Abstract
Although atypical meningioma recurs frequently in spite of total resection and/or radiotherapy, no consensus on optimal adjuvant management was found. However, several retrospective studies analysed the additional effect of adjuvant radiotherapy in atypical meningioma with inconsistent results. Therefrom, the purpose of this study was to evaluate prognostic factors influencing the recurrence/progression and progression-free survival (PFS) rates of atypical meningioma, particularly focused on the role of postoperative adjuvant radiotherapy. Between February 2001 and March 2015, 161 atypical meningioma resections were performed in our Department of Neurosurgery, of which, 128 cases underwent surgical treatment alone and 33 cases underwent surgery and radiotherapy. Kaplan–Meier analysis was used to provide median point estimates and PFS rates. The Cox-regression model was used in the univariate and multivariate analysis to identify significant factors associated with treatment. The extent of resection (Simpson grade I and II) significantly influenced the risk of recurrence (hazard ratio = 1.8, CI (95%) 1.3–2.6, p-value = 0.0004). There was no significant benefit for progression-free survival after adjuvant radiotherapy (hazard ratio = 1.48, CI (95%) 0.76–2.86, p-value = 0.22). Additionally, meningioma located at the anterior and posterior fossa showed a significantly longer PFS compared to other locations (p-value = 0.03). Adjuvant postoperative radiotherapy had no significant impact on recurrence/progression rate or PFS. The extent of resection according to Simpson grade remains the most important prognostic factor associated with lower recurrence/progression rates and longer PFS in patients with atypical meningioma. The location of the tumours at the anterior or posterior fossa was an independent factor associated with improved PFS.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Meningioma are common brain tumours in adults, with an incidence of 7.61 cases per 100 000 people in the United States [29]. The majority of meningioma are benign tumours (90%) mainly treated by total resection, followed by an innocent clinical course [28]. However, a subset of meningioma (4.7–34%) are defined as atypical meningioma [13, 18] which frequently recur even after a total resection (Simpson grad I–II) and/or adjuvant radiotherapy.
The 2016 World Health Organisation (WHO) classification classified meningioma into three histological grades (I–III) and described 16 histopathological subtypes [26, 27]. Meningioma (WHO grade I) are mainly observed in women and are associated with a low risk of recurrence after resection (~ 10% at 5 years) [27]. Atypical meningioma (WHO grade II) show an intermediate clinical progression with recurrence rates of 30–40% [26]. They are histopathologically characterised by increased mitotic activity (at least 4 mitotic figures per 10 high power fields) and/or three or more of the following features: architectural sheeting, necrosis, prominent nucleoli, hypercellularity and small cells with a high nuclear-to-cytoplasma ratio. Anaplastic meningioma are defined as WHO grade III and are associated with an aggressive growth pattern resembling clinical and histopathological features of malignancy, with a recurrence rate of 50–80% [33]. Additionally, metastatic dissemination were found in patients with anaplastic meningioma [8].
Surgical resection is the hallmark of meningioma treatment, including all grades of meningioma. Until now, the extension of surgical resection is still the most important prognostic factor in relation to oncological outcome [1, 6, 7, 12, 14, 15, 20, 37]. However, meningioma with a malignant potential (atypical and anaplastic) or non-resectable tumour-residual require additional adjuvant therapies. At present, the additional benefit of postoperative adjuvant radiotherapy for atypical meningioma is still unclear and needs further evaluation.
Several retrospective studies reported controversial results on different adjuvant therapy options [2, 5, 7, 14, 21, 30]. In a recent retrospective case study of 45 patients with atypical meningioma, Endo et al. showed no additional benefit of adjuvant radiotherapy concerning the long-term tumour control [14]. The benefit of adjuvant radiotherapy in a subgroup of patients with atypical meningioma, in whom a total resection was not possible, was discussed in several studies, critically underlining the uncertain benefit of additional postoperative radiotherapy [7, 15, 16, 23]. In contrast, Lee et al. [25] described a partial improvement of PFS in patients with subtotal resection and adjuvant radiotherapy. This findings were confirmed in further studies [1,2,3, 5, 21, 30, 31].
The aim of this retrospective study was to investigate the influence of additional adjuvant radiotherapy after surgical resection on progression-free survival in patients with atypical meningioma and to evaluate prognostic factors that affect the outcome and the clinical course of atypical meningioma.
Materials and methods
The present study was conducted as a single-centre retrospective study. All patients underwent surgery at the Department of Neurosurgery (Medical Center Freiburg, University of Freiburg, Germany) between 2001 and 2015. Only patients that fulfilled the following criteria were included in the study: (1) aged older than 18 years, (2) histopathological diagnosis of grade II meningioma at time of surgery, and (3) secondary WHO grade II meningioma that progressed from grade I. The study was approved by the local ethics committee of the University of Freiburg, Germany. Informed consent was obtained from all patients.
Data acquisition
The following parameters were recorded: patient sex, age at time of surgery, symptoms at diagnosis, tumour location, the extent of resection and recurrence/progression. Extent of surgical resection was evaluated according to the Simpson grading-scale using surgical reports and the 3-month follow-up MR-Imaging [34]. Gross total resection was defined as Simpson grades I and II, and incomplete resection or subtotal resection as Simpson grades III, IV and V. The mean follow up was 5.2 ± 3.5 years. All patients underwent frequent MRI scans 3 months postoperatively and at a regular interval of 1 year. Karnofsky Performance Scale (KPS) and progression-free survival (PFS) were used to assess oncological/neurological outcome. New lesions or growing residual tumour on a follow up MRI scan were defined as tumour progression. MRI scans were assessed by two independent investigators. Patients with incomplete record data were excluded.
Proliferation index
Tissue samples were fixed using 4% phosphate buffered formaldehyde and paraffin-embedded according to standard procedures. H&E staining was performed on 4 µm paraffin sections using standard protocols. Immunohistochemistry was applied using an autostainer (Dako) after heat-induced epitope retrieval in citrate buffer. Expression of MIB1 and GFAP was assessed by immunohistochemistry using an anti-MIB1 (1:50, Dako) and anti-GFAP antibody (1:1000, Zytomed Systems). Immunohistochemistry was performed on 3 µm paraffin-embedded tissue sections after deparaffinization and heat-induced epitope retrieval in citrate buffer. Proliferation index was achieved in 6 high-fields (×40 magnification) per slide and compared to the total number of cells in each field [17]. Brain invasion was determined in case of GFAP positive cells within the meningioma.
Statistical analysis
In this study, the primary endpoint was progression-free survival (PFS). PFS was defined as the time-interval between surgery and tumour recurrence/progression diagnosed on the follow-up MRI scans. Univariate and multivariate Cox-regression analyses were performed. The alpha-level was defined as 5% and required no adjustment to reach a statistical power at a minimum of 80%. All statistical analyses were performed using R-software tool (package: survival, ggplot2, MANOVA) and IBM SPSS statistics version 22. Plots were performed by R-software package ggplot2. Differences were considered significant at p < 0.05. Matched cohorts were computed by nonparametric pre-processing for parametric causal interference integrated in r-software [24]. The following parameters were taken into consideration: Age, Simpson Grade. KPS, MIB1-Index, Localisation and Sex. Distribution and variances of all data was tested by Shapiro–Wilk test (p > 0.05) to confirm normality. We tested the difference between both groups by Wilcoxon signed-rank test (unpaired) for numeric variables, Chi square test or Fishers-Exact test for nominal variable and determined a 5% alpha-level.
Results
Patient data
A total of 161 patients with atypical meningioma were treated at the Department of Neurosurgery between 2001 and 2015. The sex ratio (male/female) was 1:1.11. The Surgery group included 128 patients (55 males and 73 females) with a median age of 70.95 years (confidence interval 95% 51.5–90.3) and the Surgery plus radiotherapy group included 33 patients (21 males and 12 females) with a median age of 68.9 years (confidence interval 95% 56.4–83.4). Meningioma were located at the cerebral convexity in 65 cases (40%), on the falcine site in 34 cases (21%), on the sphenoid ridge in 33 cases (20%), in the posterior fossa in 11 cases (6%), and in the anterior fossa in 18 cases (11%). Anterior fossa was defined by the localisation: frontobasis, planum sphenoidale and clinoid process. In contrast, posterior fossa contained the following localisations: infratentorial tumours including petroclival meningioma.
Frequent symptoms at presentation were headache, visual deficits, gait disturbance, aphasia, seizures and dizziness. A detailed overview of all parameters is given in Table 1.
Extent of tumour resection according to Simpson grade
All patients underwent surgical resection of the tumour. In 102 cases (63.3%), gross total resection (Simpson grade I and II) was achieved and 59 cases (36.6%) received a subtotal resection (Simpson grade III, IV and V). Tumour recurrence was diagnosed in 17 cases (10.5%) after gross total resection (Simpson grade I and II). In contrast, 35 cases (21.7%) showed progression after subtotal resection (Simpson grade III, IV and V) (Table 2). Simpson grade I and II patients had a progression-free survival rate of 91% and 83% at 3 and 5 years respectively, compared to 52 and 45% in the subtotal resection group (Simpson grades III and IV) (Table 2). The difference between both groups was statistically significant (p = 0.0004) (Fig. 1a). The difference was also significant in univariate analysis (p ≤ 0.001) and multivariate analysis (p ≤ 0.001) (Table 3). In addition, the proliferation index (MIB-1) was taken into consideration. Highly proliferative meningioma were defined as an MIB1 index over 10% (Table1). Patients with high-proliferative tumours showed a significant worse outcome (HR 2.4 CI 95% 2.05–2.8, p = 0.0047) (Fig. 1b).
Additional adjuvant radiotherapy
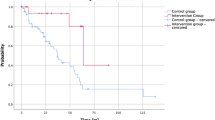
Thirty-three cases (20%) were treated postoperatively with conventional radiotherapy and 128 cases received surgery alone, of which, 14 cases treated with radiotherapy after surgery showed tumour recurrence/progression (42%). All tumors achieved a radiation between 55 and 57 gray. The 3, 5, and 10 year PFS rates were 76, 64, and 57% respectively. In the group of patients with surgery alone, tumour recurrence/progression was observed in 38 cases (29.6%). The 3, 5, and 10 year PFS rates were 80, 73, and 70%, respectively (Table 1). The statistical analysis showed no significant differences between both groups (patients treated with surgery and radiotherapy vs. surgery alone) (Fig. 2a). The additional univariate analysis (HR 0.69 CI 95% 0.37–1.27, p = 0.23) and multivariate analysis (HR 1.09 CI 95% 0.57–2.1, p = 0.77) showed also no significant differences between both groups (Table 3). However, as shown in Table 1, an increased number of subtotal resected cases (Simpson grade > 3) were obtained in the surgery plus radiotherapy group. Therefore, we computed matched cohorts to overcome the resection bias and to validate the statistically non-significant differences that were found so far. A Cox-regression analysis of the matched cohorts confirmed the non-significant survival benefit of adjuvant radiotherapy. Nevertheless, a clear trend towards an improved outcome of adjuvant radiotherapy was obtained. In an additional analysis, we stratified the influence of the proliferation index on the outcome of adjuvant radiotherapy without a statistically significant difference (Supplementary Fig. 1A, B).
Other factors
Additional univariate and multivariate Cox-regression analysis of patients with progressive disease was performed to identify potential prognostic factors for tumour recurrence/progression. The preoperative KPS greater than 70 may be a potential factor for improved PFS in univariate analysis (HR 0.47 CI 95% 0.27–0.82, p = 0.008). However, no difference was shown in multivariate analysis (HR 0.6 CI 95% 0.33–1.07, p = 0.08). Age and sex were also included in this analysis, but Cox-regression analysis detected no difference. The univariate analysis (p = 0.017) and multivariate analysis (p = 0.03) indicated that tumours located in the anterior and posterior fossa compared with other locations were associated with improved PFS (Table 3).
Discussion
This study retrospectively reviewed patients with atypical meningioma (WHO grade II) operated on between 2001 and 2015, being one of the largest single-institutional series published in the literature regarding atypical meningiomas. The study database contained 161 cases of atypical meningioma, 128 were treated with surgery alone and 33 additionally received conventional radiotherapy after surgical resection. The purpose of this study was to investigate the role of adjuvant radiotherapy after WHO grade II meningioma resection to determine possible prognostic factors for tumour recurrence/progression in this cohort.
Radiotherapy
The use of adjuvant radiotherapy after surgical resection of WHO II meningioma is still controversially. A total of 20% of cases received adjuvant radiotherapy after surgery in this study, which is comparable to other series within reported ranges of 7.4–59.1%. In the present study, no significant benefit was found in the group with postoperative radiotherapy vs. surgical treatment alone (hazard ratio = 1.48, p-value = 0.22). These findings are consistent with other recently reported results [6, 8, 10, 11, 13, 14, 17]. As described earlier, Endo et al. [14] implied that additional postoperative radiotherapy could not improve long-term tumour control. Other studies [7, 15, 16, 23] underline the doubtful benefit of additional postoperative radiotherapy of atypical meningioma, underlining the unambiguous results of our study. In contrast, several studies recommended the usefulness of postoperative treatment with radiotherapy in the past [1,2,3, 5, 16, 25]. Other studies [18, 24] implied that radiotherapy could offer good tumour control in atypical meningioma following only a subtotal resection. Bagshaw et al. [5] supported the application of adjuvant radiotherapy even after a gross total resection of atypical meningioma. Nonetheless, the benefit of adjuvant radiotherapy versus observation after surgical resection of atypical meningioma is still unclear and needs to be prospectively assessed. The ongoing ROAM trial and the Phase III trial of observation versus irradiation for gross totally resected grade II meningioma will evaluate the advantage of radiotherapy after a gross total resection in the future [19].
Stereotactic radiosurgery
Radiosurgery is a common treatment strategy for small inoperative or partially resected meningioma (grade I) predominantly located in the skull base. Cohen-Inbar et al. showed that stereotactic radiosurgery offers high rates of growth control with a low incidence of neurological deficits in benign parasellar and skull base meningioma [10, 11]. Additionally, recent studies explored the role of stereotactic radiosurgery for atypical meningioma (grade II). Refaat et al. demonstrated that postoperative gamma knife radiosurgery could improve the long-term clinical outcome for atypical meningioma [32].
Surgery
The extent of resection is known to influence the recurrence rates of all meningioma grades [14, 30]. Our results corroborate with those of other studies [1,2,3,4, 6, 7, 14, 20, 37]. A gross total resection is associated with improved PFS in comparison to a subtotal resection [1,2,3,4, 6, 7, 14, 20, 37]. Our findings emphasize the fact that the extent of resection remains an important factor in minimizing early recurrence in atypical meningioma (Fig. 1).
Predictors of progression-free survival
The median age at surgery was 69.9 years in our cohort, with older patients (> 70 years) showing a tendency for a poorer PFS. However, the differences between “younger” and “older” patients could not be confirmed as an independent predictive factor in the univariate and multivariate regression model. Champeux et al. defined a “62 years” cut-off as an independent predictor of overall survival [7] and other authors found that older age (> 70 years) was associated with worse overall survival, being a predictive factor of recurrence risk [2, 12, 36]. The preoperative KPS < 70 indicated the potential for recurrent tumour in univariate analysis (HR 0.47 CI 95% 0.27–0.82, p = 0.008). However, no difference was detected in multivariate analysis (HR 0.6 CI 95% 0.33–1.07, p = 0.08). Sex was also included in our analysis, but Cox-regression analysis revealed no significant difference.
Proliferation-index (MIB-1)
A high proliferation-index (MIB-1) with a cutoff value of 10% was significantly correlated with lower PFS (HR 2.4 CI 95% 2.05–2.8, p = 0.0045). These findings are coherent with other recently reported results [21, 30]. Champeaux et al. showed that MIB-1 is a useful predictor of risk of recurrence. Atypical meningioma with proliferation-index (MIB-1) greater than 15% was associated with a higher recurrence rate [8], also Lee et al. showed that MIB-1 is an independent predictive factor for PFS [25]. However, Aghi et al. reported that proliferation-index (MIB-1) was not a predicting factor of recurrence after a gross total resection of atypical meningioma [2].
Tumour location
Atypical meningioma located at the anterior or posterior fossa were associated with significantly longer PFS compared with tumours located in other locations (HR 4.4 CI 95% 1.08–18.44, p = 0.03) (Table 3). Kane et al. showed that primary atypical meningioma with non-skull base locations were associated with poor prognosis and higher recurrence risk [22]. Vranic et al. reported that the parasagittal–falcine location was associated with a higher recurrence rate in their series of 76 cases of atypical meningioma [35]. Zaher et al. associated the location at the convexity with a better non-significant survival of their patients [36]. In other published series, the location of the atypical meningioma did not influence the clinical outcome [1,2,3,4,5, 7, 11, 14, 15, 17, 20, 21, 26, 32]. It appears that the location of meningioma may have an influence on the prognosis. However, the underlying mechanisms are not fully understood. Clark et al. [9] examined the correlation between mutation spectrum and anatomical distribution, finding that meningioma located at the midline und middle fossa carried both TNF receptor-associated factor 7 (TRAF7) and Kruppel-like factor 4 (KLF4) mutations and were associated with more aggressive clinical course owing to increased brain swelling. Different mutations were detected in other locations, but they were not shown to be associated with prognosis.
Limitations of the study
This study is limited by its retrospective nature and the limited external validity within a single-institution. A further limitation is the relatively small number of patients treated with surgery plus radiotherapy, which may not fully represent the prognosis of patients treated with adjuvant radiotherapy. However, the percentage of patients treated with adjuvant radiotherapy is within reported ranges and our study is one of the largest series in comparison to published reports in the literature to date. Moreover, grade III meningioma patients were excluded due to different tumour biology and behaviour; consequently, WHO grade III meningioma should not be compared with WHO grade II meningioma when assessing clinical outcome [1, 11]. Additionally, the study is limited by a restricted follow-up time (5 years: 64% and 10 years: 31%).
Conclusions
The most important prognostic factor in determining recurrence was extent of resection according to Simpson grading. Additional adjuvant radiotherapy did not significantly increase progression-free survival after complete or incomplete resection. The location of the tumours at the anterior or posterior fossa was an independent factor associated with improved PFS. The use of adjuvant radiotherapy remains controversial in the management of atypical meningioma. Prospective randomized trials, such as the ongoing ROAM trial, are required to define the role of radiotherapy in the management of patients with atypical and malignant meningioma, beyond the conflicting evidence from the existing retrospective series.
References
Adeberg S, Hartmann C, Welzel T, Rieken S, Habermehl D, von Deimling A et al (2011) Long-term outcome after radiotherapy in patients with atypical and malignant meningiomas-clinical results in 85 patients treated in a single institution leading to optimized guidelines for early radiation therapy. Int J Radiat Oncol Biol Phys 83:859–864. doi:10.1016/j.ijrobp.2011.08.010
Aghi MK, Carter BS, Cosgrove GR, Ojemann RG, Amin-Hanjani S, Martuza RL et al (2009) Long-term recurrence rates of atypical meningiomas after gross total resection with or without postoperative adjuvant radiation. Neurosurgery 64:56–60. doi:10.1227/01.NEU.0000330399.55586.63
Aizer AA, Arvold ND, Catalano P, Claus EB, Golby AJ, Johnson MD et al (2014) Adjuvant radiation therapy, local recurrence, and the need for salvage therapy in atypical meningioma. Neuro Oncol 16:1547–1553. doi:10.1093/neuonc/nou098
Andric M, Dixit S, Dubey A, Jessup P, Hunn A (2012) Atypical meningiomas—a case series. Clin Neurol Neurosurg 114:699–702. doi:10.1016/j.clineuro.2011.11.023
Bagshaw HP, Burt LM, Jensen RL, Suneja G, Palmer CA, Couldwell WT et al (2016) Adjuvant radiotherapy for atypical meningiomas. J Neurosurg. doi:10.3171/2016.5.JNS152809
Cao X, Hao S, Wu Z, Wang L, Jia G, Zhang L et al (2015) Treatment response and prognosis after recurrence of atypical meningiomas. World Neurosurg 84:1014–1019. doi:10.1016/j.wneu.2015.05.032
Champeaux C, Dunn L (2016) World Health Organization grade II meningiomas. Acta Neurochir 158:921–929. doi:10.1007/s00701-016-2771-y
Champeaux C J V (2016) World Health Organization grade III meningiomas. A retrospective study for outcome and prognostic factors assessment. Neurochirurgie 62:203–208
Clark VE, Erson-omay EZ, Serin A, Yin J, Cotney J, Avşar T et al (2016) Genomic analysis of non-NF2 meningiomas reveals mutations in TRAF7, KLF4, AKT1, and SMO. Science 339:1077–1080. doi:10.1126/science.1233009.Genomic
Cohen-inbar O, Lee C, Schlesinger D, Xu Z, Sheehan JP (2016) Long-term results of stereotactic radiosurgery for skull base meningiomas. Neurosurgery 79:58–68. doi:10.1227/NEU.0000000000001045
Cohen-inbar O, Tata A, Moosa S, Lee C, Sheehan JP (2017) Stereotactic radiosurgery in the treatment of parasellar meningiomas: long-term volumetric evaluation. J Neurosurg. doi:10.3171/2016.11.JNS161402
Durand A, Labrousse F, Jouvet A, Bauchet L, Kalamaridès M, Menei P, Deruty R, Moreau JJ, Fèvre-Montange M, Guyotat J (2009) WHO grade II and III meningiomas: a study of prognostic factors. J Neurooncol 95:367–375
Dziuk TW, Woo S, Butler EB, Thornby J, Grossman R, Dennis WS, Lu H, Carpenter LS Chiu JK (1998) Malignant meningioma: an indication for initial aggressive surgery and adjuvant radiotherapy. J Neurooncol 37:177–188
Endo T, Narisawa A, Ali HSM, Murakami K, Watanabe T, Watanabe M et al (2016) A study of prognostic factors in 45 cases of atypical meningioma. Acta Neurochir 158:1661–1667. doi:10.1007/s00701-016-2900-7
Hammouche S, Clark S, Wong AH, Eldridge P FJ (2014) Long-term survival analysis of atypical meningiomas: survival rates, prognostic factors, operative and radiotherapy treatment. Acta Neurochir 156:1475–1481
Hasan S, Young M, Albert T, Shah AH, Okoye C, Bregy A et al (2015) The role of adjuvant radiotherapy after gross total resection of atypical meningiomas. World Neurosurg 83:808–815. doi:10.1016/j.wneu.2014.12.037
Heynckes S, Gaebelein A, Haaker G, Grauvogel J, Franco P, Mader I et al (2017) Expression differences of programmed death ligand 1 in de-novo and recurrent glioblastoma multiforme. Oncotarget 1:1–8
Iley KRR (2008) Hitting a moving target: evolution of a treatment paradigm for atypical meningiomas amid changing diagnostic criteria. Neurosurg Focus 24:1–8. doi:10.3171/FOC/2008/24/5/E3
Jenkinson MD, Javadpour M, Haylock BJ, Young B, Gillard H, Vinten J et al (2015) The ROAM/EORTC-1308 trial: radiation versus observation following surgical resection of atypical meningioma: study protocol for a randomised controlled trial. Trials 16:519. doi:10.1186/s13063-015-1040-3
Jenkinson MD, Waqar M, Farah JO, Farrell M, Barbagallo GM V, McManus R et al (2016) Early adjuvant radiotherapy in the treatment of atypical meningioma. J Clin Neurosci 28:87–92. doi:10.1016/j.jocn.2015.09.021
Jo K, Park HJ, Nam DH, Lee J Il, Kong DS, Park K et al (2010) Treatment of atypical meningioma. J Clin Neurosci 17:1362–1366. doi:10.1016/j.jocn.2010.03.036
Kane AJ, Sughrue ME, Rutkowski MJ, Shangari G, Fang S (2011) Anatomic location is a risk factor for atypical and malignant meningiomas. Cancer 117:1272–1278. doi:10.1002/cncr.25591
Kaur G, Sayegh ET, Larson A, Bloch O, Madden M, Sun MZ et al (2014) Adjuvant radiotherapy for atypical and malignant meningiomas: a systematic review. Neuro Oncol 16:628–636. doi:10.1093/neuonc/nou025
King G, Stuart EA (2011) MatchIt: nonparametric preprocessing for parametric causal inference. J Stat Softw 42:1–28
Lee KD, DePowell JJ, Air EL, Dwivedi AK, Kendler A, McPherson CM (2013) Atypical meningiomas: is postoperative radiotherapy indicated? Neurosurg Focus 35:E15. doi:10.3171/2013.9.FOCUS13325
Louis DN, Perry A, Reifenberger G, von Deimling A, Figarella-Branger D, Cavenee WK et al (2016) The 2016 World Health Organization classification of tumors of the central nervous system: a summary. Acta Neuropathol 131:803–820. doi:10.1007/s00401-016-1545-1
Louis DN, Ohgaki H, Wiestler OD, Cavenee WK, Burger PC, Jouvet A, Scheithauer BW KP (2007) The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol 114:97–109
Modha A GP (2005) Diagnosis and treatment of atypical and anaplastic meningiomas: a review. Neurosurgery 57:538–550
Ostrom QT, Gittleman H, Liao P, Rouse C, Chen Y, Dowling J et al (2014) CBTRUS statistical report: primary brain and central nervous system tumors diagnosed in the United States in 2007–2011. Neuro Oncol 16(Suppl 4):iv1–iv63. doi:10.1093/neuonc/nou223
Park HJ, Kang HC, Kim IH, Park SH, Kim DG, Park CK et al (2013) The role of adjuvant radiotherapy in atypical meningioma. J Neurooncol 115:241–247. doi:10.1007/s11060-013-1219-y
Pisćević I, Villa A, Milićević M, Ilić R, Nikitović M, Cavallo LM et al (2015) The influence of adjuvant radiotherapy in atypical and anaplastic meningiomas: a series of 88 patients in a single institution. World Neurosurg 83:987–995. doi:10.1016/j.wneu.2015.02.021
Refaat T, Gentile M, Sachdev S, Dalal P, Butala A, Gutiontov S, Helenowksi I, Lee P, Sathiaseelan V, Bloch O, Chandler J (2017) Gamma Knife stereotactic radiosurgery for grade 2 meningiomas. J Neurol Surg 78:288–294
Saraf S, McCarthy BJ, Villano JL (2011) Update on meningiomas. Oncologist 16:1604–1613. doi:10.1634/theoncologist.2011-0193
Simpson D (1957) The recurrence of intracranial meningiomas after surgical treatment. J Neurol Neurosurg Psychiatry 20:22–39
Vranic A, Popovic M, Cör A, Prestor B, Pizem J (2010) Mitotic count, brain invasion, and location are independent predictors of recurrence-free survival in primery atypical and malignant meningiomas: a study of 86 patients. Neurosurgery 67:1124–1132
Zaher A, Abdelbari Mattar M, Zayed DH, Ellatif RA, Ashamallah SA (2013) Atypical meningioma: a study of prognostic factors. World Neurosurg 80:549–553. doi:10.1016/j.wneu.2013.07.001
Zhao P, Hu M, Zhao M, Ren X (2015) Prognostic factors for patients with atypical or malignant meningiomas treated at a single center. Neurosurg Rev 38:101–107. doi:10.1007/s10143-014-0558-2
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Authors declare no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Masalha, W., Heiland, D.H., Franco, P. et al. Atypical meningioma: progression-free survival in 161 cases treated at our institution with surgery versus surgery and radiotherapy. J Neurooncol 136, 147–154 (2018). https://doi.org/10.1007/s11060-017-2634-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11060-017-2634-2