Abstract
Main conclusion
Overexpression of miR172a and b in tomato ( Solanum lycopersicum ) Zaofen No. 2 increased resistance to Phytophthora infestans infection by suppressing of an AP2/ERF transcription factor.
The miR172 family has been shown to participate in the growth phase transition, flowering time control, abiotic and biotic stresses by regulating the expression of a small group of AP2/ERF transcription factors. In this study, the precursors of miR172a and b were cloned from tomato, Solanum pimpinellifolium L3708. We used the degradome sequencing to determine the cleavage site of miR172 to a member of the AP2/ERF transcription factor family (Solyc11g072600.1.1). qRT-PCR results showed that the expression of AP2/ERF was negatively correlated with the expression of miR172 in S. pimpinellifolium L3708 infected with Phytophthora infestans. Overexpression of miR172a and b in S. lycopersicum Zaofen No. 2 conferred greater resistance to P. infestans infection, as evidenced by decreased disease index, lesion sizes, and P. infestans abundance. The SOD and POD play important roles in scavenging late massive ROS in plant–pathogen interaction. Malonaldehyde (MDA) is widely recognized as an indicator of lipid peroxidation. Membrane damage in plants can be estimated by measuring leakage of electrolytes, which is evaluated by determining relative electrolyte leakage (REL). Less H2O2 and O2 −, higher activities of POD and SOD, less MDA content and REL, and higher chlorophyll content and photosynthetic rate were also shown in transgenic plants after inoculation with P. infestans. Our results constitute the first step towards further investigations into the biological function and molecular mechanism of miR172-mediated silencing of AP2/ERF transcription factors in S. lycopersicum–P. infestans interaction and provide a candidate gene for breeding to enhance biotic stress-resistance in S. lycopersicum.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Late blight (LB) of Solanum lycopersicum is one of the most devastating diseases caused by one oomycete pathogen, Phytophthora infestans (Park et al. 2013; Zhang et al. 2014). Symptoms of LB include the formation and development of brown–black, water-soaked lesions on leaves and stems with pathogen sporulation, which can kill the foliage, stems, and tubers or fruits of the crops at any time during the growing season (Fry et al. 1992; Park et al. 2013). LB, which has a worldwide occurrence, has caused serious economic loss for field-grown S. lycopersicum, and, therefore, is regarded as a major threat to S. lycopersicum production (Kim and Mutschler 2005; Miranda et al. 2010; Park et al. 2013; Huang et al. 2010). To date, studies on LB resistance genes in S. lycopersicum are limited. Several resistance-P. infestans genes in S. pimpinellifolium, including Ph-1, Ph-2, Ph-3, Ph-4 and Ph-5, have been reported and mapped to chromosomes 7, 10, 9, 1 and 10, respectively (Bonde and Murphy 1952; Black et al. 1996; Kim and Mutschler 2005; Foolad et al. 2008; Merk et al. 2012). But there is still no proven cure for this disease because the mechanisms of LB are not clear yet (Luan et al. 2015).
MicroRNAs (miRNAs) are a class of small, endogenous non-coding RNAs of 20–24 nt that have emerged as key post-transcriptional regulators of genes in most eukaryotes (Janga and Vallabhaneni 2011; Yan et al. 2014). More and more evidence indicated that miRNAs were involved in plant–pathogen interaction (Navarro et al. 2006; Jin 2008; Yang and Huang 2014; Ouyang et al. 2014; Wong et al. 2014; Yang et al. 2015; Luan et al. 2015). Plant miRNAs (miR482, miR6024, miR5300, etc.) regulate an important component of the plant immune systems, effector-triggered immunity (ETI) by silencing nucleotide binding site and leucine-rich repeat (NBS-LRR) defense genes (Li et al. 2012; Shivaprasad et al. 2012; Ouyang et al. 2014; Yang et al. 2015; Fei et al. 2015). miR393 was identified to contribute to pathogen-associated molecular pattern-triggered immunity (PTI) of plant immune systems through auxin signaling (Navarro et al. 2006; Yang et al. 2015). In our previous works, we identified and characterized a number of miRNAs by high-throughput sequencing and homology-based computational research in S. pimpinellifolium L3708–P. infestans interaction (Luan et al. 2014, 2015; Sun et al. 2014). Of these miRNAs, the expression levels of miR169, miR398, miR482, miR6024, miR6026, miR6027, and other miRNAs showed a change after P. infestans infection. Plant miR482, as an important miRNA, functions in resistance against pathogens. In Solanaceae, the distribution of miR482 family members is different. For example, seven miR482 family members were identified in S. peruvianum, S. chilense, S. corneliomulleri, four members in S. ochranthum, three members in S. lycopersicoides, and three members for S. melongena (de Vries et al. 2015). Between S. lycopersicum and S. pimpinellifolium, most of the mature miR482 family members showed no species-specific differences in expression, except for miR482f (de Vries et al. 2015).
The miR172 family is a class of conserved miRNA and first isolated in Arabidopsis using small RNA sequencing (Park et al. 2002; Li et al. 2016). Previous studies showed that the miR172 targeted a small group of AP2/ERF transcription factors, down-regulating their expression by transcript cleavage or translation repression (Aukerman and Sakai 2003; Jung et al. 2007). The miR172-AP2/ERF module was involved in many biological processes, including plant flowering time control, abiotic and biotic stresses (Naqvi et al. 2010; Lee et al. 2014; Spanudakis and Jackson 2014; Li et al. 2016). The role of miR172 in controlling flowering time has been reported for Arabidopsis, maize, barley, rice, and soybean (Chen 2004; Lauter et al. 2005; Nair et al. 2010; Yoshikawa et al. 2013; Lee et al. 2014). Plant miR172 also played a critical role in abiotic stress responses (Pan et al. 2016). For example, rice miR172 was significantly decreased under drought stress; in contrast, it was up-regulated in abundance under osmotic, salt, and cold stresses in wheat (Zhou et al. 2010; Gupta et al. 2014). The expression level of miR172 was changed in leaf and root of Helianthus annuus after drought, heat and cadmium stresses (Ebrahimi Khaksefidi et al. 2015). In addition, overexpression of soybean miR172c in Arabidopsis enhanced tolerance to water deficit and salt stress (Li et al. 2016). The role of miR172 in different biotic stresses was also examined. The accumulation of miR172 was observed to increase with the days post inoculation (dpi) of Tomato leaf curl virus (ToLCV) agroinfection in S. lycopersicum cv Pusa Ruby because miR172 might be associated with leaf curl symptoms (Naqvi et al. 2010). The expression levels of miR172, down-regulated in mulberry with yellow dwarf disease, were up-regulated in grapevine affected by grapevine leafroll disease and rice inoculated with blast fungus Magnaporthe oryzae (Alabi et al. 2012; Li et al. 2014; Gai et al. 2014).
In S. lycopersicum, the miR172 family contains two members, miR172a and miR172b (Zhang et al. 2008). Our previous work showed that the transcripts per million clean tags (TPM) of miR172 in tomato, S. pimpinellifolium L3708 inoculated with P. infestans is lower than WT after analysis of the miRNA-Seq data. In addition, the promotor regions of miR172 were analyzed, which indicated the presence of biotic stress-related cis-elements in promoter regions (Li et al. 2013). However, whether miR172 affected S. lycopersicum resistance to P. infestans has not been determined. Here, the miR172a and b—overexpressed S. lycopersicum Zaofen No. 2 were generated to further illustrate the function of miR172 in S. lycopersicum–P. infestans interaction. The decreased disease index, lesion sizes, and P. infestans abundance were shown in transgenic plants after inoculation with P. infestans. The levels of biotic stress-related physiological indicators (POD and SOD activities, MDA content, REL, chlorophyll content and photosynthetic rate) and the expression of precursor miR172a and b, mature miR172 and its target gene were altered in transgenic plants after inoculation with P. infestans. These results suggest that miR172 could be involved in S. lycopersicum defense responses to P. infestans stresses.
Materials and methods
Inoculation of S. pimpinellifolium with P. infestans
Tomato, Solanum pimpinellifolium L3708, which had been reported as being highly resistant to a wide range of P. infestans isolates was chosen as the host plant and was grown in a greenhouse under 16 h light within a temperature range of 22–28 °C. P. infestans strain P12103 was used in the experiments and provided by Prof. Shan from Northwest A&F University of China. The treated samples (5–6 leaf stage) were inoculated with a suspension of P. infestans spores (106 zoospores/mL; 9 individuals each) before being placed at 100% relative humidity in the dark to ensure spore germination at 20 ± 1 °C. The leaves of each sample were collected at the indicated times (0, 1, 2, 3, 4 and 5 dpi). All samples were quickly frozen in liquid nitrogen and stored at −80 °C for storage until DNA and RNA isolation.
Cloning of pre-miR172s, sequence analysis and identification of target gene
Genomic DNA was extracted from the leaves of S. pimpinellifolium L3708 using Plant Genomic DNA Kit (Tiangen). The precursor of miR172a and b (pre-miR172a and b) were cloned using S. pimpinellifolium L3708 DNA as template and two pairs of primers c-miR172aF/R and c-miR172bF1/R1 (Table S1), which were designed according to the tomato (S. lycopersicum Heinz 1706) genome sequences using the Primer Premier 5 software. The pre-miR172a and b sequences were cloned into the PMD19-T vector (TaKaRa, Dalian, China). The sequences of the amplified DNA fragments were verified by sequencing. Multiple nucleic acid sequence alignments were performed with Clustalx 1.83. Their secondary structures were predicted using RNAshapes (Steffen et al. 2006). We used psRNAtarget (Schema V2, http://plantgrn.noble.org/psRNATarget/) to predict the target genes of miR172. Tomato transcripts (cDNA library, version 2.4) was used as data sets. The parameters were as follow: (1) maximum expectation: 1.5; (2) length for complementarity scoring (hspsize): 19; (3) range of central mismatch leading to translational inhibition: 10–11nt. The degradome sequencing library of S. pimpinellifolium L3708 inoculated with P. infestans was constructed in our previous work (unpublished). The target genes of miR172a and b were identified by analysis of the degradome sequencing library. The CleaveLand software was used to find the cleaved target genes of miR172, and the T-plot figures were produced based on R language (Addo-Quaye et al. 2008, 2009).
Overexpression vector construction, transformation and identification of transgenic tomato
The pre-miR172b fragment was linked to the XbaI and SacI restriction sites at 5′ and 3′ terminal using c-miR172bF2/R2. Then, the fragments containing pre-miR172a and b sequences were subcloned into BamHI–SacI and XbaI–SacI restriction sites of pBI121 to generate recombinant plasmids in which the pre-miR172a and b sequences were under the control of the strong constitutive CaMV35S promoter. The constructs were transformed into Agrobacterium tumefaciens strain GV3101 by the freeze–thaw method (Li et al. 2015a).
Transformed S. lycopersicum plants were produced according to the method of Li et al. (2015a). Throughout the experiments, we used S. lycopersicum Zaofen No. 2, a cultivated tomato, which was a susceptible accession to a variety of pathogens including P. infestans. The seeds were surface sterilized in 75% ethanol for 30 s and in 2.5% sodium hypochlorite for twice 15 min, then rinsed four times with sterile double distilled water. After surface sterilization, seeds were cultured on 1/2 Murashige and Skoog (MS) medium. The cultures were maintained at 25 ± 3 °C under a 16 h light and 8 h dark photoperiod. Cotyledonary leaves were carefully excised from 1-week-old seedlings and used as explants in transformation experiments. The A. tumefaciens-mediated leaf disk method was used to generate transgenic S. lycopersicum Zaofen No. 2 plants.
Putative transgenic plants were selected on MS agar medium containing 50 mg/L kanamycin. The expression levels of pre-miR172a and b, mature-miR172 and the target gene in these selected positive transgenic lines were examined by qRT-PCR.
Analysis of transgenic tomato resistance against P. infestans
The detached-leaf inoculation was performed according to the method of Li et al. (2015b). The detached leaves (fifth real leaf) from WT and transgenic plants, were inoculated with 10 μL of zoospore suspension of P. infestans (106 zoospores/mL). The inoculated leaves were maintained in the dark at high humidity for 24 h, and then moved to the greenhouse at 20 ± 1 °C with a 16 h light and 8 h dark photoperiod cycle. The areas of necrosis surrounding inoculation sites and disease indices were recorded at 4, 6, 8 and 10 dpi, respectively. In addition, we used the P. infestans actin gene to quantify the abundance of P. infestans in WT and transgenic lines after P. infestans infection. Disease grades (DG) were categorized from 0 to 5 based on the lesion area: 0, no symptoms; (1) lesion area smaller than 5% of the total leaf area; (2) 5–25% of leaves area infected; (3) 25–50% of leaves area infected; (4) 50–75% of leaves area infected; (5) larger than 75% leaves area infected. The resistance of a plant was indicated by the disease index (DI):
where DG imax is the maximum value of disease grades, n i is the number of leaves with each disease grade, and n is the total number of leaves inoculated in the plant. The leaf surface area and lesion area in each leaf was quantified upon image acquisition using IMAGEJ software (http://imagej.net/Welcome). Each experiment was carried out at least thrice.
Measurements of MDA, REL, chlorophyll and photosynthetic rates
MDA is widely recognized as an indicator of lipid peroxidation. Membrane damage in plants can be estimated by measuring leakage of electrolytes, which are evaluated by determining relative electrolyte leakage (REL) (Huang et al. 2011). The MDA content and REL was measured according to a previously described method (Li et al. 2015a). Chlorophyll was extracted using 80% (v/v) acetone and analyzed using UV spectrophotometry. The Portable Photosynthesis System CIRAS-2 was used to measure photosynthetic rate (Li et al. 2015b).
Measurements of reactive oxygen species (ROS)
In plant–pathogen interaction, low levels of ROS can act as signaling molecules in response to pathogen infection, but late massive ROS generation is toxic to the cell and may lead to peroxidation of lipids, damage to cellular membranes, disease susceptibility and ultimately cell death (Kotchoni and Gachomo 2006; Wi et al. 2012). The transgenic S. lycopersicum Zaofen No. 2 lines and WT (5–6 leaf stage) were inoculated with suspension of P. infestans spores (106 zoospores/mL). The leaves were collected for measuring H2O2 and O2 − and examining the expression levels of superoxide dismutase gene (SOD, Genbank accession no. M37151), peroxidase gene (POD, Genbank accession no. L13654), pre-miR172a and b, miR172 and the target gene at 5 dpi. The POD and SOD activities were measured according to the method described previously (Chen et al. 2013). The nitro blue tetrazolium (NBT) and diamino benzidine (DAB) staining were performed to measuring H2O2 and O2 − according to previously described method of Lee et al. (2002).
RNA isolation and reverse transcription
Total RNA were extracted from S. pimpinellifolium L3708 and S. lycopersicum Zaofen No. 2 using RNAiso Plus (TaKaRa) and the corresponding cDNAs were synthesized with SYBR PrimeScriptTM miRNA RT-PCR Kit (TaKaRa) and PrimeScriptTM RT Master Mix (TaKaRa) according to the manufacturers’ instructions.
qRT-PCR analysis
The expression level of mature-miR172 was examined by reverse transcription-quantitative polymerase chain reaction (qRT-PCR). The specific forward primers for miR172, miR172-p was designed following a previously described method (Varkonyi-Gasic et al. 2007). The reverse primers is Uni-miR qRT-PCR primer from SYBR® PrimeScriptTM miRNA RT-PCR kit (TaKaRa). The qRT-PCRs were performed using a SYBR® PrimeScriptTM miRNA RT-PCR kit following the manufacturer’s protocol with an ABI 7300 Fast Real-time PCR machine (Applied Biosystems, Foster City, CA, USA). The primers (TGF and TGR) for the target gene (Solyc11g072600.1.1) were designed. The miRNA-mediated cleavage of the target gene at a site located between TGF and TGR was ensured. The pre-miR172a and b, SOD gene and POD gene were also examined by qRT-PCR following the manufacturer’s instructions for the SYBR® Premix Ex TaqTM II kit (TaKaRa). Information on all primers were shown in Table S1. S. lycopersicum actin was used as reference control gene for qRT-PCR analysis. Of the nine leaves sampled in each experiment, three leaves were pooled into one biological replicate, resulting in three biological replicates.
Statistical analysis
All statistical analyses of the data was performed with SPSS19.0, and all data were expressed as the means ± SDs from three independent experiments. We used Duncan’s multiple range test to estimate significance (P < 0.05).
Results
Sequence analysis, identification of target gene and expression patterns
The pre-miR172a and b sequences were obtained from S. pimpinellifolium L3708. After the analysis of multiple nucleic acid sequence alignments and their secondary structures, we found that precursor sequences and the secondary structures between pre-miR172a and b were different, but their mature sequences were identical (Fig. 1). The mature miR172, pre-miR172a and b of S. pimpinellifolium L3708 and S. lycopersicum Zaofen No. 2 showed 100% sequence identity (Fig. S1).
Identification of the target gene is essential to reveal the regulatory networks of a miRNA (Yang et al. 2015). To explore possible regulation mechanisms miR172a and b in S. lycopersicum–P. infestans interaction, the target genes of miR172 were predicted by psRNAtarget. Three target genes (maximum expectation ≤1.5), Solyc11g072600.1.1, Solyc04g049800.2.1, and Solyc06g075510.2.1 were selected for next analysis. After analyzed the degradome sequencing library of the interaction of S. pimpinellifolium L3708 and P. infestans. The gene, Solyc11g072600.1.1, which was annotated as an AP2/ERF transcription factor, was identified as the target of miR172a and b. As shown in Fig. 2a and b, the cleavage site was between 1287 nt and 1288 nt.
Identification of the target gene of miR172 in S. pimpinellifolium L3708 and expression analysis of mature-miR172, target gene and pre-miR172a and b in S. pimpinellifolium L3708–P. infestans interaction. a The regions targeted by miR172 in S. lycopersicum transcripts. b The identification of the target gene by degradome sequencing. Solyc11g072600.1.1, a member of AP2/ERF transcription factors was identified as the target gene of miR172 in S. pimpinellifolium L3708–P. infestans interaction. The cleavage site might be between 1287 nt and 1288 nt. The expression profiles of mature-miR172 (c), target gene (d) and pre-miR172a and b (e, f) after P. infestans inoculation. The Y-axis represents normalized relative expression values. Time points of P. infestans inoculation are labeled along the X-axis (n = 3 per each time point). Actin expression was used as a control
The qRT-PCR was performed to measure the effect of P. infestans stress on miR172a and b expression and verify the target gene of miR172a and b. The S. lycopersicum actin gene, the expression level of which is constant in each sample, was used as reference control gene (Fig. S2). The expression level of mature-miR172 in S. pimpinellifolium L3708 was down-regulated gradually during 0–3 dpi, then moderately up-regulated (Fig. 2c–f). In contrast, the target gene was increased, reaching the highest point at 2 dpi after P. infestans stress. These results indicated the target gene (Solyc11g072600.1.1) was efficiently cleaved by miR172. In addition, it was interesting that the expression trends of pre-miR172a and b were different. The pre-miR172a was down-regulated expressed from 0 to 1dpi, then moderately up-regulated during 1-2 dpi and declined again during 2–5 dpi, and expression level of the pre-miR172b had a peak value at 1 dpi.
Identification of transgenic plants with high miR172 expression levels
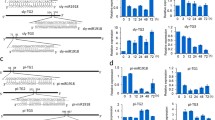
To investigate whether miR172 was involved in plant resistance to P. infestans infection, two sequences, pre-miR172a and b were cloned into the vector pBI121 driven by the CaMV 35S promoter and transformed into S. lycopersicum Zaofen No. 2 (Fig. 3a). The nine lines of transgenic S. lycopersicum Zaofen No. 2 plants were confirmed by their abilities to be rooted in medium containing kanamycin. The qRT-PCR was used to check the expression levels of pre-miR172a and b and mature-miR172 in these transgenic lines. The S. lycopersicum actin gene was used as reference control gene (Fig. S2). Compared to WT, the pre-miR172a and b transcripts were increased in all transgenic lines except for OEa1 (Fig. 3b, c). The line OEa-2, OEa-5, OEb-3 and OEb-6 were selected as candidates because of their highest expression levels of pre-miR172a and b. Subsequently, the mature-miR172 expression was also examined in these four lines. The miR172 overexpression was found to be approximately 1.49 times in line OEa-2, 2.2 times in line OEa-5, 3.3 times in line OEb-3 and 2.8 times in line OEb-6 (Fig. 3d). Hence, three lines with high expression of miR172, OEa-5, OEb-3 and OEb-6, were selected to multiply for further analyses in tissue culture and rapid propagation. In addition, the target gene of miR172a and b were down-regulated compared with the WT (Fig. 3e).
Schematic diagram of gene cassette the overexpression of pre-miR172a and b in S. lycopersicum Zaofen No 2 (a) and the qRT-PCR analysis of the transgenic lines for determination of the expression level of pre-miR172a (b) and b (c), mature-miR172 (d) and target gene (e). The Y-axis represents normalized relative expression values. WT and transgenic lines are labeled along the X-axis (n = 3 per each time point). S. lycopersicum Zaofen No 2 actin expression was used as a control
Overexpression of miR172a and b increases P. infestans resistance
The tests of resistance to P. infestans were performed on the three transgenic lines. At 10 dpi, it was observed that the transgenic plants exhibited more resistance to P. infestans, whereas the WT plants showed more disease symptoms (Fig. 4a). The transcript levels of P. infestans actin gene were used to indicate P. infestans growth in plant by qRT-PCR and result showed a significant increase in abundance of P. infestans in WT control compared to the transgenic lines (Jiang et al. 2016) (Fig. 4b). The ratio of lesion area to leaf area was compared between WT and transgenic lines (Fig. 4c). After inoculation, the relative lesion area of transgenic plants were 2.7 and 16% on average at 6 and 10 dpi, respectively, while the WT were 6.6 and 41%. The disease index of three transgenic plants was calculated at 4, 6, 8 and 10 dpi (Fig. 4d). At each time point, the transgenic lines had a lower disease index than WT (Fig. 4d). For instance, the disease index of WT was 53% at 10 dpi, while the transgenic plant was 36% on average. These results indicated that overexpression of miR172 in S. lycopersicum Zaofen No. 2 resulted in increased resistance to P. infestans. The expression levels of mature-miR172 and the target genes were also detected in the WT and three transgenic plant lines after 10 days P. infestans-inoculation using qRT-PCR. The mature-miR172 were significantly up-regulated in the three transgenic lines (P < 0.05) (Fig. 4e). Consequently, the expression level of the target gene (Solyc11g072600.1.1) was down-regulated in three transgenic lines (Fig. 4f). The expression level of Solyc04g049800.2.1 and Solyc06g075510.2.1 genes had no change in transgenic tomato compared to WT. After P. infestans inoculation, the expression levels of these two targets is down-regulated and also had no change between transgenic tomatoes and WT (Fig. S3). These results suggest that miR172 may be involved in silencing AP2/ERF (Solyc11g072600.1.1), which further affects S. lycopersicum resistance to P. infestans.
Overexpression of miR172 in transgenic S. lycopersicum Zaofen No. 2 enhanced P. infestans resistance. a Disease symptoms at 10 dpi. b Transcript accumulation of P. infestans actin gene in these inoculated plants. c The lesion size. d Disease index of WT and transgenic lines at 4, 6, 8 and 10 dpi. The expression patterns of mature-miR172 (e) and its target gene (f) in S. lycopersicum Zaofen No. 2 leaves from WT and transgenic lines before and after inoculation with P. infestans. g MDA content. h Relative electrolyte leakage. i Chlorophyll content. j Photosynthetic rate. The data are presented as the mean ± SD of three independent experiment. Samples marked with different letters are significantly different (P < 0.05). Af. inocu after inoculation, Bef. inocu before inoculation
Changes in MDA, REL, chlorophyll and photosynthetic rates of WT and transgenic plants in response to P. infestans inoculation
The MDA and REL can estimate the membrane damage after pathogen inoculated (Huang et al. 2011). Once the cellular structure is damaged, it would result in increased MDA and REL. As shown in Fig. 4g and h, the accumulations of MDA content and REL were significantly lower in the transgenic lines than in the WT after P. infestans inoculation. In addition, both the chlorophyll contents and photosynthetic rate, which serve as good indicators of photosynthetic function, were higher in transgenic lines than in WT plants, suggesting the photosynthesis was less affected in transgenic plants compared to WT (Fig. 4i, j).
Measurements of physiological indicators
Reactive oxygen species (ROS) played an important role in plant–pathogen interaction. The main species of ROS, H2O2 and O2 − were detected using DAB and NBT staining in WT and transgenic plants at 5 dpi, respectively. The transgenic plants had significantly lower H2O2 and O2 − than WT after the treatment (Fig. 5a, b). The SOD (EC 1.15.1.1) and POD (EC 1.11.1.7) activity, which are key antioxidant enzymes of ROS-scavenging system were higher in transgenic plants than those in WT (Fig. 5c, d). Similarly, the expression levels of SOD and POD genes showed no significant change between WT and transgenic lines, but after inoculation, their expression levels were significantly up-regulated in miR172-overexpressed S. lycopersicum Zaofen No. 2 plants (Fig. 5e, f).
The ROS levels of WT and transgenic lines before and after inoculation with P. infestans. a DAB staining for H2O2. b NBT staining for O2 −. c SOD activity. d POD activity. e The expression level of SOD gene. f The expression level of POD gene. The data are presented as the mean ± SD of three independent experiment. Samples marked with different letters are significantly different (P < 0.05). Af. inocu after inoculation, Bef. inocu before inoculation
Discussion
LB caused by P. infestans, is one of S. lycopersicum diseases that is difficult to control because the molecular mechanism of S. lycopersicum–P. infestans interaction is still poorly understood. In our previous work, many miRNAs were identified in response to P. infestans infection by high-throughput sequencing. Of these, the miR172 was lowly expressed after P. infestans infection. To further characterize the functions of miR172, transgenic S. lycopersicum Zaofen No. 2 plants that overexpressed miR172a and b were generated. Through analysis of the expression levels of mature-miR172 and target gene, the compartment of disease index and measurements of various physiological indicators in S. lycopersicum–P. infestans interaction, it was shown that miR172 was involved in S. lycopersicum defense response to P. infestans.
miR172 has been extensively studied in the growth phase transition, cleistogamy, the stem cell niche, flower development, and flowering time control in previous studies (Aukerman and Sakai 2003; Chen 2004; Lauter et al. 2005; Würschum et al. 2006; Nair et al. 2010; Li et al. 2016). Recently, some studies have suggested roles for miR172 in response to biotic stress conditions (Naqvi et al. 2010; Alabi et al. 2012; Li et al. 2014; Gai et al. 2014). In this study, qRT-PCR analysis showed that expression level of mature-miR172 in S. pimpinellifolium L3708 was changed after P. infestans infection (Fig. 2c). The results reported in other studies also showed that the expression level of miR172 was changed by different pathogen infection. The expression level of miR172 in grapevine affected by grapevine leafroll disease, S. lycopersicum–ToLCV interaction and rice inoculated with blast fungus Magnaporthe oryzae were up-regulated; in contrast, in mulberry with yellow dwarf disease were down-regulated (Naqvi et al. 2010; Alabi et al. 2012; Li et al. 2014; Gai et al. 2014). Thus, the change of miR172 expression level in various plants with different pathogen infection suggests that miR172 may play crucial and various roles in responses to pathogens including P. infestans.
In this study, the expression level of miR172 was down-regulated gradually during 0–3 dpi. Recently, it was found that RNA silencing suppressors from pathogens might suppress the accumulation of miRNA in plant–pathogen interaction. Previous studies showed Phytophthora encoded RNA silencing suppressors (PSRs), which play an important virulence role during infection, likely through their inhibitory effects on host small RNA-mediated defense in plant–Phytophthora interaction (Ye and Ma 2016). For example, two effectors form the soybean pathogen P. sojae, named PsPSR1 and PsPSR2 suppressed transgene silencing by inhibiting the accumulation of plant sRNAs (Qiao et al. 2013). Arabidopsis transgenic plants expressing PsPSR2 also showed hypersusceptibility to P. capsici (Xiong et al. 2014). The PsPSR1 virulence target in Arabidopsis and soybean was identified and characterized. This target contains the aspartate-glutamate-alanine-histidine-box RNA helicase domain and appears to be involved in the assembly of sRNA processing complexes (Qiao et al. 2015). PSR2 homologues was also identified from P. infestans, indicting PSR2 is a conserved effector that acts as a master switch to modify plant gene regulation early during infection for the pathogen’s benefit (de Vries et al. 2017). Thus, these suggest that the expression level of miR172 may be suppressed by PSRs.
An important step to understand the biological functions of miRNAs is the identification of their target genes. Degradome sequencing based on high-throughput sequencing technology has been used to identify the target genes of miRNAs and understand the miRNA regulator (Chen et al. 2016). The miRNAs regulate their target genes by miRNA-mediated gene silencing. In the present study, it was found that the S. lycopersicum miR172 family contained two members (miR172a and b), which had identical mature sequence (Fig. 1a). After analysis of the degradome sequencing library, Solyc11g072600.1.1, a member of the AP2/ERF transcription factor family was identified as the target gene of miR172 (Fig. 2a, b). A number of studies showed many members of AP2/ERF transcription factor family in various plants were involved in plant–pathogen interaction (Mo et al. 2011). In S. lycopersicum, a S. lycopersicum AP2/ERF transcription factor gene, TSRF1, activated the expression of GCC box—containing genes and significantly enhanced the resistance to Ralstonia solanacearum in S. lycopersicum (Zhou et al. 2008). Arabidopsis plants overexpressing S. lycopersicum Pti4 displayed increased resistance to the fungal pathogen Erysiphe orontii and increased tolerance to the bacterial pathogen Pseudomonas syringae pv tomato (Gu et al. 2002). In Arabidopis, 10 members of the AP2/ERF transcription factor family were induced by both the pathogen Fusarium oxysporum and jasmonic acid (JA) (McGrath et al. 2005). Overexpression analysis of ORA59, AtERF2, AtERF1 and AtERF15 revealed that they acted as positive regulators of resistance to pathogens such as Fusarium oxysporum, P. syringae pv. tomato DC3000 and Botrytis cinerea (Berrocal-Lobo et al. 2002; Berrocal-Lobo and Molina 2004; McGrath et al. 2005; Pré et al. 2008; Zhang et al. 2015). Besides S. lycopersicum and Arabidopis, AP2/ERF transcription factors from other plants were positive regulators, such as rice OsBIERF3, wheat TiERF1, Medicago truncatula MTERF1-1 and others (Cao et al. 2006; Chen et al. 2008; Anderson et al. 2010). However, some AP2/ERF transcription factors can also enhance plant sensitivity to pathogen infection. In Arabidopis, functional analysis of AtERF4 revealed that AtERF4 acts as a novel negative regulator of JA-responsive defense gene expression and resistance to the necrotrophic fungal pathogen F. oxysporum (McGrath et al. 2005). Similarly, the rice ERF transcription factor OsERF922 negatively regulates resistance to Magnaporthe oryzae (Liu et al. 2012). In this study, the miR172 target gene, Solyc11g072600.1.1, which was annotated as AP2/ERF transcription factor was down-regulated in miR172-overexpressed S. lycopersicum Zaofen No. 2 plants, which enhanced S. lycopersicum Zaofen No. 2 resistance to P. infestans (Figs. 4d, 5e). The miR172-overexpressed S. lycopersicum Zaofen No. 2 plants had less disease symptoms, lesion area and disease index after P. infestans infection (Fig. 4a–c). This suggested that miR172 act as a positive regulator of resistance to P. infestans; in contrasts, its target gene, a member of the AP2/ERF transcription factor family is a negative regulator because miR172 may be involved in silencing the AP2/ERF gene.
In plant–pathogen interactions, ROS was produced rapid and early to reduce the hypersensitive response (HR) (Kotchoni and Gachomo 2006; Wi et al. 2012). But the late massive ROS lead to cell death (Li et al. 2015a). To avoid this phenomenon, the ROS-scavenging systems, such as POD and SOD enzymes, may scavenge excess ROS and protect the membrane against lipid peroxidation and damage (Shi et al. 2014; Li et al. 2015b). In a study on soybean miR172c, it was found that the SOD activity in miR172c-overexpressing plants was higher than in WT plants under ABA and dehydration conditions, indicating that miR172c might regulate ROS accumulation and enhance drought stress tolerance (Li et al.2016). A number of studies indicated that AP2/ERF transcription factors, such as the target gene of miR172, were involved in ROS-related pathways (Wu et al. 2008; Tian et al. 2011). The previous study showed that Arabidopsis ERF6 is possibly either a negative regulator of ROS production or a positive regulator of ROS detoxification (Sewelam et al. 2013). In this study, miR172—mediated silencing of AP2/ERF was a positive regulator of ROS detoxification. As shown in Fig. 5, the miR172-overexpressed S. lycopersicum Zaofen No. 2 plants had less H2O2 and O2 −, higher activities of POD and SOD after inoculation with P. infestans. These results suggested that miR172-AP2/ERF module in S. lycopersicum may regulate antioxidants to reduce the accumulation of ROS and prevent cell membrane injury after P. infestans infection.
In conclusion, it was found that miR172 and Solyc11g072600.1.1 transcripts in S. pimpinellifolium L3708 were regulated by P. infestans. The degradome and qRT-PCR analysis showed that the expression of Solyc11g072600.1.1 was negatively correlated with the expression of miR172. Through the phenotypic, physiological, and molecular analyses of the miR172-overexpressed S. lycopersicum Zaofen No. 2 plant that were conducted in this study, miR172 was found to probably be involved resistance to P. infestans. Our results contributed relevant information to plant–pathogens interaction studies, thereby providing guidance for molecular breeding to improve biotic stress tolerance, especially P. infestans in the future.
Author contribution statement
YL and JM conceived and designed the experiments. JL, JC, NJ and PL preformed the experiment. JL and JC analyzed the data. JC, YL and JM wrote the paper.
Abbreviations
- dpi:
-
Days post inoculation
- MDA:
-
Malonaldehyde
- POD:
-
Peroxidase
- REL:
-
Relative electrolyte leakage
- ROS:
-
Reactive oxygen species
- SOD:
-
Superoxide dismutase
References
Addo-Quaye C, Eshoo TW, Bartel DP, Axtell MJ (2008) Endogenous siRNA and miRNA targets identified by sequencing of the Arabidopsis degradome. Curr Biol 18:758–762
Addo-Quaye C, Miller W, Axtell MJ (2009) CleaveLand: a pipeline for using degradome data to find cleaved small RNA targets. Bioinformatics 25:130–131
Alabi OJ, Zheng Y, Jagadeeswaran G, Sunkar R, Naidu RA (2012) High-throughput sequence analysis of small RNAs in grapevine (Vitis vinifera L.) affected by grapevine leafroll disease. Mol Plant Pathol 13:1060–1076
Anderson JP, Lichtenzveig J, Gleason C, Oliver RP, Singh KB (2010) The B-3 ethylene response factor MtERF1-1 mediates resistance to a subset of root pathogens in Medicago truncatula without adversely affecting symbiosis with rhizobia. Plant Physiol 154:861–873
Aukerman MJ, Sakai H (2003) Regulation of flowering time and floral organ identity by a microRNA and its APETALA2-like target genes. Plant Cell 15:2730–2741
Berrocal-Lobo M, Molina A (2004) Ethylene response factor 1 mediates Arabidopsis resistance to the soilborne fungus Fusarium oxysporum. Mol Plant Microbe Interact 17:763–770
Berrocal-Lobo M, Molina A, Solano R (2002) Constitutive expression of ETHYLENE-RESPONSE-FACTOR1 in Arabidopsis confers resistance to several necrotrophic fungi. Plant J 29:23–32
Black LL, Wang TC, Hanson PM, Chen JT (1996) Late blight resistance in four wild tomato accessions: effectiveness in diverse locations and inheritance of resistance. Phytopathology 86:S24
Bonde R, Murphy EF (1952) Resistance of certain tomato varieties and crosses to late blight. Maine Agric Exp Stn Bull 497:5–15
Cao Y, Wu Y, Zheng Z, Song F (2006) Overexpression of the rice EREBP-like gene OsBIERF3 enhances disease resistance and salt tolerance in transgenic tobacco. Physiol Mol Plant Pathol 67:202–211
Chen X (2004) A microRNA as a translational repressor of APETALA2 in Arabidopsis flower development. Science 303:2022–2025
Chen L, Zhang Z, Liang H, Liu H, Du L, Xu H, Xin Z (2008) Overexpression of TiERF1 enhances resistance to sharp eyespot in transgenic wheat. J Exp Bot 59:4195–4204
Chen HY, Ling JG, Wu FH, Zhang LJ, Sun ZD, Yang HQ (2013) Effect of hypobaric storage on flesh lignification, active oxygen metabolism and related enzyme activities in bamboo shoots. LWT Food Sci Technol 51:190–195
Chen J, Zheng Y, Qin L, Wang Y, Chen L, He Y, Fei Z, Lu G (2016) Identification of miRNAs and their targets through high-throughput sequencing and degradome analysis in male and female Asparagus officinalis. BMC Plant Biol 16:80
de Vries S, Kloesges T, Rose LE (2015) Evolutionarily dynamic, but robust, targeting of resistance genes by the miR482/2118 gene family in the Solanaceae. Genome Biol Evol 7:3307–3321
de Vries S, von Dahlen JK, Uhlmann C, Schnake A, Kloesges T, Rose LE (2017) Signatures of selection and host-adapted gene expression of the Phytophthora infestans RNA silencing suppressor PSR2. Mol Plant Pathol 18:110–124
Ebrahimi Khaksefidi R, Mirlohi S, Khalaji F, Fakhari Z, Shiran B, Fallahi H, Rafiei F, Budak H, Ebrahimie E (2015) Differential expression of seven conserved microRNAs in response to abiotic stress and their regulatory network in Helianthus annuus. Front Plant Sci 6:741
Fei Q, Li P, Teng C, Meyers BC (2015) Secondary siRNAs from Medicago NB-LRRs modulated via miRNA-target interactions and their abundances. Plant J 83:451–465
Foolad MR, Merk HL, Ashrafi H (2008) Genetics, genomics and breeding of late blight and early blight resistance in tomato. Crit Rev Plant Sci 27:75–107
Fry WE, Goodwin SB, Matuszak JM, Spoelman LJ, Milgroom MG, Drenth A (1992) Population genetics and intercontinental migration of Phytophthora infestans. Annu Rev Phytopathol 30:107–129
Gai YP, Li YQ, Guo FY, Yuan CZ, Mo YY, Zhang HL, Wang H, Ji XL (2014) Analysis of phytoplasma-responsive sRNAs provide insight into the pathogenic mechanisms of mulberry yellow dwarf disease. Sci Rep 4:5378
Gu YQ, Wildermuth MC, Chakravarthy S, Loh YT, Yang C, He X, Han Y, Martin GB (2002) Tomato transcription factors Pti4, Pti5, and Pti6 activate defense responses when expressed in Arabidopsis. Plant Cell 14:817–831
Gupta OP, Meena NL, Sharma I, Sharma P (2014) Differential regulation of microRNAs in response to osmotic, salt and cold stresses in wheat. Mol Biol Rep 41:4623–4629
Huang X, Wang A, Xu X, Li J, Li N (2010) Construction of genetic linkage map and QTL analysis of Phytophthora infestans resistant gene Ph-2 in tomato. Acta Hortic Sin 37:1085–1092
Huang XS, Luo T, Fu XZ, Fan QJ, Liu JH (2011) Cloning and molecular characterization of a mitogen-activated protein kinase gene from Poncirus trifoliata whose ectopic expression confers dehydration/drought tolerance in transgenic tobacco. J Exp Bot 62:5191–5206
Janga SC, Vallabhaneni S (2011) MicroRNAs as post-transcriptional machines and their interplay with cellular networks. Adv Exp Med Biol 722:59–74
Jiang Y, Guo L, Liu R, Jiao B, Zhao X, Ling Z, Luo K (2016) Overexpression of poplar PtrWRKY89 in transgenic Arabidopsis leads to a reduction of disease resistance by regulating defense-related genes in salicylate-jasmonate-dependent signaling. PLoS One 11:e0149137
Jin H (2008) Endogenous small RNAs and antibacterial immunity in plants. FEBS Lett 582:2679–2684
Jung JH, Seo YH, Seo PJ, Reyes JL, Yun J, Chua NH, Park CM (2007) The GIGANTEA-regulated microRNA172 mediates photoperiodic flowering independent of CONSTANS in Arabidopsis. Plant Cell 19:2736–2748
Kim MJ, Mutschler MA (2005) Transfer to processing tomato and characterization of late blight resistance derived from Solanum pimpinellifolium L. L3708. J Am Soc Hortic Sci 130:877–884
Kotchoni SO, Gachomo EW (2006) The reactive oxygen species network pathways: an essential prerequisite for perception of pathogen attack and the acquired disease resistance in plants. J Biosci 31:389–404
Lauter N, Kampani A, Carlson S, Goebel M, Moose SP (2005) microRNA172 down-regulates glossy15 to promote vegetative phase change in maize. Proc Natl Acad Sci USA 102:9412–9417
Lee B, Lee H, Xiong L, Zhu JK (2002) A mitochondrial complex I defect impairs cold-regulated nuclear gene expression. Plant Cell 14:1235–1251
Lee YS, Lee DY, Cho LH, An G (2014) Rice miR172 induces flowering by suppressing OsIDS1 and SNB, two AP2 genes that negatively regulate expression of Ehd1 and florigens. Rice (N Y) 7:31
Li F, Pignatta D, Bendix C, Brunkard JO, Cohn MM, Tung J, Sun H, Kumar P, Baker B (2012) MicroRNA regulation of plant innate immune receptors. Proc Natl Acad Sci USA 109:1790–1795
Li J, Luan Y, Zhai J, Liu P, Xia X (2013) Bioinformatic analysis of functional characteristics of miR172 family in tomato. J Northeast Agric Univ (English Edition) 20:19–27
Li Y, Lu YG, Shi Y, Wu L, Xu YJ, Huang F, Guo XY, Zhang Y, Fan J, Zhao JQ, Zhang HY, Xu PZ, Zhou JM, Wu XJ, Wang PR, Wang WM (2014) Multiple rice microRNAs are involved in immunity against the blast fungus Magnaporthe oryzae. Plant Physiol 164:1077–1092
Li J, Luan Y, Liu Z (2015a) SpWRKY1 mediates resistance to Phytophthora infestans and tolerance to salt and drought stress by modulating reactive oxygen species homeostasis and expression of defense-related genes in tomato. Plant Cell Tissue Organ Cult 123:67–81
Li J, Luan Y, Liu Z (2015b) Overexpression of SpWRKY1 promotes resistance to Phytophthora nicotianae and tolerance to salt and drought stress in transgenic tobacco. Physiol Plant 155:248–266
Li W, Wang T, Zhang Y, Li Y (2016) Overexpression of soybean miR172c confers tolerance to water deficit and salt stress, but increases ABA sensitivity in transgenic Arabidopsis thaliana. J Exp Bot 67:175–194
Liu D, Chen X, Liu J, Ye J, Guo Z (2012) The rice ERF transcription factor OsERF922 negatively regulates resistance to Magnaporthe oryzae and salt tolerance. J Exp Bot 63:3899–3911
Luan Y, Wang W, Liu P (2014) Identification and functional analysis of novel and conserved microRNAs in tomato. Mol Biol Rep 41:5385–5394
Luan Y, Cui J, Zhai J, Li J, Han L, Meng J (2015) High-throughput sequencing reveals differential expression of miRNAs in tomato inoculated with Phytophthora infestans. Planta 241:1405–1416
McGrath KC, Dombrecht B, Manners JM, Schenk PM, Edgar CI, Maclean DJ, Scheible WR, Udvardi MK, Kazan K (2005) Repressor- and activator-type ethylene response factors functioning in jasmonate signaling and disease resistance identified via a genome-wide screen of Arabidopsis transcription factor gene expression. Plant Physiol 139:949–959
Merk HL, Ashrafi H, Foolad MR (2012) Selective genotyping to identify late blight resistance genes in an accession of the tomato wild species Solanum pimpinellifolium. Euphytica 187:63–75
Miranda BEC, Suassuna ND, Reis A (2010) Mating type, mefenoxam sensitivity, and pathotype diversity in Phytophthora infestans isolates from tomato in Brazil. Pesq Agropec Bras 45:671–679
Mo J, Li D, Zhang H, Song F (2011) Roles of ERF transcription factors in biotic and abiotic stress response in plants. Plant Physiol J 47:1145–1154
Nair SK, Wang N, Turuspekov Y, Pourkheirandish M, Sinsuwongwat S, Chen G, Sameri M, Tagiri A, Honda I, Watanabe Y, Kanamori H, Wicker T, Stein N, Nagamura Y, Matsumoto T, Komatsuda T (2010) Cleistogamous flowering in barley arises from the suppression of microRNA-guided HvAP2 mRNA cleavage. Proc Natl Acad Sci USA 107:490–495
Naqvi AR, Haq QM, Mukherjee SK (2010) MicroRNA profiling of tomato leaf curl new delhi virus (tolcndv) infected tomato leaves indicates that deregulation of mir159/319 and mir172 might be linked with leaf curl disease. Virol J 7:281
Navarro L, Dunoyer P, Jay F, Arnold B, Dharmasiri N, Estelle M, Voinnet O, Jones JDG (2006) A plant miRNA contributes to antibacterial resistance by repressing auxin signaling. Science 312:436–439
Ouyang S, Park G, Atamian HS, Han CS, Stajich JE, Kaloshian I, Borkovich KA (2014) MicroRNAs suppress NB domain genes in tomato that confer resistance to Fusarium oxysporum. PLoS Pathog 10:e1004464
Pan WJ, Tao JJ, Cheng T, Bian XH, Wei W, Zhang WK, Ma B, Chen SY, Zhang JS (2016) Soybean miR172a improves salt tolerance and can function as a long-distance signal. Mol Plant 9:1337–1340
Park W, Li J, Song R, Messing J, Chen X (2002) CARPEL FACTORY, a dicer homolog, and HEN1, a novel protein, act in microRNA metabolism in Arabidopsis thaliana. Curr Biol 12:1484–1495
Park Y, Hwang J, Kim K, Kang J, Kim B (2013) Development of the gene-based SCARs for the Ph-3 locus, which confers late blight resistance in tomato. Sci Hortic 164:9–16
Pré M, Atallah M, Champion A, De Vos M, Pieterse CM, Memelink J (2008) The AP2/ERF domain transcription factor ORA59 integrates jasmonic acid and ethylene signals in plant defense. Plant Physiol 147:1347–1357
Qiao Y, Liu L, Xiong Q, Flores C, Wong J, Shi J, Wang X, Liu X, Xiang Q, Jiang S, Zhang F, Wang Y, Judelson HS, Chen X, Ma W (2013) Oomycete pathogens encode RNA silencing suppressors. Nat Genet 45:330–333
Qiao Y, Shi J, Zhai Y, Hou Y, Ma W (2015) Phytophthora effector targets a novel component of small RNA pathway in plants to promote infection. Proc Natl Acad Sci USA 112:5850–5855
Sewelam N, Kazan K, Thomas-Hall SR, Kidd BN, Manners JM, Schenk PM (2013) Ethylene response factor 6 is a regulator of reactive oxygen species signaling in Arabidopsis. PLoS One 8:e70289
Shi WN, Hao LL, Li J, Liu DD, Guo XQ, Li H (2014) The Gossypium hirsutum WRKY gene GhWRKY39-1 promotes pathogen infection defense responses and mediates salt stress tolerance in transgenic Nicotiana benthamiana. Plant Cell Rep 33:483–498
Shivaprasad PV, Chen HM, Patel K, Bond DM, Santos BA, Baulcombe DC (2012) A microRNA superfamily regulates nucleotide binding site-leucine-rich repeats and other mRNAs. Plant Cell 24:859–874
Spanudakis E, Jackson S (2014) The role of microRNAs in the control of flowering time. J Exp Bot 65:365–380
Steffen P, Voss B, Rehmsmeier M, Reeder J, Giegerich R (2006) RNAshapes: an integrated RNA analysis package based on abstract shapes. Bioinformatics 22:500–503
Sun G, Luan Y, Cui J (2014) Mining and characterization of miRNAs closely associated with the pathogenicity in tomato. Yichuan 36:69–76
Tian Y, Zhang H, Pan X, Chen X, Zhang Z, Lu X, Huang R (2011) Overexpression of ethylene response factor TERF2 confers cold tolerance in rice seedlings. Transgenic Res 20:857–866
Varkonyi-Gasic E, Wu R, Wood M, Walton EF, Hellens RP (2007) Protocol: a highly sensitive RT-PCR method for detection and quantification of microRNAs. Plant Methods 3:12
Wi SJ, Ji NR, Park KY (2012) Synergistic biosynthesis of biphasic ethylene and reactive oxygen species in response to hemibiotrophic Phytophthora parasitica in tobacco plants. Plant Physiol 159:251–265
Wong J, Gao L, Yang Y, Zhai J, Arikit S, Yu Y, Duan S, Chan V, Xiong Q, Yan J, Li S, Liu R, Wang Y, Tang G, Meyers BC, Chen X, Ma W (2014) Roles of small RNAs in soybean defense against Phytophthora sojae infection. Plant J 79:928–940
Wu L, Zhang Z, Zhang H, Wang XC, Huang R (2008) Transcriptional modulation of ethylene response factor protein JERF3 in the oxidative stress response enhances tolerance of tobacco seedlings to salt, drought, and freezing. Plant Physiol 148:1953–1963
Würschum T, Gross-Hardt R, Laux T (2006) APETALA2 regulates the stem cell niche in the Arabidopsis shoot meristem. Plant Cell 18:295–307
Xiong Q, Ye W, Choi D, Wong J, Qiao Y, Tao K, Wang Y, Ma W (2014) Phytophthora suppressor of RNA silencing 2 is a conserved RxLR effector that promotes infection in soybean and Arabidopsis thaliana. Mol Plant Microbe Interact 27:1379–1389
Yan J, Zhang H, Zheng Y, Ding Y (2014) Comparative expression profiling of miRNAs between the cytoplasmic male sterile line Meixiang A and its maintainer line Meixiang B during rice anther development. Planta 241:109–123
Yang L, Huang H (2014) Roles of small RNAs in plant disease resistance. J Integr Plant Biol 56:962–970
Yang L, Mu X, Liu C, Cai J, Shi K, Zhu W, Yang Q (2015) Overexpression of potato miR482e enhanced plant sensitivity to Verticillium dahliae infection. J Integr Plant Biol 57:1078–1088
Ye W, Ma W (2016) Filamentous pathogen effectors interfering with small RNA silencing in plant hosts. Curr Opin Microbiol 32:1–6
Yoshikawa T, Ozawa S, Sentoku N, Itoh J, Nagato Y, Yokoi S (2013) Change of shoot architecture during juvenile-to-adult phase transition in soybean. Planta 238:229–237
Zhang J, Zeng R, Chen J, Liu X, Liao Q (2008) Identification of conserved microRNAs and their targets from Solanum lycopersicum Mill. Gene 423:1–7
Zhang C, Liu L, Wang X, Vossen J, Li G, Li T, Zheng Z, Gao J, Guo Y, Visser RGF, Li J, Bai Y, Du Y (2014) The Ph-3 gene from Solanum pimpinellifolium encodes CC-NBS-LRR protein conferring resistance to Phytophthora infestans. Theor Appl Genet 127:1353–1364
Zhang H, Huang L, Dai Y, Liu S, Hong Y, Tian L, Huang L, Cao Z, Li D, Song F (2015) Arabidopsis AtERF15 positively regulates immunity against Pseudomonas syringae pv. tomato DC3000 and Botrytis cinerea. Front. Plant Sci 6:686
Zhou J, Zhang H, Yang Y, Zhang Z, Zhang H, Hu X, Chen J, Wang XC, Huang R (2008) Abscisic acid regulates TSRF1-mediated resistance to Ralstonia solanacearum by modifying the expression of GCC box-containing genes in tobacco. J Exp Bot 59:645–652
Zhou L, Liu Y, Liu Z, Kong D, Duan M, Luo L (2010) Genome wide identification and analysis of drought-responsive microRNAs in Oryza sativa. J Exp Bot 61:4157–4168
Acknowledgements
This work is supported by Grants from the National Natural Science Foundation of China (Nos. 31471880 and 61472061).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Luan, Y., Cui, J., Li, J. et al. Effective enhancement of resistance to Phytophthora infestans by overexpression of miR172a and b in Solanum lycopersicum . Planta 247, 127–138 (2018). https://doi.org/10.1007/s00425-017-2773-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00425-017-2773-x