Abstract
The tanaka’s snailfish Liparis tanakae (Gilbert & Burke) (Scorpaeniformes: Liparidae) is an economically important marine fish species in China. However, the helminth parasites of this fish are still poorly known. During a helminthological survey of Chinese marine fishes from 2011 to 2012, we revealed that L. tanakae was heavily infected with third-stage larvae and adults of ascaridoid nematodes (total prevalence 100 % and mean intensity 82.3 nematodes per fish). Four species of third-stage larvae Hysterothylacium liparis Li, Xu & Zhang, 2007, H. aduncum (Rudolphi, 1802), Hysterothylacium fabri (Rudolphi, 1819), and Anisakis pegreffii (Campana-Rouget & Biocca, 1955) and a single species of adults H. liparis were differentiated and identified by morphological and molecular methods. The detailed morphology of the four species of third-stage larvae was also studied using light microscopy and scanning electron microscopy. The morphological and molecular characterization of the third-stage larvae of H. liparis was reported. Liparis tanakae represents a new host record for A. pegreffii and H. fabri. In addition, a new name, Hysterothylacium zhoushanense nom. nov. was also given to Hysterothylacium zhoushanensis Li, Liu & Zhang, 2012 to make the latinized specific epithet agree with this neuter generic name.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The tanaka’s snailfish Liparis tanakae (Gilbert & Burke) (Scorpaeniformes: Liparidae) is endemic to Northwest Pacific and only distributes in the Yellow Sea, East China Sea, Sea of Japan, Sea of Okhotsk and Pacific coast of Japan to northern Kuril Islands (Yamada et al. 1995). During a helminthological survey of Chinese marine fishes by two of the authors of the present study (Li and Zhang) from 2011 to 2012, large numbers of third-stage larvae and adults of ascaridoid nematodes were collected from L. tanakae in the Yellow Sea and East China Sea. The specific identification of any developmental stage and sex of ascaridoid nematodes is central to exploring and understanding their life cycles, epidemiology, ecology, population genetics and control (Gasser 2006; Zhang et al. 2007). However, it is often problematic to achieve the objective purely on the basis of morphological features. In recent years, many studies have proved that integrating DNA data and morphological features can overcome the limitations of traditional approaches and attain maximum efficiency for species separation and identification of ascaridoid nematodes (Zhu et al. 2007; Mattiucci et al. 2008; Shamsi et al. 2009, 2012, 2013; Mašová et al. 2010; Garbin et al. 2011; Testini et al. 2011; Xu et al. 2012; Li et al. 2012a, b, c, d; Liu et al. 2013; Zhang et al. 2013; Silva et al. 2013). Consequently, these third-stage larvae and adults of ascaridoid nematodes collected from L. tanakae were exactly identified utilizing morphological examination and molecular methods by sequencing and analyzing the internal transcribed spacer (ITS) of nuclear ribosomal DNA (rDNA).
Materials and methods
Light and scanning electron microscopy
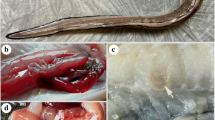
Fishes caught by commercial trawlers from the Yellow Sea (off Shidao, Shandong Province) and East China Sea (off Zhoushan Island, Zhejiang Province), respectively, were examined for parasites. Nematodes recovered from the abdominal cavity, subcutaneous layer of the skin, muscle, and digestive tract of L. tanakae (Fig. 1a–d) were washed in physiological saline and then fixed and stored in 80 % ethanol until studied. Light and scanning electron microscopical studies were prepared following the methods used by Li et al. (2012a). Drawings were made with the aid of Nikon microscope drawing attachment. Measurements (the range, followed by the mean in parentheses) are given in micrometres unless otherwise stated. Voucher specimens are deposited in College of Life Science, Hebei Normal University, Hebei Province, China.
Molecular procedures
Adult nematodes were identified as Hysterothylacium liparis on the basis of diagnostic characters including the body size, the morphology of the lips, the length of esophagus, intestinal caecum, ventricular appendix, spicules, the number and arrangement of caudal papillae, and the morphology of the tail tip. Larval specimens were identified to different morphotypes or generic level based on the morphological characters including the position of the excretory pore, the absence or presence of the intestinal caecum, and ventricular appendix, the shape of the tail, the length and ratio of the intestinal caecum, and ventricular appendix; and then some adults and larval individuals of each morphotype were randomly selected for molecular analysis (for details, see Table 1). Genomic DNA from individual worms was extracted using a Column Genomic DNA Isolation Kit (Shanghai Sangon, China) according to the manufacturer’s instructions. DNA was eluted in elution buffer and kept at -20 °C until use. The ITS region was amplified by PCR using the primers and cycling conditions described previously (Li et al. 2012a). PCR products were checked on GoldView-stained 1.5 % agarose gel and purified by the Column PCR Product Purification Kit (Shanghai Sangon, China). Sequencing was carried out using a DyeDeoxyTerminator Cycle Sequencing Kit (v.2, Applied Biosystems, California, USA) and an automated sequencer (ABI-PRISM 377). Sequencing for each sample was carried out for both strands. Sequences were aligned using ClustalW2 (Thompson et al. 1994) and adjusted manually. The ITS sequences determined were compared (using the algorithm BLASTn) with those available in the National Center for Biotechnology Information (NCBI) database (http://www.ncbi.nlmnih.gov).
Results
Third-stage larvae of Hysterothylacium liparis Li, Xu and Zhang, 2007
Diagnosis (n = 10)
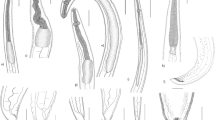
Body is small, whitish, with finely transversely striated cuticle, 5.6–11.3 (9.2) mm long; maximum width is 97–223 (183). It’s cephalic end rounded, with small ventral cuticular tooth (Figs. 2a, b and 3a, b). Oral aperture is triangular, surrounded by weakly developed anlagen of lips (Fig. 3a, b). Esophagus is almost cylindrical, muscular, 796–1,505 (1,182) long, and 49–97 (76) in maximum width, representing 11.4–15.6 (13.0)% of body length (Fig. 2b). Nerve ring is 252–388 (320) and excretory pore is 291–437 (375) from anterior extremity, respectively. Ventriculus oval to oblong, almost as wide as posterior region of esophagus, is 49–127 (89) long, and 44–98 (67) wide. Ventricular appendix is slightly shorter than intestinal caecum, 441–578 (460) long, 39–74 (53) in maximum width. Intestinal caecum is 583–971 (781) long and 49–78 (62) wide, representing 57.1–75.0 % esophageal length. Ratio of intestinal caecum to ventricular appendix 1.3–1.9:1 (1.7:1) (Fig. 2b). Tail is 87–146 (102) long; tip of tail is rounded, without any ornament (Figs. 2c, d and 3c).
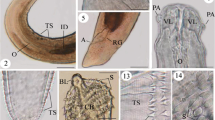
Scanning electron micrographs of third-stage larva from Liparis tanakae. Hysterothylacium liparis (a–c): a cephalic end, apical view (cuticular tooth arrowed); b cephalic end, subapical view (cuticular tooth arrowed); c posterior end of body, ventral view. Hysterothylacium fabri (d–f): d cephalic end, lateral view (cuticular tooth arrowed); e cephalic end, apical view; f. tip of tail, lateral view
Host and locality of third-stage larvae
Host is Tanaka’s snailfish L. tanakae (Gilbert & Burke) (Scorpaeniformes: Liparidae) found at Yellow Sea (off Shidao, Shandong Province) and East China Sea (off Zhoushan Island, Zhejiang Province), China.
Site of infection
Abdominal cavity, subcutaneous layer of the skin, muscle, and intestine are the sites of infection.
Prevalence and intensity of infection
Six out of ten fishes were infected with intensity of 1–118 (mean = 24.5) specimens.
Voucher specimens
There were 140 specimens (HBNU-F13043L).
Adults of Hysterothylacium liparis Li, Xu & Zhang, 2007
Diagnosis
The diagnostic characters of the adults—see Li et al. (2007).
Host and locality of adults
Host is Tanaka’s snailfish L. tanakae (Gilbert & Burke) (Scorpaeniformes: Liparidae) found at Yellow Sea (off Shidao, Shandong Province) and East China Sea (off Zhoushan Island, Zhejiang Province), China.
Site of infection
Intestine and stomach are the sites of infection
Prevalence and intensity of infection
Ten out of ten fishes were infected with intensity of 1–46 (mean = 20.1) specimens.
Voucher specimens
There were 201 specimens (HBNU-F13044L).
DNA characterization
There were six different sequence types obtained for the ITS region of the partial rDNA based on the present material examined herein and the length are all 1,007 bp. The different sequence types showed very low level of nucleotide variability (0.1–0.2 % nucleotide difference, details see Table 2). There are 16 species of Hysterothylacium ITS sequences registered in GenBank and pairwise comparison between H. liparis and the other species of Hysterothylacium (expect Hysterothylacium sp. HS1) registered in GenBank displayed 7.5 % (H. thalassini) to 22.3 % (Hysterothylacium zhoushanense) nucleotide difference. Thus, we could consider the low level of nucleotide difference among the six different genotypes obtained herein as intraspecific nucleotide difference because of the level far lower than the interspecific nucleotide difference. In addition, there was only 0.2 % of nucleotide difference between H. liparis and Hysterothylacium sp. HS1 (AM706344) in the ITS region. We considered the species Hysterothylacium sp. HS1 collected from Conger myriaster (Brevoort) (Anguilliformes: Congridae) also from the Yellow sea, China (Off Weihai, Shangdong Province) should belong to H. liparis. The ITS sequence of H. liparis are deposited in the GenBank database (http://www.ncbi.nlmnih.gov) under accession numbers (KF601895–KF601900).
Remarks
Li et al. (2007) described Hysterothylacium liparis and its fourth-stage larvae from the digestive tract of L. tanakae in the Yellow Sea, China. However, the third-stage larvae have not been found until now. The accurate identification of the third-stage larvae of Hysterothylacium purely based on morphological features is almost impossible because of the third-stage larvae of Hysterothylacium usually with very limited morphological features of taxonomic significance and their morphology commonly evidently different from the adults (Li et al. 2012a, b). The morphology and measurements of adults agree very well with the type material of H. liparis (deposited in College of Life Science, Hebei Normal University, Hebei Province, China), and the present material was also from the type host, L. tanakae, in the same or very neighboring region of the Northwest Pacific. Consequently, we considered our present specimens to be conspecific with L. tanakae. The morphology of the cephalic end and the tail of the present third-stage larvae is distinctly different from the adults of H. liparis and the relative oesophageal length of the third-stage larvae is much longer than the adults (representing 11.4–15.6 % of body length in the third-stage larvae vs. 7.1–10.9 % of body length in adults of H. liparis). However, the ratio of intestinal caecum and ventricular appendix of the present third-stage larvae is very similar to the adults, and they are collected from the same host L. tanakae. We therefore speculated that the present third-stage larvae might be congeneric with H. liparis. For further clarifying the taxonomic status of the present material, the ITS region of the paratypes of H. liparis and the present adults and putative third-stage larvae was sequenced and analyzed. The result of molecular analysis confirmed that the present adult and larval nematodes and the paratypes of H. liparis are homogeneous genetically and all belong to the same species.
Third-stage larvae of Hysterothylacium fabri (Rudolphi, 1819)
Diagnosis (n = 10)
Body is very variable in length, whitish, with finely transversely striated cuticle, 6.0–15.7 (10.8) mm long; maximum width is 243–437 (322). It’s cephalic end rounded, with very small ventral cuticular tooth (Figs. 2e, f and 3d, e). Oral aperture is triangular, surrounded by weakly developed anlagen of lips (Fig. 3d,e). Esophagus is almost cylindrical, muscular, 631–1068 (866) long, 68–107 (83.0) in maximum width, representing 6.5–10.6 (8.3)% of body length (Fig. 2f). Nerve ring is 223–350 (288) and excretory pore is 262–417 (341) from anterior extremity, respectively. Ventriculus is oval to oblong, slightly wider than posterior region of esophagus, 88–172 (115) long, 64–123 (85.0) wide. Ventricular appendix is much longer than intestinal caecum, 757–1233 (1015) long, and 49–136 (87.0) in maximum width. Intestinal caecum is very short, 181–368 (267) long, and 44–78 (64.0) wide, representing 1.9–4.0 (3.1)% esophageal length. Ratio of intestinal caecum to ventricular appendix is 0.2–0.4:1 (0.3:1) (Fig. 2f). Tail is 107–175 (125) long; tip of tail is with a very small cuticular spike (Figs. 2g and 3f).
Host and locality of third-stage larvae
Host is Tanaka’s snailfish L. tanakae (Gilbert & Burke) (Scorpaeniformes: Liparidae) found at Yellow Sea (off Shidao, Shandong Province) and East China Sea, (off Zhoushan Island, Zhejiang Province), China.
Site of infection
Abdominal cavity is the site of infection.
Prevalence and intensity of infection
Three out of ten fishes were infected with intensity of 1–15 (mean = 8.0) specimens.
Voucher specimens
There were 19 specimens (HBNU-F13045L).
DNA characterization
There were six different sequence types obtained for the ITS region of the partial rDNA based on the present material examined herein and the length are from 1,001 to 1,002 bp. The different sequence types showed 0.1–0.4 % nucleotide variability (details see Table 3). There are three ITS sequences of H. fabri registered in GenBank (nos. KC852206, JQ520158, JX974558) and pairwise comparison between the present data and the three ITS sequences of H. fabri registered in GenBank displayed very low level of nucleotide variability (0–0.4 % nucleotide difference). Thus, we considered the present material to be conspecific with H. fabri. The ITS sequences of H. fabri are deposited in the GenBank database (http://www.ncbi.nlmnih.gov) under accession numbers (KF736939–KF736944).
Remarks
H. fabri is a common parasite found in various Mediterranean fishes (Petter et al. 1984; Petter and Radujkovic 1986; Petter and Maillard 1987; Martín-Sánchez et al. 2003). Li et al. (2008a) reported it from three marine fishes in the Chinese waters. However, H. fabri is still a poorly known species, especially about its taxonomic status. Martín-Sánchez et al. (2003) studied its population genetic diversity and revealed that H. fabri collected from the Mediterranean fishes should be a complex comprising at least three sibling species. Our unpublished molecular data also proved that there were some significant genetic variations detected in the ITS region of H. fabri based on the adults collected from the Uranoscopidae fish in the Chinese waters and considered at least two sibling species to be in existence within the H. fabri complex (Li et al., unpublished). Nevertheless, the present molecular data supported that the third-stage larvae of H. fabri parasitizing L. tanakae maybe represent a single species. Moravec (1994) described the third-stage larvae of H. fabri based on the specimens collected from Salmo trutta fario Linnaeus (Salmoniformes: Salmonidae), but his material is distinctly smaller than ours in body size (1.8–2.2 mm of Moravec’s material vs. 6.0–15.7 mm of ours) and the morphology of the tail tip of his material is also different from ours (tail tip without the small spine in Moravec’s material). Moravec (1994, 1998) considered that a species identification of Hysterothylacium larvae purely on the basis of their morphology is often problematic. Therefore, the taxonomic status of the third-stage larvae identified as H. fabri by Moravec (1994) remains unclear. In the present study, the adult nematodes identified morphologically as H. fabri collected from Uranoscopus japonicus Houttuyn (Perciformes: Uranoscopidae) was firstly characterized using molecular methods by sequencing and analyzing the ITS region of rDNA, and then the ITS sequences of the randomly selected, identified morphologically as putative third-stage larvae of H. fabri were sequenced and compared with the sequence of adults. The results confirmed these third-stage larvae to be conspecific with the adults and all belong to H. fabri. L. tanakae represents a new host record for H. fabri.
Third-stage larvae of Hysterothylacium aduncum (Rudolphi, 1802)
Diagnosis (n = 10)
Body is relatively large, very variable in length, whitish, with finely transversely striated cuticle, 11.5–28.6 (17.2) mm long; maximum width is 233–437 (350). It is cephalic end rounded, with small ventral cuticular tooth, sharply pointed. (Figs. 4a, b and 5a, b). Oral aperture is triangular, surrounded by weakly developed anlagen of lips (Fig. 5a,b). Esophagus is almost cylindrical, muscular, 1,260–2,720 (1,760) long, and 68–146 (115) in maximum width, representing 9.3–11.3 (10.4)% of body length (Fig. 4a). Nerve ring is 340–534 (433) and excretory pore is 427–612 (499) from anterior extremity, respectively. Ventriculus is oval to oblong, slightly wider than posterior region of esophagus, 68–175 (124) long, and 68–146 (102) wide. Ventricular appendix is almost as long as intestinal caecum, 680–1,136 (870) long, and 78–107 (96.0) in maximum width. Intestinal caecum is 583–1505 (915) long and 58–126 (92) wide, representing 46.2–57.6 % esophageal length. Ratio of intestinal caecum to ventricular appendix is 0.8–1.3:1 (1.0:1) (Fig. 4a). Tail is 146–291 (198) long, and tip of tail is with a long cuticular spike (Figs. 4c, d and 5c).
Scanning electron micrographs of third-stage larva from Liparis tanakae. Hysterothylacium aduncum (a–c): a Cephalic end, apical view (cuticular tooth arrowed); b cephalic end, lateral view (cuticular tooth arrowed); c posterior end of body, subventral view. Anisakis pegreffii (d–f): d cephalic end, apical view (cuticular tooth arrowed); e cephalic end, apical view (excretory pore and cuticular tooth arrowed); f tip of tail, lateral view. d Dorsal lip; v Ventrolateral lip
Host and locality of third-stage larvae
Host is Tanaka’s snailfish L. tanakae (Gilbert & Burke) (Scorpaeniformes: Liparidae) found at East China Sea, off Zhoushan Island, Zhejiang Province, China.
Site of infection
Abdominal cavity is the site of infection.
Prevalence and intensity of infection
Ten out of ten fishes were infected with intensity of 1–83 (mean = 26.7) specimens.
Voucher specimens
There were 270 specimens (HBNU-F13046L).
DNA characterization
There were three different sequence types obtained for the ITS region of the partial rDNA based on the present material examined herein and the length are all 987 bp. The different sequence types showed 0.1–0.2 % nucleotide variability (for details, see Table 4). There are 35 ITS sequences of H. aduncum registered in GenBank and pairwise comparison between the present data and the sequences of H. aduncum registered in GenBank displayed very low level of nucleotide variability (0–0.5 % nucleotide difference). Thus, we considered the present material to be conspecific with H. aduncum. The ITS sequences of H. aduncum are deposited in the GenBank database (http://www.ncbi.nlmnih.gov) under accession numbers (KF736936–KF736938).
Remarks
Hysterothylacium aduncum is one of the most thoroughly studied ascaridoid nematodes parasitic in fish with respect to taxonomy (Hartwich 1975; Moravec et al. 1985; Moravec and Nagasawa 2000; Petter and Maillard 1987; Li et al. 2013), morphology (Soleim and Berland 1981), life cycles (Yoshinaga et al. 1987; Koie 1993; González 1998; Klimpel and Rückert 2005), ecology (Andersen 1993; Kalay et al. 2009), development (Balbuena et al. 1998, 2000; Iglesias et al. 2002), and population genetic diversity (Klimpel et al. 2007; Amor et al. 2011; Pekmezci et al. 2013). Recent molecular studies supported that H. aduncum parasitizing different marine teleosts worldwide only represents a single species, despite the existence of considerable intraspecific morphological variability and a low level of intraspecific genetic difference in some cases (Klimpel et al. 2007; Amor et al. 2011; Li et al. 2013; Pekmezci et al. 2013). The morphology of the larval forms of H. aduncum has been previously studied by Moravec et al. (1985), Shih and Jeng (2002), and Iglesias et al. (2002). The morphology of the present larval specimens agrees well with the descriptions by Moravec et al. (1985) and Iglesias et al. (2002), especially the relative length and ratio of the intestinal caecum, ventricular appendix and esophagus, and the morphology of the tail tip. However, the accuracy of morphological identification of larval stages of Hysterothylacium spp. is often incredible. For example, the specimens identified morphologically as H. aduncum by Shih and Jeng (2002), collected from the rabbitfish Siganus fuscescens (Houttuyn) (Perciformes: Siganidae) from off the Taiwan Stait, is not the real H. aduncum. On the basis of the morphology, measurements (especially the length and ratio of the intestinal caecum and ventricular appendix) and the host information, we considered that the material collected from S. fuscescens by Shih and Jeng (2002) should be H. longilabrum Li, Liu & Zhang, 2012. Consequently, the ITS region of rDNA were sequenced and analyzed for the accurate identification of the present larval nematodes. Comparison of the ITS sequences obtained herein with those available in GenBank confirmed that the present third-stage larval specimens from L. tanakae should belong to H. aduncum.
Third-stage larvae of Anisakis pegreffii Campana-Rouget & Biocca, 1955
Diagnosis (n = 3)
Body is large, whitish, with remarkablely transversely striated cuticle, 15.2–20.1 (18.3) mm long; maximum width is 340–495 (440). It is cephalic end rounded, with very large ventral cuticular tooth, sharply pointed (Figs. 4e, f and 5d, e). Oral aperture triangular, surrounded by weakly developed anlagen of lips (Fig. 5d, e). Excretory pore just at base of subventral lips (Figs. 4f and 5e). Esophagus is almost cylindrical, muscular, 1,379–1,748 (1570) long, and 97–136 (123) in maximum width, representing 8.1–9.1 (8.6)% of body length (Fig. 4e). Nerve ring is 291–311 (298) from anterior extremity. Ventriculus more or less cylindrical, much wider than esophagus, 534–728 (621) long, and 194–204 (197) wide (Fig. 4e). Intestinal caecum and ventricular appendix are absent. Tail is 97–117 (107) long, with a finger-like tip (Figs. 4g and 5f).
Host and locality of third-stage larvae
Host is Tanaka’s snailfish L. tanakae (Gilbert & Burke) (Scorpaeniformes: Liparidae) found at East China Sea, off Zhoushan Island, Zhejiang Province, China.
Site of infection
Abdominal cavity is the site of infection.
Prevalence and intensity of infection
One out of ten fishes was infected with intensity of three specimens.
Voucher specimens
There were three specimens (HBNU-F13047L).
DNA characterization
There was only one sequence type obtained for the ITS region of the partial rDNA based on the present material examined herein and the length is 918 bp. There are 67 ITS sequences of A. pegreffii registered in GenBank and pairwise comparison between the present data and the ITS sequences of A. pegreffii registered in GenBank showed very low level of nucleotide variability (0–0.4 % nucleotide difference). Thus, we considered the present material to be conspecific with A. pegreffii. The ITS sequence of A. pegreffii is deposited in the GenBank database (http://www.ncbi.nlmnih.gov) under accession number (KF736935).
Remarks
Due to the importance as disease agent in humans and marine organisms, A. pegreffii has received much attention. The third-stage larvae of A. pegreffii have been reported from various fishes and cephalopods worldwide (Abe et al. 2005; Mattiucci and Nascetti 2006; Umehara et al. 2006, 2010; Du et al. 2010; Shamsi et al. 2011; Kuhn et al. 2011; Quiazon et al. 2011; Smrzlic et al. 2012; Zhang et al. 2007, 2013; Setyobudi et al. 2013), but there has been no report of A. pegreffii from the fish host L. tanakae. In the Chinese waters, Zhang et al. (2007, 2013), Du et al. (2010) reported this species from various fishes. The morphology of the third-stage larvae of A. pegreffii has been studied by Zhang et al. (2013). We compared our present material with the larval specimens of Zhang et al. (2013) deposited in College of Life Science, Hebei Normal University, Hebei Province, China. The measurements and morphology of the material of Zhang et al. (2013) agree very well with ours. The molecular characterization of ITS region of A. pegreffii has been studied by Abe et al. (2005), Kuhn et al. (2011), Quiazon et al. (2011), Smrzlic et al. (2012), Setyobudi et al. (2013) and Zhang et al. (2013). Pairwise comparison between the present data and the ITS sequences of A. pegreffii registered in GenBank further confirmed the present larval material collected from L. tanakae belong to A. pegreffii.
Hysterothylacium zhoushanense nom. nov., synonym Hysterothylacium zhoushanensis Li, Liu & Zhang, 2012
Remarks
H. zhoushanensis was described as a new species from the flatfish Pseudorhombus oligodon (Bleeker) (Pleuronectiformes: Paralichthyidae) in the East China Sea (Li et al. 2012b). They proposed the latinized specific epithet zhoushanensis, which referred to the name of the type locality, Zhoushan Island. However, the generic names Hysterothylacium is in the neuter gender. Li et al. (2012b) were unaware of changing the ending of zhoushan-ensis to zhoushan-ense to make it agree with this neuter generic name. The correct ending is given now.
Discussion
The tanaka’s snailfish L. tanakae (Gilbert & Burke) (Scorpaeniformes: Liparidae) is an economically important fish species and the annual harvests have been increasing in the east coast of China (Zhang et al. 2011; Zhou et al. 2012). However, the helminth parasites of this fish are still poorly known. To our knowledge, only H. liparis and H. aduncum has previously been reported parasitic in L. tanakae (Li et al. 2008b). In the present study, the high levels of infection and helminth diversity in L. tanakae were revealed for the first time. The prevalence and mean intensity of infection of third-stage larvae of H. aduncum were 100 % and 26.7 individuals per fish, respectively, which are much higher than the other three species H. liparis, H. fabri, A. pegreffii. Analogously, we could consider that H. aduncum is the dominant parasite species in L. tanakae in the larval stage. In contrast, we cannot find the adults of H. aduncum and H. fabri in the alimentary tract of L. tanakae and only the adults of H. liparis occur in intestine and stomach of L. tanakae in this helminthological survey. Li et al. (2008b) reported the adults of H. aduncum occurred in the alimentary tract of L. tanakae in the Yellow Sea, China; however, the levels of prevalence and mean intensity of infection of H. aduncum adults were far lower than those of H. liparis. This situation possibly indicates that L. tanakae may not be the suitable definitive host of H. aduncum and H. fabri, but for H. liparis. The high levels of infection and helminth diversity in this fish can be easily explained when we considered that L. tanakae is a benthic, voracious predator, and large numbers of small fishes, benthic crustaceans and cephalopods are present in its diet (Xue et al. 2010; Zhang et al. 2011; Zhou et al. 2012), most of which are paratenic hosts or intermediate hosts of the species of Hysterothylacium and Anisakis. Meanwhile, the heavily infected level of third-stage larvae of Hysterothylacium and Anisakis in L. tanakae also reminds us that we need to be constantly alert to anisakidosis when we eat raw or undercooked meat of this marine fish.
Our present knowledge of the third-stage larvae of ascaridoid nematodes is still very limited. With the assistance of molecular techniques, for example, utilizing the ITS of nuclear rDNA, mitochondrial cytochrome coxidase subunit I (CO I) and subunit II (CO II) as genetic marker, the different larval forms of ascaridoid nematodes can be easily distinguished or separated; nevertheless, most of them hardly could be identified to the species level due to the absence of similar sequences for adult parasites. Within the genus Hysterothylacium, as far as we are aware, only the third-stage larvae of H. geschei Torres, Andrade & Silva, 1998, H. patagonense Moravec, Urawa & Coria, 1997, H. cenotae (Pearse, 1936), H. aduncum, H. longilabrum, and H. zhoushanense were accurately identified and described (Torres et al. 1998; Moravec et al. 1985, 1997; Iglesias et al. 2002; Li et al. 2012a, b). Most of the previous studies can only assign the larval forms of Hysterothylacium to different morphotypes or to generic level (Cannon 1977; Chai et al. 1986; Borges et al. 2012; Shamsi et al. 2011, 2013; Jabbar et al. 2013). The growing molecular data (i.e., ITS of rDNA, CO I and CO II of mtDNA) of the already described adults of ascaridoid nematodes will undoubtedly contribute to solve the problem.
References
Abe N, Ohya N, Yanagiguchi R (2005) Molecular characterization of Anisakis pegreffii larvae in Pacific cod in Japan. J Helminthol 79:303–306
Amor N, Farjallah S, Merella P, Said K, Slimane BB (2011) Molecular characterization of Hysterothylacium aduncum (Nematoda: Raphidascaridae) from different fish caught off the Tunisian caught off the Tunisian coast based on nuclear ribosomal DNA sequences. Parasitol Res 109:1429–1437
Andersen K (1993) Hysterothylacium aduncum (Rudolphi, 1862) infection in cod from the Oslofjord: seasonal occurrence of third- and fourth-stage larvae as well as adult worms. Parasitol Res 79:67–72
Balbuena JA, Karlsbakk E, Saksvik M, Kvenseth AM, Nylund A (1998) New data on the early development of Hysterothylacium aduncum (Nematoda, Anisakidae). J Parasitol 84:615–617
Balbuena JA, Karlsbakk E, Kvenseth AM, Saksvik M, Nylund A (2000) Growth and emigration of third-stage larvae of Hysterothylacium aduncum (Nematoda: Anisakidae) in larval herring Clupea harengus. J Parasitol 86:1271–1275
Borges JN, Cunha LFG, Santos HLC, Monteiro-Neto C, Santos CP (2012) Morphological and molecular diagnosis of anisakid nematode larvae from cutlassfish (Trichiurus lepturus) off the Coast of Rio de Janeiro, Brazil. PLoS One 7:1–14
Cannon LRG (1977) Some larval ascaridoids from south-eastern Queensland marine fishes. Int J Parasitol 7:233–243
Chai J-Y, Chu Y-M, Sohn W-M, Lee S-H (1986) Larval anisakids collected from the Yellow Corvina in Korea. Korean J Parasitol 24:1–11
Du C-X, Zhang L-P, Shi M-Q, Ming Z, Hu M, Gasser RB (2010) Elucidating the identity of Anisakis larvae from a broad range of marine fishes from the Yellow Sea, China, using a combined electrophoretic-sequencing approach. Electrophoresis 31:654–658
Garbin L, Mattiucci S, Paoletti M, González-Acuña D, Nascetti G (2011) Genetic and morphological evidences for the existence of a new species of Contracaecum (Nematoda: Anisakidae) parasite of Phalacrocorax brasilianus (Gmelin) from Chile and its genetic relationships with congeners from fish-eating birds. J Parasitol 97:476–492
Gasser RB (2006) Molecular tools—advances, opportunities and prospects. Vet Parasitol 136:69–89
González L (1998) The life cycle of Hysterothylacium aduncum (Nematoda: Anisakidae) in Chilean marine farms. Aquaculture 162:173–186
Hartwich G (1975) Schlauchwürmer, Nemathelminthes, Rund-oder Fadenwürmer, Nematoda parasitische Rundwürmer von Wirbeltieren: I. Rhabditida und Ascaridida. Tierwelt Deutschl 62:1–256
Iglesias L, Valero A, Galvez L, Benitez R, Adroher FJ (2002) In vitro cultivation of Hysterothylacium aduncum (Nematoda: Anisakidae) from 3rd-stage larvae to egg-laying adults. Parasitology 125:467–475
Jabbar A, Fong RWJ, KoK KX, Lopata AL, Gasser RB, Beveridge I (2013) Molecular characterization of anisakid nematode larvae from 13 species of fish from Western Australia. Int J Food Microbiol 161:247–253
Kalay M, Dönmez AE, Koyuncu CE, Genc E, Sahin G (2009) Seasonal variation of Hysterothylacium aduncum (Nematoda: Raphidascarididae) infestation in sparid fishes in the Northeast Mediterranean Sea. Turk J Vet Anim Sci 33:517–523
Klimpel S, Rückert S (2005) Life cycle strategy of Hysterothylacium aduncum to become the most abundant anisakid fish nematode in the North Sea. Parasitol Res 97:141–149
Klimpel S, Kleinertz S, Hanel R, Ruckert S (2007) Genetic variability in Hysterothylacium aduncum, a raphidascarid nematode isolated from spat (Sprattus sprattus) of different geographicl reas of the northeastern Atlantic. Parasitol Res 101:1425–1430
Koie M, (1993) Aspects of the life-cycle and morphology of Hysterothylacium aduncum (Rudolphi, 1802) (Nematoda, Ascaridoidea, Anisakidae). Can J Zool 71:1289–1296
Kuhn T, Garcia-Marquez J, Klimpel S (2011) Adaptive radiation within marine anisakid nematodes: a zoogeographical modeling of cosmopolitan, zoonotic parasites. PLoS One 6:1–6
Li L, Xu Z, Zhang L-P (2007) A new species of genus Hysterothylacium Ward et Magath, 1917 (Nematoda, Anisakidae) from Liparis tanakae (Scorpaeniformes, Liparidae) from the Yellow Sea, China. Acta Parasitol 52:371–375
Li L, Xu Z, Zhang L-P (2008a) Redescription of three species of Hysterothylacium (Nematoda: Anisakidae) from marine fishes from the Yellow Sea, China, with the synonymy of Hysterothylacium muraenesoxin (Luo, 1999). Zootaxa 1878:55–67
Li L, Xu Z, Zhang L-P (2008b) Investigation of nematodes in fishes from the Yellow Sea. Fish Sci 27:283–285
Li L, Xu Z, Zhang L-P (2012a) Goezia nankingensis Hsü, 1933 (Nematoda: Raphidascarididae) from the critically endangered Chinese paddlefish Psephurus gladius (Martens) (Acipenseriformes: Polyodontidae). Syst Parasitol 82:39–48
Li L, Liu Y-Y, Zhang L-P (2012b) Morphological and molecular identification of Hysterothylacium longilabrum sp. nov. and larvae of different stages (Nematoda: Anisakidae) from marine fishes in the South China Sea. Parasitol Res 111:767–777
Li L, Liu Y-Y, Zhang L-P (2012c) Morphological and genetic characterization of Hysterothylacium zhoushanensis sp. nov. (Ascaridida: Anisakidae) from the flatfish Pseudorhombus oligodon (Bleeker) (Pleuronectiformes: Paralichthyidae) in the East China Sea. Parasitol Res 111:2393–2401
Li L, Gibson DI, Liu Y-Y, Zhang L-P (2012d) Morphological and molecular study of the poorly known species Pseudanisakis rajae (Yamaguti, 1941) (Nematoda: Acanthocheilidae) from elasmobranchs in the Yellow Sea and Taiwan Strait off the coast of China. Syst Parasitol 81:115–123
Li L, Zhang L-P, Liu Y-Y (2013) Hysterothylacium simile n. sp. (Nematoda: Raphidascarididae) and H. aduncum (Rudolphi, 1802) from marine fishes in the Bohai and Yellow Sea, China, with comments on the record of H. paralichthydis (Yamaguti, 1941) in the Chinese waters. Syst Parasitol 84:57–69
Liu Y-Y, Xu Z, Zhang L-P, Li L (2013) Redescription and genetic characterization of Hysterothylacium thalassini Bruce, 1990 (Nematoda: Anisakidae) from marine fishes in the South China Sea. J Parasitol 99:655–661
Martín-Sánchez J, Díaz M, Artacho ME, Valero A (2003) Molecular arguments for considering Hysterothylacium fabri (Nematoda: Anisakidae) a complex of sibling species. Parasitol Res 89:214–220
Mašová Š, Moravec F, Baruš V, Seifertová M (2010) Redescription, systematic status and molecular characterization of Multicaecum heterotis Petter, Vassiliadès et Marchand, 1979 (Nematoda: Heterocheilidae), an intestinal parasite of Heterotis niloticus (Osteichthyes: Arapaimidae) in Africa. Fol Parasitol 57:280–288
Mattiucci S, Nascetti G (2006) Molecular systematics, phylogeny and ecology of anisakid nematodes of the genus Anisakis Dujardin, 1845: an update. Parasite 13:99–113
Moravec F (1994) Parasitic nematodes of freshwater fishes of Europe. Academia, Prague, 473 pp
Moravec F (1998) Nematodes of freshwater fishes of the Neotropical Region. Academia, Praha, 464 pp
Moravec F, Nagasawa K (2000) Some anisakid nematodes from marine fishes of Japan and the North Pacific Ocean. J Nat Hist 34:1555–1574
Moravec F, Nagasawa K, Urawa S (1985) Some fish nematodes from fresh water in Hokkaido, Japan. Fol Parasitol 32:305–316
Moravec F, Urawa S, Coria CO (1997) Hysterothylacium patagonense n. sp. (Nematoda: Anisakidae) from freshwater fishes in Patagonia, Argentina, with a key to the species of Hysterothylacium in American freshwater fishes. Syst Parasitol 36:31–38
Pekmezci GZ, Bolukbas CS, Gurler AT, Onuk EE (2013) Occurrence and molecular characterization of Hysterothylacium aduncum (Nematoda: Anisakidae) from Merlangius merlangus euxinus and Trachurus trachurus off the Turkish coast of Black Sea. Parasitol Res 112:1031–1037
Petter AJ, Maillard C (1987) Ascarides de poissons de Méditerranée occidentale. Bull Mus Hist Nat 9:773–798
Petter AJ, Radujkovic BM (1986) Nematodes parasites de poisons de la mer Adriatique. Bull Mus Hist Nat 8:489–499
Petter AJ, Lébre C, Radujkovic BM (1984) Nématodes parasites de poissons osteichthyens de l’Adriatique méridionale. Acta Adriat 25:205–221
Quiazon KMA, Yoshinaga T, Ogawa K (2011) Distribution of Anisakis species larvae from fishes of the Japanese waters. Parasitol Int 60:223–226
Setyobudi E, Jeon C-H, Choi K, Lee S II, Lee C II, Kim J-H (2013) Molecular identification of anisakid nematodes third stage larvae isolated from common squid (Todarodes pacificus) in Korea. Ocean Sci J 48:197–205
Shamsi S, Norman R, Gasser RB, Beveridge I (2009) Redescription and genetic characterization of selected Contracaecum spp. (Nematoda: Anisakidae) from various hosts in Australia. Parasitol Res 104:1507–1525
Shamsi S, Eisenbarth A, Saptarshi S, Beveridge I, Gasser RB, Lopata AL (2011) Occurrence and abundance of anisakid nematode larvae in five species of fish from southern Australian waters. Parasitol Res 108:927–934
Shamsi S, Gasser RB, Beveridge I (2012) Genetic characterisation and taxonomy of species of Anisakis (Nematoda: Anisakidae) parasitic in Australian marine mammals. Invertebr Syst 26:204–212
Shamsi S, Gasser RB, Beveridge I (2013) Description and genetic characterisation of Hysterothylacium (Nematoda: Raphidascarididae) larvae parasitic in Australian marine fishes. Parasitol Int 62:320–328
Shih HH, Jeng MS (2002) Hysterothylacium aduncum (Nematoda: -Anisakidae) infecting a herbivorous fish, Siganus fuscescens, off the Taiwanese coast of the northwest Pacific. Zool Stud 41:185–189
Silva JP, Melo FTV, Silva LCN, Gonçalves EC, Giese EG, Furtado AP, Santos JN (2013) Morphological and molecular characterization of Ortleppascaris sp. larvae, parasites of the cane toad Rhinella marina from eastern Amazonia. J Parasitol 99:118–123
Smrzlic IV, Valic D, Kapetanovic D, Kurtovic B, Teskeredzic E (2012) Molecular characterisation of Anisakidae larvae from fish in Adriatic Sea. Parasitol Res 111:2385–2391
Soleim O, Berland B (1981) The morphology of Thynnascaris adunca (Rudolphi) (Nematoda, Ascaridoidea). Zool Scr 10:167–182
Testini G, Papini R, Lia RP, Parisi A, Dantas-Torres F, Traversa D, Otranto D (2011) New insights into the morphology, molecular characterization and identification of Baylisascaris transfuga (Ascaridida, Ascarididae). Vet Parasitol 175:97–102
Thompson JD, Higgins DG, Gibson TJ (1994) CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res 22:4673–4680
Torres P, Andrade P, Silva R (1998) On a new species of Hysterothylacium (Nematoda: Anisakidae) from Cauque mauleanum (Pisces: Atherinidae) by brightfield and scanning electron microscopy. Mem I Oswaldo Cruz 93:745–752
Umehara A, Kawakami Y, Matsui T, Araki J, Uchida A (2006) Molecular identification of Anisakis simplex sensu stricto and Anisakis pegreffii (Nematoda: Anisakidae) from fish and cetacean in Japanese waters. Parasitol Int 55:267–271
Umehara A, Kawakami Y, Ooi HK, Uchida A, Ohmae H, Sugiyama H (2010) Molecular identification of Anisakis type I larvae isolated from hairtail fish off the coasts of Taiwan and Japan. Int J Food Microbiol 143:161–165
Xu Z, Zhang L-P, Liu B-C, Li L (2012) Morphological and molecular characterization of Raphidascaris (Ichthyascaris) lophii (Wu, 1949) (Nematoda, Anisakidae) from marine fishes from China, with a key to the species of the subgenus Ichthyascaris. Acta Parasitol 57:316–322
Xue Y, Xu B-D, Gao T-X, Qiu T-L, Lin L-S (2010) Preliminary study on feeding ecology of Liparis tanakae in north Yellow Sea. J Fish Sci Chin 17:1066–1074
Yamada U, Shirai S, Irie T, Tokimura M, Deng S, Zheng Y, Li C, Kim YU, Kim YS (1995) Names and illustrations of fishes from the East China Sea and the Yellow Sea. Overseas Fishery Cooperation Foundation, Tokyo, Japan, 288 pp
Yoshinaga T, Ogawa K, Wakabayashi K (1987) Experimental life cycle of Hysterothylacium aduncum Nematoda: Anisakidae in freshwater. Fish Pathol 22:243–251
Zhang B, Jin X-S, Dai F-Q (2011) Feeding habits and their variation of seasnail (Liparis tanakae) in the central and southern Yellow Sea. J Fish Sci Chin 35:1199–1207
Zhang L-P, Hu M, Shamsi S, Beveridge I, Li H-M, Xu Z, Li L, Cantacessi C, Gasser RB (2007) The specific identification of anisakid larvae from fishes from the Yellow Sea, China, using mutation scanning-coupled sequence analysis of nuclear ribosomal DNA. Mol Cell Probe 21:386–390
Zhang L-P, Du X-J, An R-Y, Li L, Gasser RB (2013) Identification and genetic characterization of Anisakis larvae from marine fishes in the South China Sea using an electrophoretic-guided approach. Electrophoresis 34:888–894
Zhou Z-P, Jin X-S, Shan X-J, Li Z-L, Dai F-Q (2012) Seasonal variations in distribution and biological characteristics of snailfish Liparis tanakae in the central and southern Yellow Sea. Acta Ecol Sin 32:5550–5561
Zhu X-Q, Podolska M, Liu J-S, Yu H-Q, Chen H-H, Lin Z-X, Luo C-B, Song H-Q, Lin R-Q (2007) Identification of anisakid nematodes with zoonotic potential from Europe and China by single-strand conformation polymorphism analysis of nuclear ribosomal DNA. Parasitol Res 101:1703–1707
Acknowledgments
This study was supported by the National Natural Science Foundation of China (Nos. 31101615, 31272269), the Natural Science Foundation of Hebei Province (No. C2012205007), the China Postdoctoral Science Foundation (No. 2012 M520593), the Natural Science Foundation of Hebei Education Department (No. Y2012012), the Postdoctoral Research Projects Merit Subsidy of Human Resources and Social Security Department of Hebei Province, the Natural Science Foundation and the Biology Postdoctoral Program of Hebei Normal University (Nos. L2010B13, 111008).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Guo, YN., Xu, Z., Zhang, LP. et al. Occurrence of Hysterothylacium and Anisakis nematodes (Ascaridida: Ascaridoidea) in the tanaka’s snailfish Liparis tanakae (Gilbert & Burke) (Scorpaeniformes: Liparidae). Parasitol Res 113, 1289–1300 (2014). https://doi.org/10.1007/s00436-014-3767-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00436-014-3767-2