Abstract
A new ascaridoid nematode Hysterothylacium longilabrum sp. nov. collected from the intestine and stomach of the marine fishes Siganus fuscescens (Houttuyn) and Siganus canaliculatus (Park) (Perciformes: Siganidae) in the South China Sea is described and illustrated. The new species differs from its congeners by the unusually long lips, the very short intestinal caecum and relatively long ventricular appendix (ratio of intestinal caecum to ventricular appendix, 1:2.38–5.50), the long spicules (1.96–3.28 mm long, representing 7.42–11.4% of the body length), the number and arrangement of male caudal papillae [38–43 pairs in total, arranged as: 31–34 pairs precloacal, 1 pair of paracloacal and 4–6 pairs postcloacal (the second or fourth pair double)] and the presence of a particular medioventral precloacal papilla in the male. Molecular analyses by sequencing and comparing the internal transcribed spacer (ITS) of the ribosomal DNA of H. longilabrum sp. nov. with the closely related nematode sequences seem to support the validity of the new species based on the morphological observation. In addition, the third- and fourth-stage larvae of the new species are also exactly identified and described by analysing and comparing the ITS sequence with the adult, and the result is a substantial step toward elucidating its life cycle.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
With 67 described species worldwide, Hysterothylacium Ward & Magath, 1917 is one of the largest groups of ascaridoid nematodes (Bruce et al. 1994; Moravec et al. 1996, 1997; Moravec and Nagasawa 1998, 2000; Torres et al. 1998; Torres and Soto 2004; Gopar-Merino et al. 2005; Li et al. 2007a, b, 2008; Raffel and Anderson 2009; Rossin et al. 2011). Adults of Hysterothylacium commonly occur in the digestive tract of marine, estuarine and freshwater fishes (Deardorff and Overstreet 1980; Bruce and Cannon 1989; Bruce et al. 1994). However, Hysterothylacium burtti Raffel and Anderson 2009 was also reported parasitic in amphibian (Raffel and Anderson 2009). Up to 2012, a total of ten species of Hysterothylacium were recorded from marine fishes in the Chinese waters (Hsü 1933; Parukhin 1966; Yin and Zhang 1982, 1983; Pan et al. 1990; Li et al. 2007a, b, c, 2008). During a helminthological survey of Chinese marine fishes from May 2005 to June 2011, large numbers of larvae and adults of ascaridoid nematodes were collected from marine fishes in the Yellow Sea, East China Sea, Taiwan Strait and South China Sea. The results of systematic evaluations of some of this material have already been published (Zhang et al. 2007; Li et al. 2007a, b, c, 2008, 2011, 2012; Du et al. 2010). In the present paper, a new species Hysterothylacium longilabrum sp. nov. collected from the marine fishes Siganus fuscescens (Houttuyn) and Siganus canaliculatus (Park) in the South China Sea is described and illustrated by light and scanning electron microscopy.
Accurate identification of a parasite at any stage of its development has very important implications for studying parasite epidemiology and resolving systemic problems (Kijewska et al. 2002). However, proper identification of adults of ascaridoid nematodes, only based on morphological characters, is sometimes problematic because of the existence of sibling or cryptic species appearing to be ubiquitous in ascaridoid nematodes, which nearly cannot be distinguished from each other only by morphological studies (Mattiucci et al. 1997; Zhu et al. 2002; Martín-Sánchez et al. 2003; Li et al. 2005; Mattiucci et al. 2008, 2010). In addition, morphological identification of ascaridoid larvae is also unreliable and unpractical as they are usually very similar to each other morphologically, and many significant taxonomic morphological features of the larvae are not fully developed (Moravec 2009). Recently, molecular techniques, utilising the internal transcribed spacer (ITS) of nuclear ribosomal DNA (rDNA) as a genetic marker, have proved to be particularly useful for the accurate identification of ascaridoid nematodes at the species level for eggs, larvae and adults (Zhu et al. 2007; for a review see Mattiucci and Nascetti 2008; Mašová et al. 2010; Fang et al. 2010; Testini et al. 2011; Li et al. 2011). Consequently, specimens of the adults, together with the identified morphologically as putative third- and fourth-stage larvae, of the new species collected from the two different hosts S. fuscescens and S. canaliculatus are characterised using molecular methods by sequencing and analysing the internal transcribed spacer (ITS) of the ribosomal DNA to assess the validity of the new species genetically, and to exactly recognize the larvae of different stages.
Materials and methods
Light and scanning electron microscopy
Fishes collected from the South China Sea were examined for parasites. Nematodes recovered from the digestive tract of fishes were washed in physiological saline and then fixed and stored in 70 % ethanol until studied. For light microscopical studies, nematodes were cleared in lactophenol. Drawings were made with the aid of a Nikon microscope drawing attachment. For scanning electron microscopy, specimens were fixed in 4 % formaldehyde solution, post-fixed in 1 % OsO4, dehydrated via an ethanol series and acetone, and then critical point dried. The specimens were coated with gold and examined using a Hitachi S-570 scanning electron microscope at an accelerating voltage of 15 kV.
Measurements (the range, followed by the mean in parentheses) are given in micrometres unless otherwise stated. Type specimens are deposited in the College of Life Science, Hebei Normal University, Hebei Province, China.
Molecular procedures
Twenty-four nematodes (details in Table 1) were subjected to molecular analysis. Genomic DNA from individual worms was extracted using a Column Genomic DNA Isolation Kit (Shanghai Sangon, China) according to the manufacturer’s instructions. DNA was eluted in an elution buffer and kept at −20 °C until use. The ITS region was amplified by PCR using the primers A (forward: 5′-GTCGAATTCGTAGGTGAACCTGCGGAAGGATCA-3′) and B (reverse: 5′-GCCGGATCCGAATCCTGGTTAGTTTCTTTTCCT-3′) (D’Amelio et al. 2000). PCR was performed in 50 μl of PCR reaction buffer with 10 mM Tris–HCl at pH 8.4, 50 mM KCl, 3.0 mM MgCl2, 250 μM of each dNTP, 50 pmol of each primer and 1.5 U of Taq polymerase (Takara) in a thermocycler (2720, Applied Biosystems) under the following conditions: 94 °C, 5 min (initial denaturation), followed by 30 cycles of 94 °C, 30 s (denaturation), 55 °C, 30 s (annealing), 72 °C, 70 s (extension), and a final extension of 72 °C for 7 min. PCR products were checked on GoldView-stained 1.5 % agarose gel and purified by the Column PCR Product Purification Kit (Shanghai Sangon, China). Sequencing was carried out using a DyeDeoxyTerminator Cycle Sequencing Kit (v.2, Applied Biosystems, CA, USA) and an automated sequencer (ABI PRISM 377). Sequencing for each sample was carried out for both strands. Sequences were aligned using ClustalW2 (Thompson et al. 1994) and adjusted manually. The ITS sequences determined were compared (using the algorithm BLASTn) with those available in the National Center for Biotechnology Information database (http://www.ncbi.nlm.nih.gov).
Results
Hysterothylacium longilabrum sp. nov.
Description
Medium to large, whitish nematodes with finely transversely striated cuticle. Maximum width of body at about mid-body. Lateral alae absent (Fig. 4a). Anterior end with three very long lips, approximately equal in size, with deep postlabial grooves and prominent lateral flanges (Figs. 1a, 3c, 4b). Proximal part of each lip with four lobes (Fig. 3a). Dorsal lip with two lateral double papillae (Figs. 1a, 3c), ventrolateral lips each with one lateral amphid, one single papilla and one double papilla (Figs. 1c, 4b). Interlabia well developed, about 1/3 length of lips (Figs. 1b, 4b). Oesophagus relatively long, slightly broader posteriorly than anteriorly. Nerve ring at 20–35 % of oesophageal length. Excretory pore just posterior to nerve ring (Fig. 1c). Ventriculus oval to oblong, slightly narrower than posterior region of oesophagus. Intestinal caecum very short, slightly longer or shorter than ventriculus (Fig. 1d, e). Ventricular appendix usually narrower and longer than intestinal caecum (Fig. 1d, e). Rectum hyaline, tubular, surrounded by three large, unicellular rectal glands. Tail of both sexes conical, tip with numbers of nodular protuberances (Figs. 1g, k, l, 3d, g).
H. longilabrum sp. nov. from S. fuscescens (Houttuyn) in the South China Sea: a cephalic end of male, dorsal view; b cephalic end of male, ventral view; c. anterior part of male, lateral view; d, e region of ventriculus; f region of vulva; g posterior end of female, lateral view; h eggs; i. posterior end of male (showing ejaculatory duct and spicules), lateral view; k posterior end of male (showing caudal papillae), lateral view; k, l tip of male tail. Scale bars: a, b 200 μm; c, d, e, f, i, j 400 μm; g 500 μm; h 50 μm; k, l 100 μm
Male (based on 18 mature specimens)
Body 26.4–31.5 (27.6) mm long; maximum width of 637–833 (755). Dorsal and ventrolateral lips almost equal in size, 392–490 (441) long, 196–275 (237) wide. Interlabia 98–147 (127) long, 196–245 (223) wide. Oesophagus 2.65–3.43 (2.98) mm long, 343–441 (402) in maximum width, representing 9.02–12.6 (10.8) % of body length. Nerve ring and excretory pore 833–1,058 (947) and 882–1,098 (1,007), respectively, from anterior extremity. Ventriculus 196–314 (241) long, 225–294 (270) wide. Ventricular appendix 882–1,274 (1,053) long, 49–98 (68.6) wide. Intestinal caecum 225–392 (280) long, 98–176 (139) wide, representing 7.81–13.8 (9.47) % oesophageal length. Ratio of intestinal caecum to ventricular appendix 1:2.50–5.20 (1:3.74). Posterior end of body curves ventrally (Fig. 1i, j). Ejaculatory duct 1.86–2.74 (2.33) mm long. Spicules slender, alate, subpointed apically, of almost equal or slightly unequal length, 1.96–3.28 (2.72) mm long, representing 101–129 (108) % of ejaculatory duct and 7.42–11.4 (9.59) % of body length (Figs. 1i, 3e). Gubernaculum absent. Caudal papillae very small, 38–43 pairs in total, arranged as follows: 31–34 pairs precloacal, 1 pair of paracloacal and 4–6 pairs postcloacal (the second or fourth pair double) (Figs. 1j, 2, 3b, d, f, 4c). Medioventral precloacal papilla present (Figs. 3b, 4d, e). Tail 167–245 (206) long. Small lateral phasmids present at base of tail tip (Fig. 3b, d, g).
Third- and fourth-stage larvae of H. longilabrum sp. nov. from S. fuscescens (Houttuyn) in the South China Sea: a, b anterior part of third-stage larva, lateral view; c cephalic end of third-stage larva, lateral view; d posterior end of third-stage larva, lateral view; e tip of tail of third-stage larva (showing tip of tail of fourth-stage larva inside); f anterior part of fourth-stage larva, lateral view; g cephalic end of fourth-stage larva, dorsal view; h cephalic end of fourth-stage larva, lateral view; i posterior end of fourth-stage larva, lateral view; j region of ventriculus. Scale bars: a, b, f, j 400 μm; c, d, e, g, h, i 100 μm
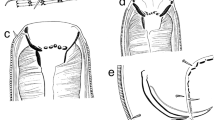
Scanning electron micrographs of H. longilabrum sp. nov. from S. fuscescens (Houttuyn) in the South China Sea: a cephalic extremity of male, apical view; b posterior end of male, ventral view, showing four postcloacal and single paracloacal papillae (black arrows) and lateral phasmid (white arrow); c cephalic extremity of male, dorsal view; d posterior end of male, lateral view, showing six postcloacal papillae (black arrows) and lateral phasmid (white arrow); e distal end of left spicule; f postcloacal double papilla; g tip of male tail, showing lateral phasmid (black arrow)
Scanning electron micrographs of H. longilabrum sp. nov. from S. fuscescens (Houttuyn) in the South China Sea: a anterior part of male, apical view (lateral alae absent); b cephalic end of male, ventral view, showing interlabium (black arrow); c paracloacal papilla (white arrow); d posterior end of male, ventral view, showing six postcloacal papillae (black arrows), single medioventral precloacal papilla and lateral phasmid (white arrows); e medioventral precloacal papilla
Female (based on 20 gravid specimens)
Body 23.4–47.2 (35.6) mm long; maximum width 588–1,274 (850). Dorsal and ventrolateral lips almost equal in size, 441–637 (497) long, 245–372 (307) wide. Interlabia 147–245 (181) long, 225–392 (298) wide. Oesophagus 2.55–5.68 (3.88) mm long, 372–539 (448) in maximum width, representing 10.0–12.0 (10.8) % of body length. Nerve ring 804–1,372 (1,003) and excretory pore 882–1,519 (1,105) from anterior extremity, respectively. Ventriculus 245–392 (315) long, 245–392 (312) wide. Ventricular appendix 882–1,078 (973) long, 58–98 (86.8) wide. Intestinal caecum 196–392 (307) long, 127–245 (185) wide, representing 6.67–13.5 (8.64) % oesophageal length. Ratio of intestinal caecum to ventricular appendix 1:2.38–5.50 (1:3.40). Vulva slit-like, situated anteriorly, 6.27–11.8 (9.47) mm from anterior extremity, at 25.0–28.0 (26.6) % of body length (Fig. 1f). Vagina muscular, directed posteriorly. Uteri form coils in region posterior to vagina, extend posteriorly to level of rectum. Eggs suboval to almost rounded, 29–49 (41.2) long, 29–49 (37.2) wide (Fig. 1h). Tail 490–853 (670) long. Small lateral phasmids present at base of tail tip (Fig. 1g).
Type host and type locality: S. fuscescens (Houttuyn) (Perciformes: Siganidae); South China Sea, off Sanya (109°30′E; 18°12′N), Hainan Province, China.
Other host and locality: S. canaliculatus (Park) (Perciformes: Siganidae); South China Sea, off Sanya (109°30′E; 18°12′N), Hainan Province, China.
Site of infection: Intestine and stomach.
Type specimens: Holotype: male collected from S. fuscescens, South China Sea, off Sanya (HBNU-F1143); allotype: female collected from S. fuscescens, South China Sea, off Sanya (HBNU-F1144); paratypes: 133 males and 324 females collected from S. fuscescens, South China Sea, off Sanya (HBNU-F1145); 10 males and 11 females collected from S. canaliculatus, South China Sea, off Sanya (HBNU-F1146).
Prevalence and intensity of infection: S. fuscescens: 87.5 % (35 out of 40 fishes) were infected off Sanya with intensity of 1–35 (mean = 13.1) specimens; S. canaliculatus: 33.3 % (one out of three fishes) were infected off Sanya with intensity of 21 specimens.
Etymology: The specific epithet is derived from a combination of the Latin words longus- (long) and labrum- (lip), and refers to the unusually long dorsal and ventrolateral lips.
Fourth-stage larvae (based on 14 specimens)
Body 16.2–18.9 (17.5) mm long; maximum width 490–588 (549). Dorsal and ventrolateral lips distinctly shorter than lips of adult, almost equal in size, 157–196 (180) long, 118–147 (135) wide (Fig. 2g, h). Interlabia 59–78 (70.6) long, 98–147 (113) wide (Fig. 2h). Oesophagus almost cylindrical, muscular, slightly broader posteriorly than anteriorly, 1.81–2.16 (1.96) mm long, 196–245 (229) in maximum width, representing 9.84–12.6 (11.3) % of body length (Fig. 2f). Nerve ring 490–519 (505) and excretory pore 539–568 (546) from anterior extremity, respectively (Fig. 2f). Ventriculus 147–245 (176) long, 163–245 (182) wide. Ventricular appendix 588–785 (667) long, 49–69 (56.8) wide (Fig. 2j). Intestinal caecum slightly longer than ventriculus, 98–294 (186) long, 78–137 (98.0) wide, representing 5.26–13.9 (9.31) % oesophageal length. Ratio of intestinal caecum to ventricular appendix 1:2.20–7.00 (1:4.25) (Fig. 2j). Tail 353–412 (388) long, tip with numbers of nodular protuberances (Fig. 2i). Small lateral phasmids not observed.
Host and locality of fourth-stage larvae: S. fuscescens (Houttuyn) (Perciformes: Siganidae); South China Sea, off Sanya (109°30′E; 18°12′N), Hainan Province, China.
Site of infection: Intestine.
Prevalence and intensity of infection: 20.0 % (8 out of 40 fishes) were infected with intensity of 3–25 (mean = 14.8) specimens.
Voucher specimens: 118 specimens (HBNU-F1147).
Third-stage larvae (based on ten specimens)
Body 8.20–13.1 (10.2) mm long; maximum width 196–392 (304). Cephalic end rounded, with prominent ventral cuticular tooth, sharply pointed (Fig. 2a–c). Anlagen of lips weakly developed (Fig. 2c). Oesophagus almost cylindrical, muscular, slightly broader posteriorly than anteriorly. Oesophagus almost cylindrical, muscular, slightly broader posteriorly than anteriorly, 931–1,274 (1,098) long, 98–176 (124) in maximum width, representing 10.1–11.4 (10.8) % of body length (Fig. 2a, b). Nerve ring 294–490 (392) and excretory pore 314–539 (426) from anterior extremity, respectively (Fig. 2a, b). Ventriculus 59–98 (78.6) long, 98–127 (108) wide. Ventricular appendix 488–841 (704) long, 29–49 (35.3) wide. Intestinal caecum slightly longer than ventriculus, 108–196 (135) long, 58–98 (76.4) wide, representing 9.47–12.9 (11.3) % oesophageal length (Fig. 2a, b). Ratio of intestinal caecum to ventricular appendix 1:2.28–5.78 (1:4.30). Tail 140–245 (196) long, tip without nodular protuberances (Fig. 2d, e). Small lateral phasmids not observed.
Host and locality of third-stage larvae: S. fuscescens (Houttuyn) (Perciformes: Siganidae); South China Sea, off Sanya (109°30′E; 18°12′N), Hainan Province, China.
Site of infection: Abdominal cavity.
Prevalence and intensity of infection: 12.5 % (5 out of 40 fishes) were infected with intensity of 1–5 (mean = 2.8) specimens.
Voucher specimens: 14 specimens (HBNU-F1148).
DNA characterisation
The sequences of the samples of adult and identified morphologically as putative third- and fourth-stage larvae, obtained for the ITS region of the partial rDNA, were all 1,007 bp in length and showed no intraspecific nucleotide variability among the different developmental specimens collected from the two different hosts examined. There are nine species of Hysterothylacium ITS sequences registered in GenBank and pairwise comparison between H. longilabrum sp. nov. and the other species of Hysterothylacium (expect Hysterothylacium sp. JYW-2010, HM545895) displayed 9.59 (JQ520158) to 27.27 % (AM706344) nucleotide differences (details in Table 2). The low level of sequence variation was found between H. longilabrum sp. nov. and Hysterothylacium sp. JYW-2010 (HM545895) (i.e. only two nucleotide alterations were detected at alignment positions 197 and 318, respectively). The ITS sequence of H. longilabrum sp. nov. is deposited in the GenBank database (http://www.ncbi.nlm. nih.gov) under accession number (JQ520159).
Discussion
The gross morphology of the specimens from S. fuscescens and S. canaliculatus, especially the position of the excretory pore and the presence of both intestinal caecum and ventricular appendix, clearly shows they should belong to Hysterothylacium. H. longilabrum sp. nov. remarkably differs from all other congeners in Hysterothylacium by the unusually long lips. As far as we are aware, Hysterothylacium ogcocephali (Olsen 1952) appears most similar to H. longilabrum sp. nov. in having the elongated lips (Olsen 1952; Deardorff and Overstreet 1980). But the lips of H. longilabrum sp. nov. are much larger than H. ogcocephali (0.44–0.64 mm in the former vs 0.17–0.31 mm in the latter, in the female). From H. ogcocephali, it also differs by having much longer spicules (1.96–3.28 mm long, representing 7.42–11.4 % of body length in the new species vs 0.33–0.66 mm long, representing 1.0–2.0 % of body length in H. ogcocephali), a much greater number of postcloacal papillae (four to six pairs in H. longilabrum sp. nov. vs three pairs in H. ogcocephali) and the absence of lateral alae (presence of distinct lateral alae in H. ogcocephali). Within Hysterothylacium, the new species is similar to the following species in having a very short intestinal caecum (slightly longer or shorter than ventriculus) and relatively long ventricular appendix (about two to six times longer than intestinal caecum): Hysterothylacium habena (Linton, 1900) from Opsanus tau (Linneaus) (Batrachoidiformes: Batrachoididae) in the American waters (Norris and Overstreet 1975); Hysterothylacium rhacodes (Deardorff and Overstreet 1978) from Pelates quadrilineatus (Blochin) (Perciformes: Terapontidae), Solea aegyptiaca Chabanaud (Pleuronectiformes: Soleidae), Boops boops (Linnaeus) (Perciformes: Sparidae), Diplodus sargus sargus (Linnaeus) (Perciformes: Sparidae), Diplodus vulgaris (Geoffroy Saint-Hilaire) (Perciformes: Sparidae), Lithognathus mormyrus (Linnaeus) (Perciformes: Sparidae), Oblada melanura (Linnaeus) (Perciformes: Sparidae) and Sparus aurata Linnaeus (Perciformes: Sparidae) in the eastern Mediterranean Sea (Deardorff and Overstreet 1978); Hysterothylacium pelagicum Deardorff and Overstreet 1982 from Coryphaena hipputus Linnaeus (Perciformes: Coryphaenidae) in the Pacific and Atlantic oceans (Deardorff and Overstreet 1982; Bruce and Cannon 1989); Hysterothylacium physiculi Moravec and Nagasawa 2000 from Physiculus japonicus Hilgendorf [as Physiculus maximowiczi (Herzenstein)] (Gadiformes: Moridae) in the Japanese waters (Moravec and Nagasawa 2000); Hysterothylacium cornutum (Stossich, 1904) from Thunnus thynnus (Linneaus), Thunnus maccoyii (Castelnau), Thunnus albacares (Bonnaterre) and Thunnus alalunga (Bonnaterre) (Perciformes: Scombridae) in the Pacific and Atlantic oceans (Yamaguti 1941; Bruce and Cannon 1989; Moravec and Nagasawa 2000); Hysterothylacium sebae Bruce 1990 from Lutjanus sebae (Cuvier) (Perciformes: Lutjanidae) in the Australian waters (Bruce 1990); Hysterothylacium reliquens (Norris and Overstreet 1975) from Archosargus probatocephalus (Walbaum) (Perciformes: Sparidae), Halichoeres bivittatus (Bloch) (Perciformes: Labridae), Opsanus beta (Goode & Bean) (Batrachoidiformes: Batrachoididae), Chilomycterus schoepfii (Walbaum) (Tetraodontiformes: Diodontidae), Micropogonias undulatus (Linnaeus) [as Micropogon undulatus (Linnaeus)] (Perciformes: Sciaenidae), Gymnothorax nigromarginatus (Girard) (Anguilliformes: Muraenidae), Sciaenops ocellatus (Linnaeus) (Perciformes: Sciaenidae) and Ogcocephalus cubifrons (Richardson) (Lophiiformes: Ogcocephalidae) in the American waters, and Batrachoides surinamensis (Bloch & Schneider) (Batrachoidiformes: Batrachoididae) in the Brazilian waters (Norris and Overstreet 1975; Deardorff and Overstreet 1980); Hysterothylacium scomberoidei Bruce and Cannon 1989 from Scomberoides commersonianus Lacepède (Perciformes: Carangidae) in the Australian waters (Bruce and Cannon 1989); Hysterothylacium fortalezae (Klein 1973) from Scomberomorus brasiliensis Collette, Russo & Zavala-Camin and Scomberomorus cavalla (Cuvier) (Perciformes: Serranidae) in the Brazilian waters (Klein 1973), and Sco mberomorus maculates (Mitchill) (Perciformes: Scombridae), Mycteroperca bonaci (Poey) (Perciformes: Serranidae) and Oligoilites saurus (Bloch & Schneider) (Perciformes: Carangidae) in the American waters and Mediterranean Sea (Deardorff and Overstreet 1980); Hysterothylacium fabri (Rudolphi 1819) from Uranoscopus scaber Linnaeus (Perciformes: Uranoscopidae) and Zeus faber Linnaeus (Zeiformes: Zeidae) in the Mediterranean Sea (Petter and Maillard 1987; Bruce et al. 1994), and Trachurus japonicus (Temminck & Schlegel) (Perciformes: Carangidae), Pennahia argentata (Houttuyn) [as Argyrosomus argentatus (Houttuyn)](Houttuyn (Perciformes: Sciaenidae), Conger myriaster (Brevoort) [as Astroconger myriaster (Brevoort)] (Anguilliformes: Congridae) and Chelidonichthys kumu (Cuvier) in the Chinese waters (Li et al. 2008). H. longilabrum sp. nov. can be readily distinguished from H. physiculi, H. scomberoidei, H. fortalezae, H. sebae, H. pelagicum and H. fabri by having much longer spicules (representing 7.42–11.4 % of body length in the former vs 1.8–5.0 % of body length in the latter six species) and the absence of lateral alae (presence of distinct lateral or caudal alae in the latter six species). The new species differs from H. cornutum by different arrangement of caudal papillae (31–34 pairs precloacal, 1 pair of paracloaca and 4–6 pairs postcloacal in the former vs 22–24 pairs precloacal, 1 pair of paracloacal and 7–10 pairs postcloacal in the latter) and different morphology of tail tip (absence of nodular protuberances in H. cornutum). From H. reliquens and H. habena, the new species differs by the presence of medioventral precloacal papilla and the absence of lateral alae (lateral alae prominent at posterior end of body in the latter two species). H. longilabrum sp. nov. is different from H. rhacodes by the presence of one pair of paracloacal papillae and a single particular, medioventral precloacal papilla and much longer spicules (representing 7.42–11.4 % of body length in the former vs 3.0–6.0 % of body length in the latter species).
Although over 60 species of Hysterothylacium have been described until now (Bruce et al. 1994; Moravec et al. 1996, 1997; Moravec and Nagasawa 1998, 2000; Torres et al. 1998; Torres and Soto 2004; Gopar-Merino et al. 2005; Li et al. 2007a, b; Raffel and Anderson 2009; Rossin et al. 2011), the life cycles of most species are still poorly known. In our opinion, this is mainly due to our very limited knowledge of the development, morphogenesis and identification of the larvae. The larvae of Hysterothylacium commonly parasitise various tissues of numerous fishes and invertebrates (Norris and Overstreet 1976; González 1998). During this helminthological survey of S. fuscescens, the prevalence and intensity of infection with third-stage larvae are very low [(12.5 % (5 out of 40 fishes) were infected with intensity of 1–5 (mean = 2.8) specimens], in contrast to the high prevalence and intensity of infection with adults [87.5 % (35 out of 40 fishes) were infected with intensity of 1–35 (mean = 13.1) specimens]. In addition, the third-stage larvae collected from the abdominal cavity of S. fuscescens are all aged because of the anlagen of lips and the nodular protuberances of tail tip well developed in most of specimens. The result may indicate that S. fuscescens only acts as the natural definitive host of H. longilabrum sp. nov.. S. fuscescens is a herbivorous fish and mainly feeds on filamentous algae, leafy algae and seagrasses, and sometimes also eats crustaceous invertebrates, such as gastropods and amphipods in the wild (Lavina and Alcala 1973; Wassef and Hady 1997). Consequently, we speculate the crustaceous invertebrates may be the intermediate hosts of H. longilabrum sp. nov..
The accurate identification of anisakid larvae is a key step for understanding their life cycles, epidemiology and disease surveillance and control (Zhang et al. 2007). However, it is often problematic to exactly recognize the different larvae developmental stages of Hysterothylacium spp. only based on morphological characters as there are usually remarkable morphological differences between the different larvae and adult developmental stages; for example, the tail tip of the third-stage larvae and the lips of the fourth-stage larvae of H. longilabrum sp. nov. are distinctly different from the adults. In addition, the larvae always lack some morphological features of taxonomic significance including the number and arrangement of cloacal papillae, the length of spicules and the position of the vulva, which result to a difficulty in distinguishing the different larvae of Hysterothylacium spp. morphologically. Therefore, in the present paper, the internal transcribed spacer (ITS) of the ribosomal DNA of the samples identified morphologically as putative third- and fourth-stage larvae of H. longilabrum sp. nov. are sequenced and compared with the sequence of adult, and no nucleotide difference was detected. The result strongly proved that the samples identified morphologically as putative third- and fourth-stage larvae and the adults are homogeneous genetically and all belong to the same species H. longilabrum sp. nov.. Although there are some morphological varieties between some individuals of H. longilabrum sp. nov., for instance, the length of intestinal caecum (Fig. 1d, e), the number of postcloacal papillae (Fig. 3b, d) and the morphology of tip of male tail (Fig. 1k, l), the molecular analyses for adult specimens of H. longilabrum sp. nov. collected from the two different fish hosts (S. fuscescens and S. canaliculatus, respectively) using ITS of rDNA showed only one genotype (i.e. no intraspecific variation was detected). This result was predictable when we considered that all the morphological varieties between individuals were just the intraspecific variation. Interspecific variation between the ITS sequences of H. longilabrum sp. nov. herein generated and those species of Hysterothylacium available in the GenBank (expect Hysterothylacium sp. JYW-2010, HM545895) was detected from 9.59 (JQ520158) to 27.27 % (AM706344) nucleotide differences, which is a very high level of interspecific sequence variation. This result supported the validity of this new nematode species based on the morphological observation and suggested that it is a useful approach to utilise the ITS of rDNA as a genetic marker for the distinction between species of Hysterothylacium spp.. The low level of ITS sequence variation (only two nucleotide alterations) detected between H. longi labrum sp. nov. and Hysterothylacium sp. JYW-2010 (HM545895) was also expected because the specimen of Hysterothylacium sp. JYW-2010 was collected from the same fish host S. fuscescens, in a neighbouring region of the South China Sea (off Zhanjiang, Guangdong Province, China). Therefore, we have no hesitation in considering the species Hysterothylacium sp. JYW-2010 (HM545895) to be conspecific with H. longilabrum sp. nov., and the two nucleotide differences should be considered as intraspecific variation because of the different geographical locations.
References
Bruce NL (1990) Hysteroyhylacim Ward and Magath, 1917, and Ichthyascaris Wu, 1949, ascaridoid nematodes from Australian demersal fishes. Mem Queensl Mus 28:389–426
Bruce NL, Cannon LRG (1989) Hysterothylacium, Iheringascaris and Maricostula new genus, nematodes (Ascaridoidea) from Australian pelagic marine fishes. J Nat Hist 23:1397–1441
Bruce NL, Adlard RD, Cannon LRG (1994) Synoptic checklist of ascaridoid parasites (Nematoda) from fish hosts. Invertebr Taxon 8:583–674
D’Amelio S, Mathiopoulos KD, Santos CP, Pugachev ON, Webb SC, Picanco M, Paggi L (2000) Genetic markers in ribosomal DNA for the identification of members of the genus Anisakis (Nematoda: Ascaridoidea) defined by polymerase-chain-reaction-based restriction fragment length polymorphism. Int J Parasitol 30:223–226
Deardorff TL, Overstreet RM (1978) Thynnascaris rhacodes n. sp. (Nematoda: Ascaridoidea) in fishes from the Israeli Mediterranean coast. Ann Parasitol Hum Comp 53:519–525
Deardorff TL, Overstreet RM (1980) Review of Hysterothylacium, and Iheringascaris (both previously = Thynnascaris) (Nematoda: Ascaridoidea) from the Northern Gulf of Mexico. P Biol Soc Wash 93:1035–1079
Deardorff TL, Overstreet RM (1982) Hysterothylacium pelagicum sp. n. and H. cornutum (Stossich, 1904) (nematode: Anisakidae) from marine fishes. P Biol Soc Wash 49:246–251
Du C-X, Zhang L-P, Shi M-Q, Ming Z, Hu M, Gasser RB (2010) Elucidating the identity of Anisakis larvae from a broad range of marine fishes from the Yellow Sea, China, using a combined electrophoretic-sequencing approach. Electrophoresis 31:654–658
Fang W-Z, Xu S-S, Zhang S-L, Wang Y-N, Chen X-B, Luo D-M (2010) Multiple primer PCR for the identification of anisakid nematodes from Taiwan Strait. Exp Parasitol 124:197–201
González L (1998) The life cycle of Hysterothylacium aduncum (Nematoda: Anisakidae) in Chilean marine farms. Aquaculture 162:173–186
Gopar-Merino L, Osorio-Sarabia D, García-Perieto L (2005) A new species of Hysterothylacium (Nematoda: Anisakidae) parasite of Ariopsis guatemalensis (Osteichthyes: Ariidae) from Tres Palos lagoon, Mexico. J Parasitol 91:909–914
Hsü H-F (1933) On some species of parasitic nematodes from fishes in China. Pek Nat Hist Bull 8:147–154
Kijewska A, Rokicki J, Sitko J, Wegrzyn G (2002) Ascaridoidea: a simple DNA assay for identification of 11 species infecting marine and freshwater fish, mammals, and fish-eating birds. Exp Parasitol 101:35–39
Klein VLM (1973) Helmintos parasites das espécies Scomberomoms cavalla (Cuvier) e Scomberomoms maculatus (Mitchill) do litoral Cearense. Contracaecum fortalezae sp. n. (Nematoda: Ascaridoidea). Mem I Oswaldo Cruz 71:199–202
Lavina M, Alcala AC (1973) Ecological studies on Phillipine siganid fishes in southern Negros, Phillipine: Abstract (No. Mss/ABA/2/1) submitted to the Marine Sciences Special Symposium, Hong Kong (7–14 December, 1973)
Li A-X, D’Amelio S, Paggi L, He F, Gasser RB, Lun Z-R, Abollo E, Turchetto M, Zhu X-Q (2005) Genetic evidence for the existence of sibling species within Contracaecum rudolphii (Hartwich, 1964) and the validity of Contracaecum septentrionale (Kreis, 1955) (Nematoda: Anisakidae). Parasitol Res 96:361–366
Li L, Xu Z, Zhang L-P (2007a) A new species of genus Hysterothylacium Ward et Magath, 1917 (Nematoda, Anisakidae) from Liparis tanakae (Scorpaeniformes, Liparidae) from the Yellow Sea, China. Acta Parasitol 52:371–375
Li L, An R-Y, Zhang L-P (2007b) A new species of Hysterothylacium (Nematoda: Anisakidae) from marine fishes from Yellow Sea, China, with a key to the species of the genus Hysterothylacium. Zootaxa 1614:43–52
Li L, Xu Z, Zhang L-P (2007c) Investigation on the nematode of Hysterothylacium aduncum (Anisakidae) from Bohai Sea and Yellow Sea in China. Chin J Parasitol Parasit Dis 25:364–367, In Chinese, English summary
Li L, Xu Z, Zhang L-P (2008) Redescription of three species of Hysterothylacium (Nematoda: Anisakidae) from marine fishes from the Yellow Sea, China, with the synonymy of Hysterothylacium muraenesoxin (Luo, 1999). Zootaxa 1878:55–67
Li L, Liu Y-Y, Liu B-C, Zhang L-P (2011) Morphological and molecular evidence for a new species of the genus Raphidascaris (Nematoda: Anisakidae) from marine fishes from the South China Sea. Parasitol Res. doi:10.1007/s00436-011-2650-7
Li L, Gibson DI, Liu Y-Y, Zhang L-P (2012) Morphological and molecular study of the poorly known species Pseudanisakis rajae (Yamaguti, 1941) (Nematoda: Acanthocheilidae) from elasmobranchs in the Yellow Sea and Taiwan Strait off the coast of China. Syst Parasitol 81:115–123
Martín-Sánchez J, Díaz M, Artacho ME, Valero A (2003) Molecular arguments for considering Hysterothylacium fabri (Nematoda: Anisakidae) a complex of sibling species. Parasitol Res 89:214–220
Mašová Š, Moravec F, Baruš V, Seifertová M (2010) Redescription, systematic status and molecular characterization of Multicaecum heterotis Petter, Vassiliadès et Marchand, 1979 (Nematoda: Heterocheilidae), an intestinal parasite of Heterotis niloticus (Osteichthyes: Arapaimidae) in Africa. Folia Parasitol 57:280–288
Mattiucci S, Nascetti G (2008) Advances and trends in the molecular systematics of anisakid nematodes, with implications for their evolutionary ecology and host–parasite coevolutionary processes. Adv Parasit 66:47–148
Mattiucci S, Nascetti G, Cianchi R, Paggi L, Arduino P, Margolis L (1997) Genetic and ecological data on the Anisakis simplex complex, with evidence for a new species (Nematoda, Ascaridoidea, Anisakidae). J Parasitol 83:401–416
Mattiucci S, Paoletti M, Olivero-Verbel J, Baldiris R, Arroyo-Salgado B, Garbin L, Navone G, Nascetti G (2008) Contracaecum bioccai n. sp. from the brown pelican Pelecanus occidentalis (L.) in Colombia (Nematoda: Anisakidae): morphology, molecular evidence and its genetic relationship with congeners from fish-eating birds. Syst Parasitol 69:101–121
Mattiucci S, Paoletti M, Solorzano AC, Nascetti G (2010) Contracaecum gibsoni n. sp. and C. overstreeti n. sp. (Nematoda: Anisakidae) from the Dalmatian pelican Pelecanus crispus (L.) in Greek waters: genetic and morphological evidence. Syst Parasitol 75:207–224
Moravec F (2009) Experimental studies on the development of Contracaecum rudolphii (Nematoda: Anisakidae) in copepod and fish paratenic hosts. Folia Parasitol 56:185–193
Moravec F, Nagasawa K (1998) Hysterothylacium japonicum sp. n. (Nematoda: Anisakidae) from the rare marine fish Trachipterus ishikawai in Japan. Acta Parasitol 43:39–42
Moravec F, Nagasawa K (2000) Some anisakid nematodes from marine fishes of Japan and the North Pacific Ocean. J Nat Hist 34:1555–1574
Moravec F, Scholz T, Vivas RC (1996) Systematic status and first description of Dujardinia cenotae Pearse, 1936 [=Hysterothylacium cenotae (Pearse, 1936) Moravec et al., 1995] (Nematoda: Anisakidae). Syst Parasitol 33:143–148
Moravec F, Urawa S, Coria CO (1997) Hysterothylacium patagonense n. sp. (Nematoda: Ascaridoidae) from freshwater fishes in Patagonia, Argentina, with a key to the species of Hysterothylacium in American freshwater fishes. Syst Parasitol 36:31–38
Norris DE, Overstreet RM (1975) Thynnascaris reliquens sp. n. and T. habena (Linton, 1900) (Nematoda: Ascaridoidea) from fishes in the northern Gulf of Mexico and eastern U. S. seaboard. J Parasitol 61:330–336
Norris DE, Overstreet RM (1976) The public health implications of larval Thynnascaris nematodes from shellfish. Milk Food Technol 39:47–54
Olsen LS (1952) Some nematodes parasitic in marine fishes. Publ Inst Mar Sci Univ Tex 2:173–215
Pan JH, Zhang JY, Li CM (1990) Fish parasitology. Science Press, Beijing, pp 291–335
Parukhin AM (1966) On the study of the helminth fauna of the family Carangidae from the South China Sea. Biol Morya 19:80–96
Petter AJ, Maillard C (1987) Ascarides de poissons de Mediterranee occidentale. Bulletin du Muséum National d’Histoire Naturelle, Section A, Zoologie, Biol Ecol Anim 9: 773–798
Raffel TR, Anderson TK (2009) A new species of Hysterothylacium (Nematoda: Anisakidae) from the stomach of the red-spotted newt, Notophthalmus viridescens, from Pennsylvania fishless ponds. J Parasitol 95:1503–1506
Rossin MA, Datri LL, Incorvaia IS, Timi JT (2011) A new species of Hysterothylacium (Ascaridoidea, Anisakidae) parasitic in Zenopsis conchifer (Zeiformes, Zeidae) from Argentinean waters. Acta Parasitol 56:310–314
Testini G, Papini R, Lia RP, Parisi A, Dantas-Torres F, Traversa D, Otranto D (2011) New insights into the morphology, molecular characterization and identification of Baylisascaris transfuga (Ascaridida, Ascarididae). Vet Parasitol 175:97–102
Thompson JD, Higgins DG, Gibson TJ (1994) CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res 22:4673–4680
Torres P, Soto MS (2004) Hysterothylacium winteri sp. n. (Nematoda: Anisakidae), a parasite of Chilean rock cod, Eleginops maclovinus (Perciformes: Eleginopidae), from South Chile. Folia Parasitol 51:55–60
Torres P, Andrade P, Silva R (1998) On a new species of Hysterothylacium (Nematoda: Anisakidae) from Cauque mauleanum (Pisces: Atherinidae) by brightfield and scanning electron microscopy. Mem I Oswaldo Cruz 93:745–752
Wassef EA, Hady HAA (1997) Breeding biology of rabbitfish Siganus canaliculatus (Siganidae) in mid Arabian Gulf. Fish Res 33:159–166
Yamaguti S (1941) Studies on the helminth fauna of Japan, part 33. Nematodes of fishes. II. Jap J Zool 9:343–396
Yin W-Z, Zhang N-X (1982) New records of parasitic nematodes in marine fishes from China. Acta Zootaxonomica Sinica 4:371, In Chinese
Yin W-Z, Zhang N-X (1983) On the occurrence of Contracaecum marinum (Linnaeus, 1767) in China. Acta Zootaxonomica Sinica 2:119, In Chinese
Zhang L-P, Hu M, Shamsi S, Beveridge I, Li H-M, Xu Z, Li L, Cantacessi C, Gasser RB (2007) The specific identification of anisakid larvae from fishes from the Yellow Sea, China, using mutation scanning-coupled sequence analysis of nuclear ribosomal DNA. Mol Cell Probe 21:386–390
Zhu X-Q, D’Amelio S, Palm HW, Paggi L, George-Nascimento M, Gasser RB (2002) SSCP-based identification of members within the Pseudoterranova decipiens complex (Nematoda: Ascaridoidea: Anisakidae) using genetic markers in the internal transcribed spacers of ribosomal DNA. Parasitology 124:615–623
Zhu X-Q, Podolska M, Liu J-S, Yu H-Q, Chen H-H, Lin Z-X, Luo C-B, Song H-Q, Lin R-Q (2007) Identification of anisakid nematodes with zoonotic potential from Europe and China by single-strand conformation polymorphism analysis of nuclear ribosomal DNA. Parasitol Res 101:1703–1707
Acknowledgments
The authors wish to thank Associate Professor Rui-Yong An (College of Life Science, Hebei Normal University) for identifying the fishes. This study was supported by the National Natural Science Foundation of China (nos. 31101615 and 30970318) and the Science Foundation of Hebei Normal University (no. L2010B13).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Li, L., Liu, YY. & Zhang, LP. Morphological and molecular identification of Hysterothylacium longilabrum sp. nov. (Nematoda: Anisakidae) and larvae of different stages from marine fishes in the South China Sea. Parasitol Res 111, 767–777 (2012). https://doi.org/10.1007/s00436-012-2897-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00436-012-2897-7