Abstract
Atypical and anaplastic meningiomas (AAM) are aggressive tumors. This study is aimed at examining associations between patient and tumor-related factors and tumor-related death in patients with AAM. We conducted a population-based cohort study utilizing prospectively collected data from the Surveillance, Epidemiology, and End Results (SEER) database. Patients with diagnosis of AAM from 1973 to 2012 in the SEER database were included. Patients lacking clinical information were excluded. Multivariate analysis between patient and lesion characteristics, and AAM-related death was performed to adjust for confounding factors. We identified and included 522 patients in our study. Mean age at diagnosis was 60.8 ± 15.7 years. The majority of patients were White(73%), 15.5% Black, and 9.8% Asian. Average tumor size was 48.2 ± 20.3 mm. The tumor was locally confined in 57.1%, whereas it had intracranial extension in 29.3%, and extracranial extension in 8.8% of patients. The vast majority (94.8%) of tumors were supratentorial. Gross total resection (GTR) was documented in 65.5% of patients. Age at diagnosis (p = 0.001), tumor size (p = 0.003), surgery result (GTR vs. subtotal resection, p = 0.027), and radiation therapy (p = 0.2) were found to be significantly different between the comparison groups. In a multivariate proportional competing risk regression analysis age (HR 1.03, CI [1.01,1.04], p < 0.001), infratentorial location (HR 2.81, CI [1.20, 6.56], p = 0.017), tumor size (HR 1.01, CI [1.00,1.02], p = 0.032),and radiation treatment (HR 1.52, CI [1.11, 2.09], p = 0.01) were significantly associated with tumor-related death. The association of age at diagnosis, tumor size, location, and radiotherapy with overall survival in patients with AAM is demonstrated. The results provide a context for individualized treatment plans in patients with AAM. Additional studies focusing on issues such as the use of radiation and chemotherapy will clarify the best modality to achieve disease control.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Meningiomas account for approximately one-third of all intracranial brain tumors [1, 2]. The majority of meningiomas are WHO grade I, but approximately 5% of meningiomas are atypical and malignant (WHO II and III) [3]. WHO grade II and III are aggressive tumors and are coupled to a poor prognosis and higher mortality caused by the tumor [4]. In contrast to WHO I meningiomas that have a female predominance, atypical and malignant meningiomas have a slight predominance in male patients [3].
The management of patients with atypical and anaplastic meningiomas (AAM) remains uncertain and few studies have identified qualities that warrant more aggressive management strategies. Some of these characteristics include genetic abnormalities, extent of surgical resection, need for adjuvant radiation, and histological features such as mitotic rates; however, most of these factors have not been verified in a large cohort [5–11].
We performed a population-based study with a national cancer registry to identify important factors that determine survival of patients with AAM.
Methods
Study design and patient population
We incorporated a prospective population-based cohort study design by utilizing data from the surveillance, epidemiology, and end results (SEER) dataset actively maintained by the National Cancer Institute (NCI). Patients were prospectively enrolled in this national cancer registry, with collection of individual demographic, lesion and survival data. To align with the purpose of this study, we included cases from the “incidence-SEER 18 registries Research Data and Hurricane Katrina Impacted Louisiana Cases” dataset between 1973 and 2012. Inclusion criteria included: brain groupings labeled as “Meningioma, benign or malignant” including International Classification of Diseases for Oncology (ICD-O 3) code of 9530–9539 (meningioma), diagnosed between 1983 and 2012, primary tumor, and malignant behavior by ICD-O-3. It should be noted that we did not utilize WHO grade for inclusion of malignant or atypical meningioma as it has been modified and inconsistently recorded in SEER database; the malignant behavior of meningioma was defined by ICD-O-3 behavior code. Exclusion criteria included: non-intracranial location, lesions not confirmed histologically, lesions listed as WHO grade 1, not primary or first tumor, and missing critical information (size and survival time).
Definition of variables
Basic demographics including age, sex, race and marital status were included for each patient. Age was defined as age at diagnosis for each patient. Race was classified into four distinct categories: White, Black, Asian and Others/Unknown. Marital status was grouped into: married, single/separated, and unknown. Size of the tumor was represented in millimeters, and extension of the tumor was described as either locally confined, tumor with intracranial extensions, tumor with extracranial extensions, or unspecified. Tumor location was clustered into three categories: supratentorial, infratentorial, and overlapping/others. For treatment variables, surgery was categorized into: gross total resection (GTR), partial/unspecified extent of resection and no surgery. Year of diagnosis was described per 5-year incremental, and analyzed as a continuous variable in the multivariate analysis to prevent over-stratification of data.
Statistical analysis
A survival analysis was conducted to delineate factors associated with survival of atypical or malignant meningioma. Outcome was defined as tumor-related death. Attributable to the relatively benign nature of meningioma compared to other malignant brain tumors, we observed a significant proportion of patients with death of other causes. Therefore, a competing risk analysis was adopted to address the limitations of conventional survival analysis [12, 13]. Baseline patient and lesion characteristics were described by comparing between tumor-related death versus alive/unrelated death, and also between alive versus tumor-related death versus unrelated death. Student’s t-test or analysis of variance (ANOVA) was used for continuous variables where appropriate, and Chi square test was used for categorical variables.
For competing risk analysis, cumulative incidence of tumor-related death was computed for each factor after accounting for death of other causes. Both tumor-related death and death of other causes were included in a cumulative risk regression (CRR) model. The Fine and Gray model was selected as our primary CRR model [14, 15]. Univariate CRR was computed for each of the variable, and an all-inclusive multivariate CRR model was constructed to adjust variable confounding. Hazard ratios were reported together with 95% confidence intervals. All p values in this study were reported as two-sided. Statistical significance was defined with an alpha level of 0.05 (p < 0.05). Statistical analysis was performed using R Statistical Software (Version 3.2.3, 2015, Vienna, Austria).
Results
Patient population and baseline characteristics
We identified 522 patients from the SEER database after applying inclusion and exclusion criteria as illustrated in Supplemental Fig. 1. Baseline characteristics of the cohort as stratified by tumor related death and by survival status are presented in Supplemental Table 1. Mean age at diagnosis for all patients was 60.8 ± 15.7 years. Seventy-three percent of patients were White, 15.5% Black, and 9.8% were Asian. More than half of the patients (53.8%) were married. Mean tumor size was 48.2 ± 20.3 mm. The tumor was locally confined in 57.1% of cases, whereas it had intracranial extension in 29.3% of cases and extracranial extension in 8.8% of patients. The vast majority (94.8%) of tumors were supratentorial. Gross total resection (GTR) was documented in 65.5% of patients, partial or unspecified resection was documented in 28.2% of patients and 6.3% of patients did not undergo surgery. Radiation therapy was employed to treat 39.3% of patients. Age at diagnosis (p = 0.001), tumor size (p = 0.003), surgery result (GTR vs. subtotal resection, p = 0.027), and radiation therapy (p = 0.2) were found to be significantly different between the comparison groups.
Impact of age, tumor characteristics, and adjuvant therapy on survival
Significant factors affecting survival of patients diagnosed with atypical meningioma were identified using a competing risk analysis. In a multivariate proportional competing risk regression analysis age (HR 1.03, CI [1.01, 1.04], p < 0.001), infratentorial location (HR 2.81, CI [1.20, 6.56], p = 0.017), tumor size (HR 1.01, CI [1.00, 1.02], p = 0.032), and radiation treatment (HR1.52, CI [1.11, 2.09], p = 0.01) (Table 1). Interestingly, survival demonstrated two peaks of greatest survival describing the intersection of the influence of tumor size and age at diagnosis on survival (Fig. 1a). Briefly, patients diagnosed around the 5th and 6th decades of their life demonstrated the longest survival as compared to older patients when assessing tumor related death. When assessing the survival associated to tumor related and unrelated death patient age and tumor size demonstrated the same influence (Fig. 1b). Interestingly, a decrease in survival was noticed in the tumor size range from 50 to 100 mm both in the tumor-related cause of mortality, as well in the all cause of mortality plot (Fig. 1). In both comparisons, age abrogates the peaks in survival seen in both analyses. Analysis of the overall cumulative competing risk of death demonstrated the probability of tumor related death is higher than death due to other causes (Fig. 1c). To better understand the factors that were found to be statistically significant in the competing risk analysis (i.e. location, age, and tumor size) we modeled a predicted curve of cumulative incidence of tumor related death using the dataset where size and age cutoff were chosen based on approximately 25 and 75% percentile (Fig. 2). This shows that in a 75 year-old patient with an infratentorial 60 mm tumor the cumulative incidence of tumor related death is considerably higher when compared to that of a 50 year-old patient with a 35 mm supratentorial tumor. Interestingly, when the same model is applied to patients that received radiation, it was evident that the effect of tumor size on survival was annulled (Fig. 2b). For example, in a 50 year-old patient with a 35 mm tumor the curve of the predicted incidence of tumor related death approximated the curve for a 50 year-old patient with a 60 mm tumor. The same effect is seen for supratentorial and infratentorial tumors. Further, the modeled time to tumor related death resulted shorter in patients that required radiation than in patients that did not require radiation, consistent with the multivariate analysis (Table 1).
3D scatter plot describing the relationship of age at diagnosis, tumor size, and survival in patients with atypical and anaplastic meningiomas in the context of all cause mortality (a) and in the context of tumor related mortality (b). c Kaplan–Meier curve of the overall cumulative competing risk of death comparing tumor-related death against death of other causes
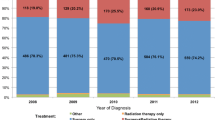
The total number of patients diagnosed with AAM increased since 1983–2007 and declined in the last period from 2007 to 2011, potentially due to the change in diagnostic criteria for WHO II and III meningiomas (Fig. 3a) [16]. The ratio of patients alive as a fraction of the total number of patients diagnosed with AAM shows a steady increase over the years since 1983–2011, whereas there is a steady decrease in the ratio of patients with tumor-related death over total number of patients diagnosed with AAM (Fig. 3b). This appears to be an artifact of the temporal course of the disease since the multivariate analysis does not show any difference in survival between the different time periods as shown in Table 1. Of note, there was no difference in survival in the different stratification categories in Supplemental Table 1. We also performed Log-rank analysis of survival for the six different time periods of diagnosis and there was no significant difference in survival in for all cause death (Fig. 3c; Table2).
Discussion
Intracranial meningiomas account for a large proportion of intracranial tumors. WHO grade II and III, or atypical or anaplastic meningiomas, account for a small fraction of meningiomas and the prognosis of patients with these tumors is grim [1–4]. We identified 522 AAM patients from the SEER database and we explored the prognostic factors that affect the survival of patients with AAM in a nationwide database. We identified factors that affect survival such as age at diagnosis, tumor size, location of the tumor, and whether the patient received radiation therapy or not. Because of the relatively long natural history of the disease, it is paramount to analyze the data with a competing risk analysis to avoid the limitations of conventional survival analysis, which includes the risk of death due to causes other than the tumor.
We found that survival was greatly influenced by older age at diagnosis when the data is analyzed by tumor-related death rather than an overall mortality, which can generate a bias given the possibility of deterioration of health with age. By analyzing this parameter in the context of tumor related death, we avoid this bias and the results suggest that the effect of age on survival is due to characteristics intrinsic to the tumor. In prior studies, survival had been analyzed in the context of all-cause mortality. However our findings are in accordance with findings from other studies where age was found to be a major determinant of survival in patients with atypical or anaplastic meningiomas [17–19] Other reports have found that patients with younger age at diagnosis were less likely to be undergo reoperation for recurrence of WHO grade II meningioma [20]. Other studies have shown that later age at diagnosis was a prognostic factor of recurrence [17–19, 20Aizer, 2015 #6]. The increased incidence in recurrence at a later age may be the explanation for significantly higher tumor related deaths in patients with later age at diagnosis. However, increased recurrence and shorter survival may be related to a more aggressive biology of these tumors in older patients.
Tumor size is another important factor determining survival and progression free survival in patients with AAM. We analyzed the interaction of age at diagnosis, tumor size and survival in the context of all cause death and tumor-related death, specifically. We found that at a younger age there are two peaks of longer survival times, one with tumors <50 mm and another peak with tumors >100 mm. This effect was abrogated at older ages suggesting differences in tumor behavior or potential invasion of adjacent critical structures [16, 19, 21, 22]. In addition to tumor size, extent of surgical resection is one of the most important prognostic factors for recurrence and survival in patients with atypical and anaplastic meningiomas [5, 19, 22–25].
Radiation therapy to treat meningiomas in general is used when there is residual tumor after surgical resection or to treat recurrence of the tumor [11, 26, 27]. In our study we found that patients who received radiation had earlier tumor related death as compared to patients who did not receive radiation. The caveat that must be considered in the interpretation of this result is that in the SEER database, it is not specified whether radiation was given as adjuvant or salvage therapy, and the radiation modality is not specified either. Also, there may be a selection bias in that clinicians may have chosen radiotherapy for patients considered high risk for recurrence or with aggressive tumors. Further, there may be large inter-institutional discrepancies in the use of radiation therapy for AAM patients. Early adjuvant radiation for AAM has been analyzed in a multicenter study and was not found to be beneficial to atypical meningioma patients [11, 28] This lack of effect on survival or PFS was independent of extent of resection, however, there was selection bias present in this study. Other studies have shown benefit of radiation therapy in PFS but not in OS [19, 29, 30]. Interestingly, one study demonstrated that patients who receive radiation therapy are more likely to undergo repeat surgery for recurrence, this is to be considered in the context that patients that receive radiation may not have gross total resection of the tumor prompting surgeons to proceed with adjuvant radiation [20]. Some authors advocate for radiation therapy in patients with gross total resection [29]. Further prospective studies with uniform dosing strategies and potentially different radiation modalities are needed to answer the question of whether radiation therapy is beneficial for AAM patients even in the context of GTR.
Using the SEER data, we found that location of the tumor is a significant factor that influences survival, in that infratentorial location represents a risk factor for poor prognosis associated with tumor related death. In a similar way, other studies have demonstrated that tumor location in the cerebral convexity is associated with improved survival as compared to other sites (parasagittal, falx, cranial base, or posterior fossa grouped together) [17, 31]. This effect is likely due to the proximity of critical structures such as the superior sagittal sinus in the falx/parasagittal location, the cranial nerves, brainstem, and venous sinuses in the posterior fossa/cranial base locations. Remarkably, a large study that included 76 WHO II and 7 WHO III meningiomas concluded that the location of the tumor in a place other than the skull base is a risk factor for higher WHO classification [32].
Molecular profiling of AAM has yielded data that may be helpful to predict recurrence and/or progression of tumors after surgical resection. In one study, authors explored the expression of pro-apoptotic and anti-apoptotic factors in 67 AAM patients, they found that low expression of CASP3 and BAX, and overexpression of survivin and MDM2 were associated with recurrence [33]. Additionally, methylation of PDCD1 and IGF2BP1 was found to correlate with increased malignant potential and associated with an aggressive phenotype [34]. Other cytogenetic alterations and specific gene mutations have been identified in patients with AAM that differ from those found in WHO grade I meningiomas [35]. Other molecular analyses have found that AAM cells express PD-L1, a checkpoint molecule capable of suppressing an effective immune response, potentially providing an immunosuppressive microenvironment and making immunotherapy an alternative therapy for these type of aggressive tumors [36]. Another important gene with effects in meningioma progression is p53-regulator PPM1D [37]. Additionally, potential targets for currently available agents have been identified in meningiomas, one of them being NY-ESO-1 [38]. Clinical trials exploring the expression of NY-ESO-1 (https://clinicaltrials.gov/ct2/show/NCT01967823) as well as trials that characterize the expression of tumor markers and treat patients according to their tumor marker profile (https://clinicaltrials.gov/ct2/show/NCT02523014). Molecular characterization of AAM may be helpful in stratifying patients to different risk categories of recurrence, directing the treatment plan to more aggressive approaches for patients with more risk factors for recurrence.
Even though the identified cohort of patients is large, there are strengths and limitations inherent to the nature of this study. The size of the cohort and the length of follow-up are among its strengths. Another strength is that this large database captures the heterogeneity of patients and treatment strategies across the institutions from which this data is accrued, yielding a realistic and potentially generalizable picture of the clinical course of the patient population. One of the limitations is the retrospective nature of the study that is accompanied by lack of details of the treatment strategy such as characteristics of radiation, details of tumor histology, use of chemotherapy in some patients, patients’ performance status and comorbidities. Potential variability in histological diagnosis and modifications to the WHO diagnostic criteria, as well as the lack of Simpson grade classification into the database are additional limitations to the study. The extent of surgical resection is not a significant predictor of survival is possibly attributable to the lack of detailed information on extent of resection such as Simpson grade, or potentially due to a difference of population as our inclusion criteria was based on ICD-O-3 instead of WHO grade. Additionally, even though the supra or infratentorial location of the tumor is available, the database does not specify factors such as venous sinus invasion and skull base location, which limit the possibility of gross-total resection. Further, the Simpson grade used to evaluate extent of resection of meningiomas is lacking in the SEER database. Despite these limitations, we believe that this study improves the current knowledge with improved analysis strategies and introduces new questions to be answered. One of these questions centers on the role of chemotherapy in the treatment of AAM, aiming at describing the outcomes of patients with these challenging tumors that have undergone chemotherapy treatment.
Conclusions
Despite the limitations of the study, we were able to clearly demonstrate the association between age at diagnosis, tumor size, location, and radiation treatment with overall survival. The results lend support to individualized treatment plans in patients with AAM and call for future studies focused on issues such as use of radiation and chemotherapy to further clarify the best therapeutic strategies to achieve disease control.
References
Dolecek TA, Propp JM, Stroup NE, Kruchko C (2012) CBTRUS statistical report: primary brain and central nervous system tumors diagnosed in the United States in 2005–2009. Neuro Oncol 14(5):1–49. doi:10.1093/neuonc/nos218
Kleihues P, Cavenee WK (2000) Pathology and genetics of tumours of the nervous system. International Agency for Research on Cancer, Lyon
Wiemels J, Wrensch M, Claus EB (2010) Epidemiology and etiology of meningioma. J Neurooncol 99:307–314. doi:10.1007/s11060-010-0386-3
Louis DN, Ohgaki H, Wiestler OD, Cavenee WK, Burger PC, Jouvet A, Scheithauer BW, Kleihues P (2007) The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol 114:97–109. doi:10.1007/s00401-007-0243-4
Aizer AA, Bi WL, Kandola MS, Lee EQ, Nayak L, Rinne ML, Norden AD, Beroukhim R, Reardon DA, Wen PY, Al-Mefty O, Arvold ND, Dunn IF, Alexander BM (2015) Extent of resection and overall survival for patients with atypical and malignant meningioma. Cancer 121:4376–4381. doi:10.1002/cncr.29639
Hammouche S, Clark S, Wong AH, Eldridge P, Farah JO (2014) Long-term survival analysis of atypical meningiomas: survival rates, prognostic factors, operative and radiotherapy treatment. Acta Neurochir (Wien) 156:1475–1481. doi:10.1007/s00701-014-2156-z
ILin CK, Tsai WC, Lin YC, Hueng DY (2012) Osteopontin predicts the behaviour of atypical meningioma. Histopathology 60:320–325. doi:10.1111/j.1365-2559.2011.04085.x
Lusis EA, Watson MA, Chicoine MR, Lyman M, Roerig P, Reifenberger G, Gutmann DH, Perry A (2005) Integrative genomic analysis identifies NDRG2 as a candidate tumor suppressor gene frequently inactivated in clinically aggressive meningioma. Cancer Res 65:7121–7126. doi:10.1158/0008-5472.CAN-05-0043
Piscevic I, Villa A, Milicevic M, Ilic R, Nikitovic M, Cavallo LM, Grujicic D (2015) The influence of adjuvant radiotherapy in atypical and anaplastic meningiomas: a series of 88 patients in a single institution. World Neurosurg 83:987–995. doi:10.1016/j.wneu.2015.02.021
Smith JS, Lal A, Harmon-Smith M, Bollen AW, McDermott MW (2007) Association between absence of epidermal growth factor receptor immunoreactivity and poor prognosis in patients with atypical meningioma. J Neurosurg 106:1034–1040. doi:10.3171/jns.2007.106.6.1034
Sun SQ, Kim AH, Cai C, Murphy RK, DeWees T, Sylvester P, Dacey RG, Grubb RL, Rich KM, Zipfel GJ, Dowling JL, Leuthardt EC, Leonard JR, Evans J, Simpson JR, Robinson CG, Perrin RJ, Huang J, Chicoine MR (2014) Management of atypical cranial meningiomas, part 1: predictors of recurrence and the role of adjuvant radiation after gross total resection. Neurosurgery 75:347–354. doi:10.1227/NEU.0000000000000461
Scrucca L, Santucci A, Aversa F (2010) Regression modeling of competing risk using R: an in depth guide for clinicians. Bone Marrow Transplant 45:1388–1395. doi:10.1038/bmt.2009.359
Scrucca L, Santucci A, Aversa F (2007) Competing risk analysis using R: an easy guide for clinicians. Bone Marrow Transplant 40:381–387. doi:10.1038/sj.bmt.1705727
Kuk D, Varadhan R (2013) Model selection in competing risks regression. Stat Med 32:3077–3088. doi:10.1002/sim.5762
Fine J, Gray R (1999) A proportional hazards model for the subdistribution of a competing risk. J Amer Statistical Assoc 94:496–509
Buttrick S, Shah AH, Komotar RJ, Ivan ME (2016) Management of atypical and anaplastic meningiomas. Neurosurg Clin N Am 27:239–247. doi:10.1016/j.nec.2015.11.003
Zaher A, Abdelbari Mattar M, Zayed DH, Ellatif RA, Ashamallah SA (2013) Atypical meningioma: a study of prognostic factors. World Neurosurg 80:549–553. doi:10.1016/j.wneu.2013.07.001
Sade B, Chahlavi A, Krishnaney A, Nagel S, Choi E, Lee JH (2007) World Health Organization grades II and III meningiomas are rare in the cranial base and spine. Neurosurgery 61:1194–1198. doi:10.1227/01.neu.0000306097.38141.65
Durand A, Labrousse F, Jouvet A, Bauchet L, Kalamarides M, Menei P, Deruty R, Moreau JJ, Fevre-Montange M, Guyotat J (2009) WHO grade II and III meningiomas:a study of prognostic factors. J Neurooncol 95:367–375. doi:10.1007/s11060-009-9934-0
Champeaux C, Dunn L (2016) World Health Organization grade II meningioma. A 10-year retrospective study for recurrence and prognostic factor assessment. World Neurosurg. doi:10.1016/j.wneu.2016.01.055
Hanft S, Canoll P, Bruce JN (2010) A review of malignant meningiomas: diagnosis, characteristics, and treatment. J Neurooncol 99:433–443. doi:10.1007/s11060-010-0348-9
Goyal LK, Suh JH, Mohan DS, Prayson RA, Lee J, Barnett GH (2000) Local control and overall survival in atypical meningioma: a retrospective study. Int J Radiat Oncol Biol Phys 46:57–61
Yang SY, Park CK, Park SH, Kim DG, Chung YS, Jung HW (2008) Atypical and anaplastic meningiomas: prognostic implications of clinicopathological features. J Neurol Neurosurg Psychiatry 79:574–580. doi:10.1136/jnnp.2007.121582
Hardesty DA, Wolf AB, Brachman DG, McBride HL, Youssef E, Nakaji P, Porter RW, Smith KA, Spetzler RF, Sanai N (2013) The impact of adjuvant stereotactic radiosurgery on atypical meningioma recurrence following aggressive microsurgical resection. J Neurosurg 119:475–481. doi:10.3171/2012.12.JNS12414
Dziuk TW, Woo S, Butler EB, Thornby J, Grossman R, Dennis WS, Lu H, Carpenter LS, Chiu JK (1998) Malignant meningioma: an indication for initial aggressive surgery and adjuvant radiotherapy. J Neurooncol 37:177–188
Santacroce A, Walier M, Regis J, Liscak R, Motti E, Lindquist C, Kemeny A, Kitz K, Lippitz B, Martinez Alvarez R, Pedersen PH, Yomo S, Lupidi F, Dominikus K, Blackburn P, Mindermann T, Bundschuh O, van Eck AT, Fimmers R, Horstmann GA (2012) Long-term tumor control of benign intracranial meningiomas after radiosurgery in a series of 4565 patients. Neurosurgery 70:32–39. doi:10.1227/NEU.0b013e31822d408a
Pollock BE, Stafford SL, Link MJ (2013) Stereotactic radiosurgery of intracranial meningiomas. Neurosurg Clin N Am 24:499–507. doi:10.1016/j.nec.2013.05.006
Jenkinson MD, Waqar M, Farah JO, Farrell M, Barbagallo GM, McManus R, Looby S, Hussey D, Fitzpatrick D, Certo F, Javadpour M (2016) Early adjuvant radiotherapy in the treatment of atypical meningioma. J Clin Neurosci 28:87–92. doi:10.1016/j.jocn.2015.09.021
Aizer AA, Arvold ND, Catalano P, Claus EB, Golby AJ, Johnson MD, Al-Mefty O, Wen PY, Reardon DA, Lee EQ, Nayak L, Rinne ML, Beroukhim R, Weiss SE, Ramkissoon SH, Abedalthagafi M, Santagata S, Dunn IF, Alexander BM (2014) Adjuvant radiation therapy, local recurrence, and the need for salvage therapy in atypical meningioma. Neuro Oncol 16:1547–1553. doi:10.1093/neuonc/nou098
Hasan S, Young M, Albert T, Shah AH, Okoye C, Bregy A, Lo SS, Ishkanian F, Komotar RJ (2015) The role of adjuvant radiotherapy after gross total resection of atypical meningiomas. World Neurosurg 83:808–815. doi:10.1016/j.wneu.2014.12.037
Palma L, Celli P, Franco C, Cervoni L, Cantore G (1997) Long-term prognosis for atypical and malignant meningiomas: a study of 71 surgical cases. J Neurosurg 86:793–800. doi:10.3171/jns.1997.86.5.0793
Cornelius JF, Slotty PJ, Steiger HJ, Hanggi D, Polivka M, George B (2013) Malignant potential of skull base versus non-skull base meningiomas: clinical series of 1663 cases. Acta Neurochir (Wien) 155:407–413. doi:10.1007/s00701-012-1611-y
Lee SH, Lee EH, Lee SH, Lee YM, Kim HD, Kim YZ (2015) Epigenetic role of histone 3 lysine methyltransferase and demethylase in regulating apoptosis predicting the recurrence of atypical meningioma. J Korean Med Sci 30:1157–1166. doi:10.3346/jkms.2015.30.8.1157
Vengoechea J, Sloan AE, Chen Y, Guan X, Ostrom QT, Kerstetter A, Capella D, Cohen ML, Wolinsky Y, Devine K, Selman W, Barnett GH, Warnick RE, McPherson C, Chiocca EA, Elder JB, Barnholtz-Sloan JS (2013) Methylation markers of malignant potential in meningiomas. J Neurosurg 119:899–906. doi:10.3171/2013.7.JNS13311
Choy W, Kim W, Nagasawa D, Stramotas S, Yew A, Gopen Q, Parsa AT, Yang I (2011) The molecular genetics and tumor pathogenesis of meningiomas and the future directions of meningioma treatments. Neurosurg Focus 30:E6. doi:10.3171/2011.2.FOCUS1116
Du Z, Abedalthagafi M, Aizer AA, McHenry AR, Sun HH, Bray MA, Viramontes O, Machaidze R, Brastianos PK, Reardon DA, Dunn IF, Freeman GJ, Ligon KL, Carpenter AE, Alexander BM, Agar NY, Rodig SJ, Bradshaw EM, Santagata S (2015) Increased expression of the immune modulatory molecule PD-L1 (CD274) in anaplastic meningioma. Oncotarget 6:4704–4716. doi:10.18632/oncotarget.3082
Fukami S, Riemenschneider MJ, Kohno M, Steiger HJ (2016) Expression and gene doses changes of the p53-regulator PPM1D in meningiomas: a role in meningioma progression? Brain Tumor Pathol 33:191–199. doi:10.1007/s10014-016-0252-x
Baia GS, Caballero OL, Ho JS, Zhao Q, Cohen T, Binder ZA, Salmasi V, Gallia GL, Quinones-Hinojosa A, Olivi A, Brem H, Burger P, Strausberg RL, Simpson AJ, Eberhart CG, Riggins GJ (2013) NY-ESO-1 expression in meningioma suggests a rationale for new immunotherapeutic approaches. Cancer Immunol Res 1:296–302. doi:10.1158/2326-6066.CIR-13-0029
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Garzon-Muvdi, T., Yang, W., Lim, M. et al. Atypical and anaplastic meningioma: outcomes in a population based study. J Neurooncol 133, 321–330 (2017). https://doi.org/10.1007/s11060-017-2436-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11060-017-2436-6