Abstract
It has been shown that swallowing involves certain attentional and cognitive resources which, when disrupted can influence swallowing function with in dysphagic patient. However, there are still open questions regarding the influence of attention and cognitive demands on brain activity during swallowing. In order to understand how brain regions responsible for attention influence brain activity during swallowing, we compared brain organization during no-distraction swallowing and swallowing with distraction. Fifteen healthy male adults participated in the data collection process. Participants performed ten 1 ml, ten 5 ml, and ten 10 ml water swallows under both no-distraction conditions and during distraction while EEG signals were recorded. After standard pre-processing of the EEG signals, brain networks were formed using the time–frequency based synchrony measure. The brain networks formed were then compared between the two sets of conditions. Results showed that there are differences in the Delta, Theta, Alpha, Beta, and Gamma frequency bands between no-distraction swallowing and swallowing with distraction. Differences in the Delta and Theta frequency bands can be attributed to changes in subliminal processes, while changes in the Alpha and Beta frequency bands are directly associated with the various levels of attention and cognitive demands during swallowing process, and changes in the Gamma frequency band are due to changes in motor activity. Furthermore, we showed that variations in bolus volume influenced the swallowing brain networks in the Delta, Theta, Alpha, Beta, and Gamma frequency bands. Changes in the Delta, Theta, and Alpha frequency bands are due to sensory perturbations evoked by the various bolus volumes. Changes in the Beta frequency band are due to reallocation of cognitive demands, while changes in the Gamma frequency band are due to changes in motor activity produced by variations in bolus volume. These findings could potentially lead to the development of better understanding of the nature of dysphagia and various rehabilitation strategies for patients with neurogenic dysphagia who have altered attention or impaired cognitive functions.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Deglutition (i.e., swallowing) is an essential neuromuscular event which provides the transportation of food and liquids from the oral cavity to the stomach. The process of swallowing has been artificially subdivided into phases to enable description of the numerous and overlapping biomechanical events that occur within the one- to two-second duration of a typical liquid swallow that does not involve oral preparation of solid food prior to transport to the pharynx and esophagus (Ertekin et al. 2003; Stevenson and Allaire 1991). This process can be divided into four distinctive phases (i.e., oral preparatory, oral transit, pharyngeal, and esophageal), all of which overlap somewhat in their sequence of occurrence. The first phase of swallowing activity consists of primarily voluntary actions, while the remaining stages are characterized by primarily involuntary actions (Dodds 1989). Swallowing under conditions requiring volitional augmentation of oral activity, posture, or other swallowing maneuvers alter the intensity of voluntary recruitment and are hypothesized to result in changes on cognitive demands during the different stages of the swallowing process (Brodsky et al. 2012a).
Dysphagia (swallowing disorders) is caused by either disruption of the swallowing mechanism itself, or by disease and brain lesions introduced by conditions such as stroke, which disrupt sensorimotor function through damage to central neural structures without focally altering the peripheral aerodigestive mechanism (Cichero and Murdoch 2006). There are a number of causes of dysphagia, but the most common in adults are neurological conditions such as stroke (Gottlieb et al. 1996), physical traumatic brain injuries (Lazarus and Logemann 1987), cerebral palsy (Rogers et al. 1994), and Parkinson’s or other neurodegenerative diseases (Murray 1999). Dysphagia often leads to the development of other adverse medical conditions, such as dehydration (Smithard et al. 1996), malnutrition (Foley et al. 2009), failure of the immune system (Curran and Groher 1990), respiratory infection (Marik and Kaplan 2003), and in general, a decreased quality of life (McHorney et al. 2000).
Although once believed to be mediated purely reflexively at the brainstem level and executed peripherally without higher level neural input, multiple and bilateral hemispheric regions have been shown to be activated before, during, and after swallowing (Hamdy et al. 1997; Michou and Hamdy 2009; Ertekin et al. 2003; Stevenson and Allaire 1991). Specifically, large swallowing neural networks involving bilateral primary motor and sensory cortices, supplemental and premotor cortices, Heschls gyri, cingulate gyri, Brocas area and the insula, and superior temporal gyri have been identified using functional magnetic resonance imaging (Mosier et al. 1999). And likewise, activation of numerous other anterior frontal and temporal cortical and subcortical structures have been observed during swallowing in healthy individuals (Hamdy et al. 1999).
Many of the cerebral areas activated during swallowing are responsible for several cognitive functions and are active during the processing of language; some, when damaged, have been strongly implicated in impaired cognitive processes such as attention (Corbetta and Shulman 2002). Hence interest in an interaction between cognition, language, and swallowing has increased speculation that central neurogenic dysphagia may have a cognitive component, has risen in the past decade. During typical swallowing clinical testing using videofluoroscopy or fiberoptic imaging, patients are instructed to swallow after receiving a verbal command from an examiner. Several studies have identified significant differences between swallows prompted by a verbal instruction compared to spontaneous swallows in healthy and dysphagic patients (Daniels et al. 2007; Matsuo and Palmer 2008; Nagy et al. 2013; Nonaka et al. 2009). Similarly, the effect of attention on swallowing function has also been implicated, with significantly altered reaction times and timing of swallow physiologic events observed in healthy and dysphagic participants when challenged by a divided attention task (Brodsky et al. 2012a, b).
Cerebral reorganization after brain damage has received much attention as it relates to the effects of rehabilitative efforts after stroke and brain injury. It has been shown that successful dysphagia rehabilitation is correlated with brain reorganization following cerebral injury and during the natural course of other specific neurological disorders (Hamdy et al. 2000). Whereas in the past dysphagia has been treated mainly using a bottom-up method of restoring peripheral function to improve swallow function, plasticity of swallowing related to reorganization of damaged central as a construct, a more top-down process, is currently receiving much attention (Doeltgen and Huckabee 2012; Robbins 2011). However, most current methods of observing brain function during swallowing and swallowing rehabilitation rely on bulky, sophisticated, and impractical instrumentation that, like language and attentional interference, introduce unnatural variables to the swallowing task. Thus, it is essential to ascertain patterns of brain activity during swallowing using more practical and meaningful methods, to provide a better understanding of neurogenic swallowing difficulties in order to generate more research into top-down rehabilitation strategies using swallowing network research that preserves natural swallowing conditions.
One way of analyzing brain activity during swallowing is to use electroencephalography (EEG) (Jestrović et al. 2014, 2015a). This portable, affordable and non-invasive technique enables the participant to swallow naturally without any imposed postural or other conditions. Unlike functional magnetic resonance imaging or near-infrared spectroscopy, EEG is characterized by a very good temporal resolution, which enables analysis of brain networks during both longer and shorter duration swallowing events. Furthermore, EEG enables simultaneous analysis of the relationships and interactions between different brain regions using statistical methods such as the graph theory approach. We have previously shown that the graph theory approach applied to EEG signals during swallowing can provide important insight into swallowing neurology (Jestrović et al. 2015b). Moreover, EEG examines the underlying transit of neural information among brain regions, rather than just observing the product of that activation (i.e. areas that are ultimately activated).
Therefore, it is germane to the investigation of neurogenic dysphagia and the effects of behavioral treatment of dysphagia, in which patients must perform volitional augmentation of oropharyngeal activities, to investigate the effects of external distraction on brain activity during swallowing, using more practical and less invasive methods. In this study, we hypothesize that the brain network is different between no-distraction swallowing and swallowing with distraction. Characterizing the brain networks during swallowing with distraction could potentially explain the reason for higher risk of aspiration within some groups of dysphagic patients (Barrett and Burkholder 2006; Mattingley et al. 1994) and potentially lead to the development of better rehabilitation strategies for dysphagia patients who also have altered attention or impaired cognitive function resulting from neurological disorders.
Methodology
Data acquisition from participants
Data were collected from 15 healthy male subjects, aged from 18 to 35. The number of 15 subjects is considered as a sample of convenience. All participants provided informed consent, and also age, height, and weight. The protocol was approved by the Institutional Review Board at the University of Pittsburgh.
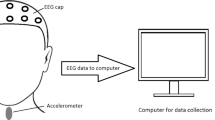
EEG signals were recorded with an array of 64 EEG electrodes. Electrodes were positioned using the actiCAP active electrodes (BrainProducts, Germany) EEG cap, which was positioned according to the 10–20 international electrode system (Jasper 1958). The EEG signals were amplified using the actiCHamp amplifier (BrainProducts, Germany). The P3 electrode was chosen as the reference electrode. Impedance of all electrodes was below 15 k\(\Omega\). Data were recorded at a sampling rate of 10 kHz and saved using PyCorder acquisition software. In order to identify the presence of occurrence of swallowing events, we concurrently recorded swallowing vibrations with a dual-axis accelerometer positioned on the anterior part of the participant’s neck. The capabilities of the dual-axis accelerometer system in the detection of swallowing events were described in detail in our previous studies (Jestrović et al. 2013).
After setting up all devices (Fig. 1) participants were asked to consume ten individual 1 ml water boluses, ten 5 ml water swallows, and ten 10 ml water swallows. Then, participants were asked to repeat the same sequence while they watching a video to occupy their attention and present cognitive demands.
Pre-processing steps
EEGlab MATLAB toolbox (Delorme and Makeig 2004) was used for pre-processing of EEG signals. First, all signals were downsampled to 256 Hz. Next, we filtered signals from 0.1 to 100 Hz using an elliptical infinite impulse response (IIR) band-pass filter. The same type of filter was also used for removing the noise from the power supply, with cut-off frequencies at 58 and 62 Hz. Next, EEG signals were segmented based on the onset and offset of separate swallows as determined by accelerometer signals. In the last step, we removed artifacts from the EEG signals using the independent component analysis (ICA) algorithm (Hyvärinen and Oja 2000).
Network constructions
The pre-processed signals were filtered into the commonly used frequency bands of interest: Delta (\(<4\) Hz), Theta (4–7 Hz), Alpha (8–15 Hz), Beta (16–31 Hz), and Gamma (>32 Hz). Connectivity networks were constructed with time-frequency based phase synchrony measures proposed by Aviyente et al. (2011) applied to the filtered signals.
Network measures
We used the brain connectivity toolbox (BCT) (Rubinov and Sporns 2010) running in MATLAB to calculate each of the following network measures:
-
The clustering coefficient describes the ratio between the number of existing edges between the nearest neighbor of the node and the maximum number of possible edges (Stam and Reijneveld 2007). In the case of the weighted network, the clustering coefficient is calculated as follows:
$$\begin{aligned} C_i=\frac{1}{S_i(D_i-1)}\sum _{\begin{array}{c} j,k \end{array}} \frac{w_{ij}+w_{ik}}{2} a_{ij}a_{ik}a_{jk} , \end{aligned}$$(1)where the parameter \({S_i(D_i-1)}\) normalizes the clustering coefficient to be in the range \(0<C_i^W<1\). \(a_{ij}, a_{ik}\), and \(a_{jk}\) all have a value of one in a case of connection between two nodes. The mean clustering coefficient is defined as follows:
$$\begin{aligned} C=\frac{1}{N}\sum _{j=1}^{N} C_j. \end{aligned}$$(2) -
The characteristic path length is the average shortest path length that connects every pair of nodes. (Strogatz 2001; Achard and Bullmore 2007). The characteristic path length contains information about connection strength between node i and node j (Rubinov and Sporns 2010), and is defined as follows:
$$\begin{aligned} L_{i}=\frac{1}{N(N-1)}\sum _{\begin{array}{c} i,j\in N,i\ne j \end{array}}d_{i,j}, \end{aligned}$$(3)Furthermore, the mean characteristic path length is equal to
$$\begin{aligned} L=\frac{1}{N}\sum _{i \in N} L_i. \end{aligned}$$(4) -
The small-worldness describes the optimal organization in the network that would provide the most efficient communication between nodes (Bassett and Bullmore 2006). The small-world network should satisfy two conditions:
$$\begin{aligned} \gamma =\frac{C}{C_{\mathrm{random}}}\gg 1, \end{aligned}$$(5)where \(\gamma\) is the normalized clustering coefficient, and
$$\begin{aligned} \lambda =\frac{L}{L_{\mathrm{random}}}\approx 1, \end{aligned}$$(6)where \(\lambda\) is the normalized characteristic path length. \(C_{\mathrm{random}}\) is the mean clustering coefficient of the random network, and \(L_{\mathrm{random}}\) is mean characteristic path length of the random network. \(C_{\mathrm{random}}\) and \(L_{\mathrm{random}}\) are calculated as the average mean clustering coefficient and average mean characteristic path length from the 100 random networks generated using the Markov-chain algorithm (Sporns and Zwi 2004; Maslov and Sneppen 2002). Finally, we can say that a network has small-world properties if its ratio,
$$\begin{aligned} S=\frac{C/C_{\mathrm{random}}}{L/L_{\mathrm{random}}}, \end{aligned}$$(7)is higher than one (\(S>1\)).
Data analysis
To examine differences in swallowing brain networks based on volume (1/5/10 ml) and task (neutral/distraction), we fit a series of linear mixed models with each network characteristic as the dependent variable (i.e. volume, task and volume by task interaction) as fixed effects of interest, and a participant random effect to account for multiple measurements from the same participant. We appropriately constructed means contrasts to make pairwise comparisons between different volumes for a given task, and between different tasks for a given volume. Next, to examine whether participant age was associated with network characteristics, we fit another set of linear mixed models stratified by task and volume. We used each network characteristic as the dependent variable, and we used age as the fixed-effect independent variable, and a participant random effect to account for multiple trials of the same participant. We used SAS® version 9.3 (SAS Institute, Inc., Cary, NC, USA) for all statistical analysis.
Results
We analyzed 900 swallows of various volumes in the no-distraction condition and during the distraction. Results of the network measures are presented with the mean values (± standard deviation) of the network measure on the vertical axis, and the frequency bands on the horizontal axis. Results are presented in colored bars that are paired based on bolus volume (blue = 1 ml, red = 5 ml, green = 10 ml) and experimental conditions (left bar = no-distraction condition, right bar = distraction condition). Black dots on the plots represent statistically significant differences between no-distraction condition swallowing and swallowing with distraction within the frequency bands of interest.
Distraction effects on brain networks
Figure 2 summarizes the mean value of the clustering coefficient for various bolus volumes consumed during the two different states. No-distraction swallowing of all bolus sizes (1, 5, and 10 ml) exhibited a higher clustering coefficient than swallowing with distraction in the Theta, Alpha, and Beta frequency bands \((p<0.03)\). Also, 10 ml no-distraction swallowing had a higher clustering coefficient than 10 ml swallowing with distraction in the Gamma frequency band \((p<0.01)\).
Figure 3 summarizes the mean value for characteristic path length for various bolus volumes consumed during the two different states. The no-distraction swallowing showed significantly higher characteristic path length than did swallowing with distraction during 5 ml swallowing within the Delta, Theta, and Beta frequency bands \((p<0.02)\). Also, the no-distraction swallowing showed higher characteristic path length than did swallowing with distraction during 10 ml swallowing within all frequency bands of interest.
Figure 4 summarizes the mean value for the small-world parameter for various bolus volumes consumed during the two different states. Swallowing with distraction for the all bolus volumes (1, 5, and 10 ml) showed a higher small-world parameter than did no-distraction swallowing in the Beta frequency band \((p<0.03)\). In the Delta frequency band, 5 ml swallowing with distraction had a higher small-world parameter than did 5 ml no-distraction swallowing \((p<0.01)\). Also, in the Alpha frequency band 10 ml swallowing with distraction had a higher small-world parameter than did the 10 ml no-distraction swallowing \((p<0.01)\).
Volume effects on brain networks
In the Beta frequency band during no-distraction swallowing, 1 ml swallowing showed a higher clustering coefficient than did the 5 ml swallowing \((p=0.04)\). During swallowing with distraction, 1 ml swallowing showed a higher clustering coefficient than did the 5 ml swallowing within all frequency bands of interest \((p<0.05)\). Also, during swallowing with distraction, 1 ml swallowing showed a higher characteristic path length than did 5 ml swallowing within the Delta, Theta, and Alpha frequency bands \((p<0.05)\).
The 1-ml swallowing with distraction showed a higher clustering coefficient than did the 10-ml swallowing with distraction within all frequency bands of interest, while the 1-ml no-distraction swallowing had a lower clustering coefficient than did the 10-ml no-distraction swallowing \((p<0.05)\). The 1-ml no-distraction swallowing showed a lower characteristic path length than did 10 ml no-distraction swallowing within the Beta and Gamma frequency bands \((p<0.01)\). The 1-ml swallowing with distraction showed a higher characteristic path length than did the 10-ml no-distraction swallowing within the Theta and Alpha frequency bands \((p<0.01)\). Furthermore, in the Gamma frequency band during the no-distraction swallowing and the swallowing with distraction, the 1-ml swallowing showed a higher small-world parameter than did the 5-ml swallowing \((p<0.01)\). Also, 1 ml swallowing with distraction had a lower small-world parameter than did 10 ml swallowing with distraction in the Theta frequency band \((p=0.02)\).
During no-distraction swallowing, 5 ml swallowing had a lower clustering coefficient and characteristic path length than did 10 ml swallowing within the Alpha, Beta, and Gamma frequency bands \((p<0.05)\). Also, during no-distraction swallowing 5 ml swallowing had a lower small-world parameter than did the 10-ml swallowing within the Theta and Gamma frequency bands \((p<0.01)\).
Age effects on brain network
Last, we investigated age dependence on the swallowing characteristics during both no-distraction swallowing and swallowing with distraction. Results did not depend on the subject’s age in any of the frequency bands of interest for both swallowing conditions.
Discussion
Our hypothesis, that the brain network is different for no-distraction swallowing compared with the brain network constructed during swallowing with distraction, was supported by our results. The significant statistical differences between no-distraction swallowing and swallowing with distraction are described for each frequency band of interest:
-
Delta and Theta: Our results showed differences in the Delta and Theta frequency bands between no-distraction swallowing and swallowing with distraction for the clustering coefficient and characteristic path length. Changes in the lower EEG frequencies (i.e. Delta and Theta) have been previously reported as important in the process of selective attention to both auditory and visual stimuli (Lakatos et al. 2008). Also, one EEG study combined with fMRI confirmed that sensory cortices such as the auditory and visual cortices are associated with the activation of the Delta and Theta EEG frequency bands (Jann et al. 2010). This means that changes in the Delta and Theta frequency bands (i.e, higher clustering coefficient, higher characteristic path length, and lower small-worldness for no-distraction swallowing in comparison with swallowing with distraction) can be attributed to the changes in the sensory cortices produced by auditory and visual stimulation during swallowing with distraction task. Furthermore, our results also showed changes in the Delta and Theta frequency bands between the swallowing of various bolus volumes. Previous studies have shown that the Delta and Theta frequency bands are activated during sensory stimulation (Yagyu et al. 1998). The swallowing process involves different types of sensory stimulation such as smell, taste, and touch in the oral areas, all of which have been shown to lead to subsequent alterations in swallowing motor activation patterns (Bastian and Riggs 1999). The various bolus volumes used in this study differently affected sensory receptors responsible for touch, kinesthesia, and proprioception in the oral cavity. Therefore, changes in the Delta and Theta frequency bands between swallowing of the various bolus volumes may be attributable to the effects of altered afferent activity entering the swallowing brain networks caused by variations in bolus volume.
-
Alpha: The Alpha frequency band is the most dominant EEG component for the conscious person. Studies showed that the activity of the Alpha frequency band is less prominent when visual stimulation is present (Klimesch 1999). Furthermore, previous studies have reported differences in the Alpha waveforms between attended and unattended stimuli (Marrufo et al. 2001; Yamagishi et al. 2003). Our results are in agreement with these findings by showing significant differences between no-distraction swallowing and swallowing with distraction for the characteristic path length and the small-world parameter in the Alpha frequency band. Thus, we can attribute these statistical differences to the different attentional demands of the no-distraction and distraction conditions while swallowing. In addition, our results showed significant statistical differences in the Alpha frequency band between swallowing of various bolus volumes. Studies have shown that EEG waveforms also exhibit changes in the Alpha frequency band during sensory stimulation (Klemm et al. 1992; Lorig et al. 1991). Therefore, changes between swallowing of different bolus volumes can be attributed to the changes in the activation of the sensory, kinesthetic, and proprioceptive receptors and pathways introduced by the variously sized stimuli employed.
-
Beta: Several previous studies have suggested that the Beta EEG frequency band is directly related to attention during sensorimotor tasks (Murthy and Fetz 1992, 1996; Feige et al. 2000; Sanes and Donoghue 1993; Kristeva-Feige et al. 1993). Our results demonstrated changes in the Beta frequency band between no-distraction swallowing and swallowing with distraction for the small-world parameter. Swallowing is a complex process that involves activation of a number of sensory receptors, as well as muscle activity in both the head and neck. Therefore, changes in the Beta EEG frequency band during swallowing with the distraction could be attributed to the reallocation of cognitive sources during this task (Sanes and Donoghue 1993; Kristeva-Feige et al. 1993).
-
Gamma: A number of studies reported changes in the Gamma EEG frequency band during various motor activities and muscle recruitment (Herrmann and Mecklinger 2001; Niedermeyer and da Silva 2005; Kristeva-Feige et al. 2002). Brodsky et al. (2012b) showed that consumption of stimuli during distraction may alter swallowing activity. Naturally, altered swallowing neural activity may also cause changes in the muscular recruitment involved in performing the swallowing act (Milnik et al. 2013). Therefore, changes in the Gamma EEG frequency band during swallowing with the distraction could be attributed to motor changes introduced by compromised attention. In addition, we found significant differences in the Gamma frequency band between the swallowing of the various bolus volumes. Alteration of bolus volume influences the kinematics of oral and pharyngeal activity, upper esophageal sphincter opening, and hyolaryngeal excursion during swallowing, all of which are motor events (Logemann 2006; Perlman et al. 1993; Massey 2006). Therefore, changes in the Gamma frequency band can be attributed to the changes in motor activity in response to manipulation of bolus volume that various bolus volumes produce.
-
Limitations of the present study: A limitation of this study is that the order of consumed stimuli was specified (i.e., 1 ml first, 5 ml second, 10 ml third), as well as the order of conditions (i.e. no-distraction swallowing first, then swallowing with the distraction). In order to overcome this limitation, future studies could randomize the order of the various manipulations of swallowing conditions. Furthermore, future studies could also investigate the influence of distraction on the swallowing of the different stimuli (i.e. nectar-thick apple juice, or solid food).
Conclusion
In this study we investigated the differences between the brain networks formed during swallowing of three bolus volumes in a no-distraction condition and during distraction. Swallowing EEG signals were collected from 15 healthy male adults aged 18–35. Each participant performed ten 1 ml swallows, ten 5 ml swallows, and ten 10 ml swallows in both conditions. Our results showed a difference between no-distraction swallowing and swallowing with distraction in all frequency bands of interest (i.e., Delta, Theta, Alpha, Beta, and Gamma). In addition, our results showed differences in the swallowing of boluses of various volumes in all frequency bands of interest.
References
Achard S, Bullmore E (2007) Efficiency and cost of economical brain functional networks. PLoS Comput Biol 3(2):174–183
Aviyente S, Bernat EM, Evans WS, Sponheim SR (2011) A phase synchrony measure for quantifying dynamic functional integration in the brain. Hum Brain Mapp 32(1):80–93
Barrett AM, Burkholder S (2006) Monocular patching in subjects with right-hemisphere stroke affects perceptual-attentional bias. J Rehabil Res Dev 43(3):337–346
Bassett DS, Bullmore E (2006) Small-world brain networks. Neuroscientist 12(6):512–523
Bastian RW, Riggs LC (1999) Role of sensation in swallowing function. Laryngoscope 109(12):1974–1977
Brodsky MB, Abbott KV, McNeil MR, Palmer CV, Grayhack JP, Martin-Harris B (2012a) Effects of divided attention on swallowing in persons with idiopathic parkinsons disease. Dysphagia 27(3):390–400
Brodsky MB, McNeil MR, Martin-Harris B, Palmer CV, Grayhack JP, Abbott KV (2012b) Effects of divided attention on swallowing in healthy participants. Dysphagia 27(3):307–317
Cichero J, Murdoch BE (2006) Dysphagia: foundation, theory and practice. Wiley, New York
Corbetta M, Shulman GL (2002) Control of goal-directed and stimulus-driven attention in the brain. Nat Rev Neurosci 3:201–215
Curran J, Groher ME (1990) Development and dissemination of an aspiration risk reduction diet. Dysphagia 5(1):6–12
Daniels SK, Schroeder MF, DeGeorge PC, Corey DM, Rosenbek JC (2007) Effects of verbal cue on bolus flow during swallowing. Am J Speech Lang Pathol 16:140–147
Delorme A, Makeig S (2004) EEGLAB: an open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. J Neurosci Methods 134(1):9–21
Dodds WJ (1989) The physiology of swallowing. Dysphagia 3(4):171–178
Doeltgen SH, Huckabee M-L (2012) Swallowing neurorehabilitation: from the research laboratory to routine clinical application. Arch Phys Med Rehabil 93(2):207–213
Ertekin C, Aydogdu I et al (2003) Neurophysiology of swallowing. Clin Neurophysiol 114(12):2226–2244
Feige B, Aertsen A, Kristeva-Feige R (2000) Dynamic synchronization between multiple cortical motor areas and muscle activity in phasic voluntary movements. J Neurophysiol 84(5):2622–2629
Foley NC, Martin RE, Salter KL, Teasell RW (2009) A review of the relationship between dysphagia and malnutrition following stroke. J Rehabil Med 41(9):707–713
Gottlieb D, Kipnis M, Sister E, Vardi Y, Brill S (1996) Validation of the 50 ml3 drinking test for evaluation of post-stroke dysphagia. Disabil Rehabil 18(10):529–532
Hamdy S, Aziz Q, Rothwell JC, Hobson A, Barlow J, Thompson DG (1997) Cranial nerve modulation of human cortical swallowing motor pathways. Am J Physiol Gastrointest Liver Physiol 272(4):G802–G808
Hamdy S, Mikulis DJ, Crawley A, Xue S, Lau H, Henry S, Diamant NE (1999) Cortical activation during human volitional swallowing: an event-related fmri study. Am J Physiol Gastrointest Liver Physiol 277(1):G219–G225
Hamdy S, Rothwell JC, Aziz Q, Thompson DG et al (2000) Organization and reorganization of human swallowing motor cortex: implications for recovery after stroke. Clin Sci 99(2):151–157
Herrmann CS, Mecklinger A (2001) Gamma activity in human EEG is related to highspeed memory comparisons during object selective attention. Vis Cogn 8(3–5):593–608
Hyvärinen A, Oja E (2000) Independent component analysis: algorithms and applications. Neural Netw 13(4):411–430
Jann K, Kottlow M, Dierks T, Boesch C, Koenig T (2010) Topographic electrophysiological signatures of fmri resting state networks. PLoS One 5(9):e12945
Jasper HH (1958) The ten twenty electrode system of the international federation. Electroencephalogr Clin Neurophysiol 10(1):371–375
Jestrović I, Coyle J, Sejdić E (2014) The effects of increased fluid viscosity on stationary characteristics of EEG signal in healthy adults. Brain Res 1589:45–53
Jestrović I, Coyle J, Sejdić E (2015a) Decoding human swallowing viaelectroencephalography: a state-of-the-artreview. J Neural Eng 12(5):1–15
Jestrović I, Coyle JL, Sejdić E (2015b) Characterizing functional connectivity patterns during saliva swallows in different head positions. J Neuroeng Rehabil 12(1):12–61
Jestrović I, Dudik JM, Luan B, Coyle JL, Sejdić E (2013) The effects of increased fluid viscosity on swallowing sounds in healthy adults. Biomed Eng Online 12(1):90–107
Klemm W, Lutes S, Hendrix D, Warrenburg S (1992) Topographical EEG maps of human responses to odors. Chem Senses 17(3):347–361
Klimesch W (1999) EEG alpha and theta oscillations reflect cognitive and memory performance: a review and analysis. Brain Res Rev 29(2):169–195
Kristeva-Feige R, Feige B, Makeig S, Ross B, Elbert T (1993) Oscillatory brain activity during a motor task. Neuroreport 4(12):1291–1294
Kristeva-Feige R, Fritsch C, Timmer J, Lücking C-H (2002) Effects of attention and precision of exerted force on beta range EEG-EMG synchronization during a maintained motor contraction task. Clin Neurophysiol 113(1):124–131
Lakatos P, Karmos G, Mehta AD, Ulbert I, Schroeder CE (2008) Entrainment of neuronal oscillations as a mechanism of attentional selection. Science 320(5872):110–113
Lazarus C, Logemann J (1987) Swallowing disorders in closed head trauma patients. Arch Phys Med Rehabil 68(2):79–84
Logemann JA (2006) Medical and rehabilitative therapy of oral, pharyngeal motor disorders. GI Motil Online. doi:10.1038/gimo50
Lorig TS, Huffman E, DeMartino A, DeMarco J (1991) The effects of low concentration odors on EEG activity and behavior. J Psychophysiol 5(1):69–77
Marik PE, Kaplan D (2003) Aspiration pneumonia and dysphagia in the elderly. CHEST J 124(1):328–336
Marrufo MV, Vaquero E, Cardoso MJ, Gómez CM (2001) Temporal evolution of alpha and beta bands during visual spatial attention. Cogn Brain Res 12(2):315–320
Maslov S, Sneppen K (2002) Specificity and stability in topology of protein networks. Science 296(5569):910–913
Massey BT (2006) Physiology of oral cavity, pharynx and upper esophageal sphincter. GI Motil Online
Matsuo K, Palmer JB (2008) Anatomy and physiology of feeding and swallowing: normal and abnormal. Phys Med Rehabil Clin N Am 19(4):691–707
Mattingley JB, Bradshaw JL, Bradshaw JA, Nettleton NC (1994) Residual rightward attentional bias after apparent recovery from right hemisphere damage: implications for a multicomponent model of neglect. J Neurol Neurosurg Psychiatry 57(5):597–604
McHorney CA, Bricker DE, Robbins J, Kramer AE, Rosenbek JC, Chignell KA (2000) The SWAL-QOL outcomes tool for oropharyngeal dysphagiain adults: II. Item reduction and preliminary scaling. Dysphagia 15(3):122–133
Michou E, Hamdy S (2009) Cortical input in control of swallowing. Curr Opin Otolaryngol Head Neck Surg 17(3):166–171
Milnik A, Nowak I, Müller NG (2013) Attention-dependent modulation of neural activity in primary sensorimotor cortex. Brain Behav 3(2):54–66
Mosier KM, Liu W-C, Maldjian JA, Shah R, Modi B (1999) Lateralization of cortical function in swallowing: a functional mr imaging study. Am J Neuroradiol 20(8):1520–1526
Murray J (1999) Manual of dysphagia assessment in adults. Singular, San Diego
Murthy VN, Fetz EE (1992) Coherent 25-to 35-hz oscillations in the sensorimotor cortex of awake behaving monkeys. Proc Natl Acad Sci 89(12):5670–5674
Murthy VN, Fetz EE (1996) Oscillatory activity in sensorimotor cortex of awake monkeys: synchronization of local field potentials and relation to behavior. J Neurophysiol 76(6):3949–3967
Nagy A, Leigh C, Hori SF, Molfenter SM, Shariff T, Steele CM (2013) Timing differences between cued and noncued swallows in healthy young adults. Dysphagia 28(3):428–434
Niedermeyer E, da Silva FL (2005) Electroencephalography: basic principles, clinical applications, and related fields. Lippincott Williams & Wilkins, Philadelphia
Nonaka T, Yoshida M, Yamaguchi T, Uchida A, Ohba H, Oka S, Nakajima I (2009) Contingent negative variations associated with command swallowing in humans. Clin Neurophysiol 120(10):1845–1851
Perlman AL, Schultz JG, VanDaele DJ (1993) Effects of age, gender, bolus volume, and bolus viscosity on oropharyngeal pressure during swallowing. J Appl Physiol 75(1):33–37
Robbins J (2011) Upper aerodigestive tract neurofunctional mechanisms: lifelong evolution and exercise. Head Neck 33(S1):S30–S36
Rogers B, Arvedson J, Buck G, Smart P, Msall M (1994) Characteristics of dysphagia in children with cerebral palsy. Dysphagia 9(1):69–73
Rubinov M, Sporns O (2010) Complex network measures of brain connectivity: uses and interpretations. Neuroimage 52(3):1059–1069
Sanes JN, Donoghue JP (1993) Oscillations in local field potentials of the primate motor cortex during voluntary movement. Proc Natl Acad Sci 90(10):4470–4474
Smithard D, O’neill P, Park C, Morris J, Wyatt R, England R, Martin D (1996) Complications and outcome after acute stroke does dysphagia matter? Stroke 27(7):1200–1204
Sporns O, Zwi JD (2004) The small world of the cerebral cortex. Neuroinformatics 2(2):145–162
Stam CJ, Reijneveld JC (2007) Graph theoretical analysis of complex networks in the brain. Nonlinear Biomed Phys 1(1):1–19
Stevenson RD, Allaire JH (1991) The development of normal feeding and swallowing. Pediatr Clin N Am 38(6):1439–1453
Strogatz SH (2001) Exploring complex networks. Nature 410(6825):268–276
Yagyu T, Kondakor I, Kochi K, Koenig T, Lehmann D, Kinoshita T, Hirota T, Yagyu T (1998) Smell and taste of chewing gum affect frequency domain EEG source localizations. Int J Neurosci 93(3–4):205–216
Yamagishi N, Callan DE, Goda N, Anderson SJ, Yoshida Y, Kawato M (2003) Attentional modulation of oscillatory activity in human visual cortex. Neuroimage 20(1):98–113
Acknowledgements
Research reported in this publication was supported by the Eunice Kennedy Shriver National Institute of Child Health & Human Development of the National Institutes of Health under Award number R01HD074819. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Jestrović, I., Coyle, J.L., Perera, S. et al. Influence of attention and bolus volume on brain organization during swallowing . Brain Struct Funct 223, 955–964 (2018). https://doi.org/10.1007/s00429-017-1535-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00429-017-1535-7