Abstract
In search of a natural antiparasitic, in vivo anthelmintic activity of petroleum ether, chloroform, ethyl acetate, methanol, and aqueous extracts of Angelica pubescens roots (Radix angelicae pubescentis), Brucea javanica fruits (Fructus bruceae), Spatholobus suberectus stems (Caulis spatholobi), Aesculus chinensis Bge. seeds (Semen aesculi), and Pharbitis purpurea (L.) Voigt seeds (Semen pharbitidis) were tested against Dactylogyrus intermedius (Monogenea) in goldfish (Carassius auratus). Among the extracts tested, the methanolic and aqueous extracts of S. aesculi were observed to be more efficient than the other plant extracts with EC50 and EC90 values of 5.23 and 7.33 mg/L and 6.48 and 12.29 mg/L after 48 h, respectively, followed by methanolic extracts of Fructus bruceae, Radix angelicae pubescentis, Caulis spatholobi, and Semen pharbitidis with EC50 49.96, 57.45, 64.92, and 309.47 mg/L. The methanolic and aqueous extracts of S. aesculi exhibited potential results and can be exploited as a preferred natural antiparasitic for the control of D. intermedius.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
During the last two decades, ornamental fish culture has emerged as a powerful income and employment generating industry. Worldwide, ornamental fish exports are worth US $278 million annually, and the wholesale trade is worth around US $1 billion per year (FAO 2005). Among freshwater fish, Carassius auratus are the most favored for aquaculture; however, losses due to parasite infections have become a major hurdle for further development of C. auratus culture, one parasitic problem recently increased in incidence and severity is the infestation with Dactylogyrus intermedius (Buchmann et al. 1995; Tóro et al. 2003).
This parasite has a direct life cycle without intermediate host. The life cycle of D. intermedius comprises an obligate adult stage, fertilized egg, and free-swimming larvae stage. The fertilized eggs develop into free-swimming ciliated larvae in the water column; the ciliated larvae are then carried to hosts by water currents as well as by their own ciliated movement (Klinger and Floyd 2002). Larvae live on the gill epithelia of fish and feed on mucus, epithelial cells, and blood from the gills. Under the appropriate temperature, the larvae shed their cilia, develop into adult, and lay eggs. The time required for maturation of D. intermedius from eggs to adult is temperature dependent. At water temperatures of 22–24°C, only a few days are required for completion of the life cycle, whereas at water temperatures of 1–3°C, generation time is extended to 5 or 6 months (Reed et al. 2009).
The parasite can cause serious problems to infected fish, such as inflamed gills, excessive mucous secretions, and accelerated respiration (Reed et al. 2009). Moreover, mixed infections with other parasites and secondary bacterial infections are common (Woo et al. 2002), resulting in a serious damage to the host such as lowered growth performance, loss of appetite, and high mortalities.
The most effective treatment of D. intermedius has been achieved by use of formalin when administrated as a prolonged bath at low dose or short-term bath at higher dose (Thoney and Hargis 1991). However, the use of formalin for the treatment of disease in dulfish has been discouraged due to its toxicity in small-scale trials, with the majority of fish dying following a 250-ppm formalin bath of 1 h duration (Diggles et al. 1993). Other chemicals, including praziquantel (Schmahl and Mehlhorn 1985), mebendazole (Treves-Brown 1999), quinaldine (Crigel et al. 1995), and toltrazuril (Schmahl et al. 1988) have been evaluated for chemotherapy of Dactylogyrus. However, the threats of anthelmintic resistance, risk of residue, environmental contamination, and toxicity to host caused by the frequently use of these drugs have led to the need of other alternative control methods (Goven et al. 1980; Klinger and Floyd 2002).
Nowadays, there have been increased research activities into the utilization of traditional plant-based medicines to control bacterial and parasitic infections in human and animal medicine (Willcox and Bodeker 2000; Iqbal et al. 2006; Eguale et al. 2007; Rahuman et al. 2008; Batabyal et al. 2009; Pavela 2009; Maciel et al. 2010), but little information is available on the use of medical plant for the treatment of parasitic diseases in fish. An attempt has therefore been made under the present work to exploit the crude extracts of Radix angelicae pubescentis (roots of Angelica pubescens), Fructus bruceae (fruits of Brucea javanica), Caulis spatholobi (stems of Spatholobus suberectus), Semen aesculi (seeds of Aesculus chinensis Bge.), and Semen pharbitidis (seeds of Pharbitis purpurea (L.) Voigt) for their anthelmintic activity against D. intermedius (Monogenea) in goldfish.
Materials and methods
Animals
One-year-old goldfish (n = 50, 4.0 ± 0.9 g), naturally infected with adult D. intermedius, were obtained from Shaan’xi Fisheries Research Institute in China and maintained in a 180-L glass aquarium at 24 ± 1°C (controlled by automatic aquarium heater) with aeration for 3 days. On the third day, ten goldfish were randomly selected, killed by spinal severance, and placed in a glass culture vase. Every lamella branchialis was separated and put into 6-wells cell culture cluster (Corning Incorporated, New York, NY, USA) containing 1.0 ml RPMI-1640 medium (Sigma–Aldrich) at 24°C for 10 h. After incubation, the D. intermedius eggs (n = 300) were collected using capillary glass tube under the stereomicroscope and subsequently placed into a 1-L glass tank containing filtered groundwater at 24 ± 1°C. After 3 days of incubation, when oncomiracidia had been hatched from eggs, ten parasite-free goldfish were then added to the tank to perform the experimental infestation. Ten days later, the larvae developed to mature adults; the ten goldfish were co-habitated with another 50 parasite-free goldfish in 180-L glass aquarium to get infected ones. The co-habitation was performed for 7–10 days, and the ratio of the infected goldfish to the parasite-free ones was 1:5. During the experiment, 2,000 were infected and obtained following the method described above. Ten goldfish were then randomly selected, killed, and checked for the presence of parasite under a light microscope (Olympus BX41, Tokyo, Japan) at 4 × 10 magnification. Fish were chosen for the tests when the mean number of the parasite on gills of each fish was 40–50.
Collection of plant materials and preparation of extracts
Fresh plant material from each of the selected species (see Table 1) was collected in 2009. These were identified by Prof. X.L. He (Northwest A&F University, Shaanxi, China), and voucher specimens have been deposited in the College of Life Science, Northwest A&F University, China. The plants were washed thoroughly, air-dried under the sunlight for a week, and finally oven-dried at 45°C for 48 h. The dried plant materials were crushed manually with a mortar and pestle and reduced to fine powder using a strainer (30–40 mesh). The powdered samples were freeze-dried at −54°C to ensure complete removal of water. Five powdered dry samples of each plant material (20.0 g) were, respectively, extracted with petroleum ether, chloroform, ethyl acetate, methanol, and water for 48 h for complete extraction, and the process was repeated three times. The ratio of sample to solvent was 1:10 (m/v). Each extract was subsequently filtered and concentrated under reduced pressure in a vacuum rotary evaporator (R-201, Shanghai Shenshen) until the solvents were completely evaporated to get more or less solidified crude extracts. The crude petroleum ether, chloroform, ethyl acetate, methanol, and aqueous extracts of R. angelicae pubescentis, F. bruceae, C. spatholobi, and A. chinensis were dissolved in 40 mL of dimethyl sulfoxide (DMSO) to get 0.5 g/mL (sample/solvent) of stocking solutions which were used for the preparations of the desired concentrations for anthelmintic efficacy assay.
In vivo anthelmintic efficacy assay
Tests were conducted in each glass tank of 5-L capacity, filled with 2 L aerated groundwater, each containing the test samples and five previously infected fish. The water pH ranged from 7.0 to 7.5, dissolved oxygen between 6.2 and 7.8 mg/L (72–85% saturation), and the water temperature was constant at 24 ± 1°C. Initial tests were conducted to get a moderate concentration range in order to avoid the mortality of fish at high concentrations. The five crude extracts of the plants were conducted at a different series of concentrations based on the initial tests, respectively, and the negative control groups containing no plant extract were set up under the same conditions as the test groups. To discard the possible effects of DMSO on the parasites, another control containing the corresponding percentage of DMSO was also included. Mebendazole was used as a positive control with a different series of concentrations of 0.6, 1.0, 1.5, 2.0, and 2.5 mg/L. All the experiments were performed in duplicate. After 48 h, all the surviving goldfish in all the treatment and control groups were killed by a spinal severance for biopsy. The lamella branchialis were placed on glass slides, and the numbers of parasites on the gills were counted under a light microscope at 4 × 10 magnification to determine the mean number of parasites per infected goldfish. The anthelmintic efficacy of each treatment and the positive control group was calculated according to the following formula (Wang et al. 2008):
Where
- E :
-
Anthelmintic efficacy
- B :
-
Average number of surviving D. intermedius in the negative control
- T :
-
Average number of surviving D. intermedius in the treatment groups
- P :
-
Average number of surviving D. intermedius in the positive control
Statistical analysis
The homogeneity of the replicates of the samples was checked by the Mann–Whitney U test. Probit analysis was used for calculating the EC50 and EC90 at the 95% confidence interval with upper confidence limit and lower confidence limit (Finney 1971).
Results
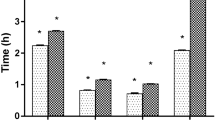
The anthelmintic efficacies and the EC50 values of different extracts of R. angelicae pubescentis, F. bruceae, C. spatholobi, S. aesculi, and S. pharbitidis are depicted in Table 2 and Figs. 1 and 2, which indicated that the crude methanolic extracts of S. aesculi was found to be the most effective with EC50 value of 5.23 mg/L and EC90 value of 7.33 mg/L after 48 h of exposure. High anthelmintic activity against D. intermedius was also observed in the aqueous extract with EC50 and EC90 values of 6.48 and 12.29 mg/L. The methanolic and aqueous extracts exhibited a 100% efficacy against D. intermedius at 10.00 and 12.00 mg/L, respectively. Followed by the chloroform and ethyl acetate extracts and the maximum anthelmintic efficacies were 88.10% (240.00 mg/L) and 75.10% (460.00 mg/L), respectively. The petroleum ether extract exhibited the least activity with the maximum anthelmintic efficacy of 49.30% at 160.00 mg/L.
Anthelmintic efficacy of different extracts of Radix angelicae pubescentis, Fructus bruceae, Caulis spatholobi, Semen aesculi, and Semen pharbitidis against Dactylogyrus intermedius after 48 h. PEE petroleum ether extract, CLE chloroform extract, EAE ethyl acetate extract, MEE methanol extract, WAE water extract
In the case of R. angelicae pubescentis and S. pharbitidis, the methanolic extracts were observed to be the most effective with EC50 and EC90 values of 49.96 and 75.93 mg/L and 309.47 and 585.29 mg/L after 48 h of post-treatment, respectively. The methanolic extracts exhibited a 100% anthelmintic efficacy against D. intermedius at 70.00 and 700.00 mg/L. The ethyl acetate R. angelicae pubescentis extract and the chloroform S. pharbitidis extract showed little activity with anthelmintic efficacies of 63.5% (500.00 mg/L) and 59.70% (800.00 mg/L). EC50 values for the ethyl acetate R. angelicae pubescentis extract and the chloroform S. pharbitidis extract were 278.55 and 602.84 mg/L after 48 h of exposure, accordingly. The petroleum ether extracts of R. angelicae pubescentis and S. pharbitidis exhibited the least activity with the maximum anthelmintic efficacy of 24.10% (140.00 mg/L) and 46.5% (1100.00 mg/L), respectively.
In the case of F. bruceae and C. spatholobi, the methanolic extracts displayed the optimal anthelmintic activity with 100% efficacy at the dose of 90.00 and 100.00 mg/L after 48 h, respectively. EC50 and EC90 values for the methanolic extracts of F. bruceae and C. spatholobi were 49.96 and 75.93 mg/L and 64.62 and 83.15 mg/L, respectively. The efficacy was followed by aqueous extract with EC50 and EC90 values at 173.03 and 274.76 mg/L and 96.09 and 145.01 mg/L for F. bruceae and C. spatholobi after 48 h of exposure, respectively. The maximum anthelmintic efficacies of the aqueous extracts of F. bruceae and C. spatholobi were 90.30% (350.00 mg/L) and 100% (250.00 mg/L), respectively.
The solvent (DMSO) acted as a control showed no anthelmintic activity when treated at the highest concentration, and the calculated EC50 and EC90 values of the positive control, mebendazole, were 1.25 and 3.68 mg/L, respectively.
Discussion
The disease caused by Dactylogyrus infestation seems to be a predominant problem in goldfish culture (Ogawa 2002). Formalin was the initially recommended treatment, but the effective levels were unpredictable, and it is now considered to be dangerous due to its side effects on hosts (Kennedy 2007). Schmahl et al. (1988) demonstrated that the antiprotozoal chemical, toltrazuril, was to be effective against Dactylogyrus, when treated using a water bath with 10 mg/L for 4 h at 20°C. A similar efficacy of quinaldine against monogenean was also reported (Crigel et al. 1995). In China, praziquantel and mebendazole are commonly used to control Dactylogyrus infection. However, because of their side effects, such as accumulation of drugs in tissues, development of drug resistance, and the potential deleterious effects on both the environment and the human consumers (Committee on Mutagenicity of Chemicals in Food, Consumer Products and the Environment 1999), it is therefore not recommended for use against Dactylogyrus. Screening and proper evaluation of medicinal plants could offer possible alternatives that may be both sustainable and environmentally acceptable. For this reason, it is necessary to test those plant extracts which could be expected to contain substances of environmental potential, and at the same time, provide adequate efficacy against Dactylogyrus. Earlier authors reported that the methanolic extracts of the seeds of Piper guineense (Piperaceae) were active against skin and gill monogenean parasites of goldfish under in vivo and in vitro conditions (Ekanem et al. 2004). In our previous work, chloroform extract of Fructus arctii (dried fruits of Arctium lappa L.) exhibited a 100% anthelmintic efficacy against D. intermedius at the lowest concentration of 240.0 mg/L after 48 h of exposure (Wang et al. 2009).
This study demonstrated the anthelmintic activity of some medical plant plants against D. intermedius using the in vivo anthelmintic efficacy assay. As far as we know, it is the first study evaluating the crude extracts of R. angelicae pubescentis, F. bruceae, C. spatholobi, S. aesculi, and S. pharbitidis against any monogenea. In all the extracts screened, there was positive correlation between the concentration of the extract and percent anthelmintic activity. The methanolic and aqueous extracts of S. aesculi, the methanolic extracts of F. bruceae and R. angelicae pubescentis, and the methanolic and aqueous extracts of S. pharbitidis presented, in this order, the best percentages of anthelmintic activity among the tested products (Fig. 2).
The Chinese horse chestnut tree (A. chinensis Bge., family Hippocastanaceae) is a medicinal plant widely distributed in northwestern China. S. aesculi dried ripe seeds have been used as a carminative, stomachic, and analgesic for the treatment of distention and pain in chest and abdomen (Qian 1996). They were also used as an herbal remedy for the treatment of mammary indurations and cancer (Harrwell 1982). Biologically active compounds of S. aesculi include triterpenoid saponins, flavonoids (Wei et al. 2004), coumarins, organic acids, sugars, and phytosterol (Wei et al. 2003). Aescin, the major active principle from S. aesculi, a natural mixture of triterpene saponins consisting of analogous pentacyclic triterpenoid oligoglycosides, has shown distinguished anti-inflammatory, anti-edematous, venotonic, anti-edema, capillary protective hypoglycemic, ethanol absorption inhibitory activities, and inhibitory activity against HIV-1 protease (Sirtori 2001; Bombardelli and Morazzoni 1996; Matsuda et al. 1997; Yoshikawa et al. 1996; Yang et al. 1999). The acid hydrolyzed product of an n-BuOH extract of S. aesculi was found to show significant in vitro cytotoxicity in the 9-KB (human nasopharyngeal carcinoma) cell culture assay (Geran et al. 1972). Further research (Konoshima and Lee 1986) demonstrated that two cytotoxic sapogenols, the new hippocaesculin and the known barringtogenol-C-21-angelate, were the active components with ED50 values of 3.6 and 3.0 μg/mL, respectively. Considering the major bioactive constituents of S. aesculi, it is supposed that the biological activity of S. aesculi extracts might be due to the aescins; these compounds may contribute jointly or independently to producing larval and adult emergence inhibition activity against D. intermedius.
Comparing the efficacies of the extracts from F. bruceae, C. spatholobi, and S. aesculi, it was observed that the crude aqueous extract showed better anthelmintic activity than the methanolic extract. The possible explanation for the better activity of the methanolic extract compared to the aqueous extract on D. intermedius in the current study could be due to easier transcuticular absorption of the methanolic extracts into the body of the parasite than the aqueous extracts. Although distinct chemical profiles of the two extracts of F. bruceae, C. spatholobi, and S. aesculi are not known, in general, methanolic extracts of plants contain some nonpolar organic chemicals with lower polarity than the aqueous extracts (Debella 2002), rendering them more lipid soluble than the aqueous extracts and hence better anthelmintic activity. Lipophilic anthelmintics have a greater capability to cross the external surface of the helminths than the hydrophilic compounds (Geary et al. 1999).
In conclusion, extracts from R. angelicae pubescentis, F. bruceae, C. spatholobi, Semen aesculi, and S. pharbitidis showed some in vivo anthelmintic activities against D. intermedius at concentrations and dose levels tested, but the observed efficacy is not to the therapeutically required level. Further phytochemical studies, toward the isolation and characterization of the active compounds, are recommended, if possible, in preparing a commercial product/formulation for use as antiparasitics.
References
Batabyal L, Sharma P, Mohan L, Maurya P, Srivastava CN (2009) Relative toxicity of neem fruit, bitter gourd, and castor seed extracts against the larvae of filaria vector, Culex quinquefasciatus (Say). Parasitol Res 105:1205–1210
Bombardelli E, Morazzoni P (1996) Aesculus hippocastanum L. Fitoterapia 67:483–511
Buchmann K, Slotved HC, Dana D (1995) Gill parasites from Cyprinus carpio in Indonesia. Aquaculture 129:437–439
Crigel P, Losson B, Defour J (1995) The antiparasitic efficacy of quinaldine, an anesthetic agent in fish. Ann Méd Vét 139:343–348
Debella A (2002) Manual for phytochemical screening of medicinal plants. Ethiopian health and Nutrition Research Institute, Addis Ababa
Diggles BK, Roubal FR, Lester RJG (1993) The influence of formalin, benzocaine and hyposalinity on the fecundity and viability of Polylabroides multispinosus (Monogenea: Microcotylidae) parasitic on the gills of Acanthopagrus australis (Pisces: Sparidiae). Int J Parasitol 23:877–884
Eguale T, Tilahun G, Debella A, Feleke A, Makonnen E (2007) In vitro and in vivo anthelmintic activity of crude extracts of Coriandrum sativum against Haemonchus contortus. J Ethnopharmacol 110:428–433
Ekanem AP, Wang M, Simon JE, Obiekezie1 AI, Morah F (2004) In vivo and in vitro activities of the seed extract of Piper guineense schum. and thonn. against skin and gill monogenean parasites of goldfish (Carassius auratus auratus). Phytother Res 18:793–797
FAO (2005) Food and Agriculture Organization. United Nations Fishery statistics. June 2005 report. http://faostat.fao.org/
Finney DJ (1971) Probit analysis, 3rd edn. Cambridge University Press, Cambridge
Geary TG, Sangster NC, Thompson DP (1999) Frontiers in anthelmintic pharmacology. Vet Parasitol 84:275–295
Geran RI, Greenberg NH, MacDonald MM, Shumacher AM, Abbott BJ (1972) Protocols for screening chemical agents and natural products against animal tumors and other biological systems. Cancer Chemother Rep 3:1–4
Goven B, Gilbert J, Gratzek J (1980) Apparent drug resistance to the organophosphate dimethyl (2, 2, 2-trichloro-1-hydroxyethyl) phosphonate by monogenetic trematodes. J Wildlife Dis 16(3):343–346
Harrwell JL (1982) Plants used against cancer. Quarterman Publications, Inc., Lawrence, p 250
Committee on Mutagenicity of Chemicals in Food, Consumer Products and the Environment (1999) Statement for COT: malachite green and leucomalachite green. Department of Health, London. http://archive.food.gov.uk/dept_health/pdf/mala.pdf
Iqbal Z, Lateef M, Jabbar A, Ghayur MN, Gilani AH (2006) In vitro and in vivo anthelmintic activity of Nicotiana tabacum L. leaves against gastrointestinal nematodes of sheep. Phytother Res 20:46–48
Kennedy CR (2007) The pathogenic helminth parasites of eels. J Fish Dis 30:319–334
Klinger R, Floyd RF (2002) Introduction to freshwater fish parasites. Document CIR716. Institute of Food and Agricultural Science, University of Florida, Florida
Konoshima T, Lee KH (1986) Antitumor agents, 82. cytotoxic sapogenols from Aesculus hippocastanum. J Nat Prod 49(4):650–656
Maciel MV, Morais SM, Bevilaqua SML, Silva RA, Barros RS, Sousa RN, Sousa LC, Brito ES, Souza-Neto MA (2010) Chemical composition of Eucalyptus spp. essential oils and their insecticidal effects on Lutzomyia longipalpisb. Vet Parasitol 167:1–7
Matsuda H, Li Y, Murakami T, Ninomiya K, Yoshikawa M (1997) Effects of escins Ia, Ib, IIa, and IIb from horse chestnut, the seeds of Aesculus hippocastanum L., on acute inflammation in animals. Biol Pharm Bull 20:1092–1095
Ogawa K (2002) Impacts of diclidophorid monogenean infections on fisheries in Japan. Int J Parasitol 32(3):373–380
Pavela M (2009) Larvicidal effects of some Euro-Asiatic plants against Culex quinquefasciatus Say larvae (Diptera: Culicidae). Parasitol Res 105:887–892
Qian XZ (1996) Colored illustrations of Chinese herbs. Part II. People’s Health Press, Beijing, p 231
Rahuman AA, Gopalakrishnan G, Venkatesan P, Geetha K (2008) Larvicidal activity of some Euphorbiaceae plant extracts against Aedes aegypti and Culex quinquefasciatus (Diptera: Culicidae). Parasitol Res 102:867–873
Reed PA, Francis-Floyd R, Klinger RC (2009) FA28/FA033: Monogenean parasites of fish. EDIS—Electronic Data Information Source—UF/IFAS Extension. University of Florida. http://edis.ifas.ufl.edu/FA033. 17 May 2009
Schmahl G, Mehlhorn H (1985) Treatment of fish parasites. 1. Praziquantel effective against Monogenea (Dactylogyrus vastator, Dactylogyrus extensus, Diplozoon paradoxum). Z Parasitenk 71:727–737
Schmahl G, Mehlhorn H, Haberkorn A (1988) Sym. Triazinone (toltrazuril) effective against fish-parasitizing Monogenea. Parasitol Res 75(1):67–68
Sirtori CR (2001) Aescin: pharmacology, pharmacokinetics and therapeutic profile. Pharmacol Res 44:183
Thoney DA, Hargis WJ (1991) Monogenea (Platyhelminthes) as hazards for fish in confinement. Annu Rev Fish Dis 1:133–153
Tóro RM, Gessner AAF, Furtado NAJC, Ceccarelli PS, Albuquerque SD, Bastos JK (2003) Activity of the Pinus elliottii resin compounds against Lernaea cyprinacea in vitro. Vet Parasitol 118:143–149
Treves-Brown KM (1999) Availability of medicines for fish. Fish Vet J 4:40–55
Wang GX, Zhou Z, Cheng C, Yao JY, Yang ZW (2008) Osthol and isopimpinellin from Fructus cnidii for the control of Dactylogyrus intermedius in Carassius auratus. Vet Parasitol 158:144–151
Wang GX, Han J, Feng TT, Li FY, Zhu B (2009) Bioassay-guided isolation and identification of active compounds from Fructus Arctii against Dactylogyrus intermedius (Monogenea) in goldfish (Carassius auratus). Parasitol Res 106:247–255
Wei Q, Ma XH, Yang XP, Zhang Q (2003) Advances in chemical composition of Aescali. J Northwest Forest Univ 18(4):126–112, in Chinese with English abstract
Wei F, Ma SC, Ma LY, But PPH, Lin RC, Khan IA (2004) Antiviral flavonoids from the seeds of Aesculus chinensis. J Nat Prod 67(4):650–653
Willcox ML, Bodeker G (2000) Plant-based malaria control: research initiative on traditional antimalarial methods. Parasitol Today 16:220–221
Woo PTK, David W, Bruno LH, Susan L (2002) Diseases and disorders of finfish in cage culture. CABI, Malaysia
Yang XW, Zhao J, Cui YX, Liu XH, Ma CM, Hattori M, Zhang LH (1999) Anti-HIV-1 protease triterpenoid saponins from the seeds of Aesculus chinensis. J Nat Prod 62:1510–1513
Yoshikawa M, Mulakami T, Matsuda H, Yamahara J, Murakami N (1996) Bioactive saponins and glycosides. III. Horse chestnut. (1): The structures, inhibitory effects on ethanol absorption, and hypoglycemic activity of escins Ia, Ib, IIa, IIb, and IIIa from the seeds of Aesculus hippocastanum L. Chem Pharm Bull 44:1454–1464
Acknowledgments
This research was supported by a World Bank Loan Project of Shaanxi province and key project program of Xi’an City (GG06114).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Liu, YT., Wang, F., Wang, GX. et al. In vivo anthelmintic activity of crude extracts of Radix angelicae pubescentis, Fructus bruceae, Caulis spatholobi, Semen aesculi, and Semen pharbitidis against Dactylogyrus intermedius (Monogenea) in goldfish (Carassius auratus). Parasitol Res 106, 1233–1239 (2010). https://doi.org/10.1007/s00436-010-1799-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00436-010-1799-9