annually, 600 million new infections occur worldwide, and at least one million of these infections are fatal. The other hard-hit tropical areas include East Asia, China and India. Experts estimated that as many as 40% of India’s malaria cases are caused by P. falciparum (Kumar 1999). Despite the existence of an array of available anti-malarial drugs, the control of this ancient infection is increasingly threatened by the emergence of drug-resistant strains of the malaria parasite, Plasmodium (Craft 2008). There is therefore an urgent need for new chemotherapeutic compounds which are easy to administer and store and which are of low cost. One possible source for such affordable treatments lies in the use of traditional herbal remedies.
The use of plants for the treatment of malaria extends over at least three continents, including several countries in Africa, America and Asia (Phillipson et al. 1987). However, scientific data on such medicinal plants are scanty. The recognition and validation of traditional medical practices and the search for plant-derived drugs could lead to new strategies in malaria control. Since many modern drugs such as quinine and artemisinin originate from plants, it is essential that other medicinal plants which have folklore reputation for anti-malarial properties are investigated in order to establish their safety and efficacy and to determine their potential as sources of new anti-malarial drugs (Gessler et al. 1994). Here we describe the anti-malarial potential of some medicinal plants collected from the Malaiyur and Javadhu hills of South India. The ethnomedical perspectives of these plants are given below.
The genus Chrysanthemum belongs to the Asteraceae family. Earlier studies have demonstrated the potential medicinal activities of Chrysanthemum indicum which exhibited good inhibitory activity against bacteria and viruses (Liu 1991), and was used in oriental traditional medicine to treat several infectious diseases such as pneumonia, colitis, stomatitis, carbuncle and fever. Its flowers were also commonly used as tea to treat some eye diseases in Chinese traditional medicine (Matsuda et al. 2002). The flower essential oils from C. indicum exhibited effective antimicrobial activity against 15 microorganisms including three yeasts and seven clinically isolated strains using disc paper and broth microdilution methods (Shunying et al. 2005), and the alcohol extract of Chrysanthemum morifolium was effective against P. falciparum (Zhao et al. 1996). The fresh flower head organic extracts of Chrysanthemum coronarium showed moderate antibacterial activity (Urzua and Mendoza 2003) and Vernonia amygdalina (Asteraceae) ethanol extracts exhibited good in vitro antiplasmodial activity against a chloroquine (CQ)-sensitive strain of P. falciparum (Tona et al. 2004). Pyrethrins, complex esters extracted from Chrysanthemum cinerariaefolium, had insecticidal effects (Cox 2000) and in vitro activity against herpes simplex virus (Stanberry et al. 1986).
Aristolochia indica is used in traditional medicine against intestinal worms and is useful in all types of poisonous bites and stings; the crude hexane, ethyl acetate and methanol extracts of A. indica were active for adulticidal, repellent and larvicidal activity against adult and early fourth-instar larvae of Culex gelidus and Culex quinquefasciatus (Kamaraj et al. 2010b); the in vitro test of petroleum ether and chloroform extracts of Aristolochia bracteolate extracts caused 100% inhibition of schizont maturation of P. falciparum (Ahmed et al. 2010) and the root hexane extract of Holostylis reniformis (Aristolochiaceae) was the most active in vivo against Plasmodium berghei and in vitro against a chloroquine-resistant strain of P. falciparum (de Andrade-Neto et al. 2007). Cassia species have been of medical interest due to their good therapeutic value in folk medicine; the acetone, chloroform, ethyl acetate, hexane, methanol and petroleum ether extracts of the leaf and flower of Cassia auriculata are known to be most active against the fourth-instar larvae of Anopheles subpictus and Culex tritaeniorhynchus (Kamaraj et al. 2009). The hexane, chloroform, ethyl acetate, acetone and methanol extracts of the leaf and flower of C. auriculata are known to have good activity against the fourth-instar larvae of the malaria vector Anopheles stephensi and the filariasis vector C. quinquefasciatus (Kamaraj et al. 2010a), and the crude ethanol extracts of Cassia alata and Cassia occidentalis have effective in vitro anti-malarial activity using the micro-dilution test against P. falciparum (Kayembe et al. 2010).
Solanum torvum (Solanaceae) is a plant intensively used worldwide in the traditional medicine system as poison antidote and for the treatment of fever, wounds, tooth decay, gastric ulceration, reproductive problems and arterial hypertension (Noumi et al. 1999; Noumi and Dibakto 2000; Ndebia et al. 2007). Rahuman et al. (2008) have reported the moderate larvicidal activity of the crude hexane, ethyl acetate, petroleum ether, acetone and methanol extracts of S. torvum against the early fourth-instar larvae of C. quinquefasciatus. The acetone, chloroform, ethyl acetate, hexane, methanol and petroleum ether extracts of the leaf and seed of S. torvum were active against the fourth-instar larvae of the malaria vector, A. subpictus, and the Japanese encephalitis vector, C. tritaeniorhynchus (Kamaraj et al. 2009). The complete egg hatching inhibition and larval development inhibition for leaf and seed ethyl acetate, acetone and methanol extracts of S. torvum indicated in vitro anthelmintic activity against the parasitic nematode of small ruminants Haemonchus contortus (Kamaraj et al. 2010d).
The dichloromethane and methanol crude extracts of Acanthaceae family plants, Justicia procumbens and Justicia balansae were screened for their antiplasmodial, cytotoxic, antioxidant and radical scavenging activities against P. falciparum (Charoenchai et al. 2010). Kamaraj et al. (2010b) reported the adulticidal, repellent and larvicidal activity of the crude hexane, ethyl acetate and methanol extracts of J. procumbens which were tested against adult and early fourth-instar larvae of C. gelidus and C. quinquefasciatus. Catharanthus roseus (Apocynaceae), commonly known as the tropical periwinkle, is a plant of medicinal importance due to its anti-cancer and antitumour activities which are attributed to the presence of the alkaloids vincristine and vinblastine in its leaves. The roots are used in various ways in traditional as well as folk medicine. The alkaloids present in the plants are also effective in the treatment of leukaemia, diabetes, hypertension, menorrhagia, etc. (Atal and Kapur 1977), and the leaf ethyl acetate, acetone and methanol extracts of C. roseus have demonstrated a complete inhibition against H. contortus (Kamaraj and Rahuman 2010).
Melia azedarach (Meliaceae), known as Chinaberry or Persian lilac, is a deciduous tree that is native to northwestern India and has long been recognized to elicit a variety of effects in insects, such as antifeedant, growth retardation, reduced fecundity, moulting disorders, anthelmintic, antiseptic, antipyretic and repellent activity (Schmid et al. 1998; Hammad et al. 2001; Gajmer et al. 2002; Banchio et al. 2003). M. azedarach extracts showed 100% parasitic inhibition against P. falciparum using an in vitro radioisotopic uptake technique (Ofulla et al. 1995); the leaves and seed aqueous and hydro-alcoholic extracts were evaluated for antihelmintic in vitro ovicidal and larvicidal activity against H. contortus (Kamaraj et al. 2010c), and the highest mortality was obtained with a combination of 20% ripe fruit extract with 10% ripe fruit oil against the head louse Pediculus humanus capitis (Carpinella et al. 2007). Gymnema sylvestre, a valuable medicinal plant belonging to Asclepiadaceae, is widely distributed in all parts of India and Africa. It has long been used in traditional medicine as a remedy for diabetes mellitus, stomach ache and diarrhoea. The methanol and dichloromethane extracts of the leaves of G. sylvestre showed good activity against P. falciparum (K1 chloroquine-resistant and NF54 chloroquine-sensitive strains) and low cytotoxic properties (Satdive et al. 2003; Irungu et al. 2007). The leaves of the shrub G. sylvestre contain a complex of pentacyclic triterpenes, gymnemines or gymnemic acids as natural deterrents of insects and herbivorous animals (Harborn 1985), and the leaf extracts of G. sylvestre showed active leishmanicidal activity against leishmanial parasites Leishmania major, Leishmania aethiopica and Leishmania tropica in in vitro conditions (Khanna et al. 2009).
Annona squamosa, commonly known as custard apple, is a native of West Indies and is cultivated throughout India mainly for its edible fruit. The major constituents identified in the members of the Annonaceae are, typically, acetogenins (Zeng et al. 1996; Liu et al. 1999; Bermejo et al. 2005) that exhibited active in vitro anti-malarial activities (Rakotomanga et al. 2004). de Mesquita et al. (2007) have reported the antiplasmodial activity of the hexane and ethanolic extracts of the leaves of Annona crassiflora, Duguetia furfuracea and Xylopia emarginata (Annonaceae) in terms of significant inhibition rates of P. falciparum (FcB1 strain) growth. The ethyl acetate and methanol extract of the leaves of A. squamosa showed moderate anti-malarial activity against CQ-sensitive (3D7) and CQ-resistant (Dd2 and INDO) strains of P. falciparum (Bagavan et al. 2011a); the methanolic extracts of the leaves of A. squamosa showed high antiplasmodial activity against the chloroquine-sensitive strain 3D7 and chloroquine resistant-strain Dd2 of P. falciparum (El Tahir et al. 1999); the hexane, chloroform, ethyl acetate, acetone and methanol extracts of the bark of A. squamosa have been reported as very active agents against the fourth-instar larvae of the malaria vector A. stephensi and the lymphatic filariasis vector C. quinquefasciatus (Kamaraj et al. 2010a), and the root extracts of Annona senegalensis showed good activity against the chloroquine-resistant strain of P. falciparum (Fall et al. 2003).
Dolichos biflorus (Fabaceae) is a branched, sub-erect and downing herb native to most parts of India. It is a fast-growing annual vine with trifoliate leaves and brown, flat, curved pods filled with seeds (Sastri 1969). In Ayurveda, the seed is used in the treatment of piles, pain, constipation, wounds, urinary calculi, cough, edoema, asthma, etc. The soup prepared from seeds is also beneficial in the treatment of enlarged liver and spleen. The seeds of D. biflorus have been reported to show antilithiatic (Garimella et al. 2001), antihepatotoxic (Laskar et al. 1998) and hypolipidemic activities (Muthu et al. 2005). The adulticidal, repellent and larvicidal activity of crude hexane, ethyl acetate and methanol extracts of D. biflorus has been found to be moderate against the adult and early fourth-instar larvae of C. gelidus and C. quinquefasciatus (Kamaraj et al. 2010b).
Ocimum gratissimum (Labiatae) is an erect small shrub, about 1 m in height, growing abundantly in India and Nigeria in deciduous forests and the savannah. It is commonly found around village huts and gardens and is cultivated in West Africa for its medicinal uses and as food flavouring (Iwu 1993). The leaf ethyl acetate extract of O. gratissimum is endowed with effective in vitro anti-malarial activity against NF54 (CQ sensitive) and K1 (CQ resistant) strains of P. falciparum (Abiodun et al. 2011); the hexane, chloroform, ethyl acetate, acetone and methanol extracts of Ocimum sanctum possess moderate activity against the chloroquine-sensitive (3D7) strain of P. falciparum (Bagavan et al. 2011b), and the aqueous extract of the leaves of O. gratissimum was screened for antidiarrhoeal effects (Offiah and Chikwendu 1999). Ocimum bacilicum and Ocimum suave extracts showed good in vitro anti-malarial activity against the chloroquine-resistant strain of P. falciparum (Nguta et al. 2010). The essential oil from O. gratissimum showed complete growth inhibition of P. berghei (Tchoumbougnang et al. 2005), and leaf decoctions of O. gratissimum were found to be moderately active against Plasmodium yoelii (Agomo et al. 1992).
Tribulus terrestris (Zygophyllaceae) is an annual plant distributed in the warm regions of India, Africa, Europe, America and Australia (Kostova et al. 2002). It is used in folk medicine as tonic, aphrodisiac, analgesic, astringent, stomachic, anti-hypertensive, diuretic, lithontriptic and urinary anti-infective (Ody 2000). Acetone extracts of the leaves and seeds from T. terrestris showed moderate activity against the third-instar larvae of Anopheles culicifacies, A. stephensi, C. quinquefasciatus and Aedes aegypti (Singh et al. 2008), and the alcoholic, methanolic, acetonitrilic, and hexanic extracts of T. terrestris showed effective in vitro antibacterial activity against Staphylococcus aureus, Escherichia coli, Proteus vulgaris, Pseudomonas aeruginosa and Klebsiella (Hussain et al. 2009).
In the present study, we describe the in vitro antiplasmodial activity of the methanolic extracts of the above mentioned 13 medicinal plant extracts tested against the chloroquine-sensitive 3D7 and chloroquine-resistant INDO strains of P. falciparum together with in vitro cytotoxicity against mammalian HeLa cell line.
Materials and methods
Collection of plant materials
The bark of A. squamosa, leaf and flower of A. indica, leaf of C. auriculata, C. roseus, C. indicum, D. biflorus, G. sylvestre, J. procumbens, M. azedarach, O. gratissimum, O. tenuiflorum, T. terrestris and leaf and seed of S. torvum were selected in the present study based upon their medicinal uses (Table 1) and biological activities (Table 2). The main target of this study was to investigate the antiplasmodial activities of 13 methanol plant extracts from Tamil Nadu, India. The plant materials were collected from Malaiyur Hills, Dharmapuri district (11°53′28″ N, 078°30′26″ E, altitude 959 m), and Javadhu Hills, Tiruvannamalai district (12°36′10′′N, 078°53'07′′E, altitude 705 m), Tamil Nadu, South India, in May 2010, and taxonomic identification was made by Dr. C. Hema, Department of Botany, Arignar Anna Government Arts College for Women, Walajapet, Vellore, India. The voucher specimens were numbered and kept in our research laboratory for further reference.
Preparation of plant extracts
The plant materials (leaf, bark, flower and seed) were air-dried for 10–25 days in the shade at environmental temperatures (27–37°C daytime), and the leaf (600 g), bark (700 g), flower (450 g) and seed (500 g) were powdered mechanically using a commercial electrical stainless steel blender and extracted with methanol (3,600 mL, Qualigens) in a Soxhlet apparatus (boiling point range 60–80°C) for 8 h. The extract was concentrated under a reduced pressure of 22–26 mmHg at 45°C, and the residue obtained was stored at 4°C.
In vitro cultivation of P. falciparum
CQ-sensitive strain 3D7 and CQ-resistant strain INDO of P. falciparum were used in in vitro blood stage culture to test the anti-malarial efficacy of different plant extracts. The culture was maintained at the Malaria Research Laboratory, International Centre for Genetic Engineering and Biotechnology, New Delhi, India. P. falciparum culture was maintained according to the method described by Trager and Jensen (1976), with minor modifications. P. falciparum (3D7) cultures were maintained in fresh O+ve human erythrocytes suspended at 4% hematocrit in RPMI 1640 (Sigma) containing 0.2% sodium bicarbonate, 0.5% albumax, 45 μg/L hypoxanthine and 50 μg/l gentamycin and incubated at 37°C under a gas mixture of 5% O2, 5% CO2 and 90% N2. Every day, infected erythrocytes were transferred into a fresh complete medium to propagate the culture. For P. falciparum (INDO strain) in culture medium, albumax was replaced by 10% pooled human serum.
Drug dilutions
Stock solutions of CQ were prepared in water (milli-Q grade). Artemisinin, and test extracts were prepared in dimethyl sulfoxide (DMSO). All stocks were then diluted with culture medium to achieve the required concentrations (in all cases except CQ, the final solution contained 0.4% DMSO, which was found to be non-toxic to the parasite). Drugs and test compounds were then placed in 96-well flat-bottom tissue culture-grade plates.
Assay for antiplasmodial activity
The methanol extracts of experimental plants were evaluated for their anti-malarial activity against P. falciparum strains 3D7 and INDO. For drug screening, SYBR green I-based fluorescence assay was used as described (Smilkstein et al. 2004). Sorbitol-synchronized parasites were incubated under normal culture conditions at 2% hematocrit and 1% parasitemia in the absence or presence of increasing concentrations of plant extracts. CQ and artemisinin were used as positive controls, while 0.4% DMSO was used as the negative control. After 48 h of incubation, 100 μl of SYBR Green I solution {0.2 μl of 10,000 X SYBR Green I (Invitrogen)/mL} in lysis buffer [Tris (20 mM; pH 7.5), EDTA (5 mM), saponin (0.008%; w/v) and Triton X-100 (0.08%; v/v)] was added to each well and mixed gently twice with a multi-channel pipette and incubated in the dark at 37°C for 1 h. Fluorescence was measured with a Victor fluorescence multi-well plate reader (Perkin Elmer) with excitation and emission wavelength bands centred at 485 and 530 nm, respectively. The fluorescence counts were plotted against the drug concentration and the 50% inhibitory concentration (IC50) was determined by an analysis of dose–response curves. Results were validated microscopically by the examination of giemsa-stained smears of extract-treated parasite cultures (Bagavan et al. 2011a).
Cytotoxic activity on HeLa cells using MTT assay
The cytotoxic effects of extracts on host cells was assessed by functional assay as described (Mosmann 1983) using HeLa cells cultured in RPMI containing 10% fetal bovine serum, 0.21% sodium bicarbonate (Sigma) and 50 μg/mL gentamycin (complete medium). Briefly, cells (104 cells/200 μl/well) were seeded into 96-well flat-bottom tissue culture plates in complete medium. Drug solutions were added after 24 h of seeding and incubated for 48 h in a humidified atmosphere at 37°C and 5% CO2. DMSO (as a positive inhibitor) was added at 10%. Twenty microliters of a stock solution of MTT (5 mg/mL in 1X phosphate-buffered saline) was added to each well, gently mixed and incubated for another 4 h. After spinning the plate at 1,500 rpm for 5 min, the supernatant was removed and 100 μl of DMSO (stop agent) was added to each well. The formation of formazon was read on a microtiter plate reader (Versa max tunable multi-well plate reader) at 570 nm. The 50% cytotoxic concentration (TC50) of drug was determined by an analysis of dose–response curves. Therapeutic index was calculated as a ratio of TC50 HeLa/IC50 3D7.
Results
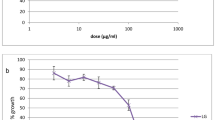
Thirteen plant methanol extracts were selected for antiplasmodial activity on the 3D7 CQ-sensitive and CQ-resistant INDO strains of P. falciparum. Promising antiplasmodial activity was found in the leaf extracts from four plants: A. indica , C. auriculata , C. indicum and D. biflorus at 50% inhibitory concentration (IC50 3D7, 10, 14, 20 and 20 μg/mL), respectively. Moderate activity was found in the bark methanol extracts of A. squamosa and leaf extracts of O. gratissimum, O. tenuiflorum, S. torvum, J. procumbens and T. terrestris (IC50 3D7, 30, 32, 31, 63 and 40 μg/mL, respectively), whereas the methanolic extracts of S. torvum (seed) and G. sylvestre (leaf) displayed poor activity (IC50 3D7, 89 and 100 μg/mL respectively). On the other hand, the methanolic extracts A. indica (flower) and C. roseus (leaf) were inactive up to 100 μg/mL. Plant extracts which showed IC50 below 20 μg/mL (A. indica, C. auriculata, C. indicum and D. biflorus) on further analysis against the CQ-resistant strain INDO of P. falciparum exhibited a similar potency with IC50 of 17, 24, 19 and 24 μg/mL, respectively (Table 3), giving resistance indices in the range of 0.9–1.7. As shown in Table 3, the leaf methanol extracts of A. indica and C. auriculata (Fig. 1) more active than the other plant extracts tested against P. falciparum. Furthermore, they were also non-toxic to mammalian cell line HeLa up to a concentration of 100 μg/mL. The growth inhibition profiles and the IC50 values of different plant extracts against 3D7 strain of P. falciparum are shown in Fig. 2a–d.
Dose-dependent growth inhibition curve of P. falciparum (3D7) by methanolic extracts of a leaf of C. indicum (P1), A. indica (P2), C. auriculata (P3) and seed of S. torvum (P4), b leaf of J. procumbens (P5), flower of A. indica (P6), leaf of S. torvum (P7) and C. roseus (P8), c leaf of M. azedarach (P9), G. sylvestre (P10), bark of A. squamosa (P11) and leaf of D. biflorus (P12), (d) leaf of Ocimum tenuiflorum (P13), O. gratissimum (P14) and T. terrestris (P15). Keys in each panel indicate extract ID (normal text) and IC50 μg/ml (subscript)
Discussion
Plants have contributed in a big way towards modern medicine and have always remained a primary subject for drug discovery. In our quest for novel drugs against malaria, we focused on the rich flora of South Indian medicinal plants. In a similar study, El Tahir et al. (1999) reported that the methanolic extract of A. squamosa leaves showed a high antiplasmodial activity with IC50 values of 2 and 30 μg/mL against the CQ-sensitive strain 3D7 and CQ-resistant strain Dd2 of P. falciparum, respectively. The leaf ethyl acetate extracts of A. squamosa displayed a moderate activity against P. falciparum (IC50 3D7, 33 μg/mL) (Bagavan et al. 2011a). The other plant extracts from the same family Annonaceae like Desmopsis panamensis, Pseudomalmea boyacana, Rollinia exsucca and Rollinia pittieri showed a good antiplasmodial activity (IC50 < 10 μg/mL) against the CQ-sensitive (F32) and CQ-resistant (W2) P. falciparum (Osorio et al. 2007). The whole plant petroleum ether/chloroform (1:1) extract of Aristolochia bracteolata produced a 100% inhibition of parasite growth at a concentration of ≤ 50 μg/ml (Ahmed et al. 2010). The extracts from H. reniformis (Aristolochiaceae) were found to be active both in vivo and in vitro. The hexane extract of the roots was the most active, causing a 67% reduction of parasitemia in vivo against P. berghei (de Andrade-Neto et al. 2007).
Traoré et al. (2008) have reported that the extracts of C. alata (leaf) showed a significant effect against P. falciparum (IC50 = 80.11 μg/mL) and P. berghei (ED50 = 112.78 mg/kg). The methanol extract of the root bark of Cassia singueana displayed an anti-malarial activity against P. berghei in an in vivo rodent model (ED50 of 847±30 mg/kg) (Adzu et al. 2003). Tona et al. (2001) found that the dichloromethane extract of C. occidentalis (200 mg/kg of body wt) showed a 60% growth suppression of P. berghei ANKA strain. Kayembe et al. (2010) isolated 20 quinones from C. alata, C. occidentalis, Garcinia kola and Ocimum basilicum and investigated the in vitro antiplasmodial activity against P. falciparum. Six of the 20 isolated quinones were found to be active with an IC50 value of below 1 μg/ml; the other quinones were bearing a moderate activity with IC50 values between 5 and 20 μg/ml.
In the present study, the leaf methanol extracts of C. roseus and G. sylvestre showed a weak antiplasmodial activity (IC50= >100 and 100 μg/mL, respectively) and the leaf methanol extract of C. indicum and D. biflorus showed a high antiplasmodial activity against the CQ-sensitive (3D7) strain of P. falciparum (IC50 = 20 μg/mL).
Plant extracts from Asteraceae family also have been reported as possessing anti-malarial properties. Four non-toxic diterpenes isolated from Aspilia pruliseta were found to possess a moderate activity (IC50, 14 to 23 μM) against the chloroquine-sensitive (D6) and chloroquine-resistant (W2) strains of P. falciparum (Sebisubi et al. 2010). The ethanolic extracts of Artemisia annua and A. absinthium showed an antiplasmodial activity against the multidrug resistant and sensitive strains of P. falciparum. A. annua and A. absinthium when examined in an in vivo rodent model displayed 94% and 83% reduction in parasitemia, respectively, at 200 mg/kg for 4 days. (Ramazani et al. 2010a; Malebo et al. 2009). Baccharis dracunculifolia extract showed a good antiplasmodial activity against P. falciparum with an IC50 value of 20 μg/mL (da Silva Filho et al. 2009).
Valadeau et al. (2009) have reported that the ethanolic extracts of Cestrum racemosum (Solanaceae), Hyptis lacustris (Lamiaceae) and Calea montana (Asteraceae) plant species displayed a good activity against the P. falciparum CQ-resistant strain (IC50 < 10 μg/ml). An in vitro screening of the dichloromethane extracts of Xanthium brasilicum (Asteraceae) and a bioactivity-guided fractionation resulted in the isolation of 8-epixanthatin 1beta, 5beta-epoxide compound having an activity against Trypanosoma brucei rhodesiense, Trypanosoma cruzi, Leishmania donovani and P. falciparum (IC50 0.09, 2.95, 0.16 and 1.71 μg/mL, respectively) (Nour et al. 2009). Samoylenko et al. (2009) demonstrated a strong in vitro anti-malarial activity in the dichloromethane and methanol extracts of Albizia schimperiana (Leguminosae). Thus, CQ-susceptible (D6) and -resistant (W2) strains of P. falciparum showed IC50 values ranging from 120 to 270 ng/mL.
Esmaeili et al. (2009) reported the in vitro antiplasmodial activity of the methanol extracts (IC50, 4.7–26.6 μg/ml) of Buxus hyrcana (aerial part), Erodium oxyrrhnchum (aerial part), Glycyrrhiza glabra (aerial part) and Ferula oopoda (roots) against the P. falciparum K1 and 3D7 strains. The chloroformic fraction of G. glabra at 10 mg/kg suppressed 86% parasitemia of an in vivo rodent model of P. berghei. The bark of Avicennia marina (Acanthaceae) extract exhibited an in vitro antiplasmodial activity against P. falciparum with a IC50 value of 49.63 μg ml−1 (Ravikumar et al. 2010). The in vitro anti-malarial activity of M. azedarach (Meliaceae) extracts against P. falciparum exhibited an IC50 of 300 μg/ml (Ofulla et al. 1995). Maneerat et al. (2008) found that the seeds of Chisocheton siamensis (Meliaceae), as anti-malarial, have IC50 values ranging from 2.06 to 6.31 μg/ml against P. falciparum. Lee et al. (2008) reported the in vitro anti-malarial activity of anthothecol (IC50, 1.4 μM) and limonoid (IC50, 0.17 μM) isolated from Khaya anthotheca (Meliaceae). The ethyl acetate extract of leaves of O. gratissimum displayed a promising antiplasmodial activity (IC50, 1.8–1.93 μg/mL) against the P. falciparum K1 strain and a low cytotoxicity (TC50 > 10 μg/mL) (Abiodun et al. 2011). The leaf hexane, chloroform, ethyl acetate, acetone and methanol extracts of O. sanctum showed a moderate antiplasmodial activity against the P. falciparum 3D7 strain (IC50 = 51, 30, 36, 36 and l45 μg/ml, respectively) (Bagavan et al. 2011b).
The five glycoalkaloids, namely, chaconine and solanine from Solanum tuberosum sprouts, solamargine and solasonine from Solanum nigrum fruit and tomatine from Lycopersicon esculentum fruit, were isolated and studied in vivo on the rodent model of P. yoelii 17XL. Chaconine, tomatine, solamargine, solasonine and solanine at a dose of 7.50 mg/kg showed 71%, 65%, 64%, 57% and 41%, respectively, suppression of parasitemia (Chen et al. 2010). The hydro-alcoholic extract of Solanum surattense was found to be active against P. falciparum K1 (CQ-resistant strain) and CY27 (CQ-sensitive strain) (IC50 = 50 and 40 μg/ml, respectively) (Ramazani et al. 2010b). Steroids isolated from Solanum nudum (Solanaceae) were found to be inhibitory to the asexual blood stages of the P. falciparum strain 7G8 with an IC50 between 20 and 87 μM (López et al. 2010). The alkaloids harmine, harmaline, vasicinone and deoxyvasicinone have been isolated from the seeds of Peganum harmala (Zygophyllaceae). Of these, harmine and harmaline showed a moderate in vitro antiplasmodial activity against P. falciparum (Astulla et al. 2008).
It would be of interest and necessary to pursue further pharmacological and phytochemical studies regarding these active plants. In our own future studies, we plan to focus on the evaluation of the in vivo activity of these plants against a mouse model of P. berghei, on the isolation and identification of active compounds through a bio-guided fractionation and on the activity and toxicity of these active isolated compounds. From these additional results, we will also consider the possibility of local valorization of active and non-toxic plants in standardized improved traditional medicine.
This preliminary study confirms the antiplasmodial activities of some plants already in medicinal usage, further justifying their use in South India as traditional medicine. The methanol extract of A. indica was more active against the chloroquine-resistant strain than the chloroquine-sensitive strain. This study indicated that A. indica, C. auriculata, C. indicum and D. biflorus have an important antiplasmodial activity and significant therapeutic index, making them good candidates for further pharmacological studies. The presence of promising anti-malarial activities in other members of the respective families to which these plants belong suggests the possibility of shared pharmacophoric metabolites with fine-tuned molecular architectures in individual species.
References
Abiodun O, Gbotosho G, Ajaiyeoba E, Happi T, Falade M, Wittlin S, Sowunmi A, Brun R, Oduola A (2011) In vitro antiplasmodial activity and toxicity assessment of some plants from Nigerian ethnomedicine. Pharm Biol 49(1):9
Adamu M, Nwosu CO, Agbede RI (2009) Anti-trypanosomal effects of aqueous extract of Ocimum gratissimum (Lamiaceae) leaf in rats infected with Trypanosoma brucei brucei. Afr J Tradit Complement Altern Med 6(3):262–267
Adzu B, Abbah J, Vongtau H, Gamaniel K (2003) Studies on the use of Cassia singueana in malaria ethnopharmacy. J Ethnopharmacol 88(2–3):261–267
Agomo PU, Idigo JC, Afolabi BM (1992) “Antimalarial” medicinal plants and their impact on cell populations in various organs of mice. Afr J Med Med Sci 21(2):39–46
Ahmed el-HM, Nour BY, Mohammed YG, Khalid HS (2010) Antiplasmodial activity of some medicinal plants used in Sudanese folk-medicine. Environ Health Insights 4:1–6
Asano J, Chiba K, Tada M, Yoshii T (1996) Antiviral activity of lignans and their glycosides from Justicia procumbens. Phytochemistry 42(3):713–717
Astulla A, Zaima K, Matsuno Y, Hirasawa Y, Ekasari W, Widyawaruyanti A, Zaini NC, Morita H (2008) Alkaloids from the seeds of Peganum harmala showing antiplasmodial and vasorelaxant activities. J Nat Med 62(4):470–472
Atal CK, Kapur BM (1977) Cultivation and utilization of medicinal and aromatic plants. Regional Research Laboratory, Jammu Tawai, p 138
Bagavan A, Kamaraj C, Elango G, Zahir AA, Rahuman AA (2009) Adulticidal and larvicidal efficacy of some medicinal plant extracts against tick, fluke and mosquitoes. Vet Parasitol 166:286–292
Bagavan A, Rahuman AA, Kaushik NK, Sahal D (2011a) In vitro antimalarial activity of medicinal plant extracts against Plasmodium falciparum. Parasitol Res 108:15–22
Bagavan A, Rahuman AA, Kamaraj C, Kaushik NK, Mohanakrishnan D, Sahal D (2011b) Antiplasmodial activity of botanical extracts against Plasmodium falciparum. Parasitol Res 108:1099–1109
Banchio E, Valladares G, Defago M, Palacios S, Carpinella C (2003) Effects of Melia azedarach (Meliaceae) fruit extracts on the leafminer Liriomyza huidobrensis (Diptera: Agromyzidae): assessment in laboratory and field experiments. Ann Appl Biol 143:187–193
Bermejo A, Figadere B, Zafra-Polo MC, Barrachina I, Estornell E, Cortes D (2005) Acetogenins from Annonaceae: recent progress in isolation, synthesis and mechanisms of action. Nat Prod Rep 22:269–303
Carpinella MC, Miranda M, Almirón WR, Ferrayoli CG, Almeida FL, Palacios SM (2007) In vitro pediculicidal and ovicidal activity of an extract and oil from fruits of Melia azedarach L. Am Acad Dermatol 56:250–256
Charoenchai P, Vajrodaya S, Somprasong W, Mahidol C, Ruchirawat S, Kittakoop P (2010) Part 1: antiplasmodial, cytotoxic, radical scavenging and antioxidant activities of Thai plants in the family Acanthaceae. Planta Med 76(16):1940–1943
Chen Y, Li S, Sun F, Han H, Zhang X, Fan Y, Tai G, Zhou Y (2010) In vivo antimalarial activities of glycoalkaloids isolated from Solanaceae plants. Pharm Biol 48(9):1018–1024
Cox NH (2000) Permethrin treatment in scabies infestation: importance of the correct formulation. Clin Rev 320:3–38
Craft JC (2008) Challenges facing drug development for malaria. Curr Opin Microbiol 11:428–433
da Silva Filho AA, Resende DO, Fukui MJ, Santos FF, Pauletti PM, Cunha WR, Silva ML, Gregório LE, Bastos JK, Nanayakkara NP (2009) In vitro antileishmanial, antiplasmodial and cytotoxic activities of phenolics and triterpenoids from Baccharis dracunculifolia D. C. (Asteraceae). Fitoterapia 80(8):478–482
de Andrade-Neto VF, da Silva T, Lopes LM, do Rosário VE, de Pilla Varotti F, Krettli AU (2007) Antiplasmodial activity of aryltetralone lignans from Holostylis reniformis. Antimicrob Agents Chemother 51(7):2346–2350
de Mesquita ML, Grellier P, Mambu L, de Paula JE, Espindola LS (2007) In vitro antiplasmodial activity of Brazilian Cerrado plants used as traditional remedies. J Ethnopharmacol 110(1):165–170
El Tahir A, Satti GM, Khalid SA (1999) Antiplasmodial activity of selected Sudanese medicinal plants with emphasis on Maytenus senegalensis (Lam.) Exell. J Ethnopharmacol 64(3):227–233
Esmaeili S, Naghibi F, Mosaddegh M, Sahranavard S, Ghafari S, Abdullah NR (2009) Screening of antiplasmodial properties among some traditionally used Iranian plants. J Ethnopharmacol 121(3):400–404
Fall D, Badiane M, Ba D, Loiseau P, Bories C, Gleye C, Laurens A, Hocquemiller R (2003) Antiparasitic effect of Senegalese Annonaceae used in traditional medicine. Dakar Méd 48(2):112–116
Gajmer T, Singh R, Saini RK, Kalidhar SB (2002) Effect of methanolic extracts of neem (Azadirachta indica A. Juss) and bakain (Melia azedarach L.) seeds on oviposition and egg hatching of Earias vittella (Fab.) (Lepidoptera: Noctuidae). J Appl Entomol 126:238–243
Garimella TS, Jolly CI, Narayanan S (2001) In vitro studies on antilithiatic activity of seeds of Dolichos biflorus Linn. and rhizomes of Bergenia ligulata Wall. Phytother Res 15:351–355
Gessler MC, Nkunya MHH, Mwasumbi LB, Heinrich M, Tanner M (1994) Screening Tanzanian medicinal plants for antimalarial activity. Acta Trop 56:65–77
Gupta S, Sharma SB, Singh UR, Bansal SK, Prabhu KM (2010) Elucidation of mechanism of action of Cassia auriculata leaf extract for its antidiabetic activity in streptozotocin-induced diabetic rats. J Med Food 13(3):528–534
Hammad AEM, Zournajian H, Talhouk S (2001) Efficacy of extracts of Melia azedarach L. callus, leaves and fruits against adults of the sweet potato whitefly Bemisia tabaci (Homoptera: Aleyrodidae). J Appl Entomol 125:483–488
Harborn J (1985) Introduction to ecological biochemistry [Russian translation]. Graevskii BM (ed). Mir, Moscow
Hay SI, Gething PW, Snow RW (2010) India's invisible malaria burden. Lancet 9754:1716–1717
Hussain AA, Mohammed AA, Ibrahim HH, Abbas AH (2009) Study the biological activities of Tribulus terrestris extracts. World Aca Sci Eng Technol 57:433–435
Irungu BN, Rukunga GM, Mungai GM, Muthaura CN (2007) In vitro antiplasmodial and cytotoxicity activities of 14 medicinal plants from Kenya. S Afr J Bot 73:204–207
Iwu MM (1993) Handbook of African medicinal plants. CRC, Boca Raton, FL, pp 214–215
Kamaraj C, Abdul Rahman A, Bagavan A, Abduz Zahir A, Elango G, Kandan P, Rajakumar G, Marimuthu S, Santhoshkumar T (2010a) Larvicidal efficacy of medicinal plant extracts against Anopheles stephensi and Culex quinquefasciatus (Diptera: Culicidae). Trop Biomed 27(2):211–219
Kamaraj C, Bagavan A, Rahuman AA, Zahir AA, Elango G, Pandiyan G (2009) Larvicidal potential of medicinal plant extracts against Anopheles subpictus Grassi and Culex tritaeniorhynchus Giles (Diptera: Culicidae). Parasitol Res 104:1163–1171
Kamaraj C, Rahuman AA (2010) Efficacy of anthelmintic properties of medicinal plant extracts against Haemonchus contortus. Res Vet Sci. doi:10.1016/j.rvsc.2010.09.018
Kamaraj C, Rahuman AA, Bagavan A, Elango G, Rajakumar G, Zahir AA, Marimuthu S, Santhoshkumar T, Jayaseelan C (2010b) Evaluation of medicinal plant extracts against blood-sucking parasites. Parasitol Res 106(6):1403–12
Kamaraj C, Rahuman AA, Bagavan A, Mohamed MJ, Elango G, Rajakumar G, Zahir AA, Santhoshkumar T, Marimuthu S (2010c) Ovicidal and larvicidal activity of crude extracts of Melia azedarach against Haemonchus contortus (Strongylida). Parasitol Res 106:1071–1077
Kamaraj C, Rahuman AA, Mahapatra A, Bagavan A, Elango G (2010d) Insecticidal and larvicidal activities of medicinal plant extracts against mosquitoes. Parasitol Res 107:1337–1349
Kamaraj C, Rahuman AA, Elango G, Bagavan A, Zahir AA (2010e) Anthelmintic activity of botanical extracts against sheep gastrointestinal nematodes, Haemonchus contortus. Parasitol Res. doi:10.1007/s00436-010-2218-y
Kayembe JS, Taba KM, Ntumba K, Tshiongo MTC, Kazadi TK (2010) In vitro anti-malarial activity of 20 quinones isolated from four plants used by traditional healers in the Democratic Republic of Congo. J Med Plant Res 4(11):991–994
Khanna VG, Kannabiran K, Getti G (2009) Leishmanicidal activity of saponins isolated from the leaves of Eclipta prostrata and Gymnema sylvestre. Indian J Pharmacol 41(1):32–5
Kostova I, Dinchev D, Rentsch GH, Dimitrov V, Ivanova A (2002) Two new sulfated furostanol saponins from Tribulus terrestris. Z Naturforsch 57(1–2):33–38
Kumar S (1999) Malaria runs amok in India. New Sci, p 9
Laskar S, Bhattarcharyya UK, Sinhababu A, Basak BK (1998) Antihepatotoxic activity of kulthi (Dolichos biflorus) seed in rats. Fitoterapia 69:401–402
Lee SE, Kim MR, Kim JH, Takeoka GR, Kim TW, Park BS (2008) Antimalarial activity of anthothecol derived from Khaya anthotheca (Meliaceae). Phytomedicine 15(6–7):533–535
Liu XX, Alali FQ, Pilarinou E, McLaughlin JL (1999) Two bioactive mono-tetrahydrofuran acetogenins, annoglacins A and B, from Annona glabra. Phytochemistry 50:815–821
Liu YG (1991) Pharmacological study and clinical apply of Chrysanthemum indicum. Shi Zhen Guo Yao 2:103
López ML, Vommaro R, Zalis M, de Souza W, Blair S, Segura C (2010) Induction of cell death on Plasmodium falciparum asexual blood stages by Solanum nudum steroids. Parasitol Int 59(2):217–225
Mahomoodally MF, Gurib-Fakim A, Subratty AH (2010) Screening for alternative antibiotics: an investigation into the antimicrobial activities of medicinal food plants of Mauritius. J Food Sci 75(3):173–177
Malebo HM, Tanja W, Cal M, Swaleh SA, Omolo MO, Hassanali A, Séquin U, Hamburger M, Brun R, Ndiege IO (2009) Antiplasmodial, anti-trypanosomal, anti-leishmanial and cytotoxicity activity of selected Tanzanian medicinal plants. Tanzan J Health Res 11(4):226–234
Maneerat W, Laphookhieo S, Koysomboon S, Chantrapromma K (2008) Antimalarial, antimycobacterial and cytotoxic limonoids from Chisocheton siamensis. Phytomedicine 12:1130–1134
Matsuda H, Morikawa T, Toguchida I, Harima S, Yoshikawa M (2002) Medicinal flowers. VI. Absolute stereostructures of two new flavanone glycosides and a phenylbutanoid glycoside from the flowers of Chrysanthemum indicum L.: their inhibitory activities for rat lens aldose reductase. Chem Pharm Bull 50:972–975
Mosmann T (1983) Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods 65:55–63
Muthu AK, Sethupathy S, Manavalan R, Karar PK (2005) Hypolipidemic effect of methanolic extract of Dolichos biflorus Linn. in high fat diet fed rats. Indian J Exp Biol 43:522–525
Nathan SS, Savitha G, George DK, Narmadha A, Suganya L, Chung PG (2006) Efficacy of Melia azedarach L. extract on the malarial vector Anopheles stephensi Liston (Diptera: Culicidae). Bioresour Technol 97(11):1316–1323
Ndebia EJ, Kamga R, Nchunga-Anye Nkeh B (2007) Analgesic and anti-inflammatory properties of aqueous extract from leaves of Solanum torvum (Solanaceae). Afr J Tradit Complement Altern Med 4(2):240–244
Nguta JM, Mbaria JM, Gakuya DW, Gathumbi PK, Kiama SG (2010) Antimalarial herbal remedies of Msambweni, Kenya. J Ethnopharmacol 128(2):424–432
Noumi E, Dibakto TW (2000) Medicinal plants used for peptic ulcer in Bangangte region in theWestern part of Cameroon. Fitoterapia 71:402–412
Noumi E, Houngue F, Lontsi D (1999) Traditional medicines in prymary health care: plants used for the treatment of hypertension in Bafia Cameroon. Fitoterapia 70(2):134–139
Nour AM, Khalid SA, Kaiser M, Brun R, Abdallah WE, Schmidt TJ (2009) The antiprotozoal activity of sixteen asteraceae species native to Sudan and bioactivity-guided isolation of xanthanolides from Xanthium brasilicum. Planta Med 75(12):1363–1368
Ody P (2000) The complete guide medicinal herbal. Dorling Kindersley, London, p 223
Offiah VN, Chikwendu UA (1999) Antidiarrhoeal effects of Ocimum gratissimum leaf extract in experimental animals. J Ethnopharmacol 68(1–3):327–330
Ofulla AV, Chege GM, Rukunga GM, Kiarie FK, Githure JI, Kofi-Tsekpo MW (1995) In vitro antimalarial activity of extracts of Albizia gummifera, Aspilia mossambicensis, Melia azedarach and Azadirachta indica against Plasmodium falciparum. Afr J Health Sci 2(2):309–311
Osorio E, Arango GJ, Jiménez N, Alzate F, Ruiz G, Gutiérrez D, Paco MA, Giménez A, Robledo S (2007) Antiprotozoal and cytotoxic activities in vitro of Colombian Annonaceae. J Ethnopharmacol 11(3):630–635
Osujih M (1993) Exploration of the frontiers of tradomedical practices: basis for development of alternative medical healthcare services in developing countries. J R Soc Health 113(4):190–194
Pandey JK, Singh DK (2009) Molluscicidal activity of Piper cubeba Linn., Piper longum Linn. and Tribulus terrestris Linn. and their combinations against snail Indoplanorbis exustus Desh. Indian J Exp Biol 47(8):643–648
Phillipson JD, O’Neill MJ, Wright CW, Bray DH, Warhurst DC (1987) Plants as sources of antimalarial and amoebicidal compounds. In: Medicinal and poisonous plants of the tropics. Proceedings of Symposium of the 14th International Botanic Congress, Berlin, pp 5–35
Prasanna R, Harish CC, Pichai R, Sakthisekaran D, Gunasekaran P (2009) Anti-cancer effect of Cassia auriculata leaf extract in vitro through cell cycle arrest and induction of apoptosis in human breast and larynx cancer cell lines. Cell Biol Int 33(2):127–134
Rahuman AA, Gopalakrishnan G, Venkatesan P, Geetha K (2008) Isolation and identification of mosquito larvicidal compound from Abutilon indicum (Linn.) Sweet. Parasitol Res 102:981–988
Rakotomanga M, Razakantoanina V, Raynaud S, Loiseau PM, Hocquemiller R, Jaureguiberry G (2004) Antiplasmodial activity of acetogenins and inhibitory effect on Plasmodium falciparum adenylate translocase. J Chemother 16:350–356
Ramazani A, Sardari S, Zakeri S, Vaziri B (2010a) In vitro antiplasmodial and phytochemical study of five Artemisia species from Iran and in vivo activity of two species. Parasitol Res 107(3):593–599
Ramazani A, Zakeri S, Sardari S, Khodakarim N, Djadidt ND (2010b) In vitro and in vivo anti-malarial activity of Boerhavia elegans and Solanum surattense. Malar J 9:124
Ravikumar S, Jacob Inbaneson S, Suganthi P, Venkatesan M, Ramu A (2010) Mangrove plants as a source of lead compounds for the development of new antiplasmodial drugs from South East coast of India. Parasitol Res. doi:10.1007/s00436-010-2184-4
Ravikumar S, Nazar S, Nuralshiefa A, Abideen S (2005) Antibacterial activity of traditional therapeutic coastal medicinal plants against some pathogens. J Environ Biol 26(2 Suppl):383–386
Samoylenko V, Jacob MR, Khan SI, Zhao J, Tekwani BL, Midiwo JO, Walker LA, Muhammad I (2009) Antimicrobial, antiparasitic and cytotoxic spermine alkaloids from Albizia schimperiana. Nat Prod Commun 4(6):791–796
Samy RP, Thwin MM, Gopalakrishnakone P, Ignacimuthu S (2007) Ethnobotanical survey of folk plants for the treatment of snakebites in southern part of Tamilnadu, India. J Ethnopharmacol 115(2):302–312
Sastri BN (1969) The wealth of India Raw Materials, Information and Publication Directorate. CSIR, New Delhi, vol III
Satdive RK, Abhilash P, Fulzele DP (2003) Antimicrobial activity of Gymnema sylvestre leaf extract. Fitoterapia 74:699–701
Schmid GH, Rembold H, Ahmed AAI, Breuer AM (1998) Effect of Melia azedarach fruit extract on juvenile hormone titer and protein content in the hemolymph of two species of noctuid lepidopteran larvae (Insecta: Lepidoptera: Noctuidae). Phytoparasitica 26:283–291
Sebisubi FM, Odyek O, Anokbonggo WW, Ogwal-Okeng J, Carcache-Blanco EJ, Ma C, Orjala J, Tan GT (2010) Antimalarial activity of Aspilia pruliseta, a medicinal plant from Uganda. Planta Med 76(16):1870–1873
Shunying Z, Yang Y, Huaidong Y, Yue Y, Guolin Z (2005) Chemical composition and antimicrobial activity of the essential oils of Chrysanthemum indicum. J Ethnopharmacol 96:151–158
Silva TM, Batista MM, Camara CA, Agra MF (2005) Molluscicidal activity of some Brazilian Solanum spp. (Solanaceae) against Biomphalaria glabrata. Ann Trop Med Parasitol 99(4):419–425
Singh SP, Raghavendra K, Singh RK, Mohanty SS, Dash AP (2008) Evaluation of Tribulus terrestris Linn (Zygophyllaceae) acetone extract for larvicidal and repellence activity against mosquito vectors. J Commun Dis 40(4):255–261
Smilkstein M, Sriwilaijaroen N, Kelly JX, Wilairat P, Riscoe M (2004) Simple and inexpensive fluorescence-based technique for high throughput antimalarial drug screening. Antimicrob Agents Chemother 48:1803–1806
Souza MMC, Bevilaqua CML, Morais SM, Costa CTC, Silva ARA, Filho RB (2008) Anthelmintic acetogenin from Annona squamosa L. seeds. Anais da Academia Brasileira de Cieˆncias 802:271–277
Stanberry LR, Bernstein DI, Myers MG (1986) Evaluation of the herpes simplex virus antiviral activity of pyrethrins. Antiviral Res 6(2):95–102
Tchoumbougnang F, Zollo PH, Dagne E, Mekonnen Y (2005) In vivo antimalarial activity of essential oils from Cymbopogon citratus and Ocimum gratissimum on mice infected with Plasmodium berghei. Planta Med 71(1):20–23
Tona L, Cimanga RK, Mesia K, Musuamba CT, De Bruyne T, Apers S, Hernans N, Miert SV, Pieters L, Totté J, Vlietinck AJ (2004) In vitro antiplasmodial activity of extracts and fractions from seven medicinal plants used in Democratic Republic of Congo. J Ethnopharmacol 93:27–32
Tona L, Mesia K, Ngimbi NP, Chrimwami B, Okond'ahoka, Cimanga K, de Bruyne T, Apers S, Hermans N, Totte J, Pieters L, Vlietinck AJ (2001) In-vivo antimalarial activity of Cassia occidentalis, Morinda morindoides and Phyllanthus niruri. Ann Trop Med Parasitol 95(1):47–57
Trager W, Jensen JB (1976) Human malaria parasites in continuous culture. Science 193:673–675
Traoré M, Diallo A, Nikièma JB, Tinto H, Dakuyo ZP, Ouédraogo JB, Guissou IP, Guiguemdé TR (2008) In vitro and in vivo antiplasmodial activity of “saye”, an herbal remedy used in Burkina Faso traditional medicine. Phytother Res 22(4):550–551
Urzua A, Mendoza L (2003) Antibacterial activity of fresh flowerheads of Chrysanthemum coronarium. Fitoterapia 74:606–608
Valadeau C, Pabon A, Deharo E, Albán-Castillo J, Estevez Y, Lores FA, Rojas R, Gamboa D, Sauvain M, Castillo D, Bourdy G (2009) Medicinal plants from the Yanesha (Peru): evaluation of the leishmanicidal and antimalarial activity of selected extracts. J Ethnopharmacol 123(3):413–422
WHO (2008) World malaria report. October 2009, www.who.int/malaria/wmr2008
Zeng L, Ye Q, Oberlies NH, Shi G, Cu ZM, He K (1996) Recent advances in annonaceous acetogenins. Nat Prod Rep 13:275–306
Zhao C, Wu Y, Lei Y, Ruan H, Voelter W, Jung A, Schick M (1996) Effect of alcohol extracts of Chrysanthemum morifolium on Plasmodium falciparum in vitro. J Tongji Med Univ 16(4):203–204
Acknowledgements
The authors are grateful to C. Abdul Hakeem of the College Management; Dr. S. Mohammed Yousuff, Principal; and Dr. K. Abdul Subhan, HOD of Zoology Department for providing the facilities to carry out this work. NKK, DM and DS thank MR4 who generously provided the chloroquine-resistant INDO strain used in the study. Thanks to X Su who deposited this strain with MR4.
Author information
Authors and Affiliations
Corresponding author
Additional information
Chinnaperumal Kamaraj and Naveen Kumar Kaushik contributed equally.
Rights and permissions
About this article
Cite this article
Kamaraj, C., Kaushik, N.K., Mohanakrishnan, D. et al. Antiplasmodial potential of medicinal plant extracts from Malaiyur and Javadhu hills of South India. Parasitol Res 111, 703–715 (2012). https://doi.org/10.1007/s00436-011-2457-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00436-011-2457-6