Abstract
In the present study, methanol extracts of 42 traditional medicinal plants with potent anthelmintic activity against Dactylogyrus intermedius (Monogenea) in goldfish (Carassius auratus) were investigated. Cinnamomum cassia, Lindera aggregata, and Pseudolarix kaempferi exhibited 100% activity and were selected for further evaluation by applying five solvents (petroleum ether, chloroform, ethyl acetate, methanol, and water) for the extraction of the samples, followed by the in vivo bioassay. Among the extracts tested, water and methanol extracts of C. cassia showed the highest efficacies with EC50 values of 13.2 and 12.3 mg L−1, showing 100% efficacy against D. intermedius at 30.0 and 40.0 mg L−1, followed by methanol extract of L. aggregata which demonstrated 100% efficacy at 60.0 mg L−1 with EC50 value of 17.1 mg L−1 after 48 h of exposure. Methanol and ethyl acetate extract of P. kaempferi, which exhibited a 100% efficacy against D. intermedius at 60.0 and 50.0 mg L−1, revealed similar activity with EC50 values of 23.5 and 23.3 mg L−1, respectively. Acute toxicity of these active extracts was investigated on goldfish for 48 h and the corresponding median lethal concentrations (LC50) of 56.9, 31.3, 88.7, 168.2, and 165.7 mg L−1, respectively. These findings indicated that these extracts of the three plants can be developed as preferred natural antiparasitic agents for the treatment of D. intermedius.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
China is a great fishery nation with a total yield of 51 million tons in 2005, which accounts for one-third of the total yield of the world, and was ranked number one in the world for the last 15 years (Zhou and Chen 2010). With the development of aquaculture industry, it has been overwhelmed with its share of diseases and problems caused by viruses, bacteria, fungi, parasites, and other undiagnosed and emerging pathogens (Bondad-Reantaso et al. 2005). Dactylogyrus spp., belonging to the family of Monogenea, are common ectoparasites living on the gills of freshwater fish and represent the largest group of metazoan fish parasites and major importance in the pathology of fishes (Woo et al. 2002). They have no intermediate hosts in their life cycle. The life cycle of D. intermedius comprises obligate adult stage, fertilized egg, and free-swimming larvae stage. The fertilized eggs develop into free-swimming ciliated larvae in the water column; the ciliated larvae are then carried to hosts by water currents as well as by their own ciliated movement (Klinger and Floyd 2002). D. intermedius attach to the gills, causing gill inflammation, excessive mucous secretions, accelerated respiration, and mixed infections with other parasites and secondary bacterial infections. Therefore, D. intermedius can cause serious damage to the host, such as loss of appetite, lowered growth performance, and high mortalities, which would result in great economic losses in aquaculture (Dove and Ernst 1998; Woo et al. 2002; Reed et al. 2009).
Chemical anthelmintics such as praziquantel, toltrazuril, and mebendazole have been used for decades throughout the world to minimize the losses caused the Dactylogyrus infection (Schmahl and Mehlhorn 1985; Schmahl et al. 1988; Goven and Amend 1982). However, the frequent use of these chemical-based agents caused serious drawbacks such as environmental contamination, toxicity to the host, and even contamination of fish products with drug residues (Goven et al. 1980; Klinger and Floyd 2002), which prompted an urgent need for alternative therapy, including natural products from medicinal plants.
In recent years, there have been increasing interests in utilizing traditional medicinal plants for the control of parasitic infection. Several studies proved that different plant extracts have significant killing effects in vitro and in vivo on nematodes, cestodes, and trematodes (Mehlhorn et al. 2011; Klimpel et al. 2011; Abdel-Ghaffar et al. 2011). Hossain et al. (2011) evaluated the anthelmintic activity of the methanol extract of Dregea volubilis leaves against Paramphistomum explanatum and observed its effect through SEM study. The current work in our laboratory is focused on screening medicinal plants with promising anthelmintic activity and isolating groups of compounds/pure compounds responsible for the activity. We have previously reported that crude extracts of several traditional medicinal plants, such as Arctium lappa L., Dioscorea zingiberensis C. H. Wright, Paris polyphylla, Angelica pubescens, Dryopteris crassirhizoma, and Cimicifuga foetida L. (Wang et al. 2009a, 2010a, b; Liu et al. 2010; Lu et al. 2011; Wu et al. 2011) and some bioactive compounds, including arctigenin, trillin, gracillin, and dioscin (Wang et al. 2009a, b, 2010b) can effectively control the D. intermedius infection in goldfish (Carassius auratus). This study screened 42 kinds of medicinal plants for anthelmintic activity against D. intermedius in goldfish (C. auratus).
Materials and methods
Infected goldfish preparation
One-year-old goldfish (mean weight 4.2 ± 0.5 g) without any record of foregone infestation with parasites were collected from a Changxing fish farm (Xian yang city, Shaanxi province, China). Then the stock was acclimatized in glass aquarium containing 180 L groundwater at 25 ± 1°C (controlled by automatic aquarium heater) with aeration for 7 days and was fed with commercial pelleted goldfish diet at 2% of body weight. One week later, all the fish were cohabitated with the ones infected with D. intermedius which were reserved in our laboratory. The parasitized procedure was described in our previous study (Wang et al. 2008). Three weeks later, ten fish were randomly sampled and killed by spinal severance, and eight gill filaments of each fish were biopsied to determine the adult D. intermedius infestation level and intensity under a light microscope (Olympus BX41, Tokyo, Japan) at 10 × 4 magnification. Fish were chosen for the assays when the infection rate was 100% and the mean number of the parasite on gills was 40–50 per fish.
Collection of plant materials
The plant materials from each of the selected species (Table 1) were collected in August 2011 and identified by Prof. X.P. Song in Northwest A&F University (Shaanxi, China). The voucher specimens have been deposited at the Herbarium of the College of Life Science, Northwest A&F University, China. After oven-dried at 45°C for 48 h, the materials were crushed and reduced to fine powder using a strainer (30–40 mesh) manually with a disintegrator. The powdered samples were freeze-dried at −45°C to ensure complete removal of water.
The extraction of screened plants
The dry powder (50.0 g) of 42 kinds of plants was extracted with methanol (500 mL three times) for 48 h. In order to get more or less solidified crude extracts, the methanol filtrates were separately filtered and evaporated under reduced pressure in a vacuum rotary evaporator (R-201, Shanghai Shenshen) until the solvents completely evaporated. The resulting extracts of different plants were dissolved in dimethyl sulfoxide (DMSO) and diluted with distilled water to obtain 0.6 g mL−1 (sample/solvent) of stocking solutions, which were used for the preparations of the desired concentrations for anthelmintic efficacy assay.
The extraction of anthelmintic plants
Three plant materials (C. cassia, L. aggregata, and P. kaempferi) which have 100% anthelmintic efficacy were selected from 42 kinds of plants. Each plant material (50.0 g) was extracted with petroleum ether, chloroform, ethyl acetate, methanol, and water for 48 h for complete extraction, and the process was repeated three times. The ratio of sample to solvent was 1:10 (m/v). All the extracts were filtered, combined, and evaporated under reduced pressure in a vacuum rotary evaporator (R-201, Shanghai Shenshen). The resulting extracts of different plants were dissolved in dimethyl sulfoxide (DMSO) and diluted with distilled water to obtain 0.6 g mL−1 (sample/solvent) of stocking solutions, which were used in the further assay.
In vivo bioassays
Tests were conducted in each glass tank of 5-L capacity, filled with 2 L aerated groundwater, each containing samples and five previously infected fish. The water pH ranged from 7.0 to 7.5, and dissolved oxygen was between 6.2 and 7.8 g mL−1 (72–85% saturation); the water temperature was constant at 24 ± 1°C. Initial tests were conducted to get a moderate concentration range in order to avoid the mortality of fish at high concentrations.
For the extracts of screened plants
The designed concentration gradients of each extract were added; the final concentrations in the test solution were 100, 200, 300, 400, 500, and 600 mg L−1. Negative control groups containing no plant extract were set up under the same conditions as the test groups. To discard the possible effects of DMSO on the parasites, another control, containing the highest percentage of DMSO, was included.
For the extracts of anthelmintic plants
The crude extracts of C. cassia, L. aggregata, and P. kaempferi were conducted at a different series of concentrations based on the initial tests, respectively, and the negative control groups containing no plant extract were set up under the same conditions as the test groups. The DMSO control was also included.
All the experiments were conducted twice. During the experiments, no food was offered to the fish. The death of fish was recorded when the opercula movement and tail beat stopped and the fish no longer responded to mechanical stimulus. To avoid the deterioration of the water quality, the observed dead fish were removed from the water in time. Forty-eight hours later, the surviving fish in all the treatments were killed by spinal severance and biopsied under a light microscope at 4 × 10 magnification (Fig. 1). The anthelmintic efficacy of each treatment and the negative control group was calculated according to the following formula:
where AE is anthelmintic efficacy, B is average number of surviving D. intermedius in the negative control, and T is average number of surviving D. intermedius in the treatment groups.
Micrographs of untreated (a, b) and treated (c, d) D. intermedius. a, b Microscopic slide with live D. intermedius detached from the gills under a light microscope at 20 × 10 and 40 × 10 magnification. c, d Microscopic slide with dead D. intermedius detached from the gills after treated with the methanol extract of C. cassia at 20 × 10 and 40 × 10 magnification
Acute toxicity test
Acute toxicity tests were performed in a 5-L capacity plastic pot with 2 L of the test solution water and ten healthy goldfish. Control groups were set under the same test conditions without extracts. Another control group containing the highest percentage of DMSO was also included. The experiments were performed twice at 24 ± 1°C. The death of fish was recorded when the opercula movement and tail beat stopped and the fish no longer responded to mechanical stimulus. To avoid the deterioration of the water quality, the observed dead fish were removed from the water in time.
Statistical analysis
The homogeneity of the replicates of the samples was checked by the Mann–Whitney U test. Probit analysis was used for calculating the median lethal concentration (LC50, LC90) and median effective concentration (EC50, EC90) at the 95% confidence interval with upper confidence limit and lower confidence limit (Finney 1971).
Result
The anthelmintic efficacies against D. intermedius (Monogenea) of selected plants were evaluated, and the results are shown in Table 1. Among the screened plants, P. kaempferi, L. aggregata, and C. cassia were found to have 100% anthelmintic efficacy at 60.0, 120.0, and 300.0 mg L−1. The solvent (DMSO) acting as a control showed no anthelmintic activity when treated at the highest concentration.
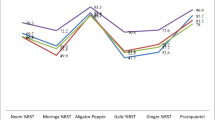
The anthelmintic efficacies of different extracts of C. cassia, L. aggregata, and P. kaempferi are depicted in Fig. 2, and the EC50 and EC90 values are shown in Fig. 3. The methanol extract of C. cassia was found to be the most effective one with EC50 and EC90 values of 12.3 and 32.1 mg L−1, respectively. After exposure for 48 h, it exhibited a 100% efficacy against D. intermedius at 30.0 mg L−1. High anthelmintic activity against D. intermedius was also observed in the water and chloroform extracts with EC50 and EC90 values of 13.2, 29.4, 39.5, and 93.6 mg L−1, respectively. The ethyl acetate and petroleum ether extracts, however, exhibited weak activity with the maximum anthelmintic efficacy of 6.1% at 40.0 mg/L and 31.1% at 100.0 mg L−1 with no fish died.
Anthelmintic efficacy of different extracts of Cinnamomum cassia, Lindera aggregata, and Pseudolarix kaempferi against Dactylogyrus intermedius after 48 h. PEE petroleum ether extract, CLE chloroform extract, EAE ethyl acetate extract, MEE methanol extract, WAE water extract. Star indicates when fish mortality first occurred
In the case of L. aggregata, the methanol extracts were observed to be the most effective with EC50 and EC90 values of 23.5 and 37.7 mg L−1 after 48 h of treatment, respectively. The methanol extracts exhibited a 100% anthelmintic efficacy against D. intermedius at 60.0 mg L−1. The extracts of water and ethyl acetate also showed high anthelmintic activity, with EC50 and EC90 values of 366.0, 632.7, 109.6, and 156.0 mg L−1, respectively. However, fish mortality occurred when the concentration reached 600.0 mg L−1 for water and 200 mg L−1 for ethyl acetate, followed by the chloroform and the petroleum ether extracts, which exhibited anthelmintic efficacies of 27.27% and 16.17% both at 50.0 mg L−1.
As for P. kaempferi, both methanol and ethyl acetate extracts displayed the optimal anthelmintic activity with 100% efficacy at the dose of 60.0 mg L−1. EC50 and EC90 values were 23.3 and 37.0 mg L−1 for methanol extract, and 17.1 and 42.8 mg L−1 for ethyl acetate extract. The remaining other plant extracts were found to exhibit weak activity with the highest anthelmintic efficacy of 0% for water, 54.6% for chloroform, and 44.4% for petroleum ether, respectively.
The results of acute toxicity assay for methanol and water extracts of C. cassia, the methanol extracts of L. aggregata, and the methanol and ethyl acetate extracts of P. kaempferi are shown in Table 2. The 48-h LC50 values of methanol extracts of C. cassia, L. aggregata, and P. kaempferi were 31.3, 165.7, and 88.7 mg L−1; the water extract of C. cassia was 56.9 mg L−1, and the ethyl acetate extract of P. kaempferi was 168.2 mg L−1.
Discussion
The monogenean trematodes are mostly parasitic in the gills and body surface of freshwater fish and treated as a serious pest in the aquaculture industry (Oliver 1977). Dactylogyrus, a main genus of monogeneans, is a common parasite in fish diseases. Traditionally, a number of chemotherapeutic agents have been used to deal with severe problems caused by Dactylogyrus. However, because of their side effects, such as accumulation of drugs in tissues, development of drug resistance, and the potential deleterious effects on the environment and the human consumers, the use of these chemicals are not recommended anymore. Screening of medicinal plants and application of their extracts to control monogenean parasites could offer possible alternatives that may be both sustainable and environmentally acceptable. For this reason, the plant-based products have been extensively studied to control Dactylogyrus infection as compared with the chemicals. In the present study, 42 medicinal plants were evaluated for the in vivo anthelmintic activity against D. intermedius (Monogenea) in goldfish (C. auratus). Three plants, namely C. cassia, L. aggregata, and P. kaempferi, were found to produce a 100% parasite elimination rate at low concentration. As far as we know, this is the first report on the anthelmintic activity of C. cassia, L. aggregata, and P. kaempferi.
Among the three potent plants, extracts from C. cassia were found to exhibit strongest efficacy with the lowest EC50 and EC90. The dried stem bark of C. cassia is a popular natural spice and a commonly used herb in traditional Chinese medicine. Shan et al. (1999) found that the water extract of C. cassia enhanced Ig production by B cells, IL-1 production by monocytes, and cytotoxic T-lymphocyte activity against allogeneic tumor cells. The ethanol extract of C. cassia exhibited the strongest antioxidant action not only in the rat homogenate model system but also in the cytochrome test. Meanwhile, the ethanol extract also displayed anti-superoxide formation activity and is treated as an excellent xanthine oxidase inhibitor (Lin et al. 2003). The biologically active constituents of C. cassia are cinnamaldehyde, cinnamon oil, eugenol, salicylaldehyde and trans-cinnamic acid. Ooi et al. (2006) pointed out that the hydro-distilled Chinese cinnamon oil and pure cinnamaldehyde of C. cassia were equally effective in inhibiting the growth of various isolates of bacteria including Gram-positive and Gram-negative, and fungi including yeasts and dermatophytes. The eugenol and salicylaldehyde revealed strong insecticidal activity, whereas trans-cinnamic acid revealed moderate activity (Park et al. 2000). Considering the major bioactive constituents of C. cassia, some of the substances mentioned earlier may contribute to the efficacy of C. cassia independently or jointly.
Radix Linderae, the root tuber of L. aggregata, is a traditional herbal medicine in both China (Wu-yao) and Japan (Uyaku) for treating several diseases including chest and abdomen pain, indigestion, regurgitation, cold hernia, and frequent urination (Jiangsu New Medical College 1979; The Editorial Committee of the Administration Bureau of Traditional Chinese Medicine 1999). The extracts of Radix Linderae have been reported to possess anti-inflammatory, analgesic, and antimicrobial properties (Chou et al. 1999). Study on Radix Linderae revealed that it contained alkaloids, volatile oils, and sesquiterpene esters. Luo et al. (2009) suggested that the total alkaloids from Radix Linderae exhibited inhibitory effects on the production of inflammatory mediators from macrophages via blocking NF-κB and MAPKs signaling pathways. In the case of Radix Linderae essential oil, it is useful to improve the immunity activities and prevent the occurrence of decubitus in aged people (Liang 2011). These findings provide a plausible explanation for the high LC50 and LC90 of the methanol extract of L. aggregata. As for P. kaempferi, it is a kind of indigenous plant in the east of China. Its root bark known as “Tu Jin Pi” is used in traditional Chinese medicine for the treatment of skin diseases caused by microbial infection. The diterpenoids, pseudolaric acid A, and pseudolaric acid B were found to be the antifungal component of this plant (Li et al. 1995; Yang et al. 2003). Zhang et al. (1990) observed that pseudolaric acid has a partial antifertility effect when it was injected ig 20 mg/kg daily to hamsters (female) for 4 days before mating. Pseudolarolides B, which belongs to triterpene lactone, showed potent cytotoxicity against three human cancer cell lines, KB (nasopharyngeal), A-549 (lung), and HCT-8 (colon), and a murine leukemia cell line (P-388) with ED50 values of 0.49, 0.67, 0.73, and 0.79 μg/mL, respectively (Chen et al. 1993).Two triterpenoids, isopseudolarifuroic acids A and B, exhibited significant cytotoxic activities against several tumor cell lines (Yang and Yue 2001). The high cytotoxic potency of triterpenoids and triterpene lactone might be involved in the eradication of the parasites. Although there are no attempts to identify the anthelmintic compound(s) in these two plants, some of the substances mentioned earlier are believed to contribute jointly or independently to the inhibition activity against D. intermedius.
Among the other 39 kinds of plants screened, a large portion exhibited high anthelmintic activity against D. intermedius but less than 100%. These may be the result of that the ingredients which are responsible for the anthelmintic activity are totally different from the ones which are toxic to the fish. Comparing the EC50 and EC90 of C. cassia and P. kaempferi, the methanol extract of C. cassia showed a close EC50 and EC90 with the water extract. Meanwhile, the similar phenomenon was also found in methanol and ethyl acetate extracts of L. aggregata. The results of acute toxicity assay for the extracts of C. cassia, L. aggregata, and P. kaempferi indicated that these extracts were safe to goldfish. The 48-h LC50 values of these extracts were higher than the corresponding EC50. For example, for the ethyl acetate extract of P. kaempferi, the toxic dose (LC50 = 168.2 mg L−1) is about ten times the effective one (EC50 = 17.1 mg L−1).These results enhance the possibility of the development and use of commercial products containing this material.
In summary, the extracts of C. cassia, L. aggregata, and P. kaempferi have the potential for the development of novel therapy for the treatment against D. intermedius infection. However, more investigations such as pharmacological evaluations before clinical trials, assessment of ecological risk posed by practical usage, and their detailed mechanism of anthelmintic (D. intermedius) activity must be performed. Further bioassay-guided isolation and purification of compound(s) responsible for the observed anthelmintic efficacy are in progress.
References
Abdel-Ghaffar F, Semmler M, AI-Rasheid, Strassen B, Aksu G, Fischer K, Klimpel S, Mehlhorn H (2011) The effects of different plant extracts on intestinal nematodes and trematodes. Parasitol Res 108(4):979–984
Bondad-Reantaso MG, Subasinghe RP, Arthur JR, Ogawa K, Chinabut S, Adlard R, Tan ZL, Shariff M (2005) Disease and health management in Asian aquaculture. Vet Parasitol 132(3–4):249–272
Chen GF, Li ZL, Pan DJ, Tang CM, He X, Xu GY, Chen K, Lee KH (1993) The isolation and structural elucidation of four novel triterpene lactones, peudolarolides A, B, C, and D, from Pseudolarix kaempferi. J Nat Prod 56(7):1114–1122
Chou GX, Wang ZT, Xu LS, Xu GJ (1999) The chemical composition and pharmacological action of Radix Linderae. Chin Wild Plant Resour 18:5–10
Dove A, Ernst I (1998) Concurrent invaders—four exotic species of Monogenea now established on exotic freshwater fishes in Australia. Int J Parasitol 28:1755–1764
Finney DJ (1971) Probit analysis, 3rd edn. Cambridge University Press, Cambridge
Goven BA, Amend DF (1982) Mebendazole/trichlorfon combination: a new anthelmintic for removing monogenetic trematodes from fish. J Fish Biol 20(4):373–378
Goven B, Gilbert J, Gratzek J (1980) Apparent drug resistance to the organophosphate dimethyl (2, 2, 2-trichloro-1-hydroxyethyl) phosphonate by monogenetic trematodes. J Wildl Dis 16(3):343–346
Hossain E, Chandra G, Nandy AP, Mandal SC, Gupta JK (2011) Anthelmintic effect of a methanol extract of leaves of Dregea volubilis on Paramphistomum explanatum. Parasitol Res doi:10.1007/s00436-011-2558-2
Jiangsu New Medical College (1979) Zhongyao dictionary (encyclopedia of Chinese materia medica). Shanghai Scientific & Technological Press, Shanghai, pp 462–463
Klimpel S, Abdel-Ghaffar F, AL-Rasheid KAS, Aksu G, Fischer K, Strassen B, Mehlhorn H (2011) The effects of different plant extracts on nematodes. Parasitol Res 108(4):1047–1054
Klinger R, Floyd RF (2002) Introduction to freshwater fish parasites. Document CIR716. Institute of Food and Agricultural Science. University of Florida, Gainesville
Li E, Clark AM, Hufford CD (1995) Hufford antifungal evaluation of pseudolaric acid B, a major constituent of Pseudolarix kaempferi. J Nat Prod 58(1):57–67
Liang ZH (2011) Radix linderae essential oil improving the immunity activities and preventing the occurrence of decubitus in aged people. J Med Plants Res 5(16):3733–3738
Lin CC, Wu SJ, Chang CH, Ng LT (2003) Antioxidant activity of Cinnamomum cassia. Phytother Res 17:726–730
Liu YT, Wang F, Wang GX, Han J, Wang Y, Wang YH (2010) In vivo anthelmintic activity of crude extracts of Radix angelicae pubescentis, Fructus bruceae, Caulis spatholobi, Semen aesculi, and Semen pharbitidis against Dactylogyrus intermedius (Monogenea) in goldfish (Carassius auratus). Parasitol Res 106:1233–1239
Lu C, Zhang HY, Ji J, Wang GX (2011) In vivo anthelmintic activity of Dryopteris crassirhizoma, Kochia scoparia, and Polygala tenuifolia against Dactylogyrus intermedius (Monogenea) in goldfish (Carassius auratus). Parasitol Res doi:10.1007/s0043601125920
Luo YB, Liu M, Yao XJ, Xia YF, Dai Y, Chou GX, Wang ZT (2009) Total alkaloids from Radix Linderae prevent the production of inflammatory mediators in lipopolysaccharide-stimulated RAW 264.7 cells by suppressing NF-κB and MAPKs activation. Cytokine 46(1):104–110
Mehlhorn H, Aksu G, Fischer K, Strassen B, Abdel-Ghaffar F, AI-Rasheid, Klimpel S (2011) The efficacy of extracts from plants—especially from coconut and onion—against tapeworms, trematodes, and nematodes. In: Mehlhorn H (ed) Parasitology research monographs 1. Springer, Berlin, pp 109–139
Oliver G (1977) Effect pathogene de la fixation de Dipleetanum aequans (Wagener 1857) Diesing, 1858 (Monogenea, Monopisthocotylea, Diplectanidae) sur les branchies de Dicentrarchus labrax (Linneaeus 1758), (Pisces, Serranidae). Parasitol Res 53(1):7–11
Ooi LS, Li YL, Kam SL, Wang H, Wong EY, Ooi VE (2006) Antimicrobial activities of cinnamon oil and cinnamaldehyde from the Chinese medicinal herb Cinnamomum cassia Blume. Am J Chinese Med 34(3):511–522
Park IK, Lee HS, Lee SG, Park JD, Ahn YJ (2000) Insecticidal and fumigant activities of Cinnamomum cassia bark-derived materials against Mechoris ursulus (Coleoptera: Attelabidae). J Agric Food Chem 48(6):2528–2531
Reed PA, Francis-Floyd R, Klinger RC (2009) FA28/FA033. Monogenean parasites of fish. EDIS—Electronic Data Information Source—UF/IFAS Extension. University of Florida. http://edis.ifas.ufl.edu/FA033. Accessed 17 May 2009
Schmahl G, Mehlhorn H (1985) Treatment of fish parasites. 1. Praziquantel effective against Monogenea (Dactylogyrus vastator, Dactylogyrus extensus, Diplozoon paradoxum). Z Parasitenk 71:727–737
Schmahl G, Mehlhorn H, Haberkorn A (1988) Sym. triazinone (toltrazuril) effective against fish-parasitizing Monogenea. Parasitol Res 75(1):67–68
Shan BE, Yoshida Y, Sugiura T, Yamashita U (1999) Stimulating activity of Chinese medicinal herbs on human lymphocytes in vitro. Int J Immunopharmaco 21(3):149–159
The Editorial Committee of the Administration Bureau of Traditional Chinese Medicine (1999) Chinese materia medica (Zhonghua Bencao), vol. 3. Shanghai Science and Technology Press, Shanghai, pp 56–59
Wang GX, Zhou Z, Cheng C, Yao JY, Yang ZW (2008) Osthol and isopimpinellin from Fructus cnidii for the control of Dactylogyrus intermedius in Carassius auratus. Vet Parasitol 158:144–151
Wang GX, Han J, Feng TT, Li FY, Zhu B (2009a) Bioassay-guided isolation and identification of active compounds from Fructus arctii against Dactylogyrus intermedius (Monogenea) in goldfish (Carassius auratus). Parasitol Res 106:247–255
Wang GX, Jiang DX, Zhou Z, Zhao YK, Shen YH (2009b) In vivo assessment of anthelmintic efficacy of ginkgolic acids (C13:0, C15:1) on removal of Pseudodactylogyrus in European eel. Aquaculture 297(1–4):38–43
Wang GX, Jiang DX, Li J, Han J, Liu YT, Liu XL (2010a) Anthelmintic activity of steroidal saponins from Dioscorea zingiberensis C. H. Wright against Dactylogyrus intermedius (Monogenea) in goldfish (Carassius auratus). Parasitol Res 107:1365–1371
Wang GX, Han J, Zhao LW, Jiang DX, Liu YT, Liu XL (2010b) Anthelmintic activity of steroidal saponins from Paris polyphylla. Phytomedicine 17:1102–1105
Woo PTK, David W, Bruno LH, Susan L (2002) Diseases and disorders of finfish in cage culture. CABI, Malaysia
Wu ZF, Zhu B, Wang Y, Lu C, Wang GX (2011) In vivo evaluation of anthelmintic potential of medicinal plant extracts against Dactylogyrus intermedius (Monogenea) in goldfish (Carassius auratus). Parasitol Res 108:1557–1563
Yang SP, Yue JM (2001) Two novel cytotoxic and antimicrobial triterpenoids from Pseudolarix kaempferi. Bioorg Med Chem Lett 11(24):3119–3122
Yang SP, Dong L, Wang Y, Wu Y, Yue JM (2003) Antifungal diterpenoids of Pseudolarix kaempferi, and their structure–activity relationship study. Bioorgan Med Chem 11(21):4577–4584
Zhang YL, Lu RZ, Yan AL (1990) Inhibition of ova fertilizability by pseudolaric acid B in hamster. Acta Pharmacol sin 11(1):60–62
Zhou YQ, Chen XJ (2010) Notes of study on development strategy of Chinese fishery to 2030. Alliance Glob Sustain Bookseries 18(3):167–176
Acknowledgments
This work was supported by the National High Technology Research and Development Program of China (863 Program) (no. 2011AA10A216) and National Natural Science Foundation of China (no. 31072242).
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Ji, J., Lu, C., Kang, Y. et al. Screening of 42 medicinal plants for in vivo anthelmintic activity against Dactylogyrus intermedius (Monogenea) in goldfish (Carassius auratus). Parasitol Res 111, 97–104 (2012). https://doi.org/10.1007/s00436-011-2805-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00436-011-2805-6