Abstract
The anthelminthic efficacy of some differently obtained extracts of several plants was tested in vivo in laboratory animals and in vitro. The extracts were obtained by ethanolic, methanolic, aqueous, or chloroform, respectively, acetonitrile polyethylenglycol (PEG) and/or propylencarbonate (PC) elution at room temperature or at 37°C. The plants used were bulbs of onions, garlic, chives, coconut, birch tree, ananas, cistrose, banana, chicory, date palm fruit, fig, pumpkin, and neem tree seeds. The worm systems tested both in vivo and in vitro were Trichuris muris and Angiostrongylus cantonensis but only in vivo Toxocara cati. The tests clearly showed that the different extraction methods eluted different components and different mass amounts, which had different efficacies against the above-cited worms. In vitro effects against A. cantonensis and T.muris were best with aqueous extracts, followed by chloroform extracts. The other plant extracts showed only low or no effects on A. cantonensis in vitro. In the case of T. muris, best results were obtained in vivo and in vitro with PEG/PC extracts of the onion followed by the aqueous extract of coconut. The complete elimination of worms in the in vivo experiments with T. muris was obtained when infected mice were treated with a 1:1 mixture of extracts of coconut and onion being produced by elutions with a mixture of 1:1 PEG and PC and fed daily for 8 days. T. cati in a naturally infected cat was eliminated by daily oral application of 6 ml coco’s fluid for 5 days. This study shows that a broad spectrum of plants has anti-nematodal activities, the intensity of which, however, depends on the mode of extraction. This implicates that, if results should be really comparable, the same extraction methods at the same temperatures have to be used. Furthermore, efficacy in in vitro systems does not guarantee as good—if at all—efficacy in vivo.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Nematodes are worldwidely distributed and are important destruents of organic materials in soil, in water, on meadows, and they help to eliminate dead bodies. However, thousands of species belonging to hundreds of genera have developed a “sophisticated parasitism” inside the different tissues of humans and animals (Mehlhorn 2008). These nematodes (round worms and threadworms) may introduce severe diseases in both host types. Some of these invaders have developed additionally a zoonotic practice so that they may wander from animals to humans and back (Eckert et al. 2008; Mehlhorn et al. 1993, 1995). Therefore, especially today, in an overcrowded world with mass production of animals, an appropriate control of such parasites must be constantly done in order to produce enough food for a healthy mankind. In early times of the eldest human societies (Babylonia, Egypt, Greece, and Rome), but also stretching from the early medieval centuries until the nineteenth century, plant diets were used to control parasites inside the house, in farm animals, as well as in humans (von Bingen 1974). However, as soon as chemotherapy was invented and as soon as it was proven that this therapy is better, the knowledge on the efficacy of several plants was forgotten since its use was mostly only orally transmitted from parents to their children. Furthermore, many nice tales on putative effects of plants were spread without any proof of truth. Therefore, it is needed today to test again plants on their efficacy against different parasites, especially since many of them have developed resistances against a broad spectrum of chemotherapeutics. Recently, a resurrection of investigations on antiparasitic effects of plants can be noted filling volumes of many journals (e.g., ed. Jabbar et al. 2007; Hussain et al. 2008; Oliveira et al. 2009; Eguale et al. 2007; Tagboto and Townson 2001; Semmler et al. 2009; Schmahl et al. 2010).
However, in many cases, the authors established in vitro studies or used peculiar laboratory models, mainly dealing with infected mice and rats. This means that only rather few studies are available on the efficacy of some plants against parasites of farm animals and even less against human parasites (lit. c. f. Mehlhorn et al. 2010). The present study examines the effects of different extracts of different plants against nematodes of mice and rats in vivo and in vitro. These experiments were done to develop a useful, non-toxic remedy against cestodes and nematodes being presented in a recent paper (Mehlhorn et al. 2010).
Materials and methods
Worms
The intestinal worms of the species Trichuris muris were kept in 8–9-week-old female laboratory mice (C 57/BL10 or NMRI) strains for years in the institute. The animals were orally infected with 250 larvae-containing eggs. This brings in mean about 75–90 adults in untreated mice (depending on the infectiosity of the eggs). T. muris has a direct life cycle, where infection occurs by oral uptake of larvae-containing eggs. Angiostrongylus cantonensis were cultivated in Wistar rats (Rattus rattus). These rats were orally infected with 40 larvae, which had been selected from the mucus or squeezed portions of infected snails (intermediate hosts = Biomphalaria species or Archachatina species). The adult worms live in the pulmonary arteries, and the larvae 1 are excreted via saliva and/or feces.
Plants
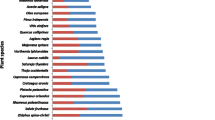
The plants used were pineapple (Ananas comosus), birch tree (Betula pendula), banana (Musa parasidaica), chicory (Cichorium intybus), date tree (Phoenix dactylifera), fig tree (Ficus carica), pumpkin (Cucurbita maxima), onion (Allium cepa), garlic (Allium sativum), neem tree (Azadirachta indica), cistrose (Cistus incanus), chives (Allium schoenoprasum), and coconut (Cocos nucifera).
Extracts
The following extracts were made at room temperature from minced and dried plant material under room temperature or 37°C and under constant shaking for 24 h:
-
Methanolic
-
Ethanolic
-
Aqueous
-
Acetonitrile
-
Chloroform
-
1:1 PEG/PC (polyethylenglycol / propylencarbonate)
In all cases, dried plant material (produced at 40°C) was mixed with the extraction fluid in a relation of 1 g plant material within 10 ml extraction solution. In the case of the Toxocara cati test, the undiluted fruit water of a coconut was used.
In vitro tests
The larvae of A. cantonensis were obtained by help of a so-called Baermann funnel from feces of infected rats and placed into the culture medium at 37°C. Adult worms of T. muris were taken from the intestines of infected adult mice and incubated like the larvae of A. cantonensis in the test media. The vitality of the incubated worms was controlled at intervals of 2 h for 24 h.
In vivo tests
Infected hosts were orally treated with different amounts of the extracted plant compounds, and the feces were controlled for the excretion of eggs. Finally, the hosts were sacrificed, and the number of surviving worms was counted and compared to the worm load of untreated mice.
In vitro dosages of test products
A broad variety of concentrations of the product was used at least in one-, two-, and triplefold steps of the initial dosage being added to the treated Tyrode solution, wherein the worms became incubated. As controls, the same worm stages were incubated in untreated Tyrode solution at 37°C.
In vivo treatment
Rats, mice, and one T. cati-infected cat were orally treated either by dosages per kilogram of body weight or—in the case of the cat—by 6 ml of undiluted coco’s fluid. Six mice were also fed for 12 days exclusively with dried coconut. The effects of the different treatments were measured either by fecal control for eggs/larvae or by intestinal/lung inspection of sacrificed animals.
Results
Extract output of the different extraction media
The different extraction media delivered different amounts of the products not only depending on their chemical composition but also depending on the temperature at which the extraction was proceeded (Table 1). The selection of some examples shows that the differences in the amounts of the obtained extract material might be considerable depending on the consistence (water content) of the plant tested (Table 1). Based on these initial studies, varying results should be obligatorily expected.
Selected examples of effects of different extracts on T. muris
In vitro results in T. muris
As seen in Tables 2, 3, 4, and 5, aqueous and chloroform extracts from different plants are able to deliver rather good results with respect to killing adult worms of T. muris or to reduce their motility. On the other hand, methanolic and acetonitrile extracts are less good (Tables 4 and 5). This antinematode effect was significantly proven by the fact that the control worms survived in extract-free Tyrode solution not only for 24 h (as documented in Tables 2, 3, 4, and 5) but for more than 5 days (equivalent to the end of testing). That the non-motile worms are really dead was proven by their transfer back into normal, extract-free Tyrode solution. However, it cannot be expected that in the intestines of worm-infected animals, similar concentrations of the plant extracts can be reached by feeding. Therefore, in vivo tests are needed to check whether the positive results of the in vitro tests can be obtained, too.
In vitro experiments with A. cantonensis
The experiments were conducted with larvae 1, which had been isolated from feces of infected rats. These larvae were incubated in Tyrode solution, into which the different plant extracts were filled at concentrations shown in Tables 6, 7, 8, and 9. Although these larvae are tough enough to survive in the feces outside the warm body of the rat until the feces are eaten by a snail (intermediate host), they were rather easily attacked by plant extracts in the present experiments. Some of these plant extracts were listed in Tables 6, 7, 8, and 9. In the tests with A. cantonensis, it was again observed that aqueous and PEG/PC extracts were superior to methanolic (Table 10). These experiments show a general sensitivity of the larvae against such plant extracts. However, from these in vitro experiments, it cannot be concluded that the plant extracts used would be successful in intestines. On the other hand, however, these experiments show that neem seed extracts, which are used as biocides against larval and adult stages of mites, ticks, and insects (Semmler et al. 2009; Mehlhorn et al. 2010; Schmahl et al. 2010), would also kill infectious larvae of nematodes if they are treated on the floors of houses and stables.
In vivo experiments
T. muris
In the first experiments, the infected mice were treated only for 3 days with the different plant extracts. On these days and on days 1, 5, 12, and 22 after the treatment, the excreted eggs were counted within one 1 g of feces and listed in Table 11, which gives the mean results of two mice of each group. The results, however, show that a 3-day treatment with the plant extracts listed in Table 11 is not sufficient to stop the egg excretion. Furthermore, the egg excretion of the control mice was also varying and decreasing (Table 11). Thus, in another series of experiments, a full coco diet was given to three infected mice for 12 days. These three mice fed daily each a mean of 1.5 g per day. The mean excreted egg amount decreased from day 2 of the treatment from a mean of 16,089 per gram of feces until 196 on day 12 of the treatment, while the controls remained rather stable. The controls started with a mean of 13,398 eggs per gram of feces and ended with 15,075 eggs on day 12 of treatment. Sacrificing the mice, it was seen that the treated four mice contained only two or four worms with signs of degeneration, while the untreated two control mice contained 68 and 72 fully motile worms without any degenerations, respectively. This result led us to the insight that a longer treatment should be done using, besides coco, other plant extracts—garlic, chives, and onion (Table 12).
However, as can be seen in Table 12, only the treatment with onion powder was able to eliminate fully the load of adult T. muris worms from the intestines of infected mice, while garlic or chives powder was not very effective. Therefore, in a further experiment, a combination treatment was done with onion powder and coconut powder (Table 13). This experiment clearly shows that the combination of 500 mg/kg body weight each of coconut and onion given for 8 days is fully successful, while 500 mg either of onion or coconut leaves residual worms in the intestine of T. muris-infected mice. The results were identical in both sexes when using female and male worms.
Pilot study on T. cati
The cat of one of the participants of the study excreted daily considerable numbers (equivalent to 360/g of feces) of eggs of the worm T. cati. The owner fed this cat for 5 days daily with 6 ml coco’s fluid (fluid endosperm) of a coconut. This resulted in the stoppage of the egg excretion after the fourth day of treatment. The egg excretion did not start again afterwards.
Discussion
The broad testing of extracts obtained with different methods from about 21 different plants in our institute and the comparison of our results with other publications (Aksu 2009; Strassen 2007; Fischer 2007; Abdel-Ghaffar et al. 2010) resulted in some not very astonishing results, but also led to some insights, which gave hope to produce soon a reliable and rather cheap product. If this thus developed plant product is added to food, deworming (e.g., of ruminants) might occur without waiting time (Mehlhorn et al. 2010).
It was not astonishing that different methods (aqueous, alcoholic, acetonitrile, and PEG/PC) delivered different extracts with different grades of efficacy. Thus, literature must be checked whether results reported there are really comparable. Not really astonishing was that several of the plants—even when extracted with the favorable aqueous extraction mode—had no or only low effects on the stages of our nematode models (T. muris and A. cantonensis; see Tables 11 and 12). On the other hand, it became clear that in vitro experiments alone will not be sufficient to find out a reliable product since—although the results in vitro might be good—in vivo, the needed concentration could never be reached.
Astonishing was that chives, onion, and garlic—although all belonging to the plant genus Allium—had completely different effects on nematodes, so that only the onion (A. cepa) was used further on in the trials.
When comparing the literature, many contradictory results are reported, e. g., Oliveira et al. (2009) obtained in their experiment an in vitro anthelminthic activity of an ethyl acetate extract of the green coconut husk fiber, which is a byproduct of the food industry, against the larvae of Haemonchus contortus. However, when feeding this extract on 3 days daily at a dose of 400 mg/kg body weight, no effects on the gastrointestinal nematodes were seen. This failure is—when looking at our results—apparently due to the fact that Oliveira et al. used another type of extract which was from another portion of the coconut, and that it was given only for 3 days, while in the present in vivo studies on mice and in our in vivo study with sheep, we fed another coconut extract for 8 days (Mehlhorn et al. 2010).
References
Abdel-Ghaffar F, Semmler M, Al-Rasheid, Strassen B, Aksu G, Fischer K, Klimpel S, Mehlhorn H (2010) The effects of different plant extracts on intestinal nematodes and trematodes. Parasitol Res. doi:10.1007/s00436-010-2167-5
Aksu G (2009) Plant extracts in the fight against worms. Diploma thesis, Duesseldorf, Germany
von Bingen H (1974) Naturkunde—reproduction of a medieval book, 2nd edn. Mueller-Wiss, Buchgesellschaft, Salzburg
Eckert J, Friedhoff KT, Zahner H, Deplazes P (2008) Lehrbuch der Parasitologie für die Tiermedizin, 2nd edn. Enke, Stuttgart
Eguale T, Tilahun G, Debella A, Feleke A, Makonnen E (2007) Haemonchus contortus: in vitro and in vivo anthelmintic activity of aqueous and hydro-alcoholic extracts of Hedera helix. Exp Parasitol 116:340–345
Fischer K (2007) The effects of plant extracts on worm stages. Diploma thesis, University of Duesseldorf
Hussain A, Khan MN, Igbal Z, Sajid MS (2008) An account of the botanical anthelminthics used in traditionally veterinary practices in Saniwal district of Punjab (Pakistan). J Ethnopharmacol 119:185–190
Jabbar A, Zaman MA, Igbal Z, Yassen M, Shamin A (2007) Anthelminthic activity of Chenopodium album and Caesalpinia crista against trichostrongylid nematodes on sheep. J Ethnopharmacol 114(1):86–91
Mehlhorn H (2008) Enyclopedia of parasitology, 3rd edn. Springer, New York
Mehlhorn H, Düwel D, Raether W (1993) Diagnosis and therapy of parasitosis of house and farm animals, 2nd edn. G Fischer, Stuttgart, in German
Mehlhorn H, Eichenlaub D, Löscher T, Peters W (1995) Diagnosis and therapy of the parsites of men, 2nd edn. G. Fischer, Stuttgart, in German
Mehlhorn H, Al-Quaraishy S, Al-Rasheid KAS, Jatzlau A, Abdel-Ghaffar F (2010) Addition of a combination of onion (Allium cepa) and coconut (Cocos nucifera) to food of sheep stops gastrointestinal helminthic infections. Parasitol Res. doi:10.1007/s00436-010-2169-3
Oliveira LMB, Bevilagne CML, Cosha CZC et al (2009) Anthelminthic activity of Cocos nucifera against sheep gastrointestinal nematodes. Vet Parasitol 159:55–59
Schmahl G, Al-Rasheid KAS, Abdel-Ghaffar F, Klimpel S, Mehlhorn H (2010) The efficacy of neem seed extracts (Tre-san®, MiteStop®) and a broad spectrum of pests and parasites. Parasitol Res 107:261–269
Semmler M, Abdel-Ghaffar F, Al-Rasheid KAS, Mehlhorn H (2009) Nature helps: from research to products against blood sucking arthropods. Parasitol Res 105:1483–1487
Strassen B (2007) Cocos nucifera investigations on the efficacy of extracts on parasites. PhD-thesis, University of Duesselorf (in German)
Tagboto S, Townson S (2001) Antiparasitic properties of medical plants and other naturally occurring products. Ad Parasitol 50:200–285
Acknowledgement
We hereby acknowledge the great support of numerous co-workers in our institute who helped us to prepare the great number of different extracts and the realization of much more experiments than presented here. Furthermore, we deeply acknowledge the strong support of the Centre of Excellence of the College of Science of the King Saud University at Riyadh, Saudi Arabia.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Klimpel, S., Abdel-Ghaffar, F., Al-Rasheid, K.A.S. et al. The effects of different plant extracts on nematodes. Parasitol Res 108, 1047–1054 (2011). https://doi.org/10.1007/s00436-010-2168-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00436-010-2168-4