Abstract
Three juvenile nematode parasites were collected naturally from 90 (75 %) out of 120 specimens of the marine greater lizard fish Saurida undosquamis captured from water coasts at Hurghada City along the Red Sea in Egypt during the period from September 2013 to April 2014. Worms were identified on the basis of light and scanning electron microscopy. Two of the recovered worms were isolated from the peritoneal cavity of the infected fish around the wall of the stomach as encapsulated larvae. The anisakid juvenile Anisakis sp. (Type II) was characterized by an anteroventrally triangular mouth, with a boring tooth; its postanal tail was rounded, without a terminal mucron or spine. The gnathostomatid Echinocephalus overstreeti was characterized by the presence of a cephalic bulb armed with six transverse rows of spines which were slightly more compact near the anterior end of bulb with maximal separation near the midbulb; the cephalic bulb terminated at a pseudolabia which situated dorsoventrally and reached its greatest width at the posterior one third of the body, The postanal tail terminated at a pointed mucron. The third juvenile species, Hysterothylacium patagonense (Anisakidae), was isolated from the intestine of the infected fish; they are characterized by a small-sized body with a conical tail provided by a nodulose apex, and the anterior end was equipped with three lips. A dorsal lip slightly smaller than the two subventrals left a deep postlabial groove and prominent lateral flanges in between, and the proximal part of each lip was smooth. The three described species were compared morphologically and morphometrically with some of the previously recorded species of the same genus. From this comparison, the similarity and variations between these species were described and concluded that the present study should be considered as a new host record in Egypt.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Nematodes comprise one of the largest and most diverse groups of helminthes mostly in freshwater, brackish water, and marine fishes; heavily infected fish show emaciation, imbalanced swimming, and reduction of their vitality (Klimpel et al. 2011; Mehlhorn et al. 2011; Morsy et al. 2012). Several species of fish nematode parasites can cause very dangerous diseases in humans. Anisakidosis is a well-known disease involving an infection with live larvae of anisakid nematodes following the consumption of infected seafood (Takahashi et al. 1998; Chai et al. 2005). Since the first reports demonstrating the pathogenic effects of Anisakis larvae in humans (Van Thiel et al. 1960), there has been an increasing awareness of fish-borne parasitic diseases. The marine nematodes are grouped into 10 orders, 78 families, 708 genera, and 5872 species in addition to 168 species which are placed in Nematoda incertae sedis (WoRMS 2012). They have been collected from many tissues and organs within fishes. However, their adults are moderately site specific within their host fishes; for example, cucullanid, gnathostomatid, and anisakid worms are typically found in the digestive tract (Olsen 1974; Moravec and Nagasawa 2000). Most species of nematodes live as adult stages in the alimentary canal of their hosts except species of the family Philometridae which inhabit the body cavities, liver, and gonads. The identification of nematodes is based on the external and internal anatomical structures. The external characteristics may include the modification of the cuticle at the anterior end, alae, cervical, or caudal papillae; location of the vulva; and the presence of a copulatory bursa in the male worms (Hoffman 1970). Most genera of the family Anisakidae live in the digestive tracts of marine fishes. They live free in the lumen of the stomach or intestine; some attach to or invade the wall of these organs and cause local tissue damage. These parasites are reported to be important for public health, including Anisakis sp. as 10 distinct species (WoRMS 2012), Pseudoterranova, Gnathostoma, Eustrongylides, Contracaecum, Phocascaris, and Hysterothylacium (Berland 2006). The genus Hysterothylacium Ward et Magath, 1917 was reported in the freshwater and marine fish farms (Moravec 1994; Hoffman 1998; Gonzalez 1998). The adult nematodes of this genus are generally restricted to the digestive tract of fishes (Machida et al. 1978). Their larvae parasitize various tissues of numerous fishes and invertebrates (Norris and Overstreet 1976). Furthermore, it is known that marine fishes can act as intermediate, paratenic, or definitive hosts (Zhu et al. 1998). Some species of adult spirurines parasitize a wide range of hosts; others exhibit a relatively narrow host specificity. Within the Gnathostomatidae, the genus Echinocephalus Molin 1858 with a few widely distributed species and the monotypic Metaleptus (Machida et al. 1982) include parasites of elasmobranchs (rays and sharks) (Machida et al. 1982; Moravec and Nagasawa 2000), whereas the only species of Ancyracanthus Diesing, 1838 from fish is known to parasitize Neotropical freshwater teleosts (characids) (Gomes and Kohn 1970; Moravec 1998). In the present study, the morphological and morphometric characterization of three juvenile nematodes infecting the greater lizard fish Saurida undosquamis was described by means of light and scanning electron microscopy as the first description from this host species in Egypt.
Materials and methods
One hundred and twenty specimens of the greater lizard fish S. undosquamis (F: Synodontidae) were collected during the period from September 2013 to April 2014 from boat landing sites and fishermen at the coasts of Hurghada City along the Red Sea in Egypt. Fish were transported alive to the Laboratory of Parasitology, Zoology Department, Faculty of Science, Cairo University, where they were morphologically identified. Skin surface, fins, and gills of fish were examined by the naked eye and dissecting microscope for any attached parasites, lesions, or external changes (Inoue et al. 2000). After dissection, nematode worms as larvae were collected from the surface of visceral organs as the stomach, intestine, and muscles; subsequently rinsed in phosphate-buffered saline (PBS); fixed in 70 % ethanol at 60 °C; and stored in the same solution. For light microscopy, fresh and fixed worms were cleared in lactophenol. Identification was based on the comparison between the morphological characteristics of larval types including the morphology of the digestive tract, the shape and the presence of the boring tooth or the lips at the anterior end, the position of the excretory pore, and the shape of the postanal tail and its terminal mucron (Hafesteinsson and Rizvi 1987; Olson et al. 1983; Smith 1983; Køie 1993; Anderson 2000; Shih and Jeng 2002). For scanning electron microscopy, specimens were fixed in 3 % buffered glutaraldehyde, washed in cacodylate buffer, and dehydrated in alcohols. After passing through an ascending series of Genesolv D, they were processed in a critical point drier “Bomer-900” with Freon 13, sputter-coated with gold–palladium in a Technics Hummer V, and examined with an Etec Autoscan at a 20-kV JEOL scanning electron microscope. All measurements are in micrometers unless otherwise stated; minimum and maximum values were given, followed in parentheses by the arithmetic mean (±SD).
Results
Two different juvenile species as encapsulated larvae were recovered from the peritoneal cavity of the infected fish. These were Anisakis sp. Type II (F: Anisakidae) and Echinocephalus overstreeti (F: Gnathostomatidae). The intensity of their infection ranged from 10 to 12 worms per fish. Hysterothylacium patagonense (F: Anisakidae) as free larvae was recovered from the intestine of infected fish as 16–20 worms per fish.
Anisakis sp. Type II, Family: Anisakidae Railliet and Henry 1912
Description
Forty two out of 120 (35 %) fish specimens were found to be naturally infected. The third-stage larvae were characterized by a body surface totally striated and were slightly large, measuring 18 ± 2 mm (16–20) in length; the maximum body width was 0.48 ± 0.2 mm (0.28–0.68). The mouth was triangular anteroventrally (Figs. 3, 17, 18) with a ventral boring tooth measuring 0.005 ± 0.002 mm in length, located ventral to the mouth projected anteroventrally (Figs. 3, 4, 17, 18). The esophagus was 1.38 ± 0.02 mm (1.36–1.4) in length, and its maximum width was 0.12 ± 0.02 mm (0.1–0.14). The excretory pore, as a transverse slit situated between the ventrolateral lips, opens at a single excretory duct. The rectum opens ventrally at the anus, with rectal glands (Fig. 5); the anus opening was separated from the posterior end of the body by 0.2 ± 0.02 mm (0.18–0.22). The postanal tail rounded without a terminal mucron (Figs. 5, 20).
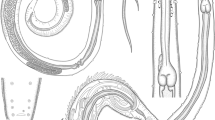
Photomicrographs showing juveniles of three nematode parasites: 1–5 Anisakis sp. Type II. 1 Whole mount preparation; bar 1.2 mm. 2–5 High magnifications of 2 the anterior part of the worm body showing its boring tooth (BT), mouth (Mo), esophagus (O), and the intestinal diverticula (ID); bar 0.24 mm. 3, 4 Boring tooth (BT), mouth (Mo), and the lateral papillae (PA); bar 0.01 mm. 5 Anal opening (A), rectal gland (RG); bar 0.11 mm. 6–10 High magnifications of H. patagonense showing 6, 7 the anterior part of the worm with the lateral expanding body cuticle (C) and three interlocked lips, one dorsal lip (DL) and two subventrals (VL). There are two lateral papillae (PA) occupying each subventral lip; bar 0.03 mm. 8–10 Posterior ends of 8 the third larval stage with a cuticular spike (CS) and the transverse striations of the cuticle (TS); bar 0.11 mm. 9, 10 The fourth larval stage with a nodulose apex (arrows); bar 0.02 mm. 11–16 High magnifications of Echinocephalus overstreeti showing 11, 12 the cephalic bulb (CB) with six rows of spines (S). The mouth is guarded by two smooth-surfaced, bulbous lips (BL); bar 0.12 mm. 13–15 Spines (S) of the cephalic bulb (CB) and transverse striation (TS). Bars: 13 0.06 mm; 14, 15 0.02 mm. 16 Posterior extremity of larva with the anus (A) and a pointed mucron (M); bar 0.01 mm
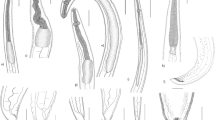
Scanning electron micrographs showing high magnifications of 17–20 Anisakis sp. Type II juveniles: 17 anterior extremity with a boring tooth (BT) and four papillae (PA) surrounding the triangular mouth (Mo). 18 Top view showing the mouth opening (Mo) and the boring tooth (BT). 19 Transverse (TS) and longitudinal (LS) striations of the cuticle and body papillae (PA). 20 The blunt posterior end. 21–24 Hysterothylacium patagonense juveniles showing 21, 22 the head region with three interlocked lips, one dorsal (DL) and two subventrals (VL) leaving an interlabium (I) in between. 23, 24 Posterior ends of 23 the third larval stage with a cuticular spike (CS). 24 The fourth larval stage with a nodulose apex (arrow). 25–30 Echinocephalus overstreeti juveniles showing 25 the cephalic bulb (CB) with six rows of spines (S) and bulbous lips (BL). 26 Bulbous lips (BL). 27, 28 Part of the spine (S) rows. 29 Transverse striations (TS) of the cuticle. 30 Posterior extremity of the larva with the anus (A) and terminating at a spiny mucron (M). Bars: 17–24, 26–28 10 μm; 25, 29, 30 50 μm
H. patagonense Moravec et al. 1997; Family: Anisakidae Railliet and Henry 1912
Description
Twenty five out of 120 (20.8 %) fish specimens were found to be naturally infected by two different types of larval stages of the same species, the fourth and third stages. The fourth stage was small and whitish measuring 5.5 ± 2 mm (3.5–7.5) long and 0.1 ± 0.02 mm (0.08–0.12) wide. The body was covered by a transversely striated cuticle, with no lateral alae; its anterior part was equipped by three lips, a single smaller dorsal lip and two subventrals, with deep postlabial grooves and prominent lateral flanges (Figs. 6, 7, 21, 22). The dorsal lip had two lateral doubled papillae (Fig. 22), while the two subventrals were equipped by mediolateral doubled papillae, a single lateral papilla, and amphids. The proximal part of each lip was smooth (Figs. 7, 21, 22). When interlocked, the three lips left an interlabium in between which was well developed and occupied one third the length of the lips. The esophagus was long, broader at its posterior bulb. The excretory pore opened posteriorly at the level of the nerve ring. The anal opening was at a distance of 0.1 mm from the posterior end of the worm. The third-stage larva was characterized by a tail terminating at a cuticular spike (Figs. 8, 23) which was different from that of the fourth stage which had a conical tail and a nodulose apex (numerous minute spines) covering its tip (Figs. 9, 10, 24).
E. overstreeti Deardorff and Ko 1983; Family: Gnathostomatidae Railliet, 1895
Description
Twenty three out of 120 (19.16 %) fish specimens were found to be naturally infected. The fourth larval stage was characterized by an unarmed body measuring 10 ± 2 mm (8–12) in length, and its greatest width at the posterior one third of the body was 0.3 ± 0.02 mm (0.28–0.32). The terminal mouth was guarded by two smooth, bulbous lips; each was 0.05 ± 0.02 mm (0.03–0.07) long (Figs. 11, 12, 25, 26). A cephalic bulb measuring 0.25 ± 0.02 mm (0.23–0.27) long was armed by six circular rows of spines (Figs. 11, 12, 25) which became compacted near the anterior end of the bulb. The spines of the first and second rows were inconspicuous; they were uncinate, larger at the posterior part of the bulb (Figs. 27, 28). The body was covered by a transversely striated cuticle (Figs. 13, 29). The esophagus was 2.71 ± 0.2 mm (2.51–2.91) long; the ventricular appendix was narrow, usually somewhat shorter than the intestinal cecum. The intestinal cecum was 7.38 ± 0.2 mm (7.18–7.58) long and 0.12 ± 0.02 mm (0.1–0.14) wide, opening at a midventral anus located approximately 0.04 mm from the posterior tip of the body which terminated at a pointed mucron (Figs. 16, 30). No papillae were observed in the pre- or postanal regions (Figs. 16, 30). Since the described worm was immature, the bursae, spicules, and fully reproductive organs were not observed.
Discussion
Nematodes are considered as the most economically important helminth parasites infecting fishes in the world (Dick and Choudhury 1995). Adult nematodes are usually found in the intestine of fish, while their larval stages are sometimes found on the flesh and viscera, causing massive diseases of fish and their economical losses. The larval stages of these parasites are infective to humans; they have the greatest impact on the consumer acceptance of fish (Post 1987; Dick and Choudhury 1995; Moravec 1994). Members of the family Anisakidae parasitize fish, mammals, birds, and reptiles (Moravec 1994; Zhu et al. 1998). They are among the most common nematodes of fish, which cause many pathological symptoms, mortalities, and a reduction of the commercial value of fish (Dick and Choudhury 1995). Anisakid nematodes were identified based on the following morphological characters: (1) the shape and the presence of the boring tooth or three lips on the anterior end; (2) the shape of the tail and the presence or absence of a mucron, caudal spine, or cactus tail; (3) the position of the excretory pore; (4) the length and shape of the ventriculus; and (5) the presence, length, and position of the anterior intestinal cecum and posterior ventricular appendix (Olson et al. 1983; Smith 1983; Køie 1993; Anderson 2000; Shih and Jeng 2002).
Anisakis sp. Type II
Since the genus Anisakis Dujardin, 1845 (Nematode: Anisakidae) is a common parasite of marine organisms worldwide, the occurrence of anisakid nematodes is of great concern for human health. Humans can be infected by anisakid nematodes by consuming raw or undercooked fish. Anisakis infection in human causes several symptoms such as sudden epigastric pain, nausea, vomiting, diarrhea, or allergic reactions (Sakanari and McKerrow 1989; Audicana and Kennedy 2008). A high percentage of these worms was recorded in the present study, and a high degree of low marketability was observed where they are found in the viscera and musculature of fish. This finding agreed with Dorny et al. (2009) and Shih et al. (2010) who concluded that the high prevalence of this nematode in economically important fish species indicates that damage to the fishing industry could occur by considerably reducing the quality of fish, leading to a loss in marketing values. Recent studies have also shown a high prevalence of Anisakis spp. in salmonid species, and the worms were mostly found in the musculature or encapsulated on the visceral organs, mesenteries, and peritoneum (Smith 1984; Sugawara et al. 2004; Noguera et al. 2009; Morsy et al. 2012). The present species is classified belonging to the genus Anisakis Dujardin, 1845 possessing all the characteristics of that genus as follows: inconspicuous lips with a prominent boring tooth on the anterior end; a straight anterior gut consisting of the esophagus, ventriculus, and intestine; and a cuticle obviously transversely striating the live larvae white or cream in color and encysting in capsules of the host origin (Olson et al. 1983; Rocka 2004; Dixon 2006). The shape of the mouth and the tail with the presence or absence of a tail mucron too distinguished between Anisakis Types I and II. The first type is characterized by an anterior head with a mouth opening rounded with dorsal and ventrolateral lips equipped with papillae; the postanal region terminated at a mucron. Anisakis sp. Type II is characterized by a triangular mouth in the head region surrounded by four papillae with no lips; the postanal tail is rounded without a terminal mucron (Shukhgalter and Nigmatullin 2001; Pardo-Gandarillas et al. 2009). A high abundance and prevalence of Anisakis Type II found in this study agreed with those reported by Shukhgalter and Nigmatullin (2001) and Pardo-Gandarillas et al. (2009), who reported a low prevalence of Anisakis Type I rather than Type II. The present-described species possesses all the structural patterns for Anisakis Type II. Table 1 shows a comparison between the present species under study and some previously reported Anisakis Types I and II. It is observed that the present-described species matches morphologically with Anisakis Type II recorded by Pardo-Gandarillas et al. (2009), with some morphometric differences as the short body length and width recorded in the present parasite herein. Since this morphologically similar species recorded previously from Dosidicus gigas by Pardo-Gandarillas et al. (2009), the present species under study should be considered as a new host record from S. undosquamis of the Red Sea in Egypt.
H. patagonense Moravec et al. 1997
Anisakid nematodes of the genus Hysterothylacium use fish as both intermediate and definitive hosts, in which they attain maturity (Costa et al. 2004). Up to 2012, there are more than 60 described species recorded from marine, estuarine, and freshwater fishes worldwide (Li et al. 2012); 24 of them are marine species (WoRMS 2012). Deardorff and Overstreet (1981) reviewed the genus, which was resurrected to include those species previously considered as members of Thynnascaris Dollfus 1933 and others placed in the genus Contracaecum Railliet and Henry 1912, namely species that mature in fishes. Similarly, Hysterothylacium and Contracaecum are closely related genera in the family Anisakidae. They have now been differentiated morphologically by the location of the excretory pore; the excretory pore of Hysterothylacium sp. is located at or near the level of the nerve ring, whereas in Contracaecum sp., it occurs at the anterior end near the base of the lips. In addition, the definitive hosts of Hysterothylacium are piscivorous fishes, not birds or mammals as for Contracaecum (Deardorff and Overstreet 1981). The geographic distributions of the various definitive hosts of these two genera differ: Contracaecum is found in shallow-water fishes, while Hysterothylacium has an intermediate distribution between inshore and open-water fishes (Moser and Hsieh 1992). Hysterothylacium are parasites of several freshwater and marine fish families (Deardorff and Overstreet 1981, 1982), and some species can occur in both (Brizzola and Tanzola 1995). Hysterothylacium sp. (Railliet and Henry 1912) is an important agent of parasitic diseases in humans (Ubeira et al. 2000). This zoonosis has gained relevance due to the popularization of food based on raw fish or insufficiently cooked meat (McCarthy and Moore 2000; Thompson 2001). Several studies regarding Hysterothylacium infection around the world were recorded including Chile (Carvajal and Gonzalez 1990; Torres et al. 1992; Torres 1995), Brazil (Eiras and Rego 1987), Yugoslavia (Petter and Radujkovic 1989), Japan (Yoshinaga et al. 1989), the USA (Moser and Hsieh 1992), Kuwait (Sey and Petter 1997), the Croatian part of the Adriatic Sea (Smrzlic et al. 2012), and the Red Sea at Yemen (Al-Zubaidy et al. 2012). In Egypt, little information is available about Hysterothylacium infection in Red Sea fishes. Recently, larval stages of Hysterothylacium aduncum from Pagrus pagrus fish of the Red Sea were recorded by Morsy et al. (2013). Numerous fourth and third larval stages which are morphologically and biometrically identical with those of H. patagonense were found in the intestine and stomach, in accordance with the study carried out by Moravec et al. (1997). The third- and fourth-stage larvae of H. patagonense can be easily distinguished one from another by the structure of the tip of their tail. While the third-stage larvae are noted for the presence of a cuticular spike on the tail terminal, the fourth-stage larvae are characterized by a rounded tip covered by a nodulose apex, a feature referred as a cactus tail. A similar morphology of the tail was previously described by Torres et al. (1998) on Hysterothylacium geschei and Moravec et al. (1997) on H. patagonense. Table 2 shows a comparison between Hysterothylacium species recorded in the present study and some previously recorded species of the same genus. It was observed that the most morphologically and morphometrically similar species is H. patagonense reported by Moravec et al. (1997) from the temperate bass Percichthys trucha in Patagonia, Argentina.
E. overstreeti Deardorff and Ko 1983
The genus Echinocephalus Molin 1858 contains nine species of distinctive gnathostomatids that are limited in host distribution primarily to marine and freshwater stingrays (Troncy 1969; Deardorff et al. 1981; Beveridge 1985). Interest in the systematic and biology of species of Echinocephalus has had both theoretical and practical components. This group has figured prominently in the development of hypotheses for the origins and biogeographic history of freshwater stingrays, Potamotrygonidae, of the Neotropics (Brooks et al. 1981; Brooks and Deardorff 1988; Brooks and McLennan 1993). Additionally, because life cycles for species of Echinocephalus involve mollusks, e.g., oysters, Crassostrea gigas (Thunberg), and abalone, Haliotis corrugata Gray, as intermediate hosts (Millemann 1951, 1963; Ko 1975), the possibility of accidental infection in humans is apparent. Juveniles of E. overstreeti were originally described from the ray Taeniura melanospilos Bleeker, 1853 (synonym of Taeniura meyeni Müller and Henle, 1841) from off the Marquesas Islands, Australian waters (Deardorff and Ko 1983). Later, adult specimens were recovered from Pastinachus sephen (Forsskål 1775), Myliobatis australis (Macleay 1881), Urogymnus asperrimus (Bloch and Schneider 1801), T. meyeni, and Heterodontus portusjacksoni (Meyer 1793), from different parts of the world (Beveridge 1987; Brooks and Deardorff 1988; Moravec and Justine 2006). As a result of studies by Millemann (1963), it is now generally agreed that the specimens of Echinocephalus studied by Molin (1858) and Baylis and Lane (1920) actually represent the larval forms of the species. Table 3 shows a comparison between Echinocephalus species recorded in the present study and some previously reported species of the same genus. Echinocephalus crassostreai serves as an example of a parasite which is of public health importance. Ko et al. (1974) demonstrated that L3 from oysters, upon ingestion by a cat and a rhesus monkey, penetrated the stomach and intestines. This is not totally surprising since the genus Echinocephalus is a member of the family Gnathostomatidae, which also includes Gnathostoma, the larvae of which genus are well known to cause gastric and other types of granulomatous lesions in humans if accidentally ingested. The morphology of specimens of the present material is mainly in accordance with the descriptions of E. overstreeti (Deardorff and Ko 1983) from Taeniura melanopilos and Moravec and Justine (2006) from T. meyeni. All the previously described species as well as the present material share the number of cephalic bulb spines (six transverse rows), with the body cuticle highly ornate; its bulbous lips do not bear teeth. Also, three different species of Echinocephalus were compared with the present material: E. crassostreai (Cheng 1975) with a cephalic bulb armed with eight rows of spines and E. multidentatus (Baylis and Lane 1920) and E. pseudouncinatus (Millemann 1951) with lips equipped by 10 and two teeth, respectively. So from this comparison, the present parasite should be classified as E. overstreeti. Since this species is recorded from S. undosquamis, the present study should be reported as a new host record from the Red Sea in Egypt.
References
Al-Zubaidy AB, (2010) Third-stage larvae of Anisakis simplex (Rudolphi, 1809) in the Red Sea fishes, Yemen coast. Journal of King Abdulaziz University, Marine Science 21:95–11
Al-Zubaidy AB, Mhaisen FT, Abker MAM (2012) Occurrence of five nematode species from some Red Sea fishes. Yemen Mesopot J Mar Sci 27(2):140–156
Anderson RC (2000) Nematode parasites of vertebrates: their development and transmission. CAB, Wallingford
Audicana MT, Kennedy MW (2008) Anisakis simplex: from obscure infectious worm to inducer of immune hypersensitivity. Clin Microbiol Rev 21(2):360–379
Baylis HA, Lane C (1920) A revision of the nematode family Gnathostomatidae. Proc Zool Soc Lond 920:245–310
Berland B (2006) Musings on nematode parasites. Havforsknings instituttet (Institute of Marine Research, Bergen), ISSN 0071-5638.
Beveridge I (1985) A redescription of Echinocephalus uncinatus Molin, 1858 (Nematoda, Gnathostomatoidea) from European rays, Dasyatis pastinaca (Linnaeus, 1758). Bulletin du Muse’um National d’Histoire Naturelle, Paris, se’r 4, Section A 7:781–790
Beveridge I (1987) Echinocephalus overstreeti Deardorff & Ko, 1983 (Nematoda: Gnathostomatoidea) from elasmobranchs and molluscs in South Australia. Trans R Soc S Aust 111:79–92
Brizzola SM, Tanzola RD (1995) H. rhamdiae n. sp. (Ascaridoidea:Anisakidae) from a Neotropical catfish, Rhamdia sapo (Pisces: Pimelodidae). Mem Instituto Oswaldo Cruz 90:349–352
Brooks DR, Deardorff TL (1988) Rhinebothrium devaneyi n. sp. (Eucestoda: Tetraphyllidea) and Echinocephalus overstreeti Deardorff and Ko, 1983 (Nematoda: Gnathostomatidae) in a thorny back ray, Urogymnus asperrimus, from Enewetak Atoll, with phylogenetic analysis of both species groups. J Parasitol 74:459–465
Brooks DR, Mclennan DA (1993) Parascript, parasites and the language of evolution. Smithsonian Institution Press, Washington, D.C., 429 p
Brooks DR, Thorson TB, Mayes MA (1981) Fresh-water stingrays (Potamotrygonidae) and their helminth parasites: testing hypotheses of evolution and coevolution. In: Funk VA, Brooks DR (eds) Advances in cladistics: proceedings of the first meeting of the Willi Hennig Society. New York Botanical Garden, New York, pp 147–175
Carvajal JR, Gonzalez L (1990) Presence of Hysterothylacium sp. (Nematoda: Anisakidae) in a cage-cultured coho salmon in Chile. Rev Chil Hist Nat 63:165–168
Chai J, Murrell KD, Lymbery AJ (2005) Fish-borne parasitic zoonoses: status and issues. Int J Parasitol 35:1233–1254
Cheng TC (1975) Echinocephalus crassostreai sp. nov., a larval nematode from the oyster Crassostrea gigas in the Orient. J Invert Pathol 26:81–90
Costa G, Madeira A, Pontes T, D’Amélio S (2004) Anisakid nematodes of the black spot seabream, Pagellus bogaraveo, from Madeiran waters, Portugal. Acta Parasitol 49:156–161
Deardorff TL, Ko RC (1983) Echinocephalus overstreeti sp. n. (Nematoda, Gnathostomatidae) in the Stingray, Taeniura melanopilos Bleeker, from the Marquesas Islands, with comments on E. sinensis Ko, 1975. Proc Helminthol Soc Wash 50(2):285–293
Deardorff TL, Overstreet RM (1981) Review of Hysterothylacium, and Iheringascaris (both previously: Thynnascaris) (Nematoda: Anisakidae) from the northern Gulf of Mexico. Proc Biol Soc Wash 93:1035–1079
Deardorff TL, Overstreet RM (1982) H. pelagicum sp. n. and H. cornutum (Stossich, 1904) (Nematoda: Anisakidae) from marine fishes. Proc Helminthol Soc Wash 49:246–251
Deardorff TL, Brooks DR, Thorson TB (1981) A new species of Echinocephalus (Nematoda: Gnathostomatidae) from Neotropical stingrays with comments on E. diazi. J Parasitol 67:433–439
Dick TA, Choudhury A (1995) Phylum Nematoda. In: Woo PTK (ed) Fish diseases and disorders, volume I protozoan and metazoan infection. Cambridge University Press, Cambridge, UK, pp 415–446
Dixon BR (2006) Isolation and identification of anisakid roundworm larvae in fish. Hea. Cana. Ottawa., OPFL-2.
Dollfus RP (1933) Thynnascaris legendrei n.gen., n.sp. de l’estomac du German, Germoalalonge (Gmel). Bull Soc Zool Fr 58:7–12
Dorny P, Praet N, Deckers N, Gabriel S (2009) Emerging food-borne parasites. Vet Parasitol 163:196–206
Eiras JC, Rego AA (1987) The histopathology of Scomber japonicas infection by Nematobothrium scombri (Trematoda: Didymozoidae) and of larval anisakid nematode infections in the liver of Pagrus pagrus. Mem Inst Oswaldo Cruz 82:155–159
Gomes DC, Kohn A (1970) Sôbre a subfamília “Ancyracanthinae” Yourke et Maplestone, 1926 (Nematoda, Spiruroidea). Atas Soc Biol Rio de Janeiro 13:83–88
Gonzalez L (1998) The life cycle of Hysterothylacium aduncum (Nematoda: Anisakidae) in Chilean marine farms. Aquaculture 162:173–186
Hafesteinsson H, Rizvi SS (1987) A review of the sealworm problem: biology, implications and solutions. J Food Prot 50:70–84
Hoffman GL (1970) Parasite of North American freshwater fishes. University of California. Press, Berkeley, 480 pp
Hoffman GL (1998) Parasites of North American freshwater fishes. University of California Press, New York
Inoue K, Oshima SI, Hirata T, Kimura I (2000) Possibility of anisakid larvae infection in farmed salmon. Fisheries Sci 66:1049–1052
Klimpel S, Abdel-Ghaffar F, Al-Rasheid KA, Aksu G, Fischer K, Strassen B, Mehlhorn H (2011) The effects of different plant extracts on nematodes. Parasitol Res 108(4):1047–1054
Ko RC (1975) Echinocephalus sinensis n. sp. (Nematoda: Gnathostomatidae) from the ray (Aetabatus flagellum) in Hong Kong, Southern China. Can J Zool 53:490–500
Ko RC, Morton B, Wong PS (1974) Echinoeephalus sp. Molin, 1858 (Spiruroidea: Gnathostomatidae), an unusual nematode from the oyster, Cmssostrea gigns Thunberg, 1793. Proc 3rd Int Congr Parasitol Munich Germany 1731–1732
Køie M (1993) Aspects of the life-cycle and morphology of H. aduncum (Rudolphi, 1802) (Nematoda, Ascaridoidea, Anisakidae). Can J Zool 71:1289–1296
Larizza A, Vovlas N (1995) Morphological observations on third-stage larvae of Anisakis simplex A (Anisakidae: Nematoda) from Adriatic and Ionian waters. J Helminthol Soc Wash 62(2):260–264
Li L, Xu Z, Zhang L (2007) A new species of genus Hysterothylacium Ward et Magath 1917 (Nematoda, Anisakidae) from Liparis tanakae (Scorpaeniformes, Liparidae) from the Yellow Sea, China. Acta Parasitol 52(4):371–375
Li L, Liu Y, Zhang L (2012) Morphological and genetic characterization of H. zhoushanensis sp. nov. (Ascaridida: Anisakidae) from the flatfish Pseudorhombus oligodon (Bleeker) (Pleuronectiformes: Paralichthyidae) in the East China Sea. Parasitol Res 111(6):2393–2401
Machida M, Takahashi K, Masuuchi S (1978) Thynnascaris haze n. sp. (Nematoda, Anisakidae) from goby in the Bay of Tokyo. Bull Nat Sci Mus Tokyo Ser A (Zoology) 4:241–244
Machida M, Ogawa K, Okiyama M (1982) A new nematode (Spirurida, Physalopteridae) from frill shark in Japan. Bull Nat Sci Mus Tokyo Ser A (Zool) 8:1–5
McCarthy J, Moore TA (2000) Emerging helminth zoonoses. Int J Parasitol 30:1351–1360
Mehlhorn H, Al-Quraishy S, Al-Rasheid KA, Jatzlau A, Abdel-Ghaffar F (2011) Addition of a combination of onion (Allium cepa) and coconut (Cocos nucifera) to food of sheep stops gastrointestinal helminthic infections. Parasitol Res 108(4):1041–1046
Millemann RE (1951) Echinocephalus pseudouncinatus n. sp., a nematode parasite of the abalone. J Parasitol 37:435–439
Millemann RE (1963) Studies on the taxonomy and life history of echinocephalid worms (Nematoda: Spiruroidea) with a complete description of Echinocephalus pseudouncinatus Millemann, 1951. J Parasitol 49:754–764
Molin R (1858) Prospectus helminthum, quae in prodromo faunae elminthological Venetiae continenlUr. Denkschriften Akad Wiss Math Naturwiss KI (Wein) 30:127–158
Moravec F (1994) Parasitic nematodes of freshwater fishes of Europe. Academia and Kluwer Academic Publishers, Prague and Dordrecht, 473 pp
Moravec F (1998) Nematodes of freshwater fishes of the Neotropical region. Academia, Prague, 464 pp
Moravec F, Justine JL (2006) Three nematode species from elasmobranchs off New Caledonia. Syst Parasitol 64:131–145
Moravec F, Nagasawa K (2000) Some anisakid nematodes from marine fishes of Japan and the North Pacific Ocean. J Nat Hist 34:1555–1574
Moravec F, Urawa S, Coria CO (1997) Hysterothylacium patagonense n. sp. (Nematoda: Anisakidae) from freshwater fishes in Patagonia, Argentina, with a key to the species of Hysterothylacium in American freshwater fishes. Syst Parasitol 36:31–38
Morsy K, Bashtar AR, Abdel-Ghaffar F, Mehlhorn H, Quraishy SA, MahdiM E, Al-Ghamdi A, Mostafa N (2012) First record of anisakid juveniles (Nematoda) in the European seabass Dicentrarchus labrax (Family: Moronidae), and their role as bio-indicators of heavy metal pollution. Parasitol Res 110(3):1131–1138
Morsy K, Bashtar AR, Abdel-Ghaffar F, Mostafa N (2013) New host and locality records of two nematode parasites Dujardinnascaris mujibii (Heterocheilidae) and Hysterothylacium aduncum (Anisakidae) from the common seabream Pagrus pagrus: a light and scanning electron microscopic study. Parasitol Res 112(2):807–815
Moser M, Hsieh J (1992) Biological tags for stock separation in Pacific herring Clupea harengus pallasi in California. J Parasitol 78:54–60
Noguera P, Collins C, Bruno D, Pert C, Turnbull A, McIntosh A, Lester K, Bricknell I, Wallace S, Cook P (2009) Red vent syndrome in wild Atlantic salmon Salmo salar in Scotland is associated with Anisakis simplex sensu stricto (Nematoda: Anisakidae). Dis Aquat Org 87:199–215
Norris DE, Overstreet RM (1976) The public health implications of larval Thynnascaris nematodes from shellfish. J Milk Food Technol 39:47–54
Olsen OW (1974) Animal parasites: their life cycles and ecology, 3rd edn. University Park Press, Baltimore, 562 pp
Olson AC, Lewis MD, Hauser ML (1983) Proper identification of anisakine worms. Am J Med Technol 49:111–114
Pardo-Gandarillas MC, Lohrmann KB, Valdivia AL, Ibáñez CM (2009) First record of parasites of Dosidicus gigas (d’ Orbigny, 1835) (Cephalopoda: Ommastrephidae) from the Humboldt Current system off Chile. Rev Biol Mar Oceanogr 44(2):397–408
Pereira J Jr, de Mattos F, Almeida NC, de Morais M, Vianna RT (2004) Hysterothylacium sp. Larvae (Nematoda: Anisakidae) in Micropogonias furnieri (Sciaenidae) from Rio Grande Do Sul Coast Brazil. Atlântica 26(1):55–60
Petter AJ, Radujkovic BM (1989) Parasites des poissons marins du Montenegro: Nematodes. Acta Adriat 30:195–236
Post G (1987) Animal parasites of fishes. In: Textbook of fish health. T.F.H. Publications Inc. USA. pp. 159–214
Railliet A, Henry A (1912) Parasitic nematodes du genera camallanus. Buf Soc Pathol 8:270
Rocka A (2004) Nematodes of the Antarctic fishes. Pol Polar Res 25(2):135–152
Sakanari JA, McKerrow JH (1989) Anisakiasis. Clin Microbiol Rev 2:278–284
Sey O, Petter AJ (1997) Incidence of ascaridoid larvae in Kuwaiti food fishes. Southeast Asian J Trop Med Publ Health 1:168–172
Shih HH, Jeng MS (2002) H. aduncum (Nematoda: Anisakidae) infecting a herbivorous fish, Siganus fuscescens, off the Taiwanese Coast of the Northwest Pacific. Zool Stud 41(2):208–215
Shih HH, Ku CC, Wang CS (2010) Anisakis simplex (Nematoda: Anisakidae) third-stage larval infections of marine cage cultured cobia, Rachycentron canadum L., in Taiwan. Vet Parasitol 171:277–285
Shukhgalter OA, Nigmatullin CM (2001) Parasitic helminths of the jumbo squid Dosidicus gigas (Cephalopoda: Ommastrephidae) in open waters of the central east Pacific. Fish Res 54:95–110
Smith JW (1983) Anisakis simplex (Rudolphi, 1809, det. Krabbe. 1878) (Nematoda: Ascaridoidea): morphology and morphometry of larvae from euphausiids and fish, and a review of the life-history and ecology. J Helminthol 57:205–224
Smith JW (1984) The abundance of Anisakis simplex L3 in the body cavity and flesh of marine teleosts. Int J Parasitol 14:491–495
Smrzlic IV, Valic D, Kapetanovic D, Kurtovic B, Teskeredzic (2012) Molecular characterization of Anisakidae larvae from fish in Adriatic Sea. Parasitol Res 111:2385–2391
Sugawara Y, Urawa S, Kaeriyama M (2004) Infection of Anisakis simplex (Nematoda: Anisakidae) larvae in chum salmon (Oncorhynchus keta) in the North Pacific Ocean, Bering Sea, and a river of Hokkaido. North Pacific Anadromous Fish Commission Doc 791. Hokkaido Tokai University, Sapporo
Takahashi S, Ishikura H, Kikuchi K (1998) Anisakidosis: global point of view. In: Ishikura H, Aikawa M, Itakura H, Kikuchi K (eds) Host response to international parasitic zoonoses. Springer Verlag, Tokyo, pp 109–120
Thompson RCA (2001) The future impact of societal and cultural factors on parasitic disease. Int J Parasitol 31:949–959
Torres P (1995) Some trematode, nematode, and acanthocephalan parasites of rainbow trout, Oncorhynchus mykiss, introduced into Chile. J Helminthol Soc Wash 62:257–259
Torres P, Contreras A, Cubillos V, Gesche W, Montefusco A, Rebolledo C, Mira A, Arenas J, Miranda JC, Asenjo S, Schlatter R (1992) Parasitismo en peces, aves piscívoras y comunidades humanas ribereñas de los lagos Yelcho y Tagua-Tagua, X Región de Chile. Arch Med Vet 24:77–92
Torres P, Andrade P, Silva R (1998) On a new species of Hysterothylacium (Nematoda: Anisakidae) from Cauque mauleanum (Pisces: Atherinidae) by brightfield and scanning electron microscopy. Mem Inst Oswaldo Cruz, Rio de Janeiro 93(6):745–752
Troncy PM (1969) Description de deux nouvelles especes de nematodes parasites de poissons. Bull du Mus Nat d’Histoire Naturelle 41:598–605
Ubeira FM, Valinas B, Lorenzo S, Iglesias R, Figueiras A, Villaescusa R (2000) anisaquiosis y alergia. Un studio soroepidemiológico en la Comunidad Autónoma Gallega. Documentos técnicos de Salud Pública, Serie B, no 24. Ed. Consellería de Sanidade e Servicios Sociais. Xunta de Galicia, España, p 102
Van Thiel PH, Kuipers FC, Roskam RT (1960) A nematode parasitic to herring, causing acute abdominal syndromes in man. Trop Geogr Med 2:97–113
World Register of Marine Species (WoRMS) (2012) marinespecies.org
Yoshinaga T, Ogawa K, Wakabayashi H (1989) Life cycle of Hysterothylacium haze (Nematoda: Anisakidae: Raphidascaridinae). J Parasitol 75(5):756–763
Zhu X, Gasser RB, Podolska M, Chilton NB (1998) Characterization of anisakid nematodes with zoonotic potential by nuclear ribosomal DNA sequences. Int J Parasitol 28:1911
Acknowledgments
The authors extend appreciations to the Faculty of Science, Cairo University, Cairo, Egypt.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Morsy, K., Bashtar, AR., Mostafa, N. et al. New host records of three juvenile nematodes in Egypt: Anisakis sp. (Type II), Hysterothylacium patagonense (Anisakidae), and Echinocephalus overstreeti (Gnathostomatidae) from the greater lizard fish Saurida undosquamis of the Red Sea. Parasitol Res 114, 1119–1128 (2015). https://doi.org/10.1007/s00436-014-4285-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00436-014-4285-y