Abstract
The MRC1 gene, encoding the human mannose receptor (MR), is a member of the C-type lectin receptors family. MR can recognize and bind to Mycobacterium tuberculosis by the extracellular structure, and play a role in antigen-presenting and maintaining a stable internal environment. This study aimed to investigate potential associations of SNPs in exon 7 of the MRC1 gene with pulmonary tuberculosis (TB). G1186A, G1195A, T1212C, C1221G, C1303T and C1323T were genotyped using PCR and DNA sequencing in 595 Chinese Uygur and 513 Kazak subjects. In the Uygur, the frequency of allele G (P = 0.031, OR = 1.29, 95 % CI = 1.02–1.62) and AA genotype (P = 0.033, OR = 1.64, 95 % CI = 1.04–2.60) for G1186A was lower in the pulmonary TB than healthy control and were significantly correlated with pulmonary TB. After adjustment for age and gender, G1186A was found to be additive models in association with pulmonary TB (P = 0.04, OR = 1.27, 95 % CI = 1.01–1.60). By calculating linkage disequilibrium, the frequency of haplotype GGTCCT (P = 0.032, OR = 0.75, 95 % CI = 0.57–0.97) and GGTCCC (P = 0.044, OR = 0.57, 95 % CI = 0.33–0.99) was significantly associated with pulmonary TB. No association was found between other SNPs and pulmonary TB. In the Kazak, all SNPs were not associated with pulmonary TB. Our results suggest that genetic factors play an important role in susceptibility to pulmonary TB at the individual level, and provide an experimental basis to clarify the pathogenesis of pulmonary TB.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
According to the latest World Health Organization statistics, there were an estimated 12.0 million prevalent cases of tuberculosis (TB) in 2011 (178 per 100,000 population) and TB kills approximately 1.45 million people each year (15 deaths per 100,000 population) [1]. In China, there were an estimated 0.45 million prevalent cases of TB (300 per 100,000 population), which accounts for one-fourth of the world’s population, and is much higher than the international average [2]. Xinjiang is one of the regions in China most seriously affected by TB. The prevalence of active TB, sputum smear-positive TB and culture-positive TB in the Uygur and Kazak populations of the Xinjiang Uygur Autonomous Region (Northwest China) have been found to be 12.4, 16.9 and 18.4 % higher than the Chinese Han population, respectively [3]. Therefore, studies to identify genes associated with pulmonary TB susceptibility in the Xinjiang Uygur and Kazak populations, and revealing the differences in susceptibility to TB among various ethnic groups will play an important role in controlling and preventing the incidence of pulmonary TB.
TB is an infectious disease caused by Mycobacterium tuberculosis (Mtb). Although about one-third of the world’s population is thought to be infected with Mtb, only 5–15 % of people develop clinically active TB during their lifetime [4]. This suggests that certain genetic factors may play important roles in susceptibility to pulmonary TB at the individual level. The genetic and environmental factors have been reported to influence human susceptibility to infectious diseases [5]. In the present study, we have confirmed that genetic mutations caused by single nucleotide polymorphisms (SNPs) are associated with increased susceptibility to pulmonary TB. Genetic polymorphisms of many candidate genes, such as, human natural resistance-associated macrophage protein gene 1 (NRAMP1) [6], Vitamin D receptor (VDR) [7], Toll-like receptors (TLR2, TLR4 and TLR8) [8–12], NOD-like receptors (NOD2) [13, 14] and cytokines (IL-10) [15, 16] have been shown to be related to pulmonary TB. To date, the predisposition of genetic variants in the mannose receptor gene (MRC1) conferring susceptibility to pulmonary TB has not yet been reported.
The MRC1 gene, encoding the human mannose receptor (MR), is a member of the pattern recognition C-type lectin receptors (CLR) family, which contributes to the activation and production of cytokines and may play an important role in TB infection[17, 18]. The MRC1 gene is located on chromosome 10p12 and comprises 30 exons. Studies have shown that the MRC1 gene has been associated with susceptibility to some diseases, such as asthma [19], sarcoidosis [20] and leprosy [21]. We speculated that the MRC1 gene may be an excellent candidate for susceptibility to pulmonary TB. This study is the first to report that genetic variants in exon 7 of the MRC1 gene can be associated with susceptibility to pulmonary TB in a Chinese Uygur population, but not in a Chinese Kazak population. The study also provides a strong experimental basis to clarify susceptibility to pulmonary TB in different ethnic groups.
Materials and methods
Patients and control subjects
Patients were diagnosed according to the diagnostic criteria for pulmonary tuberculosis of Ministry of Health, China [22]. All patients meet one of the following pulmonary tuberculosis diagnostic criteria: (1) positive sputum examination (smear or culture), (2) negative sputum examination, chest X-ray and computed tomography (CT) revealing evidence of typical active tuberculosis, (3) pathological diagnosis of tuberculosis in lung specimens, (4) suspected of having pulmonary TB after clinical follow-up and X-ray observations, and excluding other lung diseases, (5) clinically ruling out other causes of pleural effusion, and diagnosis of tuberculous pleurisy.
A total of 252 Chinese Uygur patients diagnosed with pulmonary TB (138 men and 144 women), aged 20–70 years (mean age 28.8 ± 10.1 years) were recruited from the Second People’s Hospital of Aksu, Xinjiang and Wensu County People’s Hospital between January 2008 and June 2011. For the Chinese Kazak population, a total of 188 patients diagnosed with pulmonary TB (85 men and 103 women), aged 20–70 years (mean age 37.1 ± 10.9 years) were recruited from the Tacheng District Hospital, Xinjiang between December 2007 and June 2011.
The healthy controls were recruited from the same area (Uygur and Kazak people living in the Xinjiang autonomous region). The inclusion criteria for healthy controls were: (1) no active TB lesions, pathological calcification and pathological lung shadows on chest X-ray, (2) no history of TB in the family, (3) the serum anti-body negative pulmonary TB, (4) no history of chronic obstructive pulmonary disease, asthma, lung cancer, diabetes and high blood pressure, (5) no mixed descendants within three generations, (6) no history of chronic diseases, hormone therapy and autoimmune disease.
A total of 343 Chinese Uygur healthy control individuals (185 men and 158 women), aged 20–70 years (mean age 30.1 ± 8.9 years), and 325 Chinese Kazak control individuals (147 men and 178 women), aged 20–72 years (mean age 37.5 ± 9.6 years), were included in the study (Table 1).
An early morning, fasting blood samples from the patients and controls were collected in 3.0 mL EDTA tubes and stored at −70 °C. For each gene polymorphisms, it was ensured that the final selected samples of TB patients were similar to controls in the ratio of gender, age, history of exposure to TB, vaccination and PPD skin test. The study was approved by the Ethics Committee of the Faculty of Medicine (Zhejiang University, China), and informed consents were obtained from all subjects before blood sampling.
SNP selection
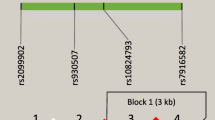
We acquired the MRC1 gene sequence by using the NCBI Gene database (http://www.ncbi.nlm.nih.gov/gene) and the Ensemble Genome Browser Database (http://asia.ensembl.org). We also obtained the information of SNPs in this gene by using dbSNP database (http://www.ncbi.nlm.nih.gov/snp/), and screened the frequency of SNPs by using HapMap database (http://www.hapmap.org). Finally, we selected six SNPs in exon 7 of the MRC1 gene, G1186A (rs34039386, Gly396Ser), G1195A (rs71497223, Gly396Ser), T1212C (rs71497224, Ile404Ile), C1221G (rs34284571, Leu407Phe), C1303T (Leu435 Phe) and C1323T (Asn441Asn).
Genotyping by PCR and DNA sequencing methods
SNPs in the MRC1 gene were analyzed by polymerase chain reaction (PCR) and direct sequencing. Genomic DNAs were extracted from the peripheral blood leukocytes with DNA extraction kit (QIAamp® DNA Blood Mini Kit, Germany) according to the manufacturer’s instruction. The PCR primer included forward: 5′-TTGAGGCTGCAATGAGACAT-3′ and reverse: 5′-AGTGTAAGGTAGACTGCTCT-3′. The PCR was performed following an amplification protocol as previously described [23]. The amplified products were purified with PCR cleaning kit (AxyPrep PCR cleaning kit, USA) and then identified by scanning with the ABI 3100 sequencer (Applied Biosystems, Carlsbad, CA, USA).
Statistical analysis
The χ 2 test was used to compare allele and genotype distribution in the TB patients and control subjects by the two-tailed Fisher exact method with the GraphPad Prism version 5.01 software. The odd ratio (OR) and 95 % confidence interval (CI) were calculated by Miettinen’s method. The value of OR is used to estimate the relative risk. If the OR is equal to 1, it indicates that the factor has no effect on the incidence of disease. If the OR is greater than 1, it indicates that the factor is a risk to the incidence of disease. If the OR is less than 1, then this indicates that the factor is protective.
Pairwise linkage disequilibrium (LD) was calculated by using SNPstats (http://bioinfo.iconcologia.net/SNPstats), and estimated based on D′ (D′ value is the relative size of the LD, which is on behalf of the differences between the expected haplotype frequency and the observed haplotype frequency and scaled in [−1,1] range) and R (R is the correlation coefficient between alleles) that are on behalf of the degree of LD between each pair of SNPs [24]. Values of D′ > 0.75 were considered to be strong pairwise LD. We constructed haplotypes between each pair of SNPs. Haplotype frequencies and associations were calculated using an expectation–maximization algorithm. Using logistic regression method, we calculated the allele combinations of OR values and 95 % CI referring to the highest frequency of haplotype allele and the additive genetic model. P value was calculated to estimate haplotype association with pulmonary TB.
Results
We investigated 6 SNPs in exon 7 of the MRC1 gene using 252 pulmonary TB cases and 343 healthy controls in the Chinese Uygur population (Fig. 1). All MRC1 SNPs were in Hardy–Weinberg equilibrium in the control group and the pulmonary TB group (P > 0.05) (Table 2). The frequencies of the G/G, A/G and A/A genotypes for the G1186A site in the control group were 0.286, 0.496 and 0.219, respectively, while, the frequencies of the three genotypes in the pulmonary TB group were 0.230, 0.480 and 0.290, respectively. The frequency of A/A genotype was lower in the control group than the pulmonary TB group and there was a significant difference between the two groups (P = 0.033, OR = 1.64, 95 % CI = 1.04–2.60). But, the frequency of A/G genotype was not significantly different between the two groups (P > 0.05). No significant differences between patients and controls were observed in the genotype distribution of G1195A, T1212C, C1221G, C1303T and C1323T (P > 0.05) (Table 3).
The DNA sequences with 6 SNPs in exon 7 of the MRC1 gene in the Chinese Uygur and Kazak population. a G1186A, a the genotype of G/G, b the genotype of G/A, c the genotype of A/A. b G1195A, a the genotype of G/G, b the genotype of G/A, c the genotype of A/A. c T1212C, a the genotype of T/T, b the genotype of C/T, c the genotype of C/C. d C1221G, a the genotype of C/C, b the genotype of C/G, c the genotype of G/G. e C1303T, a the genotype of C/C, b the genotype of C/T, c the genotype of T/T. f C1323T, a the genotype of T/T, b the genotype of C/T, c the genotype of C/C
The allele frequency of G for the G1186A was lower in the pulmonary TB group (0.470) than the control group (0.534) and there was a significant difference between the two groups (P = 0.031, OR = 1.29, 95 % CI = 1.02–1.62) (Table 2). However, the allele frequencies of G1195A, T1212C, C1221G, C1303T and C1323T were not significantly different between patients and controls (P > 0.05) (Table 2).
We performed logistic repression analysis with all SNPs using a recessive model adjusted for age and sex and found that G1186A SNP was associated with pulmonary TB in additive model (P = 0.04, OR = 1.27, 95 % CI = 1.01–1.60) (Table 4). However, the genetic model of other SNPs did not correlate with pulmonary TB (P > 0.05).
Pairwise LD between the 6 SNPs of the MRC1 gene was calculated for the cases and controls (Fig. 2). We found strong LD (D′ > 0.75, P < 0.0001) between some pairs of markers in the MRC1 gene including G1186A and G1195A, G1186A and T1212C, G1186A and C1221G, G1186A and C1303T, G1186A and C1323T, G1195A and T1212C, G1195A and G1221C, G1195A and C1303T, T1212C and C1221G, T1212C and C1303T, C1221G and C1303T. Therefore, based on logistic regression, we found that the haplotype with each pair of SNPs was associated with pulmonary TB. The frequency of the haplotype GGTCCT in the pulmonary TB group was lower than the control group and was significantly associated with pulmonary TB (P = 0.032, OR = 0.75, 95 % CI = 0.57–0.97). The frequency of the haplotype GGTCCC in the pulmonary TB group was lower than the control group and was also significantly associated with pulmonary TB (P = 0.044, OR = 0.57, 95 % CI = 0.33–0.99). Whereas, the frequency of the haplotype AGTCCT and GACGTT in the pulmonary TB group was higher than the control group, and the frequency of haplotype GGCCCT in the pulmonary TB group was lower than the control group. However, the three haplotypes with the MRC1 gene polymorphisms showed no significant association with pulmonary TB (P > 0.05) (Table 5).
Linkage disequilibrium analysis of single-nucleotide polymorphism (SNPs) in the MRC1 gene. The number at the intersection of each pair of SNPs represents the pairwise D, D′, r2 and P values between two SNPs. Values of D′ > 0.75 were considered to be strong pairwise linkage disequilibrium. a Kazak population, b Uygur population
We investigated 6 SNPs in exon 7 of the MRC1 gene in the Chinese Kazak population using 188 pulmonary TB cases and 325 healthy controls (Fig. 1). The MRC1 SNPs were in Hardy–Weinberg equilibrium in the control group and the pulmonary TB group except for the C1323T site (P > 0.05) (Table 6). No significant differences between patients and controls were observed in the genotype distribution of G1186A, G1195A, T1212C, C1221G, C1303T and C1323T (P > 0.05) (Table 7).
The allele frequencies of G1186A, G1195A, T1212C, C1221G, C1303T and C1323T were not significantly different between patients and controls (P > 0.05) (Table 6).
We performed logistic repression analysis with all SNPs using a recessive model adjusted for age and sex. The genetic model of 6 SNPs did not correlate with pulmonary TB (P > 0.05) (Tables 8, 9).
Pairwise LD between the 6 SNPs of the MRC1 gene was calculated for the cases and controls (Fig. 2). We found strong LD (D′ > 0.75, P < 0.0001) between some pairs of markers in the MRC1 gene including G1186A and G1195A, G1186A and T1212C, G1186A and C1221G, G1186A and C1303T, G1186A and C1323T, G1195A and T1212C, G1195A and G1221C, G1195A and C1303T, T1212C and C1221G, T1212C and C1303T, C1221G and C1303T. Therefore, based on logistic regression, we found that the haplotype with each pair of SNPs was associated with pulmonary TB. The frequency of the haplotype GACGTT and GGTCCC in the pulmonary TB group was lower than the control group. The frequency of the haplotype GGCCCT in the pulmonary TB group was higher than the control group. The frequency of the haplotype AGTCCT and GGTCCT in the pulmonary TB group was as much as that of the control group. However, all haplotypes with the MRC1 gene polymorphisms showed no significant association with pulmonary TB (P > 0.05) (Table 10).
Discussion
Both innate and adaptive immunity play an important role in susceptibility to pulmonary TB [17]. Therefore, genetic variability may influence host susceptibility to develop pulmonary TB. MR is predominantly present on alveolar macrophages and dendritic cells and recognizes glycan structures containing mannose, fucose and N-acetylglucosamine, which are commonly found on the cell walls of pathogenic micro-organism such as mycobacteria, fungus, parasites, and yeast [25].
MR has been shown to be an important mediator between Mtb and the host immune system [26]. Components of pathogens are recognized by MR, and then they induce cytokine production and macrophage activation to kill pathogens [26, 27]. However, polymorphisms in the MRC1 gene encoding MR have not been reported to be associated with pulmonary TB. We postulated that MRC1 polymorphisms may be associated with the development of pulmonary TB in Chinese Uygur and Kazak populations. Six SNPs in exon 7 of the MRC1 gene were analyzed in the Chinese Uygur and Kazak populations. The results showed that both the frequency of genotype (P = 0.033, OR = 1.64, 95 % CI = 1.04–2.60) and allele (P = 0.031, OR = 1.29, 95 % CI = 1.02–1.62) for the G1186A (rs34039386) site were significantly different between the pulmonary TB group and the control group in the Chinese Uygur population. The OR values of genotype and allele for G1186A were more than 1, which indicated that G1186A site in exon 7 of the MRC1 gene can be associated with susceptibility to pulmonary TB in the Chinese Uygur population and may increase the risk of being infected with pulmonary TB. No association was found between the other 5 SNPs (G1195A, T1212C, C1221G, C1303T and C1323T) and pulmonary TB (P > 0.05) in the Chinese Uygur population. We found that both the frequency of genotype and allele for the 6 SNPs (G1186A, G1195A, T1212C, C1221G, C1303T and C1323T) were not significantly different between the pulmonary TB group and the control group in the Chinese Kazak population (P > 0.05), suggesting that MRC1 polymorphisms have no effects on the development of pulmonary TB. This study is the first to report that genetic variants in exon 7 of the MRC1 gene can be associated with susceptibility to pulmonary TB in a Chinese Uygur population, but not in a Chinese Kazak population.
The G1186A site in exon 7 of the MRC1 gene is non-synonymous mutation that changes Gly to Ser. Exon 7 polymorphisms in MRC1 map to the second C-type lectin domain (CTLD2) of the MR protein. CTLD2 has been structurally assigned to a hinge domain linking the C-type lectin region to the fibronectin type II and cysteine-rich domains [28]. While CTLD2 is homologous to C-type lectins, it does not bind to ligands [29]. So, it is possible that changes at CTLD2 indirectly impact on MR ligand affinities and the mutation of this site change the function of MR and affect the binding of MR protein to polysaccharide structure on the Mtb surface, thus, contributing to the secretion of anti-inflammatory factors. However, Alter et al. [19] have studied HEK293 cells over-expressing MR constructs (293-MR) with three exon 7 haplotypes of MRC1 (G1186A, G1195A and C1221G) for their ability to bind and internalize ovalbumin and zymosan, two classical MR ligands. No difference in uptake was measured between the variants, and 293-MR failed to bind and internalize viable Mycobacterium leprae and BCG. The authors proposed that the MR–M. leprae interaction was modulated by an accessory host molecule of unknown identity. For instance, MR delivers an intracellular signal that affects the immune status of cells as well as endosome–lysosome fusion [30–32]. However, this signaling is mediated by the cytoplasmic domain of MR and would not be expected to be affected by a remote extracellular site. It was suggested that exon 7 variants have an indirect effect on overall structure and/or stability of the CTLD2 domain, likely by modulating the interaction with an additional host molecule(s). Whether the impact of the pulmonary TB-associated MRC1 alleles is via the alteration of MR ligand interactions or by changed receptor signaling or trafficking is not known. In the future, we will clarify the relationship between the changed protein (mutant protein) in the MRC1 gene and M. tuberculosis, and provide an experimental basis to clarify the pathogenesis of pulmonary TB.
In the Chinese Uygur population, the C1200T site was only found in the pulmonary TB group with the rate of occurrence being 0.39 % and minor allele frequencies (MAF) <1 %. So, this site did not belong to polymorphisms. Meanwhile, we also analyzed association between haplotypes of MRC1 gene and the incidence of pulmonary TB in the Chinese Uygur population. We found that the frequency of the haplotype GGTCCT (P = 0.032, OR = 0.75, 95 % CI = 0.57–0.97) and haplotype GGTCCC (P = 0.044, OR = 0.57, 95 % CI = 0.33–0.99) was significantly associated with pulmonary TB and may increase the risk of pulmonary TB infection. In the Chinese Kazak population, the T1191C site was only found in the pulmonary TB group with the rate of occurrence being 0.30 % and MAF < 1 %. So, this site also did not belong to polymorphisms. All haplotypes of the MRC1 gene polymorphisms showed no significant association with pulmonary TB (P > 0.05). The results suggested that the MRC1 gene polymorphisms have no effects on susceptibility to pulmonary TB and MRC1 may not be a major gene contributing to pulmonary TB infection in the Chinese Kazak population. Therefore, we proposed that the difference in genetic polymorphisms in various ethnic groups may affect susceptibility to pulmonary TB. Meanwhile, racial differences often combine with genetic and environmental factors, and make the observed difference even more complicated between different ethnic groups.
Alter et al. [19] found that the allele frequency of G1186A site in the exon 7 of MRC1 gene had significant disparity between leprosy per se cases and healthy controls in the Brazilian population (P = 0.016, OR = 1.34, 95 % CI = 1.06–1.70). The OR value was more than 1, and suggested that this polymorphism was associated with susceptibility to leprosy. Hattori et al. [20] found that G1186A site in the exon 7 of MRC1 gene was not significantly associated with asthma in both Japanese and African-American populations, suggesting that this polymorphism was not associated with susceptibility to asthma. Hattori et al. [21] also reported that the allele frequency of G1186A site in the exon 7 of MRC1 gene had no significant association with sarcoidosis in the Japanese population. According to HapMap Organization statistics, different ethnic groups have significant differences in the distribution frequency of SNPs. Zhang et al. [23] found that the frequency of allele G (P = 0.037; OR = 0.76; 95 % CI, 0.58–0.98) and AG genotypes (P < 0.01; OR = 0.57; 95 % CI, 0.37–0.87) for G1186A of the MRC1 gene in a Chinese population were significantly differences between the pulmonary TB group and the healthy control group, and may reduce the risk of infecting pulmonary TB. However, in our study, the G1186A in the Chinese Uygur population was associated with the susceptibility to pulmonary TB, and G1186A had no significant association with pulmonary TB in the Chinese Kazak population. We speculated that this is the main reason for differences in genetic background in different ethnic groups, and provides a theoretical basis for genetic epidemiology research in different ethnic groups [33].
In the present study, we analyzed alleles, genotypes and haplotypes, and concluded that the G1186A polymorphism of MRC1 gene was associated with pulmonary TB in the Chinese Uygur population, and may increase the risk of being infected with pulmonary TB. However, G1186A site in the exon 7 of MRC1 gene had no significant association with pulmonary TB in the Chinese Kazak population. In this study, our results suggest that genetic factors play an important role in susceptibility to pulmonary TB at the individual level, and provide an experimental basis to clarify the pathogenesis of pulmonary TB.
References
World Health Organization (2011) Global tuberculosis control report. Available at: http://wwwwhoint/tb/publications/global_report/en/indexhtml
National Technic Steering Group of the Epidemiological Sampling Survey for T, Duanmu H (2002) Report on fourth national epidemiological sampling survey of tuberculosis. Chin Tuberc Respir Dis 25:3–7
Xin J, Xinchun F, Huakui Y (2004) Report in the fourth epidemiological survey for tuberculosis in Xinjiang Uygur Autonomous Region. Chin J Antituberc 26:264–267
Toth A, Fackelmann J, Pigott W, Tolomeo O (2004) Tuberculosis prevention and treatment. Can Nurse 100:27–30
Cooke GS, Hill AV (2001) Genetics of susceptibility to human infectious disease. Nat Rev Genet 2:967–977
Nugraha J, Anggraini R (2011) NRAMP1 polymorphism and susceptibility to lung tuberculosis in Surabaya, Indonesia. Southeast Asian J Trop Med Public Health 42:338–341
Bornman L, Campbell SJ, Fielding K, Bah B, Sillah J et al (2004) Vitamin D receptor polymorphisms and susceptibility to tuberculosis in West Africa: a case–control and family study. J Infect Dis 190:1631–1641
Xue Y, Jin L, Li AZ, Wang HJ, Li M et al (2010) Microsatellite polymorphisms in intron 2 of the toll-like receptor 2 gene and their association with susceptibility to pulmonary tuberculosis in Han Chinese. Clin Chem Lab Med 48:785–789
Davila S, Hibberd ML, Hari Dass R, Wong HE, Sahiratmadja E et al (2008) Genetic association and expression studies indicate a role of toll-like receptor 8 in pulmonary tuberculosis. PLoS Genet 4:e1000218
Zhang YX, Xue Y, Liu JY, Zhao MY, Li FJ et al (2011) Association of TIRAP (MAL) gene polymorphisms with susceptibility to tuberculosis in a Chinese population. Genet Mol Res 10:7–15
Xue Y, Zhao ZQ, Hong D, Zhao MY, Zhang YX et al (2010) Lack of association between MD-2 promoter gene variants and tuberculosis. Genet Mol Res 9:1584–1590
Xue Y, Zhao ZQ, Wang HJ, Jin L, Liu CP et al (2010) Toll-like receptors 2 and 4 gene polymorphisms in a southeastern Chinese population with tuberculosis. Int J Immunogenet 37:135–138
Proell M, Riedl SJ, Fritz JH, Rojas AM, Schwarzenbacher R (2008) The Nod-like receptor (NLR) family: a tale of similarities and differences. PLoS One 3:e2119
Austin CM, Ma X, Graviss EA (2008) Common nonsynonymous polymorphisms in the NOD2 gene are associated with resistance or susceptibility to tuberculosis disease in African Americans. J Infect Dis 197:1713–1716
Shin HD, Park BL, Kim YH, Cheong HS, Lee IH et al (2005) Common interleukin 10 polymorphism associated with decreased risk of tuberculosis. Exp Mol Med 37:128–132
Ben-Selma W, Ben-Abderrahmen Y, Boukadida J, Harizi H (2012) IL-10R1 S138G loss-of-function polymorphism is associated with extrapulmonary tuberculosis risk development in Tunisia. Mol Biol Rep 39:51–56
Fritz JH, Girardin SE (2005) How Toll-like receptors and Nod-like receptors contribute to innate immunity in mammals. J Endotoxin Res 11:390–394
Akira S, Uematsu S, Takeuchi O (2006) Pathogen recognition and innate immunity. Cell 124:783–801
Alter A, de Leseleuc L, Van Thuc N, Thai VH, Huong NT et al (2010) Genetic and functional analysis of common MRC1 exon 7 polymorphisms in leprosy susceptibility. Hum Genet 127:337–348
Hattori T, Konno S, Hizawa N, Isada A, Takahashi A et al (2009) Genetic variants in the mannose receptor gene (MRC1) are associated with asthma in two independent populations. Immunogenetics 61:731–738
Hattori T, Konno S, Takahashi A, Isada A, Shimizu K et al (2010) Genetic variants in mannose receptor gene (MRC1) confer susceptibility to increased risk of sarcoidosis. BMC Med Genet 11:151
Ministry of Health of China (2008) Diagnostic criteria for pulmonary tuberculosis. http://wwwmohgovcn/publicfiles///business/cmsresources/zwgkzt/cmsrsdocument/doc3242pdf
Zhang X, Jiang F, Wei LL, Li FJ, Liu JY et al (2012) Polymorphic allele of human MRC1 confer protection against tuberculosis in a Chinese population. Int J Biol Sci 8:375–382
Liu CP, Li XG, Lou JT, Xue Y, Luo CF et al (2009) Association analysis of the PHOX2B gene with Hirschsprung disease in the Han Chinese population of Southeastern China. J Pediatr Surg 44:1805–1811
East L, Isacke CM (2002) The mannose receptor family. Biochim Biophys Acta 1572:364–386
Schlesinger LS (1993) Macrophage phagocytosis of virulent but not attenuated strains of Mycobacterium tuberculosis is mediated by mannose receptors in addition to complement receptors. J Immunol 150:2920–2930
Schlesinger LS, Kaufman TM, Iyer S, Hull SR, Marchiando LK (1996) Differences in mannose receptor-mediated uptake of lipoarabinomannan from virulent and attenuated strains of Mycobacterium tuberculosis by human macrophages. J Immunol 157:4568–4575
Boskovic J, Arnold JN, Stilion R, Gordon S, Sim RB et al (2006) Structural model for the mannose receptor family uncovered by electron microscopy of Endo180 and the mannose receptor. J Biol Chem 281:8780–8787
Taylor ME, Bezouska K, Drickamer K (1992) Contribution to ligand binding by multiple carbohydrate-recognition domains in the macrophage mannose receptor. J Biol Chem 267:1719–1726
Kang PB, Azad AK, Torrelles JB, Kaufman TM, Beharka A et al (2005) The human macrophage mannose receptor directs Mycobacterium tuberculosis lipoarabinomannan-mediated phagosome biogenesis. J Exp Med 202:987–999
Nigou J, Zelle-Rieser C, Gilleron M, Thurnher M, Puzo G (2001) Mannosylated lipoarabinomannans inhibit IL-12 production by human dendritic cells: evidence for a negative signal delivered through the mannose receptor. J Immunol 166:7477–7485
Shimada K, Takimoto H, Yano I, Kumazawa Y (2006) Involvement of mannose receptor in glycopeptidolipid-mediated inhibition of phagosome–lysosome fusion. Microbiol Immunol 50:243–251
Delgado JC, Baena A, Thim S, Goldfeld AE (2002) Ethnic-specific genetic associations with pulmonary tuberculosis. J Infect Dis 186:1463–1468
Acknowledgments
This work was supported by Grants from National Special Sci-Tech Projects (No. 2012ZX10005001-006, No. 2008ZX10005-010), National Natural Science Foundation of China (No. 81072724), and Zhejiang Province Special Sci-Tech Projects (No. 2009C03011-3).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhang, X., Li, X., Zhang, W. et al. The novel human MRC1 gene polymorphisms are associated with susceptibility to pulmonary tuberculosis in Chinese Uygur and Kazak populations. Mol Biol Rep 40, 5073–5083 (2013). https://doi.org/10.1007/s11033-013-2610-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-013-2610-7