Abstract
Mycobacterium tuberculosis (Mtb), the etiologic agent of tuberculosis (TB), is recognized by a number of pathogen recognition receptors (PRRs), either soluble or predominantly expressed on the surface of various cells of innate and adaptive immunity. C-type lectin receptors (CTLRs) are a class of PRRs which can recognize a variety of endogenous and exogenous ligands, thereby playing a crucial role in immunity, as well as in maintaining homeostasis. Mtb surface ligands, including mannose-capped lipoarabinomannan and cord factor, are important immune modulators which recently have been found to be directly recognized by several CTLRs. Receptor ligation is followed by cellular activation, mainly via nuclear factor κB mediated by a series of adaptors with subsequent expression of pro-inflammatory cytokines. Mtb recognition by CTLRs and their cross talk with other PRRs on immune cells is of key importance for the better understanding of the Mtb-induced complexity of the host immune responses. Epidemiological studies have shown that single nucleotide polymorphisms (SNPs) in several PRRs, as well as the adaptors in their signaling cascades, are directly involved in the susceptibility for developing disease and the disease outcome. In addition, an increasing number of CTLRs have been studied for their functional effects in the pathogenesis of TB. This review summarizes current knowledge regarding the various roles played by different CTLRs in TB, as well as the role of their SNPs associated with disease susceptibility and outcome in different human populations.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Tuberculosis (TB), a communicable infectious disease, remains the second leading cause of deaths from an infectious disease worldwide. In 2014, TB claimed about 1.5 million deaths, according to the Global Tuberculosis Report 2015 [1]. The majority of infected individuals develop asymptomatic latent TB, while ∼5–10 % of latently infected individuals will progress to active TB, resulting in about 9 million new TB cases and 1.4 million deaths per year [2].
Mtb is transmitted via inhalation of tiny droplets/aerosols containing the bacteria. Once Mtb enters the lung, it is recognized by alveolar macrophages (AMs) and phagocytosed, whereupon it proliferates until either the cell dies or is instructed by an antigen-specific T cell to kill or at least limit the growth of the bacterium [3]. However, Mtb has also correspondingly coevolved and developed mechanisms to survive inside the macrophages, mainly by inhibiting the phagosome–lysosome fusion, macrophage activation, and utilizing host molecules for its own intracellular growth [4, 5]. AMs are thought to be the primary target of the bacterium, and progress of the infection depends on the interaction between these two. If the infection is established, the migratory cells take the bacteria to a nearby lymph node, where the T cells get activated by antigen-presenting dendritic cells (DCs). The role of antigen-specific T cells and that of activated macrophages, whose activation in turn depends on the antigen-specific T cells and secretion of activating cytokines from the cells at the site of infection, have been shown to be most important in clearing or stopping early infection. In addition to these, the invariant natural killer T cells (iNKT), which produce the macrophage-activating, granulocyte macrophage colony-stimulating factor (GM-CSF), and γδT cells, which respond rapidly to the mycobacterial antigens, are also potentially involved in limiting early infection [6]. The overall outcome of infection depends on the quality of innate immune response elicited by the host and for which the innate immunity cells are armed with a plethora of receptors and other mechanisms. Mtb has a complex hydrophobic cell wall composed mainly of peptidoglycan, arabinogalactan, mycolic acids, and glycolipids which are capable of influencing and resisting the innate and adaptive immune response of the human host cells [7]. The lipids present on the cell wall are highly immunomodulatory and can influence the innate immunity in a quest to establish a niche inside the phagocytes. Molecules like phthioceroldimycoceroserate (PDIM) interfere with its recognition and thus the recruitment of activated macrophages to the infection site [6]. Trehalose-1,6-dimycolate (TDM) and lipoarabinomannan (LAM) are other powerful immunomodulators that interfere with phagosome maturation [8, 9]. Some of these bacterial surface molecules serve as pathogen-associated molecular patterns (PAMPs) that are detected by PRRs predominantly expressed on the cells of the innate immune system, such as monocytes and macrophages, DCs, as well as on some lymphocytes, and orchestrate Mtb-dependent innate and adaptive immune responses [10]. In this scenario, the best studied PRRs belong to the Toll-like receptor (TLR) family. TLR1, TLR2, TLR4, TLR6, and TLR9 have been reported to play an important initial role in the pathogenesis of TB [11–14].
Recent findings demonstrated that polymorphisms in several TLRs are associated with an increased susceptibility to Mtb, suggesting the importance of innate immunity in tackling the infection [15–20]. CTLRs are increasingly being recognized to play an important role in modulating Mtb-mediated immune responses, and several of its PAMPs are detected by this family of receptors [21–25]. In this review, we will, therefore, emphasize the role of CTLRs in Mtb recognition, TB susceptibility, and immune pathology of TB.
C-type lectin receptors: general structure, function, and signaling mechanism
CTLRs comprise a large family of receptors that are characterized by the presence of one or more carbohydrate-recognition domains (CRDs) that can bind to carbohydrate molecules in a Ca2+-dependent manner [26]. In addition, lipids, proteins, and even inorganic compounds have been found to serve as ligands for this group of receptors in a Ca2+-dependent or Ca2+-independent manner. Thus, the more general term C-type lectin-like domains (CTLDs) was introduced for these domains [27]. The CTLD structure consists of a characteristic double loop stabilized at the base by highly conserved disulfide bridges, hydrophobic and polar interactions. The second loop exhibits structural and evolutionary flexibility and can bind to different carbohydrate species in the presence of Ca2+ ions, thus providing functional versatility [26]. For example, the EPN motif confers binding to sugars such as glucose, mannose, N-acetylglucosamine, whereas the QPD motif binds to galactose and N-acetylgalactosamine. Variations in CTLD fold structure have also been described [26].
The CTLR receptor family, which includes collectins, selectins, phagocytic receptors, and proteoglycans, has been classified into 17 groups, based on domain organization and phylogenetic features [26, 28]. CTLRs have roles in maintaining homeostasis, as well as in anti-microbial host defense. They have been reported to be involved in several cellular functions, such as cell–cell adhesion, lipid scavenging, orchestrating immunity against tumors, as well as virus-infected self-cells, allergy, and the development of autoimmune reactions [29–32]. In addition, they have been shown to play an important role as PRRs in the recognition of many fungal pathogens, as has been reported in several studies in the last decade [33–38]. It is notable that the polymorphisms in many of the CTLRs and also the adaptors involved in their pathways are being increasingly associated with the susceptibility and severity of the clinical courses of several fungal infections [39–43]. Members of this receptor family have also been shown to interact with each other and to cross talk with other PRRs, for example, TLRs to modulate pathogen-associated immune responses [44–47].
CTLRs can be found in soluble form in body fluids or in transmembrane form on cell surfaces. The soluble forms which are called collectins are mainly found in oligomeric forms in serum, and in the mucosal fluids, such as in lung alveoli. Collectins enhance the aggregation, uptake, and neutralization of pathogens by cross-linking their surface carbohydrate antigens, attracting phagocytes and activating complement [48–54]. The transmembrane CTLRs, especially those expressed on myeloid cells, have also been broadly divided on the basis of the signaling effect that they exert: (1) CTLRs signaling via immunoreceptor tyrosine-based activation motif (ITAM) domains; (2) CTLRs signaling via an immunoreceptor tyrosine-based inhibitory motif (ITIM) domains; and (3) the CTLRs that do not bear any ITAM or ITIM domains [26, 55]. An ITAM motif typically consists of a two YxxL/I (Y = tyrosine, L = leucine, I = isoleucine, and x = any residue) amino acid sequences separated by six to twelve intervening residues. Receptor ligation leads to the phosphorylation of the tyrosine residue in ITAM by Src family kinases. This further leads to the recruitment of spleen tyrosine kinase (Syk) which activates transcription factors including NFκB via a complex consisting of caspase recruitment domain-containing protein 9 (CARD9), B cell lymphoma 10 (Bcl10) and mucosa-associated lymphoid tissue lymphoma translocation protein 1 (MALT1) (Fig. 1) [56, 57]. PKCδ has been shown to be important for linking Syk activation with CARD9 signaling [58]. The consensus ITIM sequence consists of a I/V/L/SxYxxI/L/V (V = valine; S = serine) motif. The tyrosine residue undergoes phosphorylation upon ligand recognition and recruits phosphatases Src homology 2 (SH2)-containing tyrosine phosphatase-1 (SHP-1), SHP-2, and/or SH2 domain-containing inositol phosphatase (SHIP-1) [59–61]. The ITIMs were previously proposed to exert an inhibitory effect on cellular activation especially those caused by the ITAM-containing receptors. However, later it was shown that ITAMs may also send inhibitory signals, while ITIMs may act in activation of certain cellular responses [62, 63]. ITIMs may also affect the TLR pathways by reducing the NFκB activity [55]. Some of the CLRs, such as Dectin-2, Mincle, MCL, C-type lectin domain family 5 member a (CLEC5a), which do not bear any signaling domains, have been shown to associate with ITAM-bearing adaptors such as FcRγ, DAP10, or DAP12 and mediate similar responses through Syk-CARD9-Bcl10-MALT-1 (Fig. 1) [27, 55]. Nevertheless, while the balance between both the activation and inhibition of immune responses is likely to play an important role in the fine-tuning of the pathogen-mediated immune response, the function of receptor clustering of several ITAM- and ITIM-bearing CTLRs on immune cells for the generation of immune responses is currently not well understood.
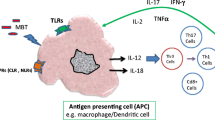
Recognition of Mtb ligands (ManLAM, PIMs, TDM) by membrane-bound CLRs and subsequent signaling pathways activated by receptor–ligand interaction. MR recognizes the mannose-containing molecules, such as ManLAM, and causes bacterial internalization, but inhibits phagosome–lysosome fusion and may also contribute to anti-inflammatory response. DC-SIGN activates Raf-1, possibly through other adaptors which, in turn, activate the NF-κB. Dectin-1 may potentially bind to α-glucans of Mtb and may cross talk with TLR2 to signal via Syk. MCL and Mincle form heterodimers and lead to pro-inflammatory cytokine production mainly via NF-κB activation. MCL also plays a role in bacterial phagocytosis [25]. Dectin-2 also probably signals through the Syk-CARD9 pathway and produces both pro- and anti-inflammatory signals (‘?’ There are conflicting or no studies yet confirming these associations/pathways) [23, 25, 128, 130, 183]
In several studies, it has been demonstrated that many CTLRs serve as important receptors for the recognition of Mtb ligands eliciting innate immune response against the pathogen (Fig. 1) [21, 23, 25, 64–67]. In the following sections, we will summarize current knowledge about each of those receptors which have been described as serving as PRR for Mtb. Taking each receptor individually, we will also collate the results of the studies associating SNPs in these CTLRs with the susceptibility to TB in different populations.
Soluble CTLRs: collectins
Collectins are collagenous soluble PRRs that mainly occur in mucosal lining fluids and in blood. They are a part of a first-line, non-specific defense system of innate immunity, that is able to recognize not only the gluco-conjugates, but also other type of ligands on the pathogen cell surface [68]. They consist of three chains, each with four domains—a cysteine-rich N-terminal which is required for oligomerization, a collagen-like region that maintains the overall structure and shape of the molecule, an α-helical neck domain that functions in protein trimerization, and a C-terminal carbohydrate-binding region which mediates ligand binding—and are generally found in multimeric forms [68, 69]. Among collectins, surfactant proteins SP-A and SP-D, and the complement protein MBL have been reported to recognize Mtb [65, 70–72].
Surfactant proteins (SP) A and D
SP-A and SP-D are large hydrophilic proteins of pulmonary surfactant that lines the alveolar epithelium in lungs and maintains the minimal surface tension preventing lung collapse. They are secreted by alveolar type II cells and Clara cells (Table 1). They can recognize a vast variety of carbohydrate ligands, such as mannose, glucose, N-acetylglucosamine (GlcNAc), N-acetylmannosamine (ManNAc), bacterial lipopolysaccharide (LPS), and pentoses. Lipid ligands include dipalmitoylphosphatidylcholine (DPPC), which is recognized by SP-A, while SP-D can bind to phosphatidylinositol (PI) [68, 69, 73]. SP-A and SP-D also serve as PRRs and are able to detect mycobacterial LAMs and other antigens of Mycobacterium tuberculosis (Table 1) [72, 74]. SP-A increased the phagocytosis of the Erdman strain, a virulent laboratory strain of Mtb, by human monocyte-derived macrophages (MDMs) which could be inhibited by anti-MR antibodies [75]. This observation was supported by another study demonstrating that SP-A upregulates the expression of mannose receptor (MR) on human MDMs [54]. SP-A levels were significantly decreased in BALF of TB patients and returned to normal in a month of therapy [65]. Low SP-A levels in patients’ BALF were also associated with increased inflammation marked by high neutrophil numbers [65], while higher SP-A levels were observed in TB patients’ serum [76]. However, the levels during the course or after the treatment were not investigated [76]. SP-A has also been shown to play a role in mediating oxidative burst of Mtb-infected macrophages. A study with rat AMs concluded that SP-A was able to suppress the production of reactive nitrogen intermediates in macrophages infected with the Mtb H37Ra strain [77]. In contrast, in another study, it was reported that SP-A enhanced the Mycobacterium bovis bacillus Calmette–Guerin (BCG)-induced production of nitric oxide synthase in rat BMMs [78]. The discrepancies in these studies might be attributed to the type of cells and the mycobacterial strains used.
SP-D can also cause agglutination of bacteria, but it has been associated with decreased adherence and phagocytosis of Erdman strain of Mtb by human MDMs. On the other hand, it has been demonstrated to enhance the fusion of bacteria-containing phagosomes with the lysosomes inside the macrophages, contributing to the intracellular mycobacterial growth control [53, 79]. Although the SP-A- and SP-D-deficient mice did not show any obvious differences compared to WT, an increased granulomatous infiltration was observed in SP-A-deficient mice. However, despite the many proved in vitro immunomodulatory roles, SP-A and SP-D seem to be dispensable in controlling immune status in response to mycobacterial infection, at least in murine infection models infected with the Erdman strain [80].
SP-A consists of two isoforms SP-A1 and SP-A2 encoded by separate genes. Polymorphisms in both of these genes as well as in SP-D have been studied for their potential role in host susceptibility toward developing TB in different populations summarized in Table 2. A study in Mexican population revealed many marker alleles flanking SP-A and SP-D that were protective or conferred susceptibility toward pulmonary TB in the given population [81]. In replication to this study, another group found that SP-A1 alleles SFTPA1 307A, SFTPA1 776T and SP-A2 alleles SFTPA2 355C and SFTPA2 751C were significantly associated with TB in Ethiopian population. The SFTPA2 751A/C polymorphism is present in the CTLD of SP-A, leading to a change of lysine to glutamine which could be influencing its binding to Mtb. The haplotype 1A3 in SP-A2, which also affects the amino acids in CTLD, was also found to be significantly associated with TB susceptibility [82]. In another study, two intronic SNPs SP-A2 G1649C and SPA2 A1660G in SP-A2 gene were identified to be associated with development of TB in an Indian population, whereas no association of SNPs in the SP-A1 gene was found [83]. In a more recent analysis, Yang et al. [84] also found a significant correlation of the SP-A2 1649G allele with pulmonary TB in Han Chinese population consistent with the results from studies in Ethiopian and Indian population. This allele leads to a transversion from proline to alanine at position 91 which may disturb the triple helical structure of SP-A, thus affecting its normal function [84]. An SP-D polymorphism, G459A in exon 7, has been reported to be significantly associated with TB susceptibility in an Indian population [85]. However, the molecular mechanism for these SNPs influencing the susceptibility to TB has not yet been elucidated.
Overall, it can be said that the surfactant proteins may play a role in the local immune responses during early infection in the lung alveoli. One of the several reasons for the discrepancy in the results from mouse models and human cell models might be that TB pathogenesis differs in mouse and human systems [86]. On the other hand, several SNPs have been shown to be associated with the regulation of SP-A1 and SP-A2 expression and others affecting their overall structure or binding affinity [82]. The association of these SNPs with TB in different populations suggests that surfactant proteins may have some immunomodulatory role in active TB infection. However, more studies with human cell or tissue models are required to establish a concrete role for these proteins in TB pathology.
Mannose-binding lectin
Mannose-binding lectin, MBL, is a member of the lectin pathway of complement system that consists of a collagenous region and a lectin domain [85]. It circulates predominantly in the serum, but has also been detected at other sites, such as the synovial fluid of inflamed joints, nasopharyngeal secretions, amniotic fluid, and subcellular compartments, including endoplasmic reticulum (Table 1) [87]. MBL structure resembles that of the surfactant proteins, with the basic structure being a trimer. In serum, it circulates as an oligomer of trimers, most often as hexamers. MBL, in association with Ca2+ ions, is able to recognize terminal sugars such as d-mannose, l-fucose, and N-acetyl-d-glucosamine, but not d-galactose and sialic acid, on the surface of a wide variety of bacteria, viruses, fungal species, parasites, as well as apoptotic and tumor cells [88, 89]. A recent study demonstrated mannosylated lipoarabinomannan (ManLAM) to be one of the main cell wall components of slow-growing mycobacteria interacting with MBL [90]. MBL can directly act as an opsonin or, in association with MBL-associated serine proteases (MASPs), it can activate the lectin pathway of the complement system cleaving complement factor C3 in an antibody-independent manner, thereby enhancing the clearance of the pathogens via phagocytosis [89, 91]. In vivo studies have shown that the deficiency of MBL and other components of complement lectin pathway increase the susceptibility to certain bacteria, fungi, as well as viruses [92–95]. However, the role of MBL in TB has not yet been studied in murine infection models.
In humans, MBL is coded by the gene MBL2 on chromosome 10. Serum levels of this protein exhibit high interindividual variations, which have been attributed mainly to the polymorphisms found in the promoter and exon 1 of the MBL2 gene, although non-genetic factors like age, hormone levels, or inflammation may also influence the serum levels [96]. The missense mutations in codons 52, 54, and 57 in exon 1, respectively, called D, B, and C alleles (the wild type is denoted as A and the three variant alleles as O), are known to disrupt the normal collagen helix, while the alleles H/L (−619), Y/X (−290), and P/Q (−66), upstream in the promoter region, affect the serum levels by regulating protein expression [88, 96]. These polymorphisms are in linkage disequilibrium and give rise to different haplotypes and genotypes, which may appear in varying frequencies among individuals, depending upon ethnicity [91, 97]. About 5–30 % of healthy individuals have MBL-deficient alleles, suggesting that these mutations may confer some selective advantage, as they have not been wiped out by natural selection [96]. Nevertheless, these polymorphisms and serum MBL levels have been associated with TB and other infectious and inflammatory diseases in different ethnic groups [91, 97]. The variant alleles have been associated with susceptibility to TB in different populations, while in others no association was observed (Table 2) [98–106]. A meta-analysis carried out by Denholm et al. comparing 17 different studies and considering the effect of MBL polymorphisms and/or serum levels found no significant association of MBL polymorphisms with TB, although a consistent increase in the serum MBL levels in TB patients was observed, which may have been an acute phase response during infection. It is worth noting that most of the studies included in this analysis did not investigate the MBL haplotypes including promoter polymorphisms and the cohort, and the study design in these investigations were of highly heterogeneous nature [107]. In contrast, a meta-analysis involving Chinese populations concluded that presence of allele B might be a risk factor for developing TB [108]. In addition, more recent studies have focused on correlating the serum MBL levels with these genotypes and studying the effect of SNPs on factors, such as treatment progression and outcome, interaction with environment, or other genes, and on TB caused by other mycobacterial species [109–114]. In a recent study in Vietnam, it was found that the YA/YA haplotype had a protective effect against developing new pulmonary TB in young adults. They also found that the MBL levels decreased over the course of treatment after seven months, again pointing to the fact that high MBL levels in TB patients may be the result of acute phase response and that this decrease was highly dependent upon the genotype [115].
Given that MBL is one of the acute phase proteins and its levels can be affected by several other factors and pathogens, as well as its complete absence in otherwise healthy individuals, it becomes difficult to ascertain a particular role for this CTLR in TB infection. Moreover, the conflicting results from the genetic studies carried out with different populations suggest that MBL may not be directly involved pathogenesis of TB.
Membrane-bound CTLRs
Transmembrane PRRs are present on the cells of the immune system and play an important role in modulating the innate immune response to pathogens. While among the CTL receptors MR, DC-SIGN, and complement receptor 3 (CR3) have long been known to recognize Mtb [116–118], some new receptors have emerged in the last decade which have been shown to be of direct importance in Mtb-mediated immune response (Fig. 1). In humans, the genes encoding for these CTLs are located on chromosome 10, in the so-called Dectin-1 and Dectin-2 clusters of genes coding for Dectin-1, Dectin-2, Mincle, and MCL, among others [32, 119].
Mannose receptor
The mannose receptor, MR (synonym CD206), is abundantly expressed on AMs, monocyte-derived DCs (MDCs), and non-vascular endothelium (Table 1). It is a type I transmembrane protein with an extracellular N-terminal consisting of three domains: a cysteine-rich domain, a fibronectin type II domain, and eight CTLDs, followed by a transmembrane domain and a tyrosine-based motif in the cytoplasmic tail involved in ligand internalization [87, 120, 121]. MR can recognize a number of endogenous and exogenous ligands and is so involved in both maintaining homeostasis and acting as a PRR. Only CTLD4 seems to be able to bind to carbohydrate ligands such as terminal mannose-containing glycoconjugates, fucose, and GlcNAc [121]. The signaling pathway downstream MR is poorly understood. It may require aid from another receptor to initiate phagocytosis [122]. It may also undergo proteolytic cleavage, forming soluble sMR, and modulate the immune response by competing with the membrane-bound form [121]. MR serves as an endocytic receptor that is involved in the internalization of ligands and antigen presentation via MHCII and CD1b [123]. MR can recognize ManLAM, higher phosphatidylinositol mannosides (PIMs), LM, and other mannosylated proteins on the Mtb cell wall (Table 1) [124–126]. MR aids in adherence of virulent Mtb strains to human MDMs [116]. In addition, some reports have shown that phagocytosis of mannosylated beads by MR, as well as MR–ManLAM engagement, interferes with phagosome maturation, which may contribute to the intracellular survival of mycobacteria [67, 127, 128]. ManLAM from both BCG and Mtb, on cross-linking with MR, can inhibit the TLR2-induced production of IL-12 by human DCs [129]. This was supported later by a study showing that ManLAM–MR engagement induced the production of anti-inflammatory factors IL-10, IL-1R antagonist, and IL-1R type II, and suppressed IL-12 secretion by human DCs [130]. Since Mtb has a plethora of ligands for different CTLRs, cross talk between these receptors is very likely. Indeed, MR together with DC-SIGN interferes with the Dectin-1-induced Th17 response and instead enhances the production of IFNγ by CD4+ cells favoring Th1 generation in the presence of Mtb-challenged human DCs [131]. Monocytes differentiated in the presence of irradiated Mtb had a low-DC-SIGN/low-MR profile associated with the loss of phagocytosis of irradiated Mtb [132]. MR was also shown to induce the production of matrix metalloproteinase-9 (MMP-9) by THP-1 cells in response to Mtb indicating a contribution to lung tissue damage during TB [133]. Together, these studies point to the fact that MR might favor Mtb in establishing its niche inside the infected phagocytes. However, in murine in vivo infection models with Mtb, MR was not involved in survival or disease progression [134].
Human MR is expressed by the MRC1 gene on chromosome 10. Only a couple of recent investigations carried out by Zhang et al. in Chinese populations focus on the detection of SNPs within MRC1 and its role in TB susceptibility. They concluded that a non-synonymous SNP, rs34039386 glycine to serine, in exon 7, coding for CTLD2 was associated with risk or protection in different groups of the Chinese population, albeit with low significance (Table 2) [135, 136]. The molecular mechanisms linked to the effect of this SNP on receptor function have not been deduced, but the authors suggest that it might affect the MR–ligand interaction [136].
Overall, the role of MR in phagocytosis of Mtb seems to be redundant as many other PRRs and complement receptors can aid in bacterial internalization. In contrast, results acquired from human cells suggest its negative immunomodulatory role by avoiding the intracellular killing of Mtb. However, to further enlighten the significance of this receptor for TB disease pathology, dedicated genetic and functional studies are required.
Dendritic cell-specific ICAM-grabbing non-integrin (DC-SIGN)
Dendritic cell-specific ICAM-grabbing non-integrin, DC-SIGN (synonym CD209), is a type II transmembrane receptor expressed mainly on myeloid DCs and some macrophage subsets including AMs [89] and also activated B cells (Table 1) [137]. The N-terminal makes the cytoplasmic tail and is endowed with three different motifs: a tyrosine-based motif, a dileucine motif, and a triacidic amino acid motif, all of which are thought to be mainly involved in endocytosis and phagocytosis. The transmembrane region consists of tandem repeats of 23 amino acids, which stabilize the tetrameric form of DC-SIGN that enhances its ligand-binding efficiency, while the extracellular C-terminus contains CTLD with an EPN motif and ligand-binding sites [89, 138]. DC-SIGN can bind to high mannose- and fucose-containing ligands and is able to bind to Mtb, as well as a variety of other pathogens. It has also been shown to bind to endogenous molecules of the human host-like intracellular adhesion molecules (ICAMs) and carcinoembryonic cell adhesion molecules CEACAM1, which is helpful in processes like DC migration, interaction with neutrophils, T cell activation, and antigen presentation [139, 140]. It can also serve as a signaling receptor. However, DC-SIGN triggering alone does not seem to initiate a response by DCs and a prior activation of NFκB by TLR signaling is required for its modulatory function [27, 55, 138, 141]. There is an evidence of TLR-independent signaling, as well [142]. It has been observed that different kinds of ligands can induce different kinds of signaling through DC-SIGN [55, 141]. Mannose-containing ligand engagement (e.g., mycobacterial ManLAM) leads to phosphorylation of Raf-1 and subsequent phosphorylation of NFκB subunit p65, increasing the production of cytokines such as IL-12, IL-10, IL-6, and CXCL8, whereas fucosylated antigens, such as Lewis antigens in LPS from H. pylori, seem to signal the DCs to suppress the pro-inflammatory cytokines production [141, 143].
In two independent studies, Geijtenbeek et al. and Tailleux et al. showed that mycobacteria directly bind to DC-SIGN to gain entry into human DCs. DC-SIGN was shown to enhance the internalization of both M. bovis BCG and Mtb [64, 117]. ManLAM–DC-SIGN binding inhibited LPS and M. bovis-induced DC maturation as well as increased IL-10 and decreased IL-12 production by LPS-activated DCs [64]. Moreover, DC-SIGN has been shown to be expressed on human lung DCs and is induced on AMs on Mtb infection [117, 144]. Further studies have demonstrated that, apart from ManLAM, DC-SIGN can also bind to other mannosylated ligands, PIMs, α-glucan on Mtb surface (Table 1) which may also contribute to host–pathogen interaction significantly [145–148]. Indeed, phagocytosis of BCG by DC-SIGN-lacking DCs [149] and the inability of mannose-dependent CLR inhibitor to inhibit Mtb infection in mice [150] suggest that the bacilli also use other modes of establishing intracellular niches and infection. Similar to MR, DC-SIGN co-stimulation also inhibits Dectin-1-induced Th17 response by Mtb-challenged DCs [131]. A transgenic mice expressing human DC-SIGN displayed higher survival rate and reduced tissue damage on high-dose infection with H37Rv advocating that DC-SIGN–Mtb interaction in vivo may in fact be beneficial to the host [151]. Also, the results obtained by knockout (KO) mice studies, which express eight different DC-SIGN homologues (SIGNR1-8), suggest that SIGNR3 (the closest human DC-SIGN orthologue)-deficient mice have impaired resistance to early Mtb infection in lungs [89, 152, 153].
A closely related CLR called L-SIGN, also known as DC-SIGNR/CD209L/CLEC4M, proposed to have originated by gene duplication of DC-SIGN has also been described [154]. L-SIGN also binds to high mannose ligands, but not fucose, and can recognize pathogens including Mtb. In fact, L-SIGN shows more than 70 % amino acid identity with DC-SIGN and has similar structure and binding properties (HIV-1 gp120, ManLAM, and ICAM-3) [155, 156]. It is expressed on a restricted set of endothelial cells. Both DC-SIGN and L-SIGN are found in a tetrameric form, with the transmembrane repeats forming the neck region to which the CRDs are flexibly attached on the extracellular part providing high-avidity binding to multivalent ligands [155, 157].
DC-SIGN and L-SIGN are expressed by the genes CD209 and CD-209L, respectively, in human chromosome 19 [158]. Association of DC-SIGN promoter polymorphisms −336A/G and −871A/G with TB has been studied in many populations (Table 2). However, the results have been contrasting. Functionally, −336G was shown to be associated with decreased expression of DC-SIGN [159]. A meta-analysis performed to compare fourteen of these studies concluded that these promoter variations were not associated with susceptibility to TB, although an analysis performed and based on ethnicity revealed an association of the 336GG genotype as a risk for developing TB in an Asian population [160]. Barreiro et al. [161] showed that the haplotype −871G/−336A conferred protection in Africans. In another study, Russian males carrying −336GG were found to be more susceptible to lethal consequences of infection with Mtb Beijing strain than non-Beijing strains [162]. Thus, not only the host, but also the genotype and characteristics of Mtb may be responsible for different pathogenicity patterns.
Polymorphisms in the neck region of L-SIGN that lead to a change in the number of repeats (ranging from four to ten) have been attributed to variations in the binding affinity to the pathogenic antigens and are associated with developing an infection. Indeed, these polymorphisms in L-SIGN have been associated with viral infections [155, 163–165]. With regard to TB, there have been conflicting results between the two studies conducted by Barreiro et al. and da Silva et al. in a South African and a Brazilian population, respectively. While, in the former, no association was found, in the Brazilian population the allele with nine repeats was related to susceptibility to develop TB, while the one with five repeats conferred protection. The authors hypothesize that low number of repeats may disrupt the binding of the receptor to Mtb, restricting its entry into the cell [166, 167].
In conclusion, the results from mice models and human cells provide conflicting results about the function of DC-SIGN in TB pathology. Also, the genetic studies with DC-SIGN promoter SNPs add to the confusion as to whether this receptor is beneficial for the host or the pathogen. As DC-SIGN recognizes a number of ligands on the Mtb surface, more in vitro and in vivo studies are required that focus on the effects of binding of these ligands with the receptor and subsequent functional responses.
Dendritic cell-associated C-type lectin 1
Dendritic cell-associated C-type lectin 1, Dectin-1 (synonym CLEC7A) was first discovered as a DC-specific receptor which binds to an unknown, probably proteinaceous, ligand on T cells increasing their proliferation and thereby acting as a co-stimulatory molecule [168]. Later, it was found to be a major receptor for β-1,3-glucans and expressed not only on DCs but also on monocytes, macrophages, neutrophils, and on eosinophils, B cells, and mast cells, lung epithelium in humans [55, 169–171]. Dectin-1 is one of the seven genes belonging to the Dectin-1 cluster in the natural killer gene complex (NKC) on chromosome 12 in humans. All the genes in this family are class V type II CLRs having a common structure consisting of an extracellular CTLD connected to the cell surface by a stalk, followed by a transmembrane region and a cytoplasmic domain having signaling motifs. Dectin-1 is one of the non-classical CLRs bearing a hemITAM, with only one YXXL motif, apart from a triacidic DED motif for signaling. Moreover, unlike many other CLRs, the β-glucan binding to Dectin-1 is Ca2+ independent [172]. Since it can bind to T cells, Dectin-1 has also been proposed to be a co-stimulatory molecule [168]. Dectin-1 can recognize Mtb, although the PAMP involved has yet to be determined. Because of its recognition of β-glucan signaling, mechanisms have been mostly studied in fungal infection models where ligand binding to Dectin-1 has been shown to induce a number of cellular processes, including the production of cytokines and chemokines, respiratory burst, ligand uptake through phagocytosis/endocytosis, and DC maturation [173]. Dectin-1-mediated signaling is complex, involving activation of various transcription factors like NFκB, MAPKs, NFAT, IRF1, IRF5, and NLRP3 inflammasome through Syk-dependent or Syk-independent pathways [174, 175]. The canonical subunits of NFκB are activated via CARD9-Bcl10-MALT1 complex formation through Syk interaction with PLCγ2 and PKCδ, leading to the production of IL-1β and IL-23 which are Th17 response-polarizing cytokines. It has also been shown to activate the non-canonical subunit NFκB RelB [27, 176–178]. A recent study demonstrated that Dectin-1 induces pro-IL-1β processing by forming non-canonical caspase-8 inflammasome [179]. The triacidic motif is also involved in phagocytosis and synergistically increasing the production of NFκB-induced cytokines, as in the case of DC-SIGN, via activation of Raf-1, and leads to Th1-favoring cytokine production [55, 180].
Dectin-1 has been shown to collaborate with TLR2 in the recognition of some mycobacterial species to induce pro-inflammatory cytokine production (Table 1) [181–183]. The TLR2-mediated release of TNFα, IL-6, RANTES, and GM-CSF by mice bone-marrow-derived macrophages (BMDMs) infected with attenuated or non-pathogenic mycobacteria such as M. smegmetis, BCG, Mtb H37Ra required Dectin-1, but not in the case of Mtb H37Rv [181]. In another study, Mtb-induced IL-12p40 production in splenic DCs of TLR2−/− mice was reduced on blocking Dectin-1 with laminarin. The Mtb-triggered IL-12p40 production was also reduced in response to inhibition of Syk, suggesting that Dectin-1 might mediate Syk-dependent response independently in these cells [184]. In experiments with human cells, as well, there seems to be an interactive role of TLR2 and Dectin-1 in inducing Mtb-specific immune response. In human lung epithelial cells, Mtb induced Dectin-1 expression, Src kinase, and ROS activation via TLR2. Dectin-1 activation, in turn, also enhanced internalization of bacteria [66]. A recent study in human DCs additionally showed similar Mtb-induced ROS production in a Dectin-1–TLR2-dependent manner [185]. Mtb–Dectin-1 engagement induces adaptive Th1/Th17 response in human MDCs, and this effect seems to be inhibited by MR and DC-SIGN co-stimulation [131]. Mtb-mediated Th17 responses in human PBMCs were studied by van de Veerdonk and colleagues, who concluded that Dectin-1 and TLR4 were responsible for these responses and IL-17A response was IL-1 pathway dependent. TLR2 blocking had less effect on IL-17A production [186]. These Dectin-1-mediated responses may contribute to mycobacterial immunity, but KO studies in mice suggest a redundant role of this receptor in Mtb infection. Although the Dectin-1−/− mice had a low bacterial burden, it was not related to animal survival on Mtb challenge compared with wild type [187]. An early stop codon polymorphism Y238X which produces a truncated Dectin-1 has been studied in some fungal diseases [40, 41, 188, 189]. However, to date, no susceptibility studies focusing on the identification of SNPs in the CLEC7A gene have been carried out in patients with TB.
In summary, these studies suggest that Dectin-1 exerts a protective role by enhancing the pro-inflammatory immune response against Mtb, at least in experiments with human cells. Genetic studies investigating Dectin-1 SNPs associated with TB would provide further insights into the functional importance of this receptor in TB development.
Dendritic cell-associated C-type lectin 2
Dendritic cell-associated C-type lectin, Dectin-2 (synonyms CLEC6A, CLECSF2), is a member of the Dectin-2 cluster in the NKC region on chromosome 12 consisting of six genes, all of which are type II transmembrane group II CTLRs. Believed to have originated by gene duplication, they share a common structure—an N-terminal cytoplasmic tail which is usually short and lacks a signaling motif, a transmembrane domain, a stalk, and a C-terminal extracellular domain with a Ca2+ binding and a CRD motif. The transmembrane domain bears a positively charged residue that aids in association with an ITAM-bearing adaptor molecule like FcRγ, DAP10, and DAP12 required for signaling [190–192]. In humans, Dectin-2 is expressed strongly in lungs, whereas weak expression is observed in spleen and lymph nodes. It is expressed on DC subsets, monocytes, tissue macrophages including AMs, and weakly on B cells (Table 1) [55, 192]. Its expression can be altered on peripheral blood cells by treatment with different stimuli, as has been observed in mice, as well as in humans [193, 194]. The EPN motif in CRD helps in the recognition of high mannose structures (albeit with low affinity) in a Ca2+-dependent manner, as the ligand binding is completely inhibited in the presence of chelators [195]. Thereby, Dectin-1 acts as a PRR for mycobacterial ManLAM apart from many fungi [23]. Signaling has been mainly studied in fungal interactions, in particular with C. albicans. Ligand binding to Dectin-2 induces cellular activation, via FcRγ recruitment activating NFκB in a Syk-dependent manner, and is important in inducing a Th17 response, as well as other cytokines including TNF, IL-1RA, IL-6, IL-12, and IL-10 [191, 196–200]. Gringhuis et al. [199] showed that Dectin-2 induces Th17 immunity by Malt-1-mediated activation of c-Rel subunits of NFκB. Also, PLCγ has been shown to be important for the proper activation of Dectin-2-mediated NFκB and MAPKs [201]. Dectin-2 can also activate the NLRP3 inflammasome and induce allergic inflammation [202, 203]. Soluble recombinant Dectin-2 is able to bind with Mtb [195]. Yonekawa et al. [23] have recently demonstrated that Dectin-2 binds directly to ManLAM. They showed that the receptor could bind to Mtb and BCG and other slow-growing mycobacteria, but not to M. abscessus and M. smegmatis whose LAM lacks capped mannose, and mediated IL-6, TNF, MIP-2, IL-2, and IL-10 production by bone-marrow-derived DCs (BMDCs) in mice. Also, DC maturation and IL-17 production by activated T cells were all abrogated in Dectin-2-deficient Clec4n−/− mice [23]. Besides, the Clec4n−/− mice displayed augmented lung pathology on infection with M. avium complex compared to WT. Moreover, IFNγ production in PBMCs from TB patients in response to mycobacterial antigenic peptides was enhanced on addition of ManLAM and was significantly inhibited on treatment with anti-hDectin-2 Mab. However, whether this signaling was also Syk dependent was not tested [23].
Dectin-2 has been predominantly studied as a fungal receptor, and knowledge of its role in bacterial infections is still in its infancy. Nevertheless, recent results from in vitro human cell studies and KO mouse models suggest a protective function for it in TB infection. To date, the role of polymorphisms in this gene has not been studied in human TB infections and further studies focusing on this aspect may reveal the importance of this receptor in TB pathology.
Macrophage-inducible C-type lectin
Macrophage-inducible C-type lectin, Mincle (synonym CLEC4E), is another member of the Dectin-2 cluster with a similar structure. It was first identified as a downstream target of NF-IL-6 transcription factor in murine macrophages and was inducible by treatment with inflammatory stimuli like LPS, IFNγ, IL-6, and TNFα, hence the name Mincle [204]. It is expressed on monocytes, macrophages, neutrophils, and myeloid DCs, and also some B cell subsets (Table 1) [205]. It binds mainly to mannose and fucose, but also shows some affinity for glucose, GlcNAc, galactose, and GalNAc [206]. Mincle also couples with ITAM-containing FcRγ and signals via the Syk-CARD9 pathway, inducing pro-inflammatory cytokine production [22, 207]. Mincle directly recognizes the mycobacterial cord factor [21] and many fungal species [35, 36]. Mycobacterial cord factor, TDM, is an important structural molecule in the cell wall of Mtb that functions as a PAMP. TDM has been shown to contribute to granuloma formation on Mtb infection in mice lungs [21]. The cord factor is also known to abrogate phagosome maturation and impairs the development of effective immune response enhancing the survival chances of the bacterium inside the cell and breaking the link between the innate and acquired arms of immunity, as the cell is unable to present the antigens to T cells [208–212]. TDM- and its synthetic analogue trehalose-6,6-dibehenate (TDB)-mediated adjuvanticity to induce Th1 and Th17 responses was lost in Mincle-deficient mice [22]. TDM-induced Mincle signaling in mice neutrophils leads to increased surface expression of CR3, ROS, and TNFα in synergy with TLR2 activation. Also, the Mincle−/− mice showed higher lung bacterial burden on Mtb infection [213]. However, Heitmann and colleagues reported that Mincle−/− mice were able to develop granuloma, generate Th1 and Th17 response, and control the infection similar to WT mice on aerosol infection with Mtb [214]. In line with this, Behler et al. [215] also obtained similar results on intratracheal BCG infection of WT and Mincle−/− mice, but the KO mice were more susceptible to intravenous administration of BCG, developing high bacterial loads in the lung, as well as other tissues. Besides, Mincle was inducible in AMs of WT mice on BCG challenge and showed increased anti-mycobacterial immune response on a secondary BCG infection in vivo [215]. The group later also investigated the role of Mincle in systemic infection in KO mice and concluded that Mincle-expressing DCs elicit a Th1 response in spleen, but not in liver [216]. It may be noted that these studies involved different Mtb strains and/or administration methods which may contribute in part to the discrepancy in results. Mincle recognition of TDB has also been shown to activate the NLRP3 inflammasome via IL-1-dependent Myd88 pathway to induce a Th1 and Th17 response in mice BMDCs, and the adaptor ASC and caspase-1 were also required for this response [217–219], while in a recent work, Wook-bin Lee and colleagues demonstrated that Mincle–TDM engagement promotes nitric oxide (NO) production which in turn inhibits the inflammasome and that Mincle also has a stimulatory effect on TLR signaling [220]. Again, the results may be contrasting because of the type of cells used. Mincle shows low expression on resting macrophages, but is inducible on stimulation with TLR ligands in mice [204] and transcription factor C/EBPβ seems to play a role in the Myd88-dependent upregulation of Mincle, TDM/TDB responsiveness, and also HIF1α-mediated NO production [221, 222]. A recent study reported that human APCs respond similarly as the rodent cells to TDM/TDB, inducing cytokines like IL-6, IL-8, IL-1A, IL-1B, and G-CSF in a Syk-dependent manner [223]. Indeed, the CRDs of mice and human Mincle have been shown to have similar TDM-binding properties, but some differences have been observed with regard to other mycobacterial ligands, namely β-gentiobiosyldiacylglycerides and 2′S-stereoisomer of glycerol monomycolate (GroMM) [224–226]. TDM and BCG interaction with Mincle may also induce anti-inflammatory IL-10 cytokine production in mice BMDMs and modulate IL-12p40 production, as reported recently [227]. Besides its role as a PRR, Mincle can also recognize the damage-associated molecular patterns (DAMPs) like SAP130 released by damaged self-cells, suggesting its role in sensing necrosis [207]. More recently, cholesterol crystals have been reported as an endogenous ligand for Mincle in human monocyte-derived DCs upregulating pro-inflammatory cytokine and chemokine synthesis [228]. A recent study found no association of Mincle SNPs (Table 2) with TB in South African colored population [229].
Overall, Mincle has been associated with pro-inflammatory responses in human cell models. The new reports about additional Mtb ligands for Mincle and the anti-inflammatory effects show that our understanding of the role of this receptor is still incomplete. Further studies need to be carried out to investigate if potential SNPs in Mincle gene might contribute to the pathogenesis of TB.
Macrophage C-type lectin
Macrophage C-type lectin (MCL) (synonyms CLEC4D/Clecsf8/Dectin-3) is another newly described member of Dectin-2 family. It was first described as macrophage-restricted CLR in mice [230]. Human MCL is a type II transmembrane receptor with an intracellular N-terminal lacking any signaling motif, a transmembrane domain, and extracellular C-terminal connected via stalk to the cell surface bearing a single CRD [231]. It is expressed by neutrophils, CD14+ CD16− monocytes, and some DC subsets (Table 1). Although constitutively expressed on myeloid cells [232], monocyte differentiation into macrophages or DCs has been shown to downregulate MCL expression [233]. MCL has been proposed to have originated by Mincle duplication and, likewise, it can also recognize TDM and some fungi [45, 232], albeit with lower affinity, since the CRD of MCL lacks the conserved tripeptide motif required for carbohydrate recognition [231]. Arce et al. [231] reported that MCL lacks an intracellular positively charged residue for pairing with adaptors and that cross-linking of this receptor leads to its internalization, suggesting a role in antigen uptake, while later it was shown that it can also induce phagocytosis, respiratory burst, and cytokine production in a Syk-dependent manner [233]. More recently, MCL has been associated with Mincle-related responses. Here, MCL associates with FcRγ and is required for inducing Mincle expression on TDM stimulation. In addition, Clec4d−/− mice showed impaired TDM-induced responses, such as granuloma formation and cytokine production (TNF, MIP-2, IL-1β, IL-6) [24, 232]. Along this line, Lobato-Pascual also observed similar results for rat receptors expressed on HEK293T cells and proposed Mincle–MCL heterodimer to be a functional unit mediating Mincle-associated immune response [234]. It was later confirmed in mice and human cells that MCL indeed associates with Mincle through its stalk region [235]. Recent studies suggest that MCL expression is co-regulated with Mincle and can be induced in mice in a Myd88-dependent manner [222, 236]. However, Zhao and colleagues, in their experiments, negated any such association between MCL and Mincle to form dimers and suggested that the main function of MCL is to induce initial Mincle expression in response to TDM via CARD9/Bcl10/MALT1-dependent NFκB activation [24]. A resolution of these differing results has yet to be made. Wilson et al. [25] concluded that MCL is non-redundantly important for anti-mycobacterial immunity. Infection with BCG or H37Rv leads to higher bacterial burdens, increased production of TNFα, IFN-γ, and G-CSF, as well as higher neutrophil infiltration in lungs of Clec4d−/−mice. Moreover, the in vitro studies showed that loss of MCL affects the bacterial binding to leukocytes and thus reduces phagocytosis [25].
Only a few data are available regarding the impact on SNPs in the MCL gene for TB susceptibility. A non-synonymous SNP Ser32Gly has been associated with pulmonary TB in an Indonesian cohort with the G allele associated with disease susceptibility in a dominant model (Table 2). The polymorphism causes a missense mutation in the transmembrane region of the protein affecting its association with adaptor Fcγ and influencing its surface expression [25].
In a nutshell, MCL has an important protective function in generating anti-mycobacterial immunity by enhancing Mincle expression, Mtb recognition, and internalization. The only genetic study has further supported its role in TB, and further studies would be interesting to more deeply understand its significance in mycobacterial infections.
Complement receptor 3
Complement receptor 3, CR3 [synonyms αMβ2, CD11b/CD18, macrophage-1 antigen (Mac-1)], is a heterodimeric membrane receptor belonging to the integrin superfamily. It is expressed on neutrophils, macrophages, natural killer cells, and monocytes (Table 1) [237]. The extracellular part of its α chain, which is a type I transmembrane protein, consists of an I domain for binding a variety of ligands like iC3b (making it a major complement receptor for opsonized tubercle bacteria), mycobacterial Ag85C, ICAM-1, and many bacterial components [238, 239], while the C domain having a lectin-like site can interact with sugars like β-glucan, glucose, GlcNAc, and mycobacterial oligosaccharides, including LAM and PIMs (Table 1) [238, 240]. Thus, CR3 is capable of inducing both opsonic and non-opsonic phagocytosis [238, 241]. Increased expression of CR3 was observed in the peripheral blood phagocytes and AMs in TB patients, suggesting its role in pathogenesis [242–244]. Studies with human BALF suggest that classical complement pathway activation leads to opsonization of pathogens like Mtb and may enhance their phagocytosis by AMs [245, 246]. However, it was shown that the absence of CR3 does not influence bacterial survival inside the cell as observed in CR3- and CD11b-deficient mice [118, 247, 248]. This suggests that, in the absence of CR3, mycobacteria can still be internalized by other receptors; thus, the role of CR3 in Mtb pathogenesis seems to be redundant. Besides, no SNP studies have been performed in humans to support its role in the development of TB.
Conclusion
CTLRs have gained attention in recent decades with respect to the increasing numbers of pathogens and endogenous ligands that are recognized by these PRRs, strengthening their significance in maintaining immunity and homeostasis. While some CTLRs with newly discovered roles in mycobacterial infections, such as MCL and Mincle, are important for inducing a specific immune response against the pathogen, there are others, such as CR3, MR, DC-SIGN, which may in fact contribute to immune evasion strategies of mycobacteria. The outcome may depend on various factors which include, but are not limited to the type of cells infected, surface expression of receptors, and interactions between them, the cell’s gene expression profiles of receptors and adaptors involved in Mtb recognition, extracellular factors, and bacterial, as well as host genetics. Apart from this, standardization of the experimental procedures and practices, such as ensuring the purity of ligands used and minimizing the endotoxin contamination, is also crucially important to attain reliable results. Recent finding that MCL is indispensable for anti-mycobacterial response and that it also regulates the expression of Mincle suggests the importance of interactions between the receptors in defining a specific immune response. Genetic association studies offer further information on the relevance of a receptor and its function. However, in several genetic studies, polymorphisms are not investigated for their functional effect, and in others, the significance level for the association between the SNPs and TB is rather low. Additionally, while performing such studies, it is to be considered that complex diseases like TB do not follow the patterns of Mendelian diseases and are in fact polygenic, being largely affected by gene–gene interaction. Mtb is recognized by a number of PRRs and precise knowledge about cross talk between these receptors and their signaling, as well as the manipulation elicited by bacteria, in order to develop its niche would enable us to better understand the host–bacteria interaction and control the infection and also block further spread.
Elucidating the mechanisms of ligand recognition, adaptors involved in signaling cascades and signaling regulation by CTLRs, and other receptors involved in TB may be helpful in designing vaccine adjuvants that can specifically activate an adaptive response and help develop other protective strategies, such as targeting the key components in specific signaling pathways via immunomodulation. Along with this information, the host genetics profile data, including the SNPs in different genes involved in specific responses toward TB, should be further elucidated for their functional significance which will help in identifying prognostic markers and aid in the ambitious goal of tailoring individual specific therapies. As knowledge about the significance of Mtb-recognizing members of CTLRs is in preface, they serve as an important and promising group of receptors that have the potential to be further explored and more clearly define their roles in mycobacterial infections.
References
World Health Organisation (2015) Global tuberculosis report 2015. http://www.who.int/tb/publications/global_report/en/
Zumla A, Raviglione M, Hafner R, von Reyn CF (2013) Tuberculosis. New Engl J Med 368(8):745–755. doi:10.1056/NEJMra1200894
van Crevel R, Ottenhoff THM, van der Meer JWM (2002) Innate immunity to Mycobacterium tuberculosis. Clin Microbiol Rev 15(2):294–309. doi:10.1128/cmr.15.2.294-309.2002
Pieters J (2008) Mycobacterium tuberculosis and the macrophage: maintaining a balance. Cell Host Microbe 3(6):399–407. doi:10.1016/j.chom.2008.05.006
Ehrt S, Schnappinger D (2009) Mycobacterial survival strategies in the phagosome: defence against host stresses. Cell Microbiol 11(8):1170–1178. doi:10.1111/j.1462-5822.2009.01335.x
Orme IM, Robinson RT, Cooper AM (2015) The balance between protective and pathogenic immune responses in the TB-infected lung. Nat Immunol 16(1):57–63. doi:10.1038/ni.3048
Brennan PJ, Nikaido H (1995) The envelope of mycobacteria. Annu Rev Biochem 64:29–63. doi:10.1146/annurev.bi.64.070195.000333
Fukuda T, Matsumura T, Ato M, Hamasaki M, Nishiuchi Y, Murakami Y, Maeda Y, Yoshimori T, Matsumoto S, Kobayashi K, Kinoshita T, Morita YS (2013) Critical roles for lipomannan and lipoarabinomannan in cell wall integrity of mycobacteria and pathogenesis of tuberculosis. mBio 4(1):e00472-12. doi:10.1128/mBio.00472-12
Welsh KJ, Hunter RL, Actor JK (2013) Trehalose 6,6′-dimycolate: a coat to regulate tuberculosis immunopathogenesis. Tuberculosis 93(Suppl):S3–S9. doi:10.1016/S1472-9792(13)70003-9
Jo EK (2008) Mycobacterial interaction with innate receptors: TLRs, C-type lectins, and NLRs. Curr Opin Infect Dis 21(3):279–286. doi:10.1097/QCO.0b013e3282f88b5d
Takeuchi O, Sato S, Horiuchi T, Hoshino K, Takeda K, Dong Z, Modlin RL, Akira S (2002) Cutting edge: role of Toll-like receptor 1 in mediating immune response to microbial lipoproteins. J Immunol 169(1):10–14
Bafica A, Scanga CA, Feng CG, Leifer C, Cheever A, Sher A (2005) TLR9 regulates Th1 responses and cooperates with TLR2 in mediating optimal resistance to Mycobacterium tuberculosis. J Exp Med 202(12):1715–1724. doi:10.1084/jem.20051782
Jo EK, Yang CS, Choi CH, Harding CV (2007) Intracellular signalling cascades regulating innate immune responses to Mycobacteria: branching out from Toll-like receptors. Cell Microbiol 9(5):1087–1098. doi:10.1111/j.1462-5822.2007.00914.x
Sanchez D, Rojas M, Hernandez I, Radzioch D, Garcia LF, Barrera LF (2010) Role of TLR2- and TLR4-mediated signaling in Mycobacterium tuberculosis-induced macrophage death. Cell Immunol 260(2):128–136. doi:10.1016/j.cellimm.2009.10.007
Uciechowski P, Imhoff H, Lange C, Meyer CG, Browne EN, Kirsten DK, Schroder AK, Schaaf B, Al-Lahham A, Reinert RR, Reiling N, Haase H, Hatzmann A, Fleischer D, Heussen N, Kleines M, Rink L (2011) Susceptibility to tuberculosis is associated with TLR1 polymorphisms resulting in a lack of TLR1 cell surface expression. J Leukoc Biol 90(2):377–388. doi:10.1189/jlb.0409233
Liu Q, Li W, Li D, Feng Y, Tao C (2014) TIRAP C539T polymorphism contributes to tuberculosis susceptibility: evidence from a meta-analysis. Infect Genet Evol 27:32–39. doi:10.1016/j.meegid.2014.06.025
Dittrich N, Berrocal-Almanza LC, Thada S, Goyal S, Slevogt H, Sumanlatha G, Hussain A, Sur S, Burkert S, Oh DY, Valluri V, Schumann RR, Conrad ML (2015) Toll-like receptor 1 variations influence susceptibility and immune response to Mycobacterium tuberculosis. Tuberculosis. doi:10.1016/j.tube.2015.02.045
Qi H, Sun L, Wu X, Jin Y, Xiao J, Wang S, Shen C, Chu P, Qi Z, Xu F, Guo Y, Jiao W, Tian J, Shen A (2015) Toll-like receptor 1(TLR1) Gene SNP rs5743618 is associated with increased risk for tuberculosis in Han Chinese children. Tuberculosis 95(2):197–203. doi:10.1016/j.tube.2014.12.001
Schurz H, Daya M, Moller M, Hoal EG, Salie M (2015) TLR1, 2, 4, 6 and 9 variants associated with tuberculosis susceptibility: a systematic review and meta-analysis. PLoS One 10(10):e0139711. doi:10.1371/journal.pone.0139711
Azad AK, Sadee W, Schlesinger LS (2012) Innate immune gene polymorphisms in tuberculosis. Infect Immun 80(10):3343–3359. doi:10.1128/IAI.00443-12
Ishikawa E, Ishikawa T, Morita YS, Toyonaga K, Yamada H, Takeuchi O, Kinoshita T, Akira S, Yoshikai Y, Yamasaki S (2009) Direct recognition of the mycobacterial glycolipid, trehalose dimycolate, by C-type lectin Mincle. J Exp Med 206(13):2879–2888. doi:10.1084/jem.20091750
Schoenen H, Bodendorfer B, Hitchens K, Manzanero S, Werninghaus K, Nimmerjahn F, Agger EM, Stenger S, Andersen P, Ruland J, Brown GD, Wells C, Lang R (2010) Cutting edge: mincle is essential for recognition and adjuvanticity of the mycobacterial cord factor and its synthetic analog trehalose-dibehenate. J Immunol 184(6):2756–2760. doi:10.4049/jimmunol.0904013
Yonekawa A, Saijo S, Hoshino Y, Miyake Y, Ishikawa E, Suzukawa M, Inoue H, Tanaka M, Yoneyama M, Oh-hora M, Akashi K, Yamasaki S (2014) Dectin-2 is a direct receptor for mannose-capped lipoarabinomannan of mycobacteria. Immunity 41(3):402–413. doi:10.1016/j.immuni.2014.08.005
Zhao XQ, Zhu LL, Chang Q, Jiang C, You Y, Luo T, Jia XM, Lin X (2014) C-type lectin receptor dectin-3 mediates trehalose 6,6′-dimycolate (TDM)-induced Mincle expression through CARD9/Bcl10/MALT1-dependent nuclear factor (NF)-kappaB activation. J Biol Chem 289(43):30052–30062. doi:10.1074/jbc.M114.588574
Wilson GJ, Marakalala MJ, Hoving JC, van Laarhoven A, Drummond RA, Kerscher B, Keeton R, van de Vosse E, Ottenhoff TH, Plantinga TS, Alisjahbana B, Govender D, Besra GS, Netea MG, Reid DM, Willment JA, Jacobs M, Yamasaki S, van Crevel R, Brown GD (2015) The C-type lectin receptor CLECSF8/CLEC4D is a key component of anti-mycobacterial immunity. Cell Host Microbe 17(2):252–259. doi:10.1016/j.chom.2015.01.004
Zelensky AN, Gready JE (2005) The C-type lectin-like domain superfamily. FEBS J 272(24):6179–6217. doi:10.1111/j.1742-4658.2005.05031.x
Geijtenbeek TB, Gringhuis SI (2009) Signalling through C-type lectin receptors: shaping immune responses. Nat Rev Immunol 9(7):465–479. doi:10.1038/nri2569
Drickamer K (1999) C-type lectin-like domains. Curr Opin Struct Biol 9(5):585–590
Cambi A, Figdor CG (2003) Dual function of C-type lectin-like receptors in the immune system. Curr Opin Cell Biol 15(5):539–546
McGreal EP, Martinez-Pomares L, Gordon S (2004) Divergent roles for C-type lectins expressed by cells of the innate immune system. Mol Immunol 41(11):1109–1121. doi:10.1016/j.molimm.2004.06.013
Garcia-Vallejo JJ, van Kooyk Y (2009) Endogenous ligands for C-type lectin receptors: the true regulators of immune homeostasis. Immunol Rev 230(1):22–37. doi:10.1111/j.1600-065X.2009.00786.x
Graham LM, Brown GD (2009) The Dectin-2 family of C-type lectins in immunity and homeostasis. Cytokine 48(1–2):148–155. doi:10.1016/j.cyto.2009.07.010
Atochina EN, Gow AJ, Beck JM, Haczku A, Inch A, Kadire H, Tomer Y, Davis C, Preston AM, Poulain F, Hawgood S, Beers MF (2004) Delayed clearance of pneumocystis carinii infection, increased inflammation, and altered nitric oxide metabolism in lungs of surfactant protein-D knockout mice. J Infect Dis 189(8):1528–1539. doi:10.1086/383130
Madan T, Reid KBM, Singh M, Sarma PU, Kishore U (2005) Susceptibility of mice genetically deficient in the surfactant protein (SP)-A or SP-D gene to pulmonary hypersensitivity induced by antigens and allergens of Aspergillus fumigatus. J Immunol 174(11):6943–6954
Wells CA, Salvage-Jones JA, Li X, Hitchens K, Butcher S, Murray RZ, Beckhouse AG, Lo YL, Manzanero S, Cobbold C, Schroder K, Ma B, Orr S, Stewart L, Lebus D, Sobieszczuk P, Hume DA, Stow J, Blanchard H, Ashman RB (2008) The macrophage-inducible C-type lectin, mincle, is an essential component of the innate immune response to Candida albicans. J Immunol 180(11):7404–7413
Yamasaki S, Matsumoto M, Takeuchi O, Matsuzawa T, Ishikawa E, Sakuma M, Tateno H, Uno J, Hirabayashi J, Mikami Y, Takeda K, Akira S, Saito T (2009) C-type lectin Mincle is an activating receptor for pathogenic fungus, Malassezia. Proc Natl Acad Sci USA 106(6):1897–1902. doi:10.1073/pnas.0805177106
Balderramas HA, Penitenti M, Rodrigues DR, Bachiega TF, Fernandes RK, Ikoma MRV, Dias-Melicio LA, Oliveira SL, Soares AMVC (2014) Human neutrophils produce IL-12, IL-10, PGE2 and LTB4 in response to Paracoccidioides brasiliensis: involvement of TLR2, mannose receptor and dectin-1. Cytokine 67(1):36–43. doi:10.1016/j.cyto.2014.02.004
Holmer SM, Evans KS, Asfaw YG, Saini D, Schell WA, Ledford JG, Frothingham R, Wright JR, Sempowski GD, Perfect JR (2014) Impact of surfactant protein D, interleukin-5, and eosinophilia on Cryptococcosis. Infect Immun 82(2):683–693. doi:10.1128/IAI.00855-13
Ou XT, Wu JQ, Zhu LP, Guan M, Xu B, Hu XP, Wang X, Weng XH (2011) Genotypes coding for mannose-binding lectin deficiency correlated with cryptococcal meningitis in HIV-uninfected Chinese patients. J Infect Dis 203(11):1686–1691. doi:10.1093/infdis/jir152
Rosentul DC, Plantinga TS, Oosting M, Scott WK, Velez Edwards DR, Smith PB, Alexander BD, Yang JC, Laird GM, Joosten LA, van der Meer JW, Perfect JR, Kullberg BJ, Netea MG, Johnson MD (2011) Genetic variation in the dectin-1/CARD9 recognition pathway and susceptibility to candidemia. J Infect Dis 204(7):1138–1145. doi:10.1093/infdis/jir458
Sainz J, Lupianez CB, Segura-Catena J, Vazquez L, Rios R, Oyonarte S, Hemminki K, Forsti A, Jurado M (2012) Dectin-1 and DC-SIGN polymorphisms associated with invasive pulmonary Aspergillosis infection. PLoS One 7(2):e32273. doi:10.1371/journal.pone.0032273
Yamamoto H, Nakamura Y, Sato K, Takahashi Y, Nomura T, Miyasaka T, Ishii K, Hara H, Yamamoto N, Kanno E, Iwakura Y, Kawakami K (2014) Defect of CARD9 leads to impaired accumulation of gamma interferon-producing memory phenotype T cells in lungs and increased susceptibility to pulmonary infection with Cryptococcus neoformans. Infect Immun 82(4):1606–1615. doi:10.1128/IAI.01089-13
Qu X, Che C, Gao A, Lin J, Wang N, Du X, Liu Y, Guo Y, Chen W, Zhao G (2015) Association of Dectin-1 and DC-SIGN gene single nucleotide polymorphisms with fungal keratitis in the northern Han Chinese population. Mol Vis 21:391–402
Wang MY, Wang FP, Yang JB, Zhao DF, Wang HP, Shao F, Wang WJ, Sun RL, Ling MZ, Zhai JJ, Song SJ (2013) Mannan-binding lectin inhibits Candida albicans-induced cellular responses in PMA-activated THP-1 cells through Toll-like receptor 2 and Toll-like receptor 4. PLoS One. doi:10.1371/journal.pone.0083517
Zhu LL, Zhao XQ, Jiang C, You Y, Chen XP, Jiang YY, Jia XM, Lin X (2013) C-type lectin receptors Dectin-3 and Dectin-2 form a heterodimeric pattern-recognition receptor for host defense against fungal infection. Immunity 39(2):324–334. doi:10.1016/j.immuni.2013.05.017
Wevers BA, Kaptein TM, Zijlstra-Willems EM, Theelen B, Boekhout T, Geijtenbeek TB, Gringhuis SI (2014) Fungal engagement of the C-type lectin mincle suppresses dectin-1-induced antifungal immunity. Cell Host Microbe 15(4):494–505. doi:10.1016/j.chom.2014.03.008
Loures FV, Araujo EF, Feriotti C, Bazan SB, Calich VLG (2015) TLR-4 cooperates with Dectin-1 and mannose receptor to expand Th17 and Tc17 cells induced by Paracoccidioides brasiliensis stimulated dendritic cells. Front Microbiol. doi:10.3389/Fmicb.2015.00261
Watford WT, Wright JR, Hester CG, Jiang H, Frank MM (2001) Surfactant protein A regulates complement activation. J Immunol 167(11):6593–6600
Kostina E, Ofek I, Crouch E, Friedman R, Sirota L, Klinger G, Sahly H, Keisari Y (2005) Noncapsulated Klebsiella pneumoniae bearing mannose-containing O antigens is rapidly eradicated from mouse lung and triggers cytokine production by macrophages following opsonization with surfactant protein D. Infect Immun 73(12):8282–8290. doi:10.1128/IAI.73.12.8282-8290.2005
Malherbe DC, Erpenbeck VJ, Abraham SN, Crouch EC, Hohlfeld JM, Wright JR (2005) Surfactant protein D decreases pollen-induced IgE-dependent mast cell degranulation. Am J Physiol Lung Cell Mol Physiol 289(5):L856–L866. doi:10.1152/ajplung.00009.2005
Palaniyar N, Clark H, Nadesalingam J, Shih MJ, Hawgood S, Reid KB (2005) Innate immune collectin surfactant protein D enhances the clearance of DNA by macrophages and minimizes anti-DNA antibody generation. J Immunol 174(11):7352–7358
Holmskov U, Thiel S, Jensenius JC (2003) Collectins and ficolins: humoral lectins of the innate immune defense. Annu Rev Immunol 21:547–578. doi:10.1146/annurev.immumol.21.120601.140954
Ferguson JS, Voelker DR, McCormack FX, Schlesinger LS (1999) Surfactant protein D binds to Mycobacterium tuberculosis bacilli and lipoarabinomannan via carbohydrate–lectin interactions resulting in reduced phagocytosis of the bacteria by macrophages. J Immunol 163(1):312–321
Beharka AA, Gaynor CD, Kang BK, Voelker DR, McCormack FX, Schlesinger LS (2002) Pulmonary surfactant protein A up-regulates activity of the mannose receptor, a pattern recognition receptor expressed on human macrophages. J Immunol 169(7):3565–3573
Sancho D, Reis e Sousa C (2012) Signaling by myeloid C-type lectin receptors in immunity and homeostasis. Annu Rev Immunol 30:491–529. doi:10.1146/annurev-immunol-031210-101352
Kerrigan AM, Brown GD (2010) Syk-coupled C-type lectin receptors that mediate cellular activation via single tyrosine based activation motifs. Immunol Rev 234(1):335–352. doi:10.1111/j.0105-2896.2009.00882.x
Marakalala MJ, Graham LM, Brown GD (2010) The role of Syk/CARD9-coupled C-type lectin receptors in immunity to Mycobacterium tuberculosis infections. Clin Dev Immunol 2010:567571. doi:10.1155/2010/567571
Strasser D, Neumann K, Bergmann H, Marakalala MJ, Guler R, Rojowska A, Hopfner KP, Brombacher F, Urlaub H, Baier G, Brown GD, Leitges M, Ruland J (2012) Syk kinase-coupled C-type lectin receptors engage protein kinase C-sigma to elicit Card9 adaptor-mediated innate immunity. Immunity 36(1):32–42. doi:10.1016/j.immuni.2011.11.015
Billadeau DD, Leibson PJ (2002) ITAMs versus ITIMs: striking a balance during cell regulation. J Clin Investig 109(2):161–168. doi:10.1172/Jci14843
Marshall AS, Willment JA, Lin HH, Williams DL, Gordon S, Brown GD (2004) Identification and characterization of a novel human myeloid inhibitory C-type lectin-like receptor (MICL) that is predominantly expressed on granulocytes and monocytes. J Biol Chem 279(15):14792–14802. doi:10.1074/jbc.M313127200
Richard M, Thibault N, Veilleux P, Gareau-Page G, Beaulieu AD (2006) Granulocyte macrophage-colony stimulating factor reduces the affinity of SHP-2 for the ITIM of CLECSF6 in neutrophils: a new mechanism of action for SHP-2. Mol Immunol 43(10):1716–1721. doi:10.1016/j.molimm.2005.10.006
Barrow AD, Trowsdale J (2006) You say ITAM and I say ITIM, let’s call the whole thing off: the ambiguity of immunoreceptor signalling. Eur J Immunol 36(7):1646–1653. doi:10.1002/eji.200636195
Pinheiro da Silva F, Aloulou M, Benhamou M, Monteiro RC (2008) Inhibitory ITAMs: a matter of life and death. Trends Immunol 29(8):366–373. doi:10.1016/j.it.2008.05.001
Geijtenbeek TBH, van Vliet SJ, Koppel EA, Sanchez-Hernandez M, Vandenbroucke-Grauls CMJE, Appelmelk B, van Kooyk Y (2003) Mycobacteria target DC-SIGN to suppress dendritic cell function. J Exp Med 197(1):7–17. doi:10.1084/jem.20021229
Gold JA, Hoshino Y, Tanaka N, Rom WN, Raju B, Condos R, Weiden MD (2004) Surfactant protein A modulates the inflammatory response in macrophages during tuberculosis. Infect Immun 72(2):645–650
Lee HM, Yuk JM, Shin DM, Jo EK (2009) Dectin-1 is inducible and plays an essential role for mycobacteria-induced innate immune responses in airway epithelial cells. J Clin Immunol 29(6):795–805. doi:10.1007/s10875-009-9319-3
Astarie-Dequeker C, N’Diaye EN, Le Cabec V, Rittig MG, Prandi J, Maridonneau-Parini I (1999) The mannose receptor mediates uptake of pathogenic and nonpathogenic mycobacteria and bypasses bactericidal responses in human macrophages. Infect Immun 67(2):469–477
Gupta G, Surolia A (2007) Collectins: sentinels of innate immunity. BioEssays: news and reviews in molecular, cellular and developmental biology 29(5):452–464. doi:10.1002/bies.20573
Kishore U, Greenhough TJ, Waters P, Shrive AK, Ghai R, Kamran MF, Bernal AL, Reid KBM, Madan T, Chakraborty T (2006) Surfactant proteins SP-A and SP-D: structure, function and receptors. Mol Immunol 43(9):1293–1315. doi:10.1016/j.molimm.2005.08.004
Ferguson JS, Martin JL, Azad AK, McCarthy TR, Kang PB, Voelker DR, Crouch EC, Schlesinger LS (2006) Surfactant protein D increases fusion of Mycobacterium tuberculosis-containing phagosomes with lysosomes in human macrophages. Infect Immun 74(12):7005–7009. doi:10.1128/Iai.01402-06
Hoppe HC, deWet BJM, Cywes C, Daffe M, Ehlers MRW (1997) Identification of phosphatidylinositol mannoside as a mycobacterial adhesin mediating both direct and opsonic binding to nonphagocytic mammalian cells. Infect Immun 65(9):3896–3905
Sidobre S, Nigou J, Puzo G, Riviere M (2000) Lipoglycans are putative ligands for the human pulmonary surfactant protein A attachment to mycobacteria: critical role of the lipids for lectin-carbohydrate recognition. J Biol Chem 275(4):2415–2422
Seaton BA, Crouch EC, McCormack FX, Head JF, Hartshorn KL, Mendelsohn R (2010) Review: structural determinants of pattern recognition by lung collectins. Innate Immun 16(3):143–150. doi:10.1177/1753425910368716
Ragas A, Roussel L, Puzo G, Riviere M (2007) The Mycobacterium tuberculosis cell-surface glycoprotein apa as a potential adhesin to colonize target cells via the innate immune system pulmonary C-type lectin surfactant protein A. J Biol Chem 282(8):5133–5142. doi:10.1074/jbc.M610183200
Gaynor CD, McCormack FX, Voelker DR, McGowan SE, Schlesinger LS (1995) Pulmonary surfactant protein A mediates enhanced phagocytosis of Mycobacterium tuberculosis by a direct interaction with human macrophages. J Immunol 155(11):5343–5351
Hu H, Teng GL, Gai LZ, Yang Y, Zhu CJ (2013) Clinical value of surfactant protein-A in serum and sputum for pulmonary tuberculosis diagnosis. Genet Mol Res: GMR 12(4):4918–4924. doi:10.4238/2013.October.24.2
Pasula R, Wright JR, Kachel DL, Martin WJ (1999) Surfactant protein A suppresses reactive nitrogen intermediates by alveolar macrophages in response to Mycobacterium tuberculosis. J Clin Investig 103(4):483–490. doi:10.1172/Jci2991
Weikert LF, Lopez JP, Abdolrasulnia R, Chroneos ZC, Shepherd VL (2000) Surfactant protein A enhances mycobacterial killing by rat macrophages through a nitric oxide-dependent pathway. Am J Physiol-Lung C 279(2):L216–L223
Ferguson JS, Voelker DR, Ufnar JA, Dawson AJ, Schlesinger LS (2002) Surfactant protein D inhibition of human macrophage uptake of Mycobacterium tuberculosis is independent of bacterial agglutination. J Immunol 168(3):1309–1314
Lemos MP, McKinney J, Rhee KY (2011) Dispensability of surfactant proteins A and D in immune control of Mycobacterium tuberculosis infection following aerosol challenge of mice. Infect Immun 79(3):1077–1085. doi:10.1128/Iai.00286-10
Floros J, Lin HM, Garcia A, Salazar MA, Guo X, DiAngelo S, Montano M, Luo J, Pardo A, Selman M (2000) Surfactant protein genetic marker alleles identify a subgroup of tuberculosis in a Mexican population. J Infect Dis 182(5):1473–1478. doi:10.1086/315866
Malik S, Greenwood CM, Eguale T, Kifle A, Beyene J, Habte A, Tadesse A, Gebrexabher H, Britton S, Schurr E (2006) Variants of the SFTPA1 and SFTPA2 genes and susceptibility to tuberculosis in Ethiopia. Hum Genet 118(6):752–759. doi:10.1007/s00439-005-0092-y
Madan T, Saxena S, Murthy KJR, Muralidhar K, Sarma PU (2002) Association of polymorphisms in the collagen region of human SP-A1 and SP-A2 genes with pulmonary tuberculosis in Indian population. Clin Chem Lab Med 40(10):1002–1008. doi:10.1515/Cclm.2002.174
Yang HY, Li H, Wang YG, Xu CY, Zhao YL, Ma XG, Li XW, Chen H (2014) Correlation analysis between single nucleotide polymorphisms of pulmonary surfactant protein A gene and pulmonary tuberculosis in the Han population in China. Int J Infect Dis 26:31–36. doi:10.1016/j.ijid.2014.03.1395
Vaid M, Kaur S, Madan T, Singh H, Gupta VK, Murthy KJR, Sarma PU (2006) Association of SP-D, MNL and I-NOS genetic variants with pulmonary tuberculosis. Indian J Hum Genet 12(3):105–110
Helke KL, Mankowski JL, Manabe YC (2006) Animal models of cavitation in pulmonary tuberculosis. Tuberculosis 86(5):337–348. doi:10.1016/j.tube.2005.09.001
Turner MW (1996) Mannose-binding lectin: the pluripotent molecule of the innate immune system. Immunol Today 17(11):532–540
Ip WK, Takahashi K, Ezekowitz RA, Stuart LM (2009) Mannose-binding lectin and innate immunity. Immunol Rev 230(1):9–21. doi:10.1111/j.1600-065X.2009.00789.x
Lugo-Villarino G, Hudrisier D, Tanne A, Neyrolles O (2011) C-type lectins with a sweet spot for Mycobacterium tuberculosis. Eur J Microbiol Immunol 1(1):25–40. doi:10.1556/EuJMI.1.2011.1.6
Bartlomiejczyk MA, Swierzko AS, Brzostek A, Dziadek J, Cedzynski M (2014) Interaction of lectin pathway of complement-activating pattern recognition molecules with mycobacteria. Clin Exp Immunol 178(2):310–319. doi:10.1111/cei.12416
Takahashi K, Ezekowitz RA (2005) The role of the mannose-binding lectin in innate immunity. Clin Infect Dis 41(Suppl 7):S440–S444. doi:10.1086/431987
Shi L, Takahashi K, Dundee J, Shahroor-Karni S, Thiel S, Jensenius JC, Gad F, Hamblin MR, Sastry KN, Ezekowitz RA (2004) Mannose-binding lectin-deficient mice are susceptible to infection with Staphylococcus aureus. J Exp Med 199(10):1379–1390. doi:10.1084/jem.20032207
Genster N, Takahashi M, Sekine H, Endo Y, Garred P, Fujita T (2014) Lessons learned from mice deficient in lectin complement pathway molecules. Mol Immunol 61(2):59–68. doi:10.1016/j.molimm.2014.07.007
Moller-Kristensen M, Ip WK, Shi L, Gowda LD, Hamblin MR, Thiel S, Jensenius JC, Ezekowitz RA, Takahashi K (2006) Deficiency of mannose-binding lectin greatly increases susceptibility to postburn infection with Pseudomonas aeruginosa. J Immunol 176(3):1769–1775
Held K, Thiel S, Loos M, Petry F (2008) Increased susceptibility of complement factor B/C2 double knockout mice and mannan-binding lectin knockout mice to systemic infection with Candida albicans. Mol Immunol 45(15):3934–3941. doi:10.1016/j.molimm.2008.06.021
Heitzeneder S, Seidel M, Forster-Waldl E, Heitger A (2012) Mannan-binding lectin deficiency: good news, bad news, doesn’t matter? Clin Immunol 143(1):22–38. doi:10.1016/j.clim.2011.11.002
Thiel S, Frederiksen PD, Jensenius JC (2006) Clinical manifestations of mannan-binding lectin deficiency. Mol Immunol 43(1–2):86–96. doi:10.1016/j.molimm.2005.06.018
El Sahly HM, Reich RA, Dou SJ, Musser JM, Graviss EA (2004) The effect of mannose binding lectin gene polymorphisms on susceptibility to tuberculosis in different ethnic groups. Scand J Infect Dis 36(2):106–108
Soborg C, Madsen HO, Andersen AB, Lillebaek T, Kok-Jensen A, Garred P (2003) Mannose-binding lectin polymorphisms in clinical tuberculosis. J Infect Dis 188(5):777–782. doi:10.1086/377183
Ozbas-Gerceker F, Tezcan I, Berkel AI, Ozkara S, Ozcan A, Ersoy F, Sanal O, Ozguc M (2003) The effect of mannose-binding protein gene polymorphisms in recurrent respiratory system infections in children and lung tuberculosis. Turk J Pediatr 45(2):95–98
Cosar H, Ozkinay F, Onay H, Bayram N, Bakiler AR, Anil M, Can D, Ozkinay C (2008) Low levels of mannose-binding lectin confers protection against tuberculosis in Turkish children. Eur J Clin Microbiol Infect Dis 27(12):1165–1169. doi:10.1007/s10096-008-0573-8
Capparelli R, Iannaccone M, Palumbo D, Medaglia C, Moscariello E, Russo A, Iannelli D (2009) Role played by human mannose-binding lectin polymorphisms in pulmonary tuberculosis. J Infect Dis 199(5):666–672. doi:10.1086/596658
Selvaraj P, Narayanan PR, Reetha AM (1999) Association of functional mutant homozygotes of the mannose binding protein gene with susceptibility to pulmonary tuberculosis in India. Tuber Lung Dis 79(4):221–227. doi:10.1054/tuld.1999.0204
Bellamy R, Ruwende C, McAdam KPWJ, Thursz M, Sumiya M, Summerfield J, Gilbert SC, Corrah T, Kwiatkowski D, Whittle HC, Hill AVS (1998) Mannose binding protein deficiency is not associated with malaria, hepatitis B carriage nor tuberculosis in Africans. Qjm-Mon J Assoc Phys 91(1):13–18. doi:10.1093/qjmed/91.1.13
Wu LL, Deng HJ, Zheng YH, Mansjo M, Zheng XB, Hu Y, Xu BA (2015) An association study of NRAMP1, VDR, MBL and their interaction with the susceptibility to tuberculosis in a Chinese population. Int J Infect Dis 38:129–135. doi:10.1016/j.ijid.2015.08.003
Liu W, Zhang F, Xin ZT, Zhao QM, Wu XM, Zhang PH, de Vlas S, Richardus JH, Habbema JDF, Yang H, Cao WC (2006) Sequence variations in the MBL gene and their relationship to pulmonary tuberculosis in the Chinese Han population. Int J Tuberc Lung Dis 10(10):1098–1103
Denholm JT, McBryde ES, Eisen DP (2010) Mannose-binding lectin and susceptibility to tuberculosis: a meta-analysis. Clin Exp Immunol 162(1):84–90. doi:10.1111/j.1365-2249.2010.04221.x
Shi J, Xie M, Wang JM, Xu YJ, Xiong WN, Liu XS (2013) Mannose-binding lectin two gene polymorphisms and tuberculosis susceptibility in Chinese population: a meta-analysis. J Huazhong Univ Sci Technol Med Sci 33(2):166–171. doi:10.1007/s11596-013-1091-1
de Wit E, van der Merwe L, van Helden PD, Hoal EG (2011) Gene-gene interaction between tuberculosis candidate genes in a South African population. Mamm Genome 22(1–2):100–110. doi:10.1007/s00335-010-9280-8
Chen MS, Deng J, Su CX, Li J, Wang M, Abuaku BK, Hu SM, Tan HZ, Wen SW (2014) Impact of passive smoking, cooking with solid fuel exposure, and MBL/MASP-2 gene polymorphism upon susceptibility to tuberculosis. Int J Infect Dis 29:1–6. doi:10.1016/j.ijid.2014.08.010
Chen M, Liang Y, Li W, Wang M, Hu L, Abuaku BK, Huang X, Tan H, Wen SW (2015) Impact of MBL and MASP-2 gene polymorphism and its interaction on susceptibility to tuberculosis. BMC Infect Dis 15:151. doi:10.1186/s12879-015-0879-y
Singla N, Gupta D, Joshi A, Batra N, Singh J, Birbian N (2012) Association of mannose-binding lectin gene polymorphism with tuberculosis susceptibility and sputum conversion time. Int J Immunogenet 39(1):10–14. doi:10.1111/j.1744-313X.2011.01047.x
Thye T, Niemann S, Walter K, Homolka S, Intemann CD, Chinbuah MA, Enimil A, Gyapong J, Osei I, Owusu-Dabo E, Rusch-Gerdes S, Horstmann RD, Ehlers S, Meyer CG (2011) Variant G57E of mannose binding lectin associated with protection against tuberculosis caused by Mycobacterium africanum but not by M. tuberculosis. PLoS One. doi:10.1371/journal.pone.0020908
Chalmers JD, Matsushita M, Kilpatrick DC, Hill AT (2015) No strong relationship between components of the lectin pathway of complement and susceptibility to pulmonary tuberculosis. Inflammation 38(4):1731–1737. doi:10.1007/s10753-015-0150-0
Hijikata M, Matsushita I, Hang NT, Maeda S, Thuong PH, do Tam B, Shimbo T, Sakurada S, Cuong VC, Lien LT, Keicho N (2014) Age-dependent association of mannose-binding lectin polymorphisms with the development of pulmonary tuberculosis in Viet Nam. Hum Immunol 75(8):840–846. doi:10.1016/j.humimm.2014.06.006
Schlesinger LS (1993) Macrophage phagocytosis of virulent but not attenuated strains of Mycobacterium tuberculosis is mediated by mannose receptors in addition to complement receptors. J Immunol 150(7):2920–2930
Tailleux L, Schwartz O, Herrmann JL, Pivert E, Jackson M, Amara A, Legres L, Dreher D, Nicod LP, Gluckman JC, Lagrange PH, Gicquel B, Neyrolles O (2003) DC-SIGN is the major Mycobacterium tuberculosis receptor on human dendritic cells. J Exp Med 197(1):121–127. doi:10.1084/jem.20021468
Melo MD, Catchpole IR, Haggar G, Stokes RW (2000) Utilization of CD11b knockout mice to characterize the role of complement receptor 3 (CR3, CD11b/CD18) in the growth of Mycobacterium tuberculosis in macrophages. Cell Immunol 205(1):13–23. doi:10.1006/cimm.2000.1710
Plato A, Willment JA, Brown GD (2013) C-type Lectin-like receptors of the Dectin-1 cluster: ligands and signaling pathways. Int Rev Immunol 32(2):134–156. doi:10.3109/08830185.2013.777065
Taylor ME, Conary JT, Lennartz MR, Stahl PD, Drickamer K (1990) Primary structure of the mannose receptor contains multiple motifs resembling carbohydrate-recognition domains. J Biol Chem 265(21):12156–12162
Martinez-Pomares L (2012) The mannose receptor. J Leukoc Biol 92(6):1177–1186. doi:10.1189/jlb.0512231
Le Cabec V, Emorine LJ, Toesca I, Cougoule C, Maridonneau-Parini I (2005) The human macrophage mannose receptor is not a professional phagocytic receptor. J Leukoc Biol 77(6):934–943. doi:10.1189/jlb.1204705
Prigozy TI, Sieling PA, Clemens D, Stewart PL, Behar SM, Porcelli SA, Brenner MB, Modlin RL, Kronenberg M (1997) The mannose receptor delivers lipoglycan antigens to endosomes for presentation to T cells by CD1b molecules. Immunity 6(2):187–197
Schlesinger LS, Hull SR, Kaufman TM (1994) Binding of the terminal mannosyl units of lipoarabinomannan from a virulent strain of Mycobacterium tuberculosis to human macrophages. J Immunol 152(8):4070–4079
Torrelles JB, Azad AK, Schlesinger LS (2006) Fine discrimination in the recognition of individual species of phosphatidyl-myo-inositol mannosides from Mycobacterium tuberculosis by C-type lectin pattern recognition receptors. J Immunol 177(3):1805–1816
Diaz-Silvestre H, Espinosa-Cueto P, Sanchez-Gonzalez A, Esparza-Ceron MA, Pereira-Suarez AL, Bernal-Fernandez G, Espitia C, Mancilla R (2005) The 19-kDa antigen of Mycobacterium tuberculosis is a major adhesin that binds the mannose receptor of THP-1 monocytic cells and promotes phagocytosis of mycobacteria. Microb Pathog 39(3):97–107. doi:10.1016/j.micpath.2005.06.002
Astarie-Dequeker C, Carreno S, Cougoule C, Maridonneau-Parini I (2002) The protein tyrosine kinase Hck is located on lysosomal vesicles that are physically and functionally distinct from CD63-positive lysosomes in human macrophages. J Cell Sci 115(1):81–89
Kang PB, Azad AK, Torrelles JB, Kaufman TM, Beharka A, Tibesar E, DesJardin LE, Schlesinger LS (2005) The human macrophage mannose receptor directs Mycobacterium tuberculosis lipoarabinomannan-mediated phagosome biogenesis. J Exp Med 202(7):987–999. doi:10.1084/jem.20051239
Nigou J, Zelle-Rieser C, Gilleron M, Thurnher M, Puzo G (2001) Mannosylated lipoarabinomannans inhibit IL-12 production by human dendritic cells: evidence for a negative signal delivered through the mannose receptor. J Immunol 166(12):7477–7485
Chieppa M, Bianchi G, Doni A, Del Prete A, Sironi M, Laskarin G, Monti P, Piemonti L, Biondi A, Mantovani A, Introna M, Allavena P (2003) Cross-linking of the mannose receptor on monocyte-derived dendritic cells activates an anti-inflammatory immunosuppressive program. J Immunol 171(9):4552–4560
Zenaro E, Donini M, Dusi S (2009) Induction of Th1/Th17 immune response by Mycobacterium tuberculosis: role of dectin-1, mannose receptor, and DC-SIGN. J Leukoc Biol 86(6):1393–1401. doi:10.1189/jlb.0409242
Balboa L, Romero MM, Yokobori N, Schierloh P, Geffner L, Basile JI, Musella RM, Abbate E, de la Barrera S, Sasiain MC, Aleman M (2010) Mycobacterium tuberculosis impairs dendritic cell response by altering CD1b, DC-SIGN and MR profile. Immunol Cell Biol 88(7):716–726. doi:10.1038/icb.2010.22
Rivera-Marrero CA, Schuyler W, Roser S, Ritzenthaler JD, Newburn SA, Roman J (2002) M. tuberculosis induction of matrix metalloproteinase-9: the role of mannose and receptor-mediated mechanisms. Am J Physiol Lung Cell Mol Physiol 282(3):L546–L555. doi:10.1152/ajplung.00175.2001
Court N, Vasseur V, Vacher R, Fremond C, Shebzukhov Y, Yeremeev VV, Maillet I, Nedospasov SA, Gordon S, Fallon PG, Suzuki H, Ryffel B, Quesniaux VFJ (2010) Partial redundancy of the pattern recognition receptors, scavenger receptors, and C-type lectins for the long-term control of Mycobacterium tuberculosis infection. J Immunol 184(12):7057–7070. doi:10.4049/jimmunol.1000164
Zhang X, Jiang F, Wei L, Li F, Liu J, Wang C, Zhao M, Jiang T, Xu D, Fan D, Sun X, Li JC (2012) Polymorphic allele of human MRC1 confer protection against tuberculosis in a Chinese population. Int J Biol Sci 8(3):375–382. doi:10.7150/ijbs.4047
Zhang X, Li X, Zhang W, Wei L, Jiang T, Chen Z, Meng C, Liu J, Wu F, Wang C, Li F, Sun X, Li Z, Li JC (2013) The novel human MRC1 gene polymorphisms are associated with susceptibility to pulmonary tuberculosis in Chinese Uygur and Kazak populations. Mol Biol Rep 40(8):5073–5083. doi:10.1007/s11033-013-2610-7
Rappocciolo G, Piazza P, Fuller CL, Reinhart TA, Watkins SC, Rowe DT, Jais M, Gupta P, Rinaldo CR (2006) DC-SIGN on B lymphocytes is required for transmission of HIV-1 to T lymphocytes (vol 2, pg 3, 2004). PLoS Pathog 2(8):808. doi:10.1371/journal.ppat.0020088
Garcia-Vallejo JJ, van Kooyk Y (2013) The physiological role of DC-SIGN: a tale of mice and men. Trends Immunol 34(10):482–486. doi:10.1016/j.it.2013.03.001
Geijtenbeek TB, Krooshoop DJ, Bleijs DA, van Vliet SJ, van Duijnhoven GC, Grabovsky V, Alon R, Figdor CG, van Kooyk Y (2000) DC-SIGN-ICAM-2 interaction mediates dendritic cell trafficking. Nat Immunol 1(4):353–357. doi:10.1038/79815
van Gisbergen KPJM, Ludwig IS, Geijtenbeek TBH, van Kooyk Y (2005) Interactions of DC-SIGN with Mac-1 and CEACAM1 regulate contact between dendritic cells and neutrophils. FEBS Lett 579(27):6159–6168. doi:10.1016/j.febslet.2005.09.089
Gringhuis SI, den Dunnen J, Litjens M, van der Vlist M, Geijtenbeek TBH (2009) Carbohydrate-specific signaling through the DC-SIGN signalosome tailors immunity to Mycobacterium tuberculosis, HIV-1 and Helicobacter pylori. Nat Immunol 10(10):1081–1088. doi:10.1038/ni.1778
Hodges A, Sharrocks K, Edelmann M, Baban D, Moris A, Schwartz O, Drakesmith H, Davies K, Kessler B, McMichael A, Simmons A (2007) Activation of the lectin DC-SIGN induces an immature dendritic cell phenotype triggering Rho-GTPase activity required for HIV-1 replication. Nat Immunol 8(6):569–577. doi:10.1038/ni1470
Gringhuis SI, den Dunnen J, Litjens M, van Het Hof B, van Kooyk Y, Geijtenbeek TB (2007) C-type lectin DC-SIGN modulates Toll-like receptor signaling via Raf-1 kinase-dependent acetylation of transcription factor NF-kappaB. Immunity 26(5):605–616. doi:10.1016/j.immuni.2007.03.012
Tailleux L, Pham-Thi N, Bergeron-Lafaurie A, Herrmann JL, Charles P, Schwartz O, Scheinmann P, Lagrange PH, de Blic J, Tazi A, Gicquel B, Neyrolles O (2005) DC-SIGN induction in alveolar macrophages defines privileged target host cells for mycobacteria in patients with tuberculosis. PLoS Med 2(12):e381. doi:10.1371/journal.pmed.0020381
Pitarque S, Herrmann JL, Duteyrat JL, Jackson M, Stewart GR, Lecointe F, Payre B, Schwartz O, Young DB, Marchal G, Lagrange PH, Puzo G, Gicquel B, Nigou J, Neyrolles O (2005) Deciphering the molecular bases of Mycobacterium tuberculosis binding to the lectin DC-SIGN reveals an underestimated complexity. Biochem J 392(Pt 3):615–624. doi:10.1042/BJ20050709
Appelmelk BJ, den Dunnen J, Driessen NN, Ummels R, Pak M, Nigou J, Larrouy-Maumus G, Gurcha SS, Movahedzadeh F, Geurtsen J, Brown EJ, Eysink Smeets MM, Besra GS, Willemsen PT, Lowary TL, van Kooyk Y, Maaskant JJ, Stoker NG, van der Ley P, Puzo G, Vandenbroucke-Grauls CM, Wieland CW, van der Poll T, Geijtenbeek TB, van der Sar AM, Bitter W (2008) The mannose cap of mycobacterial lipoarabinomannan does not dominate the Mycobacterium–host interaction. Cell Microbiol 10(4):930–944. doi:10.1111/j.1462-5822.2007.01097.x
Driessen NN, Ummels R, Maaskant JJ, Gurcha SS, Besra GS, Ainge GD, Larsen DS, Painter GF, Vandenbroucke-Grauls CM, Geurtsen J, Appelmelk BJ (2009) Role of phosphatidylinositol mannosides in the interaction between mycobacteria and DC-SIGN. Infect Immun 77(10):4538–4547. doi:10.1128/IAI.01256-08
Geurtsen J, Chedammi S, Mesters J, Cot M, Driessen NN, Sambou T, Kakutani R, Ummels R, Maaskant J, Takata H, Baba O, Terashima T, Bovin N, Vandenbroucke-Grauls CM, Nigou J, Puzo G, Lemassu A, Daffe M, Appelmelk BJ (2009) Identification of mycobacterial alpha-glucan as a novel ligand for DC-SIGN: involvement of mycobacterial capsular polysaccharides in host immune modulation. J Immunol 183(8):5221–5231. doi:10.4049/jimmunol.0900768
Gagliardi MC, Teloni R, Giannoni F, Pardini M, Sargentini V, Brunori L, Fattorini L, Nisini R (2005) Mycobacterium bovis Bacillus Calmette-Guerin infects DC-SIGN- dendritic cell and causes the inhibition of IL-12 and the enhancement of IL-10 production. J Leukoc Biol 78(1):106–113. doi:10.1189/jlb.0105037
Driessen NN, Boshoff HI, Maaskant JJ, Gilissen SA, Vink S, van der Sar AM, Vandenbroucke-Grauls CM, Bewley CA, Appelmelk BJ, Geurtsen J (2012) Cyanovirin-N inhibits mannose-dependent Mycobacterium-C-type lectin interactions but does not protect against murine tuberculosis. J Immunol 189(7):3585–3592. doi:10.4049/jimmunol.1102408
Schaefer M, Reiling N, Fessler C, Stephani J, Taniuchi I, Hatam F, Yildirim AO, Fehrenbach H, Walter K, Ruland J, Wagner H, Ehlers S, Sparwasser T (2008) Decreased pathology and prolonged survival of human DC-SIGN transgenic mice during mycobacterial infection. J Immunol 180(10):6836–6845
Tanne A, Ma B, Boudou F, Tailleux L, Botella H, Badell E, Levillain F, Taylor ME, Drickamer K, Nigou J, Dobos KM, Puzo G, Vestweber D, Wild MK, Marcinko M, Sobieszczuk P, Stewart L, Lebus D, Gicquel B, Neyrolles O (2009) A murine DC-SIGN homologue contributes to early host defense against Mycobacterium tuberculosis. J Exp Med 206(10):2205–2220. doi:10.1084/jem.20090188
Tanne A, Neyrolles O (2010) C-type lectins in immune defense against pathogens: the murine DC-SIGN homologue SIGNR3 confers early protection against Mycobacterium tuberculosis infection. Virulence 1(4):285–290. doi:10.4161/viru.1.4.11967
Soilleux EJ, Barten R, Trowsdale J (2000) DC-SIGN; a related gene, DC-SIGNR; and CD23 form a cluster on 19p13. J Immunol 165(6):2937–2942
Khoo US, Chan KY, Chan VS, Lin CL (2008) DC-SIGN and L-SIGN: the SIGNs for infection. J Mol Med 86(8):861–874. doi:10.1007/s00109-008-0350-2
Koppel EA, Ludwig IS, Hernandez MS, Lowary TL, Gadikota RR, Tuzikov AB, Vandenbroucke-Grauls CMJE, van Kooyk Y, Appelmelk BJ, Geijtenbeek TBH (2004) Identification of the mycobacterial carbohydrate structure that binds the C-type lectins DC-SIGN, L-SIGN and SIGNR1. Immunobiology 209(1–2):117–127. doi:10.1016/j.imbio.2004.03.003
Feinberg H, Guo Y, Mitchell DA, Drickamer K, Weis WI (2005) Extended neck regions stabilize tetramers of the receptors DC-SIGN and DC-SIGNR. J Biol Chem 280(2):1327–1335. doi:10.1074/jbc.M409925200
Soilleux EJ (2003) DC-SIGN (dendritic cell-specific ICAM-grabbing non-integrin) and DC-SIGN-related (DC-SIGNR): friend or foe? Clin Sci 104(4):437–446. doi:10.1042/cs20020092
Sakuntabhai A, Turbpaiboon C, Casademont I, Chuansumrit A, Lowhnoo T, Kajaste-Rudnitski A, Kalayanarooj SM, Tangnararatchakit K, Tangthawornchaikul N, Vasanawathana S, Chaiyaratana W, Yenchitsomanus PT, Suriyaphol P, Avirutnan P, Chokephaibulkit K, Matsuda F, Yoksan S, Jacob Y, Lathrop GM, Malasit P, Despres P, Julier C (2005) A variant in the CD209 promoter is associated with severity of dengue disease. Nat Genet 37(5):507–513. doi:10.1038/ng1550
Chang K, Deng SL, Lu WP, Wang F, Jia SR, Li FK, Yu LL, Chen M (2012) Association between CD209-336A/G and-871A/G polymorphisms and susceptibility of tuberculosis: a meta-analysis. PLoS One. doi:10.1371/journal.pone.0041519
Barreiro LB, Neyrolles O, Babb CL, Tailleux L, Quach H, McElreavey K, Helden PD, Hoal EG, Gicquel B, Quintana-Murci L (2006) Promoter variation in the DC-SIGN-encoding gene CD209 is associated with tuberculosis. PLoS Med 3(2):e20. doi:10.1371/journal.pmed.0030020
Ogarkov O, Mokrousov I, Sinkov V, Zhdanova S, Antipina S, Savilov E (2012) ‘Lethal’ combination of Mycobacterium tuberculosis Beijing genotype and human CD209 -336G allele in Russian male population. Infect Genet Evol 12(4):732–736. doi:10.1016/j.meegid.2011.10.005
Fayssel N, Bensghir R, Ouladlahsen A, Abdelghaffar H, Sodqi M, Lahlou K, Benjelloun S, Marhoum El Filali K, Ezzikouri S, Wakrim L (2015) Association of CD209L tandem repeats polymorphism with susceptibility to human immunodeficiency virus-1 infection, disease progression, and treatment outcomes: a Moroccan cohort study. Clin Microbiol Infect 21(5):513.e1-5. doi:10.1016/j.cmi.2014.12.012
Chan VS, Chan KY, Chen Y, Poon LL, Cheung AN, Zheng B, Chan KH, Mak W, Ngan HY, Xu X, Screaton G, Tam PK, Austyn JM, Chan LC, Yip SP, Peiris M, Khoo US, Lin CL (2006) Homozygous L-SIGN (CLEC4M) plays a protective role in SARS coronavirus infection. Nat Genet 38(1):38–46. doi:10.1038/ng1698
Ezzikouri S, Rebbani K, Fakhir FZ, Alaoui R, Nadir S, Diepolder H, Thursz M, Khakoo SI, Benjelloun S (2014) The allele 4 of neck region liver-lymph node-specific ICAM-3-grabbing integrin variant is associated with spontaneous clearance of hepatitis C virus and decrease of viral loads. Clin Microbiol Infect 20(5):O325–O332. doi:10.1111/1469-0691.12403
Barreiro LB, Neyrolles O, Babb CL, van Helden PD, Gicquel B, Hoal EG, Quintana-Murci L (2007) Length variation of DC-SIGN and L-SIGN neck-region has no impact on tuberculosis susceptibility. Hum Immunol 68(2):106–112. doi:10.1016/j.humimm.2006.10.020
da Silva RC, Segat L, da Cruz HL, Schindler HC, Montenegro LM, Crovella S, Guimaraes RL (2014) Association of CD209 and CD209L polymorphisms with tuberculosis infection in a Northeastern Brazilian population. Mol Biol Rep 41(8):5449–5457. doi:10.1007/s11033-014-3416-y
Ariizumi K, Shen GL, Shikano S, Xu S, Ritter R 3rd, Kumamoto T, Edelbaum D, Morita A, Bergstresser PR, Takashima A (2000) Identification of a novel, dendritic cell-associated molecule, dectin-1, by subtractive cDNA cloning. J Biol Chem 275(26):20157–20167. doi:10.1074/jbc.M909512199
Brown GD, Taylor PR, Reid DM, Willment JA, Williams DL, Martinez-Pomares L, Wong SYC, Gordon S (2002) Dectin-1 is a major beta-glucan receptor on macrophages. J Exp Med 196(3):407–412. doi:10.1084/Jem.20020470
Taylor PR, Brown GD, Reid DM, Willment JA, Martinez-Pomares L, Gordon S, Wong SYC (2002) The beta-glucan receptor, dectin-1, is predominantly expressed on the, surface of cells of the monocyte/macrophage and neutrophil lineages. J Immunol 169(7):3876–3882
Heyl KA, Klassert TE, Heinrich A, Muller MM, Klaile E, Dienemann H, Grunewald C, Bals R, Singer BB, Slevogt H (2014) Dectin-1 is expressed in human lung and mediates the proinflammatory immune response to nontypeable haemophilus influenzae. mBio. doi:10.1128/mBio.01492-14
Kerrigan AM, Brown GD (2010) Syk-coupled C-type lectin receptors that mediate cellular activation via single tyrosine based activation motifs. Immunol Rev 234:335–352
Brown GD (2006) Dectin-1: a signalling non-TLR pattern-recognition receptor. Nat Rev Immunol 6(1):33–43. doi:10.1038/nri1745
Kerrigan AM, Brown GD (2011) Syk-coupled C-type lectins in immunity. Trends Immunol 32(4):151–156. doi:10.1016/j.it.2011.01.002
Dambuza IM, Brown GD (2015) C-type lectins in immunity: recent developments. Curr Opin Immunol 32:21–27. doi:10.1016/j.coi.2014.12.002
Gross O, Gewies A, Finger K, Schafer M, Sparwasser T, Peschel C, Forster I, Ruland J (2006) Card9 controls a non-TLR signalling pathway for innate anti-fungal immunity. Nature 442(7103):651–656. doi:10.1038/nature04926
Gringhuis SI, den Dunnen J, Litjens M, van der Vlist M, Wevers B, Bruijns SCM, Geijtenbeek TBH (2009) Dectin-1 directs T helper cell differentiation by controlling noncanonical NF-kappa B activation through Raf-1 and Syk. Nat Immunol 10(2):203–213. doi:10.1038/ni.1692
Gringhuis SI, Wevers BA, Kaptein TM, van Capel TM, Theelen B, Boekhout T, de Jong EC, Geijtenbeek TB (2011) Selective C-Rel activation via Malt1 controls anti-fungal T(H)-17 immunity by dectin-1 and dectin-2. PLoS Pathog 7(1):e1001259. doi:10.1371/journal.ppat.1001259
Gringhuis SI, Kaptein TM, Wevers BA, Theelen B, van der Vlist M, Boekhout T, Geijtenbeek TB (2012) Dectin-1 is an extracellular pathogen sensor for the induction and processing of IL-1beta via a noncanonical caspase-8 inflammasome. Nat Immunol 13(3):246–254. doi:10.1038/ni.2222
Underhill DM, Rossnagle E, Lowell CA, Simmons RM (2005) Dectin-1 activates Syk tyrosine kinase in a dynamic subset of macrophages for reactive oxygen production. Blood 106(7):2543–2550. doi:10.1182/blood-2005-03-1239
Yadav M, Schorey JS (2006) The beta-glucan receptor dectin-1 functions together with TLR2 to mediate macrophage activation by mycobacteria. Blood 108(9):3168–3175. doi:10.1182/blood-2006-05-024406
Shin DM, Yang CS, Yuk JM, Lee JY, Kim KH, Shin SJ, Takahara K, Lee SJ, Jo EK (2008) Mycobacterium abscessus activates the macrophage innate immune response via a physical and functional interaction between TLR2 and dectin-1. Cell Microbiol 10(8):1608–1621. doi:10.1111/j.1462-5822.2008.01151.x
Romero MM, Basile JI, Corra Feo L, Lopez B, Ritacco V, Aleman M (2015) Reactive oxygen species production by human dendritic cells involves TLR2 and dectin-1 and is essential for efficient immune response against Mycobacteria. Cell Microbiol. doi:10.1111/cmi.12562
Rothfuchs AG, Bafica A, Feng CG, Egen JG, Williams DL, Brown GD, Sher A (2007) Dectin-1 interaction with Mycobacterium tuberculosis leads to enhanced IL-12p40 production by splenic dendritic cells. J Immunol 179(6):3463–3471
Romero MM, Basile JI, Feo LC, Lopez B, Ritacco V, Aleman M (2016) Reactive oxygen species production by human dendritic cells involves TLR2 and dectin-1 and is essential for efficient immune response against Mycobacteria. Cell Microbiol 18(6):875–886. doi:10.1111/cmi.12562
van de Veerdonk FL, Teirlinck AC, Kleinnijenhuis J, Kullberg BJ, van Crevel R, van der Meer JW, Joosten LA, Netea MG (2010) Mycobacterium tuberculosis induces IL-17A responses through TLR4 and dectin-1 and is critically dependent on endogenous IL-1. J Leukoc Biol 88(2):227–232. doi:10.1189/jlb.0809550
Marakalala MJ, Guler R, Matika L, Murray G, Jacobs M, Brombacher F, Rothfuchs AG, Sher A, Brown GD (2011) The Syk/CARD9-coupled receptor Dectin-1 is not required for host resistance to Mycobacterium tuberculosis in mice. Microbes Infect 13(2):198–201. doi:10.1016/j.micinf.2010.10.013
Rosentul DC, Plantinga TS, Papadopoulos A, Joosten LA, Antoniadou A, Venselaar H, Kullberg BJ, van der Meer JW, Giamarellos-Bourboulis EJ, Netea MG (2011) Variation in genes of beta-glucan recognition pathway and susceptibility to opportunistic infections in HIV-positive patients. Immunol Invest 40(7–8):735–750. doi:10.3109/08820139.2011.599088
Usluogullari B, Gumus I, Gunduz E, Kaygusuz I, Simavli S, Acar M, Oznur M, Gunduz M, Kafali H (2014) The role of Human Dectin-1 Y238X Gene Polymorphism in recurrent vulvovaginal candidiasis infections. Mol Biol Rep 41(10):6763–6768. doi:10.1007/s11033-014-3562-2
Ariizumi K, Shen GL, Shikano S, Ritter R 3rd, Zukas P, Edelbaum D, Morita A, Takashima A (2000) Cloning of a second dendritic cell-associated C-type lectin (dectin-2) and its alternatively spliced isoforms. J Biol Chem 275(16):11957–11963
Sato K, Yang XL, Yudate T, Chung JS, Wu JM, Luby-Phelps K, Kimberly RP, Underhill D, Cruz PD, Ariizumi K (2006) Dectin-2 is a pattern recognition receptor for fungi that couples with the Fc receptor gamma chain to induce innate immune responses. J Biol Chem 281(50):38854–38866. doi:10.1074/jbc.M606542200
Kanazawa N, Tashiro K, Inaba K, Lutz MB, Miyachi Y (2004) Molecular cloning of human dectin-2. J Invest Dermatol 122(6):1522–1524. doi:10.1111/j.0022-202X.2004.22602.x
Gavino AC, Chung JS, Sato K, Ariizumi K, Cruz PD Jr (2005) Identification and expression profiling of a human C-type lectin, structurally homologous to mouse dectin-2. Exp Dermatol 14(4):281–288. doi:10.1111/j.0906-6705.2005.00312.x
Taylor PR, Reid DM, Heinsbroek SEM, Brown GD, Gordon S, Wong SYC (2005) Dectin-2 is predominantly myeloid restricted and exhibits unique activation-dependent expression on maturing inflammatory monocytes elicited in vivo. Eur J Immunol 35(7):2163–2174. doi:10.1002/eji.200425785
McGreal EP, Rosas M, Brown GD, Zamze S, Wong SYC, Gordon S, Martinez-Pomares L, Taylor PR (2006) The carbohydrate-recognition domain of Dectin-2 is a C-type lectin with specificity for high mannose. Glycobiology 16(5):422–430. doi:10.1093/glycob/cwj077
Robinson MJ, Osorio F, Rosas M, Freitas RP, Schweighoffer E, Gross O, SjefVerbeek J, Ruland J, Tybulewicz V, Brown GD, Moita LF, Taylor PR, Sousa CRE (2009) Dectin-2 is a Syk-coupled pattern recognition receptor crucial for Th17 responses to fungal infection. J Exp Med 206(9):2037–2051. doi:10.1084/jem.20082818
Bi L, Gojestani S, Wu W, Hsu YM, Zhu J, Ariizumi K, Lin X (2010) CARD9 mediates dectin-2-induced IkappaBalpha kinase ubiquitination leading to activation of NF-kappaB in response to stimulation by the hyphal form of Candida albicans. J Biol Chem 285(34):25969–25977. doi:10.1074/jbc.M110.131300
Saijo S, Ikeda S, Yamabe K, Kakuta S, Ishigame H, Akitsu A, Fujikado N, Kusaka T, Kubo S, Chung SH, Komatsu R, Miura N, Adachi Y, Ohno N, Shibuya K, Yamamoto N, Kawakami K, Yamasaki S, Saito T, Akira S, Iwakura Y (2010) Dectin-2 recognition of alpha-mannans and induction of th17 cell differentiation is essential for host defense against Candida albicans. Immunity 32(5):681–691. doi:10.1016/j.immuni.2010.05.001
Gringhuis SI, Wevers BA, Kaptein TM, van Capel TMM, Theelen B, Boekhout T, de Jong EC, Geijtenbeek TBH (2011) Selective C-Rel activation via Malt1 controls anti-fungal T-H-17 immunity by Dectin-1 and Dectin-2. PLoS Pathog. doi:10.1371/journal.ppat.1001259
Ishikawa T, Itoh F, Yoshida S, Saijo S, Matsuzawa T, Gonoi T, Saito T, Okawa Y, Shibata N, Miyamoto T, Yamasaki S (2013) Identification of distinct ligands for the C-type lectin receptors Mincle and Dectin-2 in the pathogenic fungus Malassezia. Cell Host Microbe 13(4):477–488. doi:10.1016/j.chom.2013.03.008
Gorjestani S, Yu M, Tang B, Zhang D, Wang D, Lin X (2011) Phospholipase Cgamma2 (PLCgamma2) is key component in Dectin-2 signaling pathway, mediating anti-fungal innate immune responses. J Biol Chem 286(51):43651–43659. doi:10.1074/jbc.M111.307389
Ritter M, Gross O, Kays S, Ruland J, Nimmerjahn F, Saijo S, Tschopp J, Layland LE, da Costa CP (2010) Schistosoma mansoni triggers Dectin-2, which activates the Nlrp3 inflammasome and alters adaptive immune responses. Proc Natl Acad Sci USA 107(47):20459–20464. doi:10.1073/pnas.1010337107
Barrett NA, Rahman OM, Fernandez JM, Parsons MW, Xing W, Austen KF, Kanaoka Y (2011) Dectin-2 mediates Th2 immunity through the generation of cysteinyl leukotrienes. J Exp Med 208(3):593–604. doi:10.1084/jem.20100793
Masumoto M, Tanaka T, Kaisho T, Sanjo H, Copeland NG, Gilbert DJ, Jenkins NA, Akira S (1999) A novel LPS-inducible C-type lectin is a transcriptional target of NF-IL6 in macrophages. J Immunol 163(9):5039–5048
Kerscher B, Willment JA, Brown GD (2013) The Dectin-2 family of C-type lectin-like receptors: an update. Int Immunol 25(5):271–277. doi:10.1093/intimm/dxt006
Lee RT, Hsu TL, Huang SK, Hsieh SL, Wong CH, Lee YC (2011) Survey of immune-related, mannose/fucose-binding C-type lectin receptors reveals widely divergent sugar-binding specificities. Glycobiology 21(4):512–520. doi:10.1093/glycob/cwq193
Yamasaki S, Ishikawa E, Sakuma M, Hara H, Ogata K, Saito T (2008) Mincle is an ITAM-coupled activating receptor that senses damaged cells. Nat Immunol 9(10):1179–1188. doi:10.1038/Ni.1651
Spargo BJ, Crowe LM, Ioneda T, Beaman BL, Crowe JH (1991) Cord factor (Alpha, Alpha-Trehalose 6,6′-Dimycolate) inhibits fusion between phospholipid-vesicles. Proc Natl Acad Sci USA 88(3):737–740. doi:10.1073/pnas.88.3.737
Indrigo J, Hunter RL, Actor JK (2002) Influence of trehalose 6,6 ‘-dimycolate (TDM) during mycobacterial infection of bone marrow macrophages. Microbiol-SGM 148:1991–1998
Indrigo J, Hunter RL, Actor JK (2003) Cord factor trehalose 6,6 ‘-dimycolate (TDM) mediates trafficking events during mycobacterial infection of murine macrophages. Microbiol-SGM 149:2049–2059. doi:10.1099/mic.0.26226-0
Hunter RL, Olsen M, Jagannath C, Actor JK (2006) Trehalose 6,6 ‘-dimycolate and lipid in the pathogenesis of caseating granulomas of tuberculosis in mice. Am J Pathol 168(4):1249–1261. doi:10.2353/ajpath.2006.050848
Axelrod S, Oschkinat H, Enders J, Schlegel B, Brinkmann V, Kaufmann SHE, Haas A, Schaible UE (2008) Delay of phagosome maturation by a mycobacterial lipid is reversed by nitric oxide. Cell Microbiol 10(7):1530–1545. doi:10.1111/j.1462-5822.2008.01147.x
Lee WB, Kang JS, Yan JJ, Lee MS, Jeon BY, Cho SN, Kim YJ (2012) Neutrophils promote mycobacterial trehalose dimycolate-induced lung inflammation via the mincle pathway. PLoS Pathog. doi:10.1371/journal.ppat.1002614
Heitmann L, Schoenen H, Ehlers S, Lang R, Holscher C (2013) Mincle is not essential for controlling Mycobacterium tuberculosis infection. Immunobiology 218(4):506–516. doi:10.1016/j.imbio.2012.06.005
Behler F, Steinwede K, Balboa L, Ueberberg B, Maus R, Kirchhof G, Yamasaki S, Welte T, Maus UA (2012) Role of Mincle in alveolar macrophage-dependent innate immunity against mycobacterial infections in mice. J Immunol 189(6):3121–3129. doi:10.4049/jimmunol.1201399
Behler F, Maus R, Bohling J, Knippenberg S, Kirchhof G, Nagata M, Jonigk D, Izykowski N, Magel L, Welte T, Yamasaki S, Maus UA (2015) Macrophage-inducible C-type lectin Mincle-expressing dendritic cells contribute to control of splenic Mycobacterium bovis BCG infection in mice. Infect Immun 83(1):184–196. doi:10.1128/IAI.02500-14
Desel C, Werninghaus K, Ritter M, Jozefowski K, Wenzel J, Russkamp N, Schleicher U, Christensen D, Wirtz S, Kirschning C, Agger EM, da Costa CP, Lang R (2013) The Mincle-activating adjuvant TDB induces MyD88-dependent Th1 and Th17 responses through IL-1R signaling. PLoS One. doi:10.1371/journal.pone.0053531
Schweneker K, Gorka O, Schweneker M, Poeck H, Tschopp J, Peschel C, Ruland J, Gross O (2013) The mycobacterial cord factor adjuvant analogue trehalose-6,6 ‘-dibehenate (TDB) activates the Nlrp3 inflammasome. Immunobiology 218(4):664–673. doi:10.1016/j.imbio.2012.07.029
Shenderov K, Barber DL, Mayer-Barber KD, Gurcha SS, Jankovic D, Feng CG, Oland S, Hieny S, Caspar P, Yamasaki S, Lin X, Ting JPY, Trinchieri G, Besra GS, Cerundolo V, Sher A (2013) Cord factor and peptidoglycan recapitulate the Th17-promoting adjuvant activity of mycobacteria through mincle/CARD9 signaling and the inflammasome. J Immunol 190(11):5722–5730. doi:10.4049/jimmunol.1203343
Lee WB, Kang JS, Choi WY, Zhang Q, Kim CH, Choi UY, Kim-Ha J, Kim YJ (2016) Mincle-mediated translational regulation is required for strong nitric oxide production and inflammation resolution. Nat Commun. doi:10.1038/ncomms11322
Schoenen H, Huber A, Sonda N, Zimmermann S, Jantsch J, Lepenies B, Bronte V, Lang R (2014) Differential control of mincle-dependent cord factor recognition and macrophage responses by the transcription factors C/EBPbeta and HIF1alpha. J Immunol 193(7):3664–3675. doi:10.4049/jimmunol.1301593
Kerscher B, Dambuza IM, Christofi M, Reid DM, Yamasaki S, Willment JA, Brown GD (2016) Signalling through MyD88 drives surface expression of the mycobacterial receptors MCL (Clecsf8, Clec4d) and Mincle (Clec4e) following microbial stimulation. Microbes Infect 18(7–8):505–509. doi:10.1016/j.micinf.2016.03.007
Ostrop J, Jozefowski K, Zimmermann S, Hofmann K, Strasser E, Lepenies B, Lang R (2015) Contribution of MINCLE-SYK signaling to activation of primary human APCs by mycobacterial cord factor and the novel adjuvant TDB. J Immunol 195(5):2417–2428. doi:10.4049/jimmunol.1500102
Rambaruth NDS, Jegouzo SAF, Marlor H, Taylor ME, Drickamer K (2015) Mouse mincle: characterization as a model for human mincle and evolutionary implications. Molecules 20(4):6670–6682. doi:10.3390/molecules20046670
Richardson MB, Torigoe S, Yamasaki S, Williams SJ (2015) Mycobacterium tuberculosis beta-gentiobiosyl diacylglycerides signal through the pattern recognition receptor Mincle: total synthesis and structure activity relationships. Chem Commun 51(81):15027–15030. doi:10.1039/c5cc04773k
van der Peet PL, Gunawan C, Torigoe S, Yamasaki S, Williams SJ (2015) Corynomycolic acid-containing glycolipids signal through the pattern recognition receptor Mincle. Chem Commun 51(24):5100–5103. doi:10.1039/c5cc00085h
Patin EC, Willcocks S, Orr S, Ward TH, Lang R, Schaible UE (2016) Mincle-mediated anti-inflammatory IL-10 response counter-regulates IL-12 in vitro. Innate Immun. doi:10.1177/1753425916636671
Kiyotake R, Oh-hora M, Ishikawa E, Miyamoto T, Ishibashi T, Yamasaki S (2015) Human mincle binds to cholesterol crystals and triggers innate immune responses. J Biol Chem 290(42):25322–25332. doi:10.1074/jbc.M115.645234
Bowker N, Salie M, Schurz H, van Helden PD, Kinnear CJ, Hoal EG, Moller M (2016) Polymorphisms in the pattern recognition receptor Mincle gene (CLEC4E) and association with tuberculosis. Lung. doi:10.1007/s00408-016-9915-y
Balch SG, McKnight AJ, Seldin MF, Gordon S (1998) Cloning of a novel C-type lectin expressed by murine macrophages. J Biol Chem 273(29):18656–18664
Arce I, Martinez-Munoz L, Roda-Navarro P, Fernandez-Ruiz E (2004) The human C-type lectin CLECSF8 is a novel monocyte/macrophage endocytic receptor. Eur J Immunol 34(1):210–220. doi:10.1002/eji.200324230
Miyake Y, Toyonaga K, Mori D, Kakuta S, Hoshino Y, Oyamada A, Yamada H, Ono KI, Suyama M, Iwakura Y, Yoshikai Y, Yamasaki S (2013) C-type lectin MCL is an FcR gamma-coupled receptor that mediates the adjuvanticity of mycobacterial cord factor. Immunity 38(5):1050–1062. doi:10.1016/j.immuni.2013.03.010
Graham LM, Gupta V, Schafer G, Reid DM, Kimberg M, Dennehy KM, Hornsell WG, Guler R, Campanero-Rhodes MA, Palma AS, Feizi T, Kim SK, Sobieszczuk P, Willment JA, Brown GD (2012) The C-type lectin receptor CLECSF8 (CLEC4D) is expressed by myeloid cells and triggers cellular activation through syk kinase. J Biol Chem 287(31):25964–25974. doi:10.1074/jbc.M112.384164
Lobato-Pascual A, Saether PC, Fossum S, Dissen E, Daws MR (2013) Mincle, the receptor for mycobacterial cord factor, forms a functional receptor complex with MCL and Fc epsilon RI-gamma. Eur J Immunol 43(12):3167–3174. doi:10.1002/eji.201343752
Miyake Y, Masatsugu OH, Yamasaki S (2015) C-type lectin receptor MCL facilitates mincle expression and signaling through complex formation. J Immunol 194(11):5366–5374. doi:10.4049/jimmunol.1402429
Kerscher B, Wilson GJ, Reid DM, Mori D, Taylor JA, Besra GS, Yamasaki S, Willment JA, Brown GD (2015) The mycobacterial receptor, Clec4d (CLECSF8, MCL) is co-regulated with Mincle and upregulated on mouse myeloid cells following microbial challenge. Eur J Immunol. doi:10.1002/eji.201545858
Arnaout MA (1990) Structure and function of the leukocyte adhesion molecules CD11/CD18. Blood 75(5):1037–1050
Velasco-Velazquez MA, Barrera D, Gonzalez-Arenas A, Rosales C, Agramonte-Hevia J (2003) Macrophage–Mycobacterium tuberculosis interactions: role of complement receptor 3. Microb Pathog 35(3):125–131
Ehlers MRW, Daffe M (1998) Interactions between Mycobacterium tuberculosis and host cells: are mycobacterial sugars the key? Trends Microbiol 6(8):328–335. doi:10.1016/S0966-842x(98)01301-8
Villeneuve C, Gilleron M, Maridonneau-Parini I, Daffe M, Astarie-Dequeker C, Etienne G (2005) Mycobacteria use their surface-exposed glycolipids to infect human macrophages through a receptor-dependent process. J Lipid Res 46(3):475–483. doi:10.1194/jlr.M400308-JLR200
Le Cabec V, Carreno S, Moisand A, Bordier C, Maridonneau-Parini I (2002) Complement receptor 3 (CD11b/CD18) mediates type I and type II phagocytosis during nonopsonic and opsonic phagocytosis, respectively. J Immunol 169(4):2003–2009
Yassin RJ, Hamblin AS (1994) Altered expression of CD11/CD18 on the peripheral blood phagocytes of patients with tuberculosis. Clin Exp Immunol 97(1):120–125
Juffermans NP, Dekkers PE, Verbon A, Speelman P, van Deventer SJ, van der Poll T (2001) Concurrent upregulation of urokinase plasminogen activator receptor and CD11b during tuberculosis and experimental endotoxemia. Infect Immun 69(8):5182–5185. doi:10.1128/IAI.69.8.5182-5185.2001
Kuo HP, Ho TC, Wang CH, Yu CT, Lin HC (1996) Increased production of hydrogen peroxide and expression of CD11b/CD18 on alveolar macrophages in patients with active pulmonary tuberculosis. Tuber Lung Dis 77(5):468–475
Watford WT, Ghio AJ, Wright JR (2000) Complement-mediated host defense in the lung. Am J Physiol-Lung C 279(5):L790–L798
Ferguson JS, Weis JJ, Martin JL, Schlesinger LS (2004) Complement protein c3 binding to Mycobacterium tuberculosis is initiated by the classical pathway in human bronchoalveolar lavage fluid. Infect Immun 72(5):2564–2573. doi:10.1128/Iai.72.5.2564-2573.2004
Hu C, Mayadas-Norton T, Tanaka K, Chan J, Salgame P (2000) Mycobacterium tuberculosis infection in complement receptor 3-deficient mice. J Immunol 165(5):2596–2602
Rooyakkers AWJ, Stokes RW (2005) Absence of complement receptor 3 results in reduced binding and ingestion of Mycobacterium tuberculosis but has no significant effect on the induction of reactive oxygen and nitrogen intermediates or on the survival of the bacteria in resident and interferon-gamma activated macrophages. Microb Pathog 39(3):57–67. doi:10.1016/j.micpath.2005.05.001
Carlson TK, Torrelles JB, Smith K, Horlacher T, Castelli R, Seeberger PH, Crouch EC, Schlesinger LS (2009) Critical role of amino acid position 343 of surfactant protein-D in the selective binding of glycolipids from Mycobacterium tuberculosis. Glycobiology 19(12):1473–1484. doi:10.1093/glycob/cwp122
Matsunaga I, Moody DB (2009) Mincle is a long sought receptor for mycobacterial cord factor. J Exp Med 206(13):2865–2868. doi:10.1084/jem.20092533
Hattori Y, Morita D, Fujiwara N, Mori D, Nakamura T, Harashima H, Yamasaki S, Sugita M (2014) Glycerol monomycolate is a novel ligand for the human, but not mouse macrophage inducible C-type lectin, Mincle. J Biol Chem 289(22):15405–15412. doi:10.1074/jbc.M114.566489
Hetland G, Wiker HG (1994) Antigen 85C on Mycobacterium bovis, BCG and M. tuberculosis promotes monocyte-CR3-mediated uptake of microbeads coated with mycobacterial products. Immunology 82(3):445–449
Selvaraj P, Jawahar MS, Rajeswari DN, Alagarasu K, Vidyarani M, Narayanan PR (2006) Role of mannose binding lectin gene variants on its protein levels and macrophage phagocytosis with live Mycobacterium tuberculosis in pulmonary tuberculosis. FEMS Immunol Med Microbiol 46(3):433–437. doi:10.1111/j.1574-695X.2006.00053.x
Naderi M, Hashemi M, Taheri M, Pesarakli H, Eskandari-Nasab E, Bahari G (2014) CD209 promoter-336 A/G (rs4804803) polymorphism is associated with susceptibility to pulmonary tuberculosis in Zahedan, southeast Iran. J Microbiol Immunol 47(3):171–175. doi:10.1016/j.jmii.2013.03.013
Vannberg FO, Chapman SJ, Khor CC, Tosh K, Floyd S, Jackson-Sillah D, Crampin A, Sichali L, Bah B, Gustafson P, Aaby P, McAdam KP, Bah-Sow O, Lienhardt C, Sirugo G, Fine P, Hill AV (2008) CD209 genetic polymorphism and tuberculosis disease. PLoS One 3(1):e1388. doi:10.1371/journal.pone.0001388
Gomez LM, Anaya JM, Sierra-Filardi E, Cadena J, Corbi A, Martin J (2006) Analysis of DC-SIGN (CD209) functional variants in patients with tuberculosis. Hum Immunol 67(10):808–811. doi:10.1016/j.humimm.2006.07.003
Ben-Ali M, Barreiro LB, Chabbou A, Haltiti R, Braham E, Neyrolles O, Dellagi K, Gicquel B, Quintana-Murci L, Barbouche MR (2007) Promoter and neck region length variation of DC-SIGN is not associated with susceptibility to tuberculosis in Tunisian patients. Hum Immunol 68(11):908–912. doi:10.1016/j.humimm.2007.09.003
Selvaraj P, Alagarasu K, Swaminathan S, Harishankar M, Narendran G (2009) CD209 gene polymorphisms in South Indian HIV and HIV-TB patients. Infect Genet Evol 9(2):256–262. doi:10.1016/j.meegid.2008.12.003
Zheng RJ, Zhou Y, Qin LH, Jin RL, Wang J, Lu JM, Wang WB, Tang SJ, Hu ZY (2011) Relationship between polymorphism of DC-SIGN (CD209) gene and the susceptibility to pulmonary tuberculosis in an eastern Chinese population. Hum Immunol 72(2):183–186. doi:10.1016/j.humimm.2010.11.004
Olesen R, Wejse C, Velez DR, Bisseye C, Sodemann M, Aaby P, Rabna P, Worwui A, Chapman H, Diatta M, Adegbola RA, Hill PC, Ostergaard L, Williams SM, Sirugo G (2007) DC-SIGN (CD209), pentraxin 3 and vitamin D receptor gene variants associate with pulmonary tuberculosis risk in West Africans. Genes Immun 8(6):456–467. doi:10.1038/sj.gene.6364410
Sadki K, Lamsyah H, Rueda B, Lahlou O, El Aouad R, Martin J (2009) CD209 promoter single nucleotide polymorphism-336A/G and the risk of susceptibility to tuberculosis disease in the moroccan population. Int J Hum Genet 9(3–4):239–243
Kobayashi K, Yuliwulandari R, Yanai H, Lien LT, Hang NT, Hijikata M, Keicho N, Tokunaga K (2011) Association of CD209 polymorphisms with tuberculosis in an Indonesian population. Hum Immunol 72(9):741–745. doi:10.1016/j.humimm.2011.04.004
Acknowledgments
This study was supported by the Deutsche Forschungsgemeinschaft (DFG) International Graduate School (IRTG) GRK1673 and by the Federal Ministry for Education and Science (Grant BMBF 03Z2JN22) to HS.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Goyal, S., Klassert, T.E. & Slevogt, H. C-type lectin receptors in tuberculosis: what we know. Med Microbiol Immunol 205, 513–535 (2016). https://doi.org/10.1007/s00430-016-0470-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00430-016-0470-1