Abstract
Meningiomas account for up to 20 % of all primary intracranial neoplasms; although the majority of these have a benign course, as many as 5–10 % can display more aggressive behavior and a higher incidence of disease progression. The benefit of immediate adjuvant radiotherapy is still being debated for atypical and malignant meningiomas. This study aimed to retrospectively assess prognostic factors and outcome in 68 patients with atypical and malignant meningiomas. Sixty-eight meningioma patients were treated with radiotherapy after initial resection or for recurrence, between January 1993 and December 2011. Surgery was macroscopically complete in 80 % of the patients; histology was atypical and malignant in 51 patients and 17 patients, respectively. Mean dose of radiotherapy was 54.6 Gy. Fifty-six percent of all patients received radiotherapy after surgical resection, 26 % at the first relapse, and 18 % at the second relapse. Median follow-up was 6.7 years, (range 1.5–19.9 years). The 5- and 10-year actuarial overall survival (OS) rates were 74.1 and 45.6 %, respectively. At univariate analysis age >60 years, radiotherapy dose >52 Gy showed statistical significance, (p = 0.04 and p = 0.03, respectively). At the multivariate analysis radiotherapy dose >52 Gy maintained the statistical significance, (p = 0.037). OS of patients treated with radiotherapy at diagnosis was longer than the survival of patients treated with salvage radiotherapy; however this difference did not reach statistical significance when tested for the entire series or for the subgroups of grade 2 and grade 3 patients. The 5- and 10-year disease-free survival (DFS) rates were 76.5 and 69.5 %, respectively, and were significantly influenced by size >5 cm (p = 0.04) and grading (p = 0.003) on univariate analysis. At multivariate analysis, size and grading both remained significant prognostic factors, p = 0.044 and p = 0.0006, respectively. Grade ≤ 2 acute side effects were seen during radiotherapy treatment in 16 % of the patients, with no ≥ grade 3 acute toxicity, based on the Common Terminology Criteria for Adverse Events. In this mono-institutional retrospective study, age and radiotherapy dose were associated with a longer OS, while preoperative size and grading of the tumor influenced DFS. Although there were some advantages in terms of OS for patients treated with postoperative radiotherapy, the benefit did not reach the significance. Multicenter prospective studies are necessary to clarify the management and the correct timing of radiotherapy in such a rare disease.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Meningiomas account for nearly 15–20 % of all primary brain tumors in adults, the vast majority of which are histologically benign World Health Organization (WHO grade 1), having a more indolent course and a lower rate of local recurrence, while atypical and malignant meningiomas account for ~5–7 and 1–3 % of all meningiomas, respectively. This aggressive variant, originally described by Cushing, Eisenhardt and coworkers [1–4], is referred to nowadays as grade 2 and grade 3 meningiomas, according to the WHO classification.
The WHO classification was revised firstly in 2000 and then in 2007, creating a standardizable set of diagnostic criteria, and since then the correlation between histological grade and subsequent tumor behavior have significantly improved. The new grading criteria have also led to a significant increase in the proportion of meningiomas categorized as atypical.
These tumors present clinical and histological features suggesting aggressive potential; early diagnosis and classification is crucial, since, differently from benign meningiomas, where postoperative radiotherapy (RT) can be withheld, adjuvant radiotherapy may have a significant role.
The benefit of immediate adjuvant radiotherapy is clearer for malignant meningiomas, while the role of radiotherapy is still under debate for atypical ones. In fact postoperative radiotherapy is frequently chosen for atypical meningiomas, even if there is no clear consensus that this treatment is indicated. More particularly, there is ongoing debate on whether all grade 2 meningiomas should receive radiotherapy or whether the treatment should be limited to incompletely resected ones [5, 6].
In the present manuscript, we have analyzed the outcome in one of the largest populations of atypical and malignant meningiomas patients treated with radiotherapy in a single institution, focusing on prognostic factors in terms of overall survival (OS) and progression-free survival.
Materials and methods
Between January 1993 and December 2011, 68 consecutive patients referred to our centre for evaluation, (51 with atypical and 17 with malignant meningiomas) were treated with radiotherapy at the Radiation Oncology Unit, Azienda Ospedaliero-Universitaria Careggi, Florence, Italy.
Patient sex, age at the time of surgery, symptoms at diagnosis, tumor location, the extent of resection, histology grade, the use of postoperative radiotherapy, the duration of follow up, and recurrence were recorded for each patient.
The degree of tumor resection was recorded as either gross total resection (GTR), in which the whole tumor was removed with dural excision or coagulation (Simpson grade 1 or 2), or subtotal resection (STR), in which some residual tumor was left (Simpson grade 3 or 4), as implied by the surgeon and as shown subsequently on postoperative imaging [7].
Patient characteristics
Sex ratio (male/female) was 75 %, and median age was 58.5 years ± 14.4. Patients presented at initial diagnosis motor/sensory deficits, headache, cognitive disturbance, and seizure in 29, 41, 20 and 10 %, respectively.
Meningiomas were located at the cerebral convexity in 33 patients (47 %), in a parasagittal/falcine site in 19 (28 %), arising from the skull base in 15 (23 %), and at other sites in 1 (2 %).
Fifty-five patients (80 %) underwent GTR (Simpson grade 1 or 2) with the remaining 13 (20 %) undergoing partial removal (Simpson grade 3 in eight patients and Simpson grade 4 in 5 patients).
Histology was atypical and malignant in 51 patients (75 %) and 17 patients (25 %), respectively.
Patients characteristics are summarized in Table 1.
Treatment characteristics
Radiotherapy was administered after initial resection in 38 patients (55.9 %) or at recurrence, with or without further resection, in 30 patients (44.1 %).
Most patients (61) were treated with three-dimensional conformal radiotherapy; only 7 patients, treated before 1995, received conventional 2-D radiotherapy. For 3-D treatment planning, patients were fixed with a custom-made mask fixation; computed tomography as well as magnetic resonance imaging, when available, were used for treatment planning.
The clinical target volume (CTV) was defined as the macroscopic lesion visible on pre-operative contrast-enhanced imaging (GTV), plus the subclinical microscopic tumor (including the preoperative tumor or postoperative tumor bed, peritumoral edema, and dural enhancement or thickening as seen in the imaging at diagnosis), adding 1 cm margin. Organs at risk such as the brainstem, optic nerves, chiasm and spinal cord were contoured.
Mean dose was 54.6 Gy (range 50–60 Gy), (2 Gy per fraction a day, five fractions a week).
Dose ≥54 Gy was administered in cases with one or more of the following factors: anaplastic histotype, infiltration of brain parenchyma, STR, diameter >5 cm.
Toxicity was assessed according to the Common Terminology Criteria for Adverse Events (CTCAE) v 4.0.
Clinical neurological assessment with contrast-enhanced imaging was scheduled 6 weeks after completion of RT, and, thereafter at 3 month intervals. No patient was lost to follow-up. Median follow-up was 6.7 years, (range 1.5–29.9 years).
Treatment characteristics are summarized in Table 1.
Characteristics of patients treated with radiotherapy for newly diagnosed cases were not significantly different from characteristics of patients treated with radiotherapy for recurrent disease [Chi square test p > 0.05 (Table 1)].
Statistical analysis
The date of first surgery was used as the start of observation for the OS analyses. The survival time was calculated from this date to the date of death or of the last follow-up for patients still alive. We also estimated disease-free survival (DFS) as the time from the start of RT to the occurrence of local relapses (LR). The probability of dying or LR occurrence was estimated by using the Kaplan–Meier method, and differences between patient groups were assessed by the log-rank test. Estimated relative risks of death or LR occurrence were expressed as hazard ratios (HR) and corresponding 95 % confidence intervals (95 % CI). Univariate Cox regression models were used to evaluate the effect of each specific parameter on death or LR occurrence. Multivariate regression models with stepwise selection were used to identify the influence of the main independent prognostic factors on specific outcome (death and LR). Differences among the various patient subgroups were calculated using the Chi square test. Statistical results were considered significant at a p value <0.05. All statistical tests were performed using the SPSS for windows software (version 8.1).
Results
Time from first surgery to first recurrence
Median time between surgery and first recurrence was 25.8 months for the 30 patients who were not treated with radiotherapy at diagnosis (range 11.8–92.6 months). Among the 38 newly diagnosed cases who were treated with postoperative radiotherapy at the time of the study there were only 5 recurrences; for these five recurrences the median time between surgery and first recurrence was 18.9 months (range 9.8–28.4 months).
Toxicity of radiotherapy
Grade ≤ 2 acute side effects were seen during radiotherapy treatment in 11 patients out of 68 (16 %), including skin irritation, alopecia, fatigue, sickness, headache. No patients experienced ≥ grade 3 acute toxicity, based on the CTCAE.
Response rate
Tumor response of patients with STR (n = 12) was assessed by imaging studies. Imaging criteria for tumor progression and regression were a 25 % increase and decrease, respectively, in MRI volume. Eight patients had stable disease, four had partial response and one had progressive disease.
Overall survival
The actuarial OS rates of the entire series were 74.1 and 45.6 % at 5 and 10 years, respectively.
We did not find a statistically significant difference (log-rank test p = 0.18) between patients treated with radiotherapy at diagnosis (OS at 5 years 75.2 % ± 0.13) compared to those treated at recurrence (OS at 5 years 72.9 % ± 0.12).
Moreover, after stratifying patients according to grade, there was no significant difference in OS between patients treated with radiotherapy at diagnosis and those treated with salvage radiotherapy (OS at 5 years for grade 2 meningiomas treated with radiotherapy at diagnosis: 83.3 % ± 0.15, OS at 5 years for grade 2 meningiomas treated with salvage radiotherapy 78.3 % ± 0.11; p = 0.99. OS at 5 years for grade 3 meningiomas treated with radiotherapy at diagnosis: 56.3 % ± 0.19, OS at 5 years for grade 3 meningiomas treated with salvage radiotherapy 48.9 % ± 0.25; p = 0.09).
The following parameters were assessed at univariate and multivariate analysis: age, sex, RT dose, grading, size of lesion, MIB-1, brain invasion, mitotic rate and radicality of surgery.
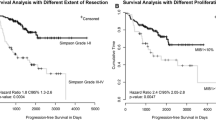
At univariate analysis age >60 years (Fig. 1) and radiotherapy dose >52 Gy showed statistical significance (Fig. 2), (p = 0.04 and p = 0.03, respectively). At multivariate analysis only radiotherapy dose >52 Gy maintained statistical significance, p = 0.037.
Disease-free survival
At last follow-up, 12 patients out of 68 (5 with grade 2 meningioma, 8 with grade 3 meningioma) had LR. The rates of 5- and 10-year DFS rates were 76.5 and 69.5 %, respectively.
Treatment of relapse after radiation treatment was surgery followed by radiosurgery, radiosurgery alone, and surgery alone in 27, 11, and 62 % patients, respectively.
DFS was significantly influenced by size >5 cm (p = 0.047) and grading (p = 0.003) at univariate analysis, (Figs. 3, 4; Table 2). At multivariate analysis size and grading both remained significant prognostic factors, p = 0.044 and p = 0.0006, respectively.
Discussion
Although the important limits of this series (its retrospective nature and the absence of a second review of the histologic specimens), to our knowledge, the present study is one of the largest mono-institutional series of atypical and malignant meningiomas ever published. The aim of the present manuscript is to analyze the influence of prognostic factors on local recurrence and survival rates and to assess outcomes in patients with non-benign meningiomas.
Prognostic factors
In our study age >60 years and dose ≤52 Gy were adverse prognostic factors for OS, whereas WHO grade 3 and size >5 cm were associated with worse DFS.
Older age has been found to be an unfavourable prognostic factor in several series [6, 8–10].
In the series by Milosevic et al. [11], younger age and dose >50 Gy were associated with a favourable outcome at multivariate analysis.
Goldsmith et al. [12]. recommended that patients with non-benign meningiomas should receive at least 53 Gy to achieve a better outcome (5-year progression free survival 63 % for doses ≥53 vs 17 % for lower doses). Dziuk et al. [13]. recommended that at least 60 Gy should be administered even after total resection [7].
Grading was found to be a relevant prognostic factor in various studies by other authors [14, 15].
In the series published by Palma et al. [14], 71 patients (WHO grade 2 n = 42 and WHO grade 3 n = 29) received radiotherapy. OS rate at 10 years was 79 and 34 % in atypical and malignant meningiomas, respectively (p = 0.001); recurrence-free survival was also significantly longer in patients with atypical than in those with malignant meningiomas: (11.9 vs 2.0 years, respectively, p = 0.001). In the Mayo Clinic series [15], estimated 5-year mortality rates in atypical and anaplastic meningiomas were 25 and 83 %, respectively. Recently, Adeberg et al. [16]. published the long-term results of sixty-two patients treated with radiotherapy (n = 39 atypical meningioma, n = 23 malignant meningioma). They found that histological grade showed a significant impact on overall and progression-free survival. OS at 5 years was 81 % for atypical and 53 % for malignant meningioma, (p = 0.022). Progression-free survival was 50 % for atypical, and 13 % for malignant meningioma at 5 years, (p = 0.017).
Several series have reported that the radicality of surgery was associated with outcome [14, 17, 18]. Dziuk et al. [13] in 1998 reported that in patients affected by atypical meningioma GTR is associated with lower recurrence rates (17 %) than STR (87 %). In cases of anaplastic meningiomas, GTR led to lower recurrence rate and increased survival, as reported by Perry et al. [15]. In our series, instead, total resection did not achieve statistically significant improvements either in terms of OS or in terms of DFS. As reported by Pasquier et al. [10], this divergent finding may be due to the difficulty of retrospectively assessing the grade of surgical exeresis.
In a few series, some histologic parameters such as brain invasion [19], mitotic rate [10] and Mib-1 [20, 21] were found to be powerful prognostic factors. However, in our study, no proliferative index achieved relevant prognostic value.
Radiotherapy timing
In our study, the OS of patients treated with radiotherapy at diagnosis was better than the survival of patients treated with salvage radiotherapy; however this difference did not reach statistical significance when tested with log-rank test, either for the entire series or for the cohorts of grade 2 and grade 3 patients.
This study does not address the value of adjuvant radiotherapy in terms of progression-free survival, as it does not include patients treated with exclusive surgery at diagnosis who did not relapse. For this reason, we had no censored cases of patients treated with surgery only, so we could not perform a Kaplan–Meier analysis for comparing progression-free survival of patients treated or not with radiotherapy at diagnosis.
Studies concerning RT for aggressive meningiomas have often been statistically limited due to the rarity of these tumors.
The majority of the authors recommend radiotherapy for grade 3 meningiomas, regardless of extent of surgery, considering the extremely high rate of local recurrence [5, 9, 11, 13, 16, 22, 23].
Dziuk et al. [13], in a series of 48 malignant meningiomas compared patients treated with postoperative radiation treatment with patients treated with exclusive surgery and found that adjuvant RT following initial surgery significantly increased the 5-year DFS rates from 15 to 80 %. Postoperative radiotherapy was found to be an independent prognostic factor by multivariate analysis.
Recently, Stessin et al. [8] examined the effect of postoperative radiotherapy on disease-specific survival in 657 patients with non-benign meningiomas from the Surveillance, Epidemiology, and End Results (SEER) database. Radiotherapy did not cause survival benefit considering the entire series, including WHO grade II (3.9 %), grade III (13.3 %) and unknown grade (82.8 %) meningiomas. When Kaplan–Meier curves were replotted only for patients with grade III histology, the median disease-specific survival was longer for patients treated with adjuvant radiotherapy versus exclusive surgery (72 vs 42 months, respectively); however, this difference was not statistically significant.
In the published literature the role of adjuvant RT in grade 2 meningiomas is more contradictory. Some authors have concluded that postoperative radiation therapy should be recommended for all cases of atypical meningiomas in order to improve outcome [6, 11, 24].
Aghi et al. [6], in a retrospective review of 108 atypical meningiomas, showed high recurrence rate of atypical meningiomas even in patients who had GTR (28 % of patients with completely resected atypical meningiomas presented a local recurrence, with an actuarial tumor recurrence rate at 5 years of 41 %). Thus, they conclude that postoperative radiotherapy could be useful.
Similarly, Kotomar et al. [25] analysed 45 cases with atypical meningiomas treated with gross total removal at Memorial Sloan-Kettering Cancer Center and found that GTR without postoperative radiotherapy was associated with a relative risk of recurrence approximately 5 times greater than with adjuvant postoperative radiotherapy. Analysing 45 cases with atypical meningiomas treated with gross total removal at Memorial Sloan-Kettering Cancer Center, they found actuarial recurrence rates at 5 years close to 42 % without radiotherapy and 20 % with radiotherapy.
Milker-Zabel et al. [24] reported very good results with a recurrence-free survival of 96 % at 3 years and 89 % at 5 years in 26 patients with atypical meningioma who received postoperative radiotherapy. They also showed a significant difference in local failure between patients treated for primary disease and patients treated for recurrence.
On the other hand, some authors recommended postoperative radiotherapy for atypical meningiomas only in selected cases.
Goyal et al. [17] and Jo et al. [26] concluded that atypical meningiomas should be treated with upfront radiotherapy only in case of STR. Goyal et al. [17], in fact, showed that incomplete resection without postoperative radiotherapy in atypical meningiomas is associated with a higher local failure rate, with a trend toward lower OS. In the series by Jo et al. [26], exclusive complete resection (Simpson grade I) was associated with no tumor recurrence, whereas significantly better progression-free survival (p = 0.011) was reached with adjuvant radiotherapy in the presence of incomplete surgical excision (Simpson II–IV).
Yang et al. [19] stated that adjuvant radiotherapy may be helpful for improved patient outcome not only when the atypical meningioma was incompletely resected but also in case of brain invasion.
Modha and Gutin [23] proposed an interesting treatment algorithm for the management of atypical meningiomas. According to them, immediate postoperative RT should be administered in all patients with atypical meningiomas subtotally resected. In case of complete excision, postoperative RT should be prescribed in case of invasion of the brain tissue, since they reported that nearly 60 % of brain-invasive tumors recur, with an approximate 25 % mortality rate. Finally, in case of a totally resected atypical meningioma without brain invasion, cases with higher proliferative index (Mib-1 >4 %) should be treated with postoperative RT.
In 2011 Mair et al. [27] investigated the role of postoperative radiotherapy in a large series of newly diagnosed patients undergoing resection of atypical meningioma. Thirty patients out of 114 were given postoperative radiotherapy. Postoperative radiotherapy did not provide a statistically significant benefit. The authors analysed the cohort of patients who had undergone STR: although there was a trend toward postoperative radiotherapy having some impact on reducing the progression rate, a log-rank test failed to demonstrate a statistically significant difference. The authors concluded that postoperative radiotherapy should be considered only in the presence of a large residual tumor, when radiosurgery or second surgery are not appropriate.
Conclusions
In the present study, age and radiotherapy dose were associated with a longer OS, while size and grading of the tumor influenced DFS. Even though the survival of patients treated with immediate RT was longer than OS of patients treated with radiation therapy at the recurrence, the benefit of postoperative RT was not statistically significant. The ongoing EORTC 22042-26042 and RTOG 0539 trials, will define the role of radiotherapy in the management of patients with atypical and malignant meningioma, beyond the conflicting evidence from the existing retrospective series.
References
Bondy M, Ligon BL (1996) Epidemiology and etiology of intracranial meningiomas: a review. J Neurooncol 29:197–205
Cushing HW, Eisenhardt LC (1938) Meningiomas. Their classification, regional behavior, life history, and surgical end results. Thomas CC, Springfield, pp 47–50, 669–719
Louis DN, Scheithauer BW, Budka H et al (2000) Meningiomas. In: Kleihues P, Cavenee WK (eds) Pathology and genetics. Tumors of the nervous system, WHO classification of tumors. IARC Press, Lyon, p 176–184
Kleihues P, Cavanee WK (eds) (2000) Pathology and genetics: tumours of the nervous system. IARC Press, Lyon, p 176
Engenhart-Cabillic R, Farhoud A, Sure U, Heinze S, Heinzel M, Mennel HD et al (2006) Clinicopathologic features of aggressive meningioma emphasizing the role if radiotherapy in treatment. Strahlenther Onkol 182:641–646
Aghi MK, Carter BS, Cosgrove GR, Ojemann RG, Amin-Hanjani S, Martuza RL et al (2009) Long-term recurrence rates of atypical meningiomas after gross total resection with or without postoperative adjuvant radiation. Neurosurgery 64:56e60
Simpson D (1957) The recurrence of intracranial meningiomas after surgical treatment. J Neurol Neurosurg Psychiatry 20:22–39
Stessin A, Schwartz A, Judanin G, Pannullo S, Boockvar J, Schwartz T et al (2012) Does adjuvant external-beam radiotherapy improve outcomes for nonbenign meningiomas? A Surveillance, Epidemiology, and End Results (SEER)–based analysis. J Neurosurg 117:669–675
Rosenberg L, Prayson R, Lee J, Reddy C, Chao S, Barnett G et al (2009) Long-term experience with World Health Organization grade III (malignant) meningiomas at a single institution. Int J Radiat Oncol Biol Phys 74(2):427–432
Pasquier D, Bijmolt S, Veninga T et al (2008) Atypical and malignant meningioma: outcome and prognostic factors in 119 irradiated patients. A multicenter, retrospective study of the rare cancer network. Int J Radiat Oncol Biol Phys 71(5):1388–1393
Milosevic MF, Frost PJ, Laperriere NJ, Wong CS, Simpson WJ (1996) Radiotherapy for atypical or malignant intracranial meningioma. Int J Radiat Oncol Biol Phys 34(4):817–822
Goldsmith BJ, Wara WM, Wilson CB et al (1994) Postoperative irradiation for subtotally resected meningiomas: a retrospective analysis of 140 patients treated from 1967 to 1990. J Neurosurg 80:195–201
Dzuik T, Woo S, Butler B et al (1998) Malignant meningioma: an indication for initial aggressive surgery and adjuvant radiotherapy. J Neurooncol 37:177–188
Palma L, Celli P, Franco C et al (1997) Long-term prognosis for atypical and malignant meningiomas: A study of 71 surgical cases. J Neurosurg 86:793–800
Perry A, Scheithauer BW, Stafford SL, Lohse CM, Wollan P (1999) ‘‘Malignancy’’ in meningiomas: a clinicopathologic study of 116 patients, with grading implications. Cancer 85:2046–2056
Adeberg S, Hartmann C, Welzel T, Rieken S, Habermehl D et al (2012) Long-term outcome after radiotherapy in patients with atypical and malignant meningiomas-clinical results in 85 patients treated in a single institution leading to optimized guidelines for early radiation therapy. Int J Radiat Oncol Biol Phys 83(3):859–864
Goyal LK, Suh JH, Mohan DS, Prayson RA, Lee J, Barnett GH et al (2000) Local control and overall survival in atypical meningioma: a retrospective study. Int J Radiat Oncol Biol Phys 46(1):57–61
Jaaskelainen J, Haltia M, Servo A (1986) Atypical and anaplastic meningiomas: radiology, surgery, radiotherapy and outcome. Surg Neurol 25:233–242
Yang S-Y, Park C-K, Park S-H, Kim D, Chung YS, Jung H-W (2008) Atypical and anaplastic meningiomas: prognostic implications of clinicopathological features. J Neurol Neurosurg Psychiatry 79:574–580
Bruna J, Brell M, Ferrer I, Gimenez-Bonafe P, Tortosa P (2007) Ki-67 proliferative index predicts clinical outcome in patients with atypical or anaplastic meningioma. Neuropathology 27:114–120
Ho M-T, Hsu C-Y, Ting L-T, Chiang H (2002) Histopathology and MIB-1 labelling index predicted recurrence of meningiomas. A proposal of diagnostic criteria for patients with atypical meningioma. Cancer 94(5):1538–1547
Pourel N, Auque J, Bracard S, Hoffstettter S, Luporsi E, Vignaud JM, Bey P (2001) Efficacy of external fractionated radiation therapy in the treatment of meningiomas: a 20-year experience. Radiat Oncol 61:65–70
Modha A, Gutin P (2005) Diagnosis and treatment of atypical and anaplastic meningiomas: a review. Neurosurgery 57:538–550
Milker-Zabel S, Zabel A, Schulz-Ertner D et al (2005) Fractionated stereotactic radiotherapy in patients with benign or atypical intracranial meningioma: long-term experience and prognostic factors. Int J Radiat Oncol Biol Phys 61:809–816
Komotar RJ, Iorgulescu JB, Raper DM, Holland EC, Beal K, Bilsky MH et al (2012) The role of radiotherapy following gross-total resection of atypical meningiomas. J Neurosurg 117:679–686
Jo K, Park HJ, Nam DH, Lee JI, Kong DS, Park K, Kim JH (2010) Treatment of atypical meningioma. J Clin Neurosci 17(11):1362–1366
Mair R, Morris K, Scott I, Carroll TA (2011) Radiotherapy for atypical meningiomas. J Neurosurg 115:811–819
Conflict of interest
The authors have no personal financial or institutional interest in any of the drugs, materials, or devices described in this article.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Detti, B., Scoccianti, S., Di Cataldo, V. et al. Atypical and malignant meningioma: outcome and prognostic factors in 68 irradiated patients. J Neurooncol 115, 421–427 (2013). https://doi.org/10.1007/s11060-013-1239-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11060-013-1239-7