Abstract
Purpose
The initial management of atypical meningiomas poses a distinct clinical challenge in that treatment protocols have not been fully established, and outcomes, especially differences by patient age, have not been broadly measured. The National Cancer Database (NCDB) allows for analysis of a large, diverse patient population to determine clinical parameters and survival outcomes based on the initial treatment of patients with atypical meningiomas.
Methods
Analysis of the NCDB yielded 3611 atypical meningioma patients treated between 2008 and 2012. Principal treatment paradigms included surgery with or without radiation. Survival estimates were calculated using Kaplan–Meier curves stratified by age at diagnosis for each treatment paradigm. Subset analysis was performed for socio-economic factors.
Results
Overall 5-year survival rate was 77.6% and declined with increasing patient age (p < 0.0001). Five-year survival for patients ≤ 45 years undergoing surgery alone was 89.3 vs. 44.4% for those > 75 years (p < 0.0001). For patients undergoing surgery with adjuvant radiation, 5-year survival was 93.7% in those ≤ 45 years and 54.1% in those > 75 years (p < 0.0001). Use of adjuvant radiation was stable over time. Private-insured patients were more likely to receive adjuvant radiation (p = 0.0001).
Conclusions
Patients treated for atypical meningioma have high rates of 5-year survival. A marginal survival benefit of adjuvant radiation was observed for patients < 55 and > 75 years, while patients between 55 and 75 years tended to have slightly improved survival with surgery alone. Though surgery remains the standard of care in the primary treatment of atypical meningioma, the decision to administer radiation post-operatively has remained controversial.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Meningiomas are the most common primary intracranial tumors in adults, accounting for more than one-third of all brain tumors (Ostrom et al. 2016). As behavior and clinical outcomes can vary considerably among these tumors, meningiomas are categorized as benign, atypical, or malignant, corresponding to grades I, II, and III, respectively, of the World Health Organization (WHO) meningioma grading classification (Combs et al. 2011; Klinger et al. 2015; Pollock 2014; Rogers et al. 2010; Zaher et al. 2013). Although the majority of meningiomas are benign, the atypical and malignant subtypes are more aggressive in their progression and have been associated with increased rates of recurrence, patient morbidity, and mortality (Combs et al. 2011; Kaur et al. 2014). Atypical meningiomas in particular pose a distinct therapeutic challenge, as there are currently no standardized treatment guidelines for these lesions (Rogers et al. 2010; Sun et al. 2015).
The WHO classification of meningiomas has undergone several revisions within the past decades (Combs et al. 2011; Kaur et al. 2014). The 2007 WHO grading scheme considers the presence of brain invasion as now part of the criteria for defining the atypical subgroup. Given the reclassification and more precise grading criteria, there has been a marked increase in the incidence of grade II atypical meningiomas in particular (Rogers et al. 2010; Kaur et al. 2014). The previous rarity of the atypical subtype defined by the previous WHO grading iterations led to many underpowered and often conflicting clinical studies regarding appropriate treatment paradigms to optimize patient outcomes (Combs et al. 2011; Klinger et al. 2015; Pollock 2014; Rogers et al. 2010; Andric et al. 2012; Cain et al. 2015; Jo et al. 2010; Palma et al. 1997). As such, the current study was restricted to patients diagnosed after the 2007 WHO classification change to represent outcomes of modern atypical meningioma cohorts. More recently, the WHO grading classification was revised in 2016 to denote brain invasion as a sole criterion that can diagnose an atypical meningioma (Louis et al. 2016).
While gross total resection of meningiomas, when feasible, remains the initial atypical and malignant meningioma treatment, the procedure may incur high rates of post-operative patient morbidity (Klinger et al. 2015; Cain et al. 2015; Goyal et al. 2000). Otherwise, if a subtotal resection is achieved, post-operative adjuvant measures including radiation therapy (RT) or re-operation may be beneficial to prevent further growth and risk of recurrence (Klinger et al. 2015; Sun et al. 2015; Cain et al. 2015; Jo et al. 2010; Wang et al. 2016). The precise role of incorporating radiation therapy (RT) in the primary management of high-grade meningioma after resection, however, remains elusive; many studies support the use of early post-surgical adjuvant RT, while other sources suggest that the risks of adjuvant treatment outweigh any potential benefit (Klinger et al. 2015; Rogers et al. 2010; Kaur et al. 2014; Sun et al. 2015; Cain et al. 2015; Jo et al. 2010; Goyal et al. 2000; Wang et al. 2016; Aghi et al. 2009; Detti et al. 2013; Hammouche et al. 2014; Hasan et al. 2015; Pasquier et al. 2008). A recent analysis of adjuvant RT for atypical meningiomas found a small survival benefit of RT after surgery only for those patients with a subtotal, but not a gross total, resection (Wang et al. 2017). Further, the age of the patient can additionally complicate the decision for adjuvant RT, due to increased prevalence of co-morbid conditions or poor functional status that may prohibit RT use (Orton et al. 2017; Farina et al. 2014). Earlier WHO criteria for diagnosing atypical and malignant meningioma subtypes have limited the number of adequately powered studies available for studying these tumors; as a result, there is a relative paucity of data on the natural history, treatment and prognosis of these meningioma subtypes.
The National Cancer Database (NCDB), a large, aggregated database consisting of approximately 70% of all newly diagnosed hospital-based cancers in the United States annually, captures not only the primary treatment management of atypical meningiomas, but also clinical outcomes and long-term patient survival (American College of Surgeons 2017). Thus, the current study utilized the NCDB, lauded as the largest clinical cancer registry in the world (Boffa et al. 2017), to elucidate the initial treatment paradigms for atypical meningioma. In particular, we assessed the effect of different primary treatment methods (surgery with or without RT) on overall survival of patients diagnosed with atypical meningiomas by age strata. This study secondarily examined if patient features, such as socio-economic factors, affected the choice of treatment.
It is important to note that, as the NCDB is not maintained to be neurosurgery- or neuro-oncology specific, the database does not include measures of tumor recurrence, grade of surgical resection, or rates of re-operation (American College of Surgeons 2017). However, as definitive treatment protocols for atypical meningioma are still controversial, we sought to characterize trends across institutions in the initial management of atypical meningioma by patient age group and evaluate its effect on overall survival and outcomes.
Methods
This study provides an analysis of publicly available de-identified data and, therefore, met criteria for exclusion of review by the institutional review board. We retrospectively analyzed the clinical parameters and treatment outcomes of patients diagnosed with atypical meningiomas and entered into the NCDB. The NCDB, established in 1989, is a nationwide, hospital-based, comprehensive clinical surveillance registry that captures approximately 70% of all newly diagnosed malignancies in the United States annually. This database is a joint project of the American Cancer Society and the Commission of Cancer of the American College of Surgeons, where the latter has implemented a data use agreement with each of its Commission on Cancer accredited hospitals, currently measuring approximately 1500 in number (American College of Surgeons 2017). Participating hospitals are subjected to systematic audits to verify accuracy and data completeness (Boffa et al. 2017). After application approval by the NCDB, HIPAA-compliant de-identified patient data were downloaded in September 2017 and analyzed in this study.
Patient selection
Patients were identified from newly diagnosed meningioma cases between 2008 and 2012 using International Classification of Diseases for Oncology (ICDO) codes 9530 to 9539, yielding a total of 84,077 cases. This cohort was further filtered to yield only atypical meningioma cases by using ICDO codes 9538/1 and 9539/1, which yielded 3611 total cases. Those records that contained invalid codes for gender, date of diagnosis, vital status, date of last contact, or date of birth were excluded from analysis.
Included study variables
Recorded information available from the registry included demographic characteristics, primary treatment data, and clinical outcomes. Demographics included: age at diagnosis, sex, race, insurance status, hospital type (academic or non-academic), Charlson–Deyo score (a weighted validated index of co-morbid conditions) (Deyo et al. 1992), date of diagnosis, primary site, laterality, and tumor grade. Treatment details included days from diagnosis to first treatment, surgical procedure at primary site, RT and modality, days from diagnosis to RT, and other treatment protocols. Clinical outcomes included mortality at 30 and 90 days, date of last contact, and vital status.
Statistical analysis
To evaluate the effects of differential treatment paradigms on survival estimate rates, cases were separated into three principal therapeutic combinations: (1) surgery only; (2) surgery and RT; (3) RT only. Five groupings were selected to stratify age at diagnosis: ≤ 45 years, 46–55 years, 56–65 years, 66–75 years, and > 75 years. Overall survival was defined as the difference in time between the date of diagnosis to the date of last contact, incorporating the binary value for patient’s reported vital status to determining appropriate censoring (Jagsi et al. 2014). Chi square estimates were used for categorical data testing. The Kaplan–Meier product-limit method was used to determine survival estimates and comparisons between estimates were made using the log-rank test. Observed survival rates were estimated and were not adjusted for deaths due to other causes. Significance value below 0.05 was considered statistically significant. The data were analyzed with Statistical Analysis System (SAS) software, Version 9.4.
Results
Demographics
Analysis of the NCDB registry yielded a total of 3611 patients diagnosed with atypical meningioma between 2008 and 2012. This filtered subset represented 4.3% of the larger cohort of all patients diagnosed with meningioma within the same time period (n = 84,077). The patient demographics and tumor characteristics are summarized in Table 1. The mean age at time of diagnosis was 57.8 years, with a median length of follow-up of 34.5 months (range 1.02–83.91 months) after diagnosis.
The 56–65 year age grouping had the largest proportion of patients at 24.8% of the total cohort. Fifty-four percent of the cohort was female, and the vast majority of included patients identified as ‘white’ (78.9%). Most of this cohort (69.8%) had a Charlson–Deyo Score of 0, suggesting an absence of common, major co-morbidities such as cardiac or renal disease in the majority of patients. For those in which the information was available, there was a nearly even distribution in the type of insurance coverage between government (e.g., Medicare, Medicaid) and private (47.4 and 44.4%, respectively). A larger number of patients received their primary treatment at an academic hospital center (58.2%) as opposed to a community clinic. Though specific tumor location is not provided in the database, tumors did not differ in right- or left-sided laterality (see Table 1).
Treatment type and incidence by year of diagnosis
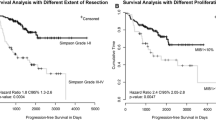
The majority of cases were treated with surgery alone (2580; 74.8%). No data was available for this cohort with respect to resection margins. Among the 3447 cases for which primary treatment data was available, 97% of cases underwent either surgical resection, RT, or a combination thereof (Table 2). A relatively stable distribution of treatment paradigms is indicated across the years included in this analysis (Fig. 1).
Survival rates
The 5-year overall survival rate for the entire cohort was 77.6% with 520 patients either deceased or censored due to loss of follow-up (Fig. 2). Overall 1- and 2-year survival was 94.5 and 91.0%, respectively. The upper limit of follow-up from date of diagnosis was approximately 84 months. At this time point (~ 7 years), the overall survival was estimated to be 63.0%. When stratified by age, the 5-year overall survival rate decreased with increasing age, ranging from 90.8% survival probability for the ≤ 45 age group to 44.3% survival probability for the > 75 age group (p < 0.0001; see Fig. 3).
Treatment paradigm by age
To determine whether treatment paradigm varied by age group, the data were stratified across these two variables. Patterns of treatment were grossly similar to values found for the entire sample, with surgery only remaining as the predominant therapeutic approach for all patients (Table 3). A significant shift was, however, observed, where surgery alone was the increasingly principal mode of therapy for patients of increasing age with a concomitant decrease in the use of adjuvant RT (p < 0.0001).
Five-year survival rates by age for each treatment type
Five-year survival rates were determined for each treatment modality by age stratum. The cohort undergoing RT alone was too small for statistically significant conclusions to be drawn (n = 25). For the remaining groups under analysis, a survival benefit was observed for younger patients: the ≤ 45 years age group had 5-year survival estimates of 89.3 and 93.7% after surgery done without and with adjuvant RT, respectively. In contrast, the > 75 years age group had a 5-year survival probability of 44.4% with surgery alone vs. 54.1% survival when RT was added. The within-group estimates for surgery with or without RT were significant (Figs. 4, 5; p < 0.0001).
Radiation treatment modalities
The most common modality for both RT alone and as adjuvant treatment was intensity-modulated radiation therapy (IMRT, 44.0% in both groups, see Table 4). Stereotactic radiosurgery was the next most common modality for patients receiving RT alone (28.0%), while the use of other external beam therapy was the second most common modality for use as adjuvant therapy (31.7%). Mean time from meningioma diagnosis to RT for patients receiving RT alone (66.4 days, SD = 64.0 days) did not differ from time to RT for patients receiving radiation as adjuvant treatment (93.4 days, SD = 75.6 days, p = 0.074). For the latter group, the average time from surgery to adjuvant RT was 76.7 days (SD = 63.0 days).
Socio-economic factors
Data on insurance status revealed that 81% of government insured patients underwent surgery alone, whereas 19% or these patients received both surgery and adjuvant RT. This compares to 75.5% of private-insured patients who underwent surgery alone vs. 24.5% with adjuvant RT (p = 0.0001). Age-related differences in insurance coverage were noted, with patients ≤ 60 years of age having 22.1% government insurance coverage vs. 77.9% private, whereas patients > 60 years of age had 74.2% government coverage vs. 25.8% private (p < 0.0001). While these findings were statistically significant, they may be confounded by Medicare eligibility at the age of 65.
Discussion
This study utilized a large, multi-center patient sample that contains collected data regarding patient demographics, tumor characteristics, primary treatment details, and follow-up clinical outcomes. The national trends in the initial atypical meningioma treatment outlined in this study further expand on the findings of prior clinical- and population-based studies performed in smaller cohorts, which, taken together, continue to address the complex issue of prognostic clinical factors and optimal treatment paradigms for atypical meningioma management (Klinger et al. 2015; Kaur et al. 2014; Sun et al. 2015; Andric et al. 2012; Cain et al. 2015; Jo et al. 2010; Goyal et al. 2000; Wang et al. 2016; Hasan et al. 2015; Pasquier et al. 2008).
From the standpoint of demographic analysis, atypical meningiomas are diagnosed at an earlier age (mean age of 57.8 years in the current study; see Table 1) than their benign counterparts (median age 66 years) (Ostrom et al. 2016; Klinger et al. 2015; Rogers et al. 2010), likely due to the more aggressive histopathologic nature of atypical meningiomas. In addition, while there is a significant female predominance for benign meningiomas, a gender disparity is not observed in atypical meningiomas (Ostrom et al. 2016; Combs et al. 2011; Klinger et al. 2015; Rogers et al. 2010). Of the 3611 cases, 54% of were female (Table 1); this value is in agreement with those of other studies such as a male:female ratio of about 9:10 reported (Klinger et al. 2015; Zaher et al. 2013; Andric et al. 2012; Aghi et al. 2009; Hammouche et al. 2014; Hasan et al. 2015). The racial composition of our cohort demonstrates white predominance, which is consistent with other studies that previously utilized the NCDB cancer registry (Table 1) (Wang et al. 2017; Killelea et al. 2015; McCarthy et al. 1998). However, a higher rate of meningioma diagnoses among black patients vs. white patients has been identified in population-based studies of primary brain tumor prevalence (Ostrom et al. 2016).
The overall 5-year survival estimate for the entire cohort was 77.6% (Fig. 2). Our analysis suggested that this rate steadily decreased with age, with the patients under 45 having more than double the survival rate of those older than 75 (90.8 and 44.3%, respectively; Fig. 2). This overall survival estimate is consistent with previously reported findings, suggesting accurate reporting of age and the validity of the outcome. The 1998 NCDB analysis by McCarthy et al., for example, reported an overall survival rate of 74.5% for atypical meningiomas, with the surgery only cohort exhibiting a survival advantage over the combined surgery and RT group (79.7 vs. 64.6%, respectively) (McCarthy et al. 1998). A more recent analysis of atypical meningiomas, however, found a survival advantage for of adjuvant RT for those with subtotal, but not gross total, resection though a 5-year survival rate was not reached (Wang et al. 2017). The inverse relationship of increasing age and survival rate is likely an expected finding attributable to the natural history of the disease and age-related morbidity secondary to treatment. Other clinical studies that have provided a meningioma 5-year survival rate, inclusive of all subtypes, range from 67.5 to 93.5% and many also identified increasing age as a significant prognostic factor for survival (Zaher et al. 2013; Kaur et al. 2014; Palma et al. 1997; Goyal et al. 2000; Wang et al. 2016; Hammouche et al. 2014; Garzon-Muvdi et al. 2017).
The relative proportion of the initial treatment modalities employed remained fairly stable across each included year with the majority of patients undergoing either surgery (75%) only or both surgery and RT (22%, see Fig. 1). A recent meta-analysis reported a similar treatment paradigm, with 72.5% of patients undergoing surgery alone, while the remaining 27.5% were treated with both surgery and adjuvant RT (Hasan et al. 2015). When stratified by age, our findings for the relative proportions of initial treatment types were supported for all except the oldest patient group (> 75 years) in which patients had the highest rate of surgery only (Table 3). Lower use of adjuvant RT in older patients was also similarly reported for anaplastic meningiomas in a recent NCDB analysis (Orton et al. 2017). The risks of pursuing a more aggressive treatment regimen in this population may have been thought to outweigh any potential benefits.
The survival trend by age observed in this study mostly persisted across treatment stratification in surgically treated patients (Figs. 4, 5). While adjuvant RT was associated with a small increase in 5-year survival for the under 45 and 46–55 years age groups, this pattern was not found for the 55–65 and 66–75 year age groups. However, a survival benefit was observed for adjuvant RT vs. surgery alone in the oldest (over 75 years) age group (54.1 vs. 44.4%, respectively). This may, however, reflect treatment bias in the elderly population in which more aggressive treatments were recommended only in older patients when prognosis is more favorable given risks of potential secondary morbidity that may be involved (Minniti et al. 2017). The group treated with primary RT only was not large enough appreciate clear age-related effects for this treatment modality.
Two conclusions may be drawn from the comparison of survival curves by primary treatment paradigm. First, the relative rarity of a RT-only paradigm reaffirms that surgery remains the standard of initial care for atypical meningiomas; indeed, over 95% of patients in this cohort underwent surgical resection, regardless of age (Table 3). Second, this data highlights one possible source of the continued controversy around the use of adjuvant RT for patients undergoing surgical resection: a modest survival benefit is suggested in patients under the age of 55; however, this tendency towards improved survival was not observed in the remaining age strata until patients were greater than 75 years.
Equivocal outcomes with adjuvant RT are also observed in recent reviews. A meta-analysis by Hasan et al. focused exclusively on the possible benefits of adjuvant RT following complete resection of atypical meningiomas (Hasan et al. 2015). A statistically significant benefit favoring adjuvant RT was appreciated in crude recurrence rates; however, no definite benefit was found in terms of local control or 5-year survival (Hasan et al. 2015). For partial resection, Wang et al. recently reported a small benefit of adjuvant RT in atypical meningioma in overall survival, but not for patients with a complete resection (Wang et al. 2017). Similarly, in a systematic literature review, Kaur et al. evaluated the role of adjuvant RT for atypical and malignant meningiomas, though the review did not evaluate patients by age. Besides a possible benefit for local tumor control, the authors were unable to demonstrate any significant clinical effect of adjuvant RT in atypical meningioma outcomes (Kaur et al. 2014). Thus, although evidence in the past studies and currently suggest potential resection grade-dependent and age-dependent effects of adjuvant RT on survival rates, well-defined benefits remain unclear in this patient population and should continue to be investigated in further studies.
The most common modality for adjuvant RT and for RT alone was found to be IMRT for almost half of the patients in this cohort. However, external beam therapy and stereotactic radiosurgery were still considerably utilized (see Table 4). While this reflects the increasing use of advanced IMRT in the past decade for atypical meningioma, availability of modalities are still largely hospital-specific (Moraes and Chung 2017). In addition, the amount of time from diagnosis to either sole RT or adjuvant RT post-resection was highly variable and did not differ between these groups, likely due to disease progression on an individual patient basis and the heterogeneity of treatment modalities.
The socio-economic analyses revealed that adjuvant RT was more available for patients with private insurance compared to government-based insurance. This finding is consistent with previous work noting lower availability of RT for lower socio-economic status and publicly insured patients (Curry and Barker 2009; McClelland et al. 2017). In particular, those greater than 60 years had a much greater proportion of government-based insurance, which was expected due to Medicare eligibility beginning at age 65.
Despite the advantages of using a large volume hospital-based registry to describe nationwide patterns in primary clinical management of atypical meningioma in relation with clinical outcomes, the study is constrained by specific limitations. First, the retrospective nature of the study prohibits clarification regarding sequence of data collection or completeness thereof. Next, despite the standardized data collection methods and quality assurance checks, there is non-uniform reporting of data which complicates controlling for biases. Finally, as previously indicated, the absence of further details regarding variables in the NCDB such as extent of surgical resection, rates of re-operation, or tumor recurrences does offer an opportunity for further characterization of the observed clinical outcomes.
Conclusion
This hospital-based analysis of patients with WHO grade II atypical meningioma elucidates nationwide primary therapeutic patterns and clinical outcomes by patient age group. While initial surgical resection was performed in the large majority of cases, use of adjuvant RT was more prevalent for younger patients. Further, the value of initial surgical resection on overall survival is supported by the multi-center data, but the role and impact of post-operative RT on clinical outcomes and survival is yet to be resolved, especially for older patients. As such, the appearance of an age-dependent effect on optimal use of adjuvant RT should be further investigated in prospective studies.
Abbreviations
- NCDB:
-
National Cancer Database
- WHO:
-
World Health Organization
- RT:
-
Radiation therapy
- ICDO:
-
International Classification of Diseases for Oncology
- IMRT:
-
Intensity-modulated radiation therapy
References
Aghi MK, Carter BS, Cosgrove GR et al (2009) Long-term recurrence rates of atypical meningiomas after gross total resection with or without postoperative adjuvant radiation. Neurosurgery 64(1):56–60
American College of Surgeons (2017) About the National Cancer Database. http://www.facs.org/quality-programs/cancer/ncdb/about. Accessed 16 Jun 2017
Andric M, Dixit S, Dubey A, Jessup P, Hunn A (2012) Atypical meningiomas—a case series. Clin Neurol Neurosurg 114(6):699–702
Boffa DJ, Rosen JE, Mallin K et al (2017) Using the National Cancer Database for outcomes research: a review. JAMA Oncol 3(12):1722–1728
Cain SA, Smoll NR, Van Heerden J, Tsui A, Drummond KJ (2015) Atypical and malignant meningiomas: considerations for treatment and efficacy of radiotherapy. J Clin Neurosci 22(11):1742–1748
Combs SE, Schulz-Ertner D, Debus J, von Deimling A, Hartmann C (2011) Improved correlation of the neuropathologic classification according to adapted world health organization classification and outcome after radiotherapy in patients with atypical and anaplastic meningiomas. Int J Radiat Oncol Biol Phys 81(5):1415–1421
Curry WT Jr, Barker FG 2nd (2009) Racial, ethnic and socioeconomic disparities in the treatment of brain tumors. J Neurooncol 93(1):25–39
Detti B, Scoccianti S, Di Cataldo V et al (2013) Atypical and malignant meningioma: outcome and prognostic factors in 68 irradiated patients. J Neurooncol 115(3):421–427
Deyo RA, Cherkin DC, Ciol MA (1992) Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol 45(6):613–619
Farina P, Lombardi G, Bergo E, Roma A, Zagonel V (2014) Treatment of malignant gliomas in elderly patients: a concise overview of the literature. Biomed Res Int 2014:734281
Garzon-Muvdi T, Yang W, Lim M, Brem H, Huang J (2017) Atypical and anaplastic meningioma: outcomes in a population based study. J Neurooncol 133(2):321–330
Goyal LK, Suh JH, Mohan DS, Prayson RA, Lee J, Barnett GH (2000) Local control and overall survival in atypical meningioma: a retrospective study. Int J Radiat Oncol Biol Phys 46(1):57–61
Hammouche S, Clark S, Wong AHL, Eldridge P, Farah JO (2014) Long-term survival analysis of atypical meningiomas: survival rates, prognostic factors, operative and radiotherapy treatment. Acta Neurochir 156(8):1475–1481
Hasan S, Young M, Albert T et al (2015) The role of adjuvant radiotherapy after gross total resection of atypical meningiomas. World Neurosurg 83(5):808–815
Jagsi R, Bekelman JE, Chen A et al (2014) Considerations for observational research using large data sets in radiation oncology. Int J Radiat Oncol Biol Phys 90(1):11–24
Jo K, Park HJ, Nam DH et al (2010) Treatment of atypical meningioma. J Clin Neurosci 17(11):1362–1366
Kaur G, Sayegh ET, Larson A et al (2014) Adjuvant radiotherapy for atypical and malignant meningiomas: a systematic review. Neuro Oncol 16(5):628–636
Killelea BK, Yang VQ, Mougalian S et al (2015) Neoadjuvant chemotherapy for breast cancer increases the rate of breast conservation: results from the National Cancer Database. J Am Coll Surg 220(6):1063–1069
Klinger DR, Flores BC, Lewis JJ et al (2015) Atypical meningiomas: recurrence, reoperation, and radiotherapy. World Neurosurg 84(3):839–845
Louis DN, Perry A, Reifenberger G et al (2016) The 2016 World Health Organization classification of tumors of the central nervous system: a summary. Acta Neuropathol 131(6):803–820
McCarthy BJ, Davis FG, Freels S et al (1998) Factors associated with survival in patients with meningioma. J Neurosurg 88(5):831–839
McClelland S, Page BR, Jaboin JJ, Chapman CH, Deville C, Thomas CR (2017) The pervasive crisis of diminishing radiation therapy access for vulnerable populations in the United States, part 1: African-American patients. Adv Radiat Oncol 2(4):523
Minniti G, Filippi AR, Osti MF, Ricardi U (2017) Radiation therapy for older patients with brain tumors. Radiat Oncol 12(1):101
Moraes FY, Chung C (2017) Radiation for skull base meningiomas: review of the literature on the approach to radiotherapy. Chin Clin Oncol 6(1):S3
Orton A, Frandsen J, Jensen R, Shrieve DC, Suneja G (2017) Anaplastic meningioma: an analysis of the National Cancer Database from 2004 to 2012. J Neurosurg 21:1–6
Ostrom QT, Gittleman H, Xu J et al (2016) CBTRUS statistical report: primary brain and other central nervous system tumors diagnosed in the United States in 2009–2013. Neuro Oncol 18(suppl_5):v1–v75
Palma L, Celli P, Franco C, Cervoni L, Cantore G (1997) Long-term prognosis for atypical and malignant meningiomas: a study of 71 surgical cases. J Neurosurg 86(5):793–800
Pasquier D, Bijmolt S, Veninga T et al (2008) Atypical and malignant meningioma: outcome and prognostic factors in 119 irradiated patients. A multicenter, retrospective study of the Rare Cancer Network. Int J Radiat Oncol Biol Phys 71(5):1388–1393
Pollock BE (2014) Defining the best management for patients with intracranial World Health Organization grade II meningiomas. World Neurosurg 81(5–6):712–713
Rogers L, Gilbert M, Vogelbaum MA (2010) Intracranial meningiomas of atypical (WHO grade II) histology. J Neurooncol 99(3):393–405
Sun SQ, Hawasli AH, Huang J, Chicoine MR, Kim AH (2015) An evidence-based treatment algorithm for the management of WHO Grade II and III meningiomas. Neurosurg Focus 38(3):E3
Wang YC, Chuang CC, Wei KC et al (2016) Long term surgical outcome and prognostic factors of atypical and malignant meningiomas. Sci Rep 6:35743
Wang C, Kaprealian TB, Suh JH et al (2017) Overall survival benefit associated with adjuvant radiotherapy in WHO grade II meningioma. Neuro Oncol 19(9):1263–1270
Zaher A, Abdelbari Mattar M, Zayed DH, Ellatif RA, Ashamallah SA (2013) Atypical meningioma: a study of prognostic factors. World Neurosurg 80(5):549–553
Funding
The authors received no financial or material support in the preparation of this manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors report no conflict of interest concerning the materials or methods used in this study or the findings specified in this paper. Ernest Barthélemy declares that he has no conflict of interest. Joshua Loewenstern declares that he has no conflict of interest. Neeraja Konuthula declares that she has no conflict of interest. Margaret Pain declares that she has no conflict of interest. Jordan Hall declares that he has no conflict of interest. Satish Govindaraj declares that he has no conflict of interest. Joshua Bederson declares that he has no conflict of interest. Raj K. Shrivastava declares that he has no conflict of interest.
Ethical approval
This article does not contain any studies with human participants performed by any other authors. Data were downloaded from a publicly available de-identified dataset.
Rights and permissions
About this article
Cite this article
Barthélemy, E., Loewenstern, J., Konuthula, N. et al. Primary management of atypical meningioma: treatment patterns and survival outcomes by patient age. J Cancer Res Clin Oncol 144, 969–978 (2018). https://doi.org/10.1007/s00432-018-2618-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00432-018-2618-4