Abstract
Rice (Oryza sativa L.) is a warm-season plant exposed to various stresses. Low temperature is an important factor limiting extension of rice cultivation areas and productivity. Previously, we have demonstrated that tomato ERF protein TERF2 enhances freezing tolerance of transgenic tobacco and tomato plants. Herein, we report that overexpression of TERF2 enhances transgenic rice tolerance to cold without affecting growth or agronomic traits. Physiological assays revealed that TERF2 could not only increase accumulation of osmotic substances and chlorophyll, but also reduce reactive oxygen species (ROS) and malondialdehyde (MDA) content and decrease electrolyte leakage in rice under cold stress. Further analysis of gene expression showed that TERF2 could activate expression of cold-related genes, including OsMyb, OsICE1, OsCDPK7, OsSODB, OsFer1, OsTrx23, and OsLti6, in transgenic rice plants under natural condition or cold stress. Thus, our findings demonstrated that TERF2 modulated expression of stress-related genes and a series of physiological adjustments under cold stress, indicating that TERF2 might have important regulatory roles in response to abiotic stress in rice and possess potential utility in improving crop cold tolerance.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
A wide array of environmental stresses seriously affect plant growth and crop yield. Among the various abiotic stresses, cold (chilling or low temperature) represents one of the most significant limitations to crop distribution and productivity. Rice (Oryza sativa L.) is a warm-season plant that is sensitive to cold, particularly at seedling stage. When exposed to cold stress, plants show changes in gene expression, biomembrane lipid composition, photosynthetic efficiency, and small-molecule and free-radical production (Beck et al. 2007; Mahajan and Tuteja 2005; Parvanova et al. 2004; Sharma et al. 2005); for example, cold-induced physiological imbalance leads to elevated levels of reactive oxygen species (ROS) in plant cells. In particular, H2O2 is generated rapidly under stress conditions, and its steady-state levels depend on the balance between synthesis and degradation, which is facilitated by the ROS-scavenging system of the plant cell (Cheng et al. 2007). Moreover, the products of cold-inducible genes may either directly protect against cold stress or further regulate expression of other genes (Yamaguchi-Shinozaki and Shinozaki 2006). Contrary to the classical breeding and marker-assisted selection approaches, increasing amounts of data demonstrate that direct introduction of genes by genetic engineering seems a more attractive and quick solution for improving cold stress tolerance. Moreover, genetic engineering of plants for tolerance to cold stresses could be achieved by regulated expression of cold-induced transcription factors, which in turn would regulate expression of a large number of relevant downstream genes (Agarwal et al. 2006; Hu et al. 2008; Ito et al. 2006; Wang et al. 2008). Thus, transcription factors are powerful tools for genetic engineering, as their overexpression can lead to upregulation of a whole array of genes under their control.
Recent research has identified several cold-regulated transcription factors such as NAM and ATAF, and CUC (NAC), Myb domain protein (MYB), and ethylene response factor (ERF) genes, which play roles in cold-response pathways by controlling downstream genes and tuning crosstalk among different signaling pathways (Agarwal et al. 2006; Chinnusamy et al. 2007; Dai et al. 2007; Fowler and Thomashow 2002; Hu et al. 2008; Su et al. 2010; Zhu et al. 2004, 2007). The ERF proteins make up a subfamily of the AP2/ERF superfamily, which also contains the C-repeat binding factor/dehydration-responsive element-binding protein (CBF/DREB) subfamily (Nakano et al. 2006). Previous studies have shown that ERF proteins regulate growth, development, and processes in responses to biotic and abiotic stresses (including due to pathogen, drought, submergence, salt, and cold) in plant (Agarwal et al. 2006; Mantiri et al. 2008; Pré et al. 2008; Quan et al. 2010; Vernié et al. 2008; Wu et al. 2008; Xu et al. 2006); for example, overexpression of CaPF1, which codes for an ERF transcription factor and is inducible by cold, subsequently activates expression of cold-responsive genes and confers tolerance against freezing temperature in Arabidopsis (Yi et al. 2004). Ectopic expression of JERF3 confers cold tolerance by decreasing accumulation of ROS in tobacco (Wu et al. 2007, 2008).
The tomato ERF member, LeERF2, has been reported to confer the typical triple response (Pirrello et al. 2006). More recently, ectopic expression of TERF2, an allele of LeERF2, has been shown to affect ethylene levels by regulating ethylene biosynthesis and to enhance tolerance to freezing in transgenic tomato and tobacco plants (Zhang et al. 2009; Zhang and Huang 2010). Herein, we report that overexpression of TERF2 in rice modulates expression of stress-related genes, subsequently resulting in increased accumulation of osmolytes and chlorophyll, reduced reactive oxygen species (ROS) and malondialdehyde (MDA) content, and decreased electrolyte leakage under cold stress, finally improving rice cold tolerance. These findings indicate that ectopic expression of TERF2 represents a viable strategy for engineering cold tolerance in rice.
Materials and methods
Plasmid construction and transformation of rice
Full-length TERF2 complementary DNA (cDNA) was cloned into the pMCG161 vector using AscI and SpeI. This pMCG-CaMV 35S-TERF2 plasmid was digested by BamHI and HindIII, and the resulting CaMV 35S:TERF2 fragment was ligated into pCAMBIA1200 vector (Supplemental Fig. 1A). Then, this construct was transformed into rice (O. sativa ssp. japonica cv. Nipponbare) following the method described by Hiei et al. (1997).
Growth conditions and cold treatment of rice seedlings
Transgenic and wild-type (WT) (O. sativa ssp. japonica cv. Nipponbare) seeds were germinated for 3 days at room temperature and then planted into soil and grown in a greenhouse (with 16/8 h light/dark) at 28°C with 60–70% relative humidity. For cold tolerance, after germinated seeds were grown for 5 days, rice seedlings were exposed to 6°C for 72 h, then 28°C for 7 days for recovery.
Measurement of soluble sugars, proline, chlorophyll, MDA, and electrolyte leakage
Ten-day-old rice seedlings were divided into two groups (natural growth and cold treatment), each group containing about 50 plants. After seedlings were exposed to 6°C for 12 h, about 0.2 g leaf tissue was harvested for each sample and extracted with different solutions according to the particular assay. Soluble sugars were determined spectrophotometrically by anthrone reagent using glucose as standard (Dubois et al. 1956). Proline was assayed using colorimetric method (Bates et al. 1973; Troll and Lindsley 1955). Chlorophyll content and MDA equivalent were correspondingly measured as previously described (Lichtenthaler 1987; Hodges et al. 1999). Electrolyte leakage of rice seedlings was detected after treatment at 6°C for 8 h, following the method described by Gilmour et al. (2000). The percentage of electrolyte leakage was calculated as the ratio of the conductivity before autoclaving to that after autoclaving. To exclude the effects of cold treatment, all physiological data are shown as relative values, namely the data for WT under normal condition were set to 1, and the other values were compared with it.
Detection of reactive oxygen species
Ten-day-old seedlings were kept at 4°C for 0, 12, 24, and 48 h, respectively. For superoxide detection, leaves were vacuum-infiltrated with 1 mg/mL nitroblue tetrazolium in 25 mM 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES) buffer, pH 7.6, and incubated at room temperature in the dark for 12 h. Then, the stained samples were transferred to boiling ethanol (95%) and incubated for 10 min before photographing (Dong et al. 2009).
Real-time PCR analysis
Total RNA was prepared from 10-day-old leaves of transgenic and WT rice seedlings grown under normal conditions or cold stress (6°C for 12 or 24 h) using Trizol reagent (Invitrogen, Carlsbad, CA, USA). For quantitative real-time analysis, 1 μg RNA was used for cDNA preparation with Moloney murine leukemia virus (M-MLV) reverse transcriptase (Promega, Madison, WI, USA). cDNA was then diluted and used for real-time polymerase chain reaction (PCR) amplification (ABI PRISM7000 real-Time PCR system, Applied Biosystems) with gene-specific primers (Supplemental Table 1). The quantitative variation between different samples was evaluated by the ∆∆C t method, and the amplification of actin was used as internal control to normalize all data. To validate our quantitative reverse-transcription (qRT)-PCR results, we repeated each experiment three times. The mean values for the expression levels of the examined genes were calculated from three independent experiments compared with that of WT (standardized to 100).
Accession numbers
NM_001067099 (OsSODB), NM_001072072 (OsFer1), NM_001065604 (OsTrx23), NM_001066925 (OsLti6a), NM_001061126 (OsLti6b), NM_001060205 (OsCDPK7), NM_001052096 (OsMyb), NM_001050547 (OsICE1), AY496704 (TERF2), and NM_001057621 (actin).
Results
Expression of TERF2 enhances rice tolerance to cold
To examine the role of TERF2 in rice, transgenic rice lines (OE) overexpressing TERF2 under control of CaMV 35S promoter were generated using Agrobacterium-mediated transformation. The transcript level of TERF2 was detected in eight independent T3 homozygous lines (Supplemental Fig. 1B). We also found that there were no obvious differences in agricultural characters among these different transgenic lines. Two independent T3 lines of OE-8 and OE-10 were randomly selected for further study. Furthermore, we observed that there were no obvious differences in yield or phenotypes of OE lines for growth at the different developmental stages and for 1,000-kernel weights compared with those of WT rice under normal growth conditions (Supplemental Fig. 1C–E). These observations suggested that ectopic expression of TERF2 had no negative effects on growth of rice plants under normal conditions.
To investigate whether overexpression of TERF2 correlated with cold tolerance in rice, 5-day-old transgenic and WT seedlings were exposed to cold stress (6°C) for 72 h, then recovered growth for 7 days under normal conditions. We found that transgenic plants recovered better than WT plants (Fig. 1). In particular, the heights and the shoot (stem and leaves) fresh weights of OE lines were 1.2- to 1.4-fold greater than those of WT. In addition, roots in OE lines were longer than those in WT plants (Supplemental Fig. 2). Together, our results indicate that overexpression of TERF2 in rice plants increases their tolerance to low-temperature stress.
Effect of cold stress on OE and WT seedlings. Two independent lines overexpressing TERF2 in rice (OE-8 and OE-10) and WT seedlings were grown in the greenhouse for 5 days, and then subjected to cold stress (6°C for 72 h), followed by recovery at 28°C for 7 days (right). The experiments were repeated at least three times, with more than 20 seedlings for WT and each independent OE line. Control seedlings were under normal growth conditions (left). Bars 1 cm
TERF2 promotes accumulation of osmolytes and reduces chlorophyll loss in rice under cold stress
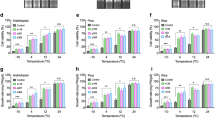
When exposed to abiotic stresses, plants can increase accumulation of osmotic substances to maintain high intracellular osmotic pressure and normal physiological functions of cells (Sharma et al. 2005). Here, we first examined accumulation of proline and soluble sugars in rice seedlings under normal and cold conditions. We found that there were no significant differences in content of soluble sugars and proline between WT and OE lines under normal conditions. However, the soluble sugars and proline in OE lines increased 1.5- and 0.5-fold after cold treatment, respectively, while there was only little change in WT seedlings (Fig. 2a, b). Analyses with Student’s t tests indicated that soluble sugars and proline contents in OE lines were different from those in WT seedlings at 95% probability. These results indicated that overexpression of TERF2 promoted accumulation of osmotic substances in rice seedlings under cold stress.
Comparison of osmotic substances and chlorophyll in OE and WT seedlings with or without cold treatment. a Sugar contents. b Free proline. c Chlorophyll. Leaf tissues for measurement were sampled directly from 10-day seedlings growing at 28°C (control) or after exposure to 6°C for 12 h (cold). Results are the average of three replicates, and 5–6 plants were taken in each replicate. Error bars represent standard error (SE)
Cold stress can decrease chlorophyll content by inhibiting chloroplast formation and the chlorophyll synthesis rate (Sharma et al. 2005), thus we examined whether overexpression of TERF2 in rice had an effect on chlorophyll content. We found that chlorophyll content was not significantly different between transgenic and WT plants under normal conditions. After transgenic and WT seedlings were subjected to cold treatment (6°C) for 12 h, the chlorophyll content of WT plants decreased to only 30% of its normal level, whereas those of OE lines were over 50% of their normal levels (Fig. 2c). Student’s t tests indicated that the chlorophyll contents of OE lines were significantly different from that of WT plants at 95% probability. These results indicated that TERF2 could maintain higher chlorophyll content in transgenic rice under cold stress compared with that of WT plants.
TERF2 decreases accumulation of ROS, MDA, and affects electrolyte leakage in rice under cold stress
Accumulated evidence indicates that stress-stimulated physiological imbalance increases the level of reactive oxygen species (ROS) in plant cells (Wu et al. 2008). To check whether overexpression of TERF2 resulted in alteration of ROS accumulation, we tested accumulation of ROS in WT and OE lines by nitroblue tetrazolium (NBT) staining for superoxide. Without stress treatment, NBT staining was slight in leaves and showed no obvious difference between WT and OE lines. After exposure to 4°C for 12, 24, and 48 h, ROS accumulated significant quantities in WT, whereas its levels in OE lines remained low, indicating that TERF2 might decrease accumulation of ROS under low temperature (Fig. 3a).
Analyses of ROS and MDA contents, and electrolyte leakage, in OE and WT seedlings. a Comparison of superoxide in rice leaves. Approximately 10 seedlings in triplicates were used for each line in this assay. Bars = 1 cm. Comparison of MDA (b) and electrolyte leakage (c) in OE and WT seedlings with or without cold treatment. Leaf tissues for measurement were sampled directly from 10-day seedlings growing at 28°C (control) or after exposure to 6°C for 12 h (cold). Results are the average of three replicates, and about 5–6 plants were taken in each replicate. For all the data, one asterisk indicates significant difference (P < 0.05) in comparison with wild type. Error bars represent SE
Accumulating research indicates that electrolyte leakage and accumulation of MDA, an end product of membrane lipid peroxidation, are indicators of free-radical production and cellular membrane injury caused by various stresses (Bates et al. 1973; Hodges et al. 1999; Lichtenthaler 1987; Parvanova et al. 2004). To determine whether overexpression of TERF2 in rice has effects on membrane stability, we investigated the MDA level and electrolyte leakage in OE lines and WT plants with or without cold treatment. There were no significant differences in MDA accumulation and electrolyte leakage between WT and OE seedlings under normal conditions. However, after 12 h of cold treatment, the MDA levels of WT increased about 75% compared with controls, but increased by only up to 25% in OE lines (Fig. 3b). Similarly, electrolyte leakage increased about two-fold in WT after cold treatment, but was only enhanced by 10–40% in OE lines (Fig. 3c).
TERF2 activates expression of genes related to redox regulation and membrane stability in rice under cold stress
Key enzymes that scavenge reactive oxygen species (ROS), such as superoxide dismutase B (SODB), are very important for membrane stability (Apel and Hirt 2004). Here, we analyzed the transcript levels of OsSODB by real-time PCR. Our results showed that the expression level of OsSODB was similar between WT and transgenic lines under normal conditions. However, the transcripts of OsSODB of different OE lines were 3- to 4-fold higher than that of WT plants after cold treatment (Fig. 4a), suggesting that TERF2 might function as a positive regulator tuning the transcript level of OsSODB, subsequently scavenging excessive ROS triggered by cold stress in transgenic rice. Ferritin and thioredoxin have oxidoreductase activity and participate in oxidation–reduction in plant under stress conditions (Nuruzzaman et al. 2008; Theil 1987). In this work, we also found that expression levels of OsFer1 and OsTrx23 were not changed significantly between WT and OE plants under normal growth conditions; however, the expression levels of these genes were much higher in OE plants under low temperature as compared with those in WT (Fig. 4b, c). These results indicated that TERF2 might affect the redox system under cold stress. Moreover, we investigated expression of OsLti6a and OsLti6b, which encode membrane proteins that contribute greatly toward membrane stability (Kim et al. 2007; Morsy et al. 2005). Their expression level in WT was similar to those in two OE lines under normal conditions, whereas the transcripts of OsLti6a and OsLti6b in OE lines were both two-fold higher than in WT plants after cold treatment (Fig. 4d, e). These findings implied that overexpression of TERF2 might be helpful in maintaining cellular membrane stability of rice by reducing MDA content and electrolyte leakage as well as upregulating expression of OsLti6a and OsLti6b under cold stress.
Analyses of genes related to redox regulation and membrane stability in OE and WT seedlings with or without cold treatment. a–e Expression analyses of SODB, OsFer1, OsTrx23, OsLti6a, and OsLti6b. About 0.2 g leaves of 10-day seedlings growing at 28°C (control) or after exposure to 6°C for 12 h (cold) were directly used to extract RNA for each sample. The expression of genes was determined using actin as internal control, calculated from three independent experiments, compared with WT controls (standardized to 100). The assay was repeated three times, and every leaves mixture from 6 plants were taken in each assay. For all data, one asterisk indicates significant difference (P < 0.05) in comparison with wild type. Error bars represent SE
TERF2 activates expression of cold-induced regulatory genes in rice
In plants, calcium (Ca) signaling and transcriptional regulation may be involved in the cold-stress signaling pathway (Chinnusamy et al. 2003; Dai et al. 2007; Lee et al. 2005; Sharma et al. 2005; Su et al. 2010). In this work, we found that expression of three regulatory genes, including OsICE, OsMyb, and OsCDPK7, were induced by cold treatment in rice (Fig. 5a). To further clarify whether TERF2 regulates their expression, we investigated expression of OsICE1 (inducer of CBF expression 1), OsMyb , and OsCDPK7 in rice under normal conditions by qRT-PCR. Our results showed that the transcript levels of these genes in OE lines were significantly higher than those in WT plants (Fig. 5b).
Analyses of expression of regulatory genes (OsICE1, OsMyb, and OsCDPK7) in rice. a Expression of OsICE1, OsMyb, and OsCDPK7 after cold stress in WT seedlings. b Expression of OsICE1, OsMyb, and OsCDPK7 in OE and WT seedlings under normal conditions. About 0.2 g leaves of 10-day seedlings growing at 28°C (control) or after exposure to 6°C for 12 and 24 h, respectively, were directly used to extract RNA for each sample. The expression of genes was determined by qRT-PCR using actin as internal control, calculated from three independent experiments, compared with controls (standardized to 100). The assay was repeated three times, and every leaves mixture from 5 plants were taken in each assay. For all the data, one asterisk indicates significant difference (P < 0.05) in comparison with wild type. Error bars represent SE
Discussion
We previously reported that TERF2 could transcriptionally modulate ethylene biosynthesis to enhance freezing tolerance in transgenic tobacco and tomato plants (Zhang et al. 2009; Zhang and Huang 2010). To investigate the effect of TERF2 on a monocotyledon, we analyzed the response of OE lines to 1-aminocyclopropane-1-carboxylic acid. The shoot and coleoptile lengths of the OE lines were not significantly different from those of WT controls (data not shown). The results indicated that TERF2 might not affect the ethylene sensitivity in rice, which differed from those in dicotyledons such as tobacco and tomato (Zhang et al. 2009; Zhang and Huang 2010). However, in this study, our results revealed that overexpression of TERF2 enhanced cold tolerance by modulating expression of stress-responsive genes and resulted in a series of physiological adjustments in transgenic rice under cold stress, indicating that TERF2 might have important regulatory roles in response to abiotic stress in rice.
Cold is one of the major abiotic stresses depressing plant growth and productivity. One mechanism of injury of cold stress involves generation and reactions of ROS in plant (Kaniuga 2008). Under various stresses, ROS production increased quickly in chloroplasts, resulting in damage to the structure of plant cells (Gechev et al. 2006). ROS are very small molecules, and they are highly reactive due to the presence of unpaired valence-shell electrons. When plants are exposed to cold stress, their ROS levels can increase dramatically, leading to lipid peroxidation, denaturation of proteins, and metabolic disorders. ROS degrade polyunsaturated lipids and subsequently generate MDA, which causes toxic stress in cells by forming covalent protein adducts. Furthermore, ROS triggered by various stresses also affect membrane integrity and cell compartmentation in plants. In this regard, electrolyte leakage is widely used as an indicator of membrane damage. In addition, low-molecular-weight metabolites, such as proline and a variety of sugars, serve as osmoprotectants and increase the ability of plant cells to retain normal physiological function (Sharma et al. 2005; Bhatnagar-Mathur et al. 2008). Moreover, they also play crucial roles in reducing plant damage by oxidative stress, protecting plant cells against accumulation of ROS (Apel and Hirt 2004). In this study, our findings showed that TERF2 could not only reduce accumulation of ROS and MDA and decrease electrolyte leakage, but also promote accumulation of proline and soluble sugars in transgenic rice under cold stress. These physiological adjustments might result in transgenic rice with enhanced cold tolerance.
It has been proved that superoxide dismutases (SODs; EC 1.15.1.1) function to defend against ROS in plants by catalyzing dismutation of superoxide anion radicals into molecular oxygen and hydrogen peroxide (Apel and Hirt 2004). Ferritin and thioredoxin are conserved proteins that are involved in cellular redox regulation (Nuruzzaman et al. 2008; Theil 1987). It has been demonstrated that ferritin could reduce accumulation of ROS in response to oxidant challenge (Orino et al. 2001). OsTrx23, one of the cold-induced Trx h proteins, plays important roles in response to oxidative stress imposed by cold (Nuruzzaman et al. 2008; Xie et al. 2009). OsLti6, a hydrophobic transmembrane protein with low molecular weight, is involved in preserving plasma membrane integrity during cold stress in rice (Kim et al. 2007; Morsy et al. 2005). In the present work, although there was no difference between expression of OsSODB, OsFer1, OsTrx23, OsLti6a, and OsLti6b in WT and OE lines under normal conditions, their transcripts in OE lines were much higher than those in WT plants after cold treatment, implying that TERF2-mediated cold tolerance is consistent with data from studies describing other transgenic plants with improved cold tolerance (Chinnusamy et al. 2007; Dai et al. 2007; Hu et al. 2008; Kim et al. 2007; Morsy et al. 2005; Su et al. 2010). It is possible that these transcript alterations might be dependent on some specific TERF2-interacting proteins induced by cold stress or TERF2 protein modification in rice under cold treatment.
Moreover, we found that overexpression of TERF2 in rice regulated expression of cold-induced regulatory genes including OsICE1, OsMyb, and OsCDPK7 under normal condition. In rice, OsCDPK7 and several members of Mybs were reported to be involved in regulating rice response to abiotic stresses (Dai et al. 2007; Saijo et al. 2000; Su et al. 2010). The calcium-dependent protein kinases (CDPKs), the most common serine/threonine protein kinases, are important calcium sensors with a regulatory domain binding to Ca2+ ions in response to stresses (Klimecka and Muszyńska 2007); for instance, OsCDPK7 can confer both cold and salt tolerance in plants (Saijo et al. 2000, 2001). Many regulatory factors, including ICE1, MYB, ERF, and NAC family members, positively regulate plant responses to cold stress by tuning the expression of stress-related genes in Arabidopsis (Chinnusamy et al. 2007; Dai et al. 2007) and rice (Hu et al. 2008; Su et al. 2010). Here, we found that transcript levels of OsICE1, OsMyb, and OsCDPK7, which were induced by cold, were upregulated in OE seedlings under normal conditions, suggesting that TERF2 might regulate cold response through different stress-related signaling pathways.
Previous studies documented that TERF2 could bind to the GCC-box and dehydration-responsive element/C-repeat (DRE/CRT) in vitro and in vivo, and regulate expression of its targeted genes in transgenic tobacco (Zhang et al. 2009). In this study, our analysis of the promoter regions revealed that there were DRE/CRT elements present in the promoters of TERF2-targeted genes except OsTrx23 (Supplemental Table 2), suggesting that TERF2 might directly upregulate expression of its targeted genes by interacting with these cis-elements in rice. Subsequently, these transcriptional changes might result in decreased accumulation of ROS and MDA and reduced membrane injury of transgenic rice. These physiological adjustments finally confer transgenic rice with cold tolerance. The present results suggest that ectopic expression of TERF2 might play important roles in modulating rice cold response and possess potential utility in genetic engineering for developing cold-tolerant rice.
References
Agarwal PK, Agarwal P, Reddy MK, Sopory SK (2006) Role of DREB transcription factors in abiotic and biotic stress tolerance in plants. Plant Cell Rep 25:1263–1274
Apel K, Hirt H (2004) Reactive oxygen species: metabolism, oxidative stress, and signal transduction. Annu Rev Plant Biol 55:373–399
Bates LS, Walden RP, Teare ID (1973) Rapid determination of free proline for water stress studies. Plant Soil 39:205–207
Beck EH, Fettig S, Knake C, Hartig K, Bhattarai T (2007) Specific and unspecific responses of plants to cold and drought stress. J Biosci 32:501–510
Bhatnagar-Mathur P, Vadez V, Sharma K (2008) Transgenic approaches for abiotic stress tolerance in plants: retrospect and prospects. Plant Cell Rep 27:411–424
Cheng C, Yun KY, Ressom HW, Mohanty B, Bajic VB, Jia Y, Yun SJ, de los Reyes BG (2007) An early response regulatory cluster induced by low temperature and hydrogen peroxide in seedlings of chilling-tolerant japonica rice. BMC Genomics 8:175
Chinnusamy V, Ohta M, Kanrar S, Lee BH, Hong X, Agarwal M, Zhu JK (2003) ICE1: a regulator of cold-induced transcriptome and freezing tolerance in Arabidopsis. Gene Dev 17:1043–1054
Chinnusamy V, Zhu J, Zhu JK (2007) Cold stress regulation of gene expression in plants. Trends Plant Sci 12:444–451
Dai X, Xu Y, Ma Q, Xu W, Wang T, Xue Y, Chong K (2007) Overexpression of an R1R2R3 MYB gene, OsMYB3R-2, increases tolerance to freezing, drought, and salt stress in transgenic Arabidopsis. Plant Physiol 143:1739–1751
Dong CH, Zolman BK, Bartel B, Lee BH, Stevenson B, Agarwal M, Zhu JK (2009) Disruption of Arabidopsis CHY1 reveals an important role of metabolic status in plant cold stress signaling. Mol Plant 2:59–72
Dubois M, Gilles KA, Hamilton JK, Rebers PA, Smith F (1956) Colorimetric method for determination of sugars and related substances. Anal Chem 28:350–356
Fowler S, Thomashow MF (2002) Arabidopsis transcriptome profiling indicates that multiple regulatory pathways are activated during cold acclimation in addition to the CBF cold response pathway. Plant Cell 14:1675–1690
Gechev TS, Van Breusegem F, Stone JM, Denev I, Laloi C (2006) Reactive oxygen species as signals that modulate plant stress responses and programmed cell death. Bioessays 28:1091–1101
Gilmour SJ, Sebolt AM, Salazar MP, Everard JD, Thomashow MF (2000) Overexpression of the Arabidopsis CBF3 transcriptional activator mimics multiple biochemical changes associated with cold acclimation. Plant Physiol 124:1854–1865
Hiei Y, Komari T, Kubo T (1997) Transformation of rice mediated by Agrobacterium tumefaciens. Plant Mol Biol 35:205–218
Hodges DM, DeLong JM, Forney CF, Prange RK (1999) Improving the thiobarbituric acid-reactive-substances assay for estimating lipid peroxidation in plant tissues containing anthocyanin and other interfering compounds. Planta 207:604–611
Hu H, You J, Fang Y, Zhu X, Qi Z, Xiong L (2008) Characterization of transcription factor gene SNAC2 conferring cold and salt tolerance in rice. Plant Mol Biol 67:169–181
Ito Y, Katsura K, Maruyama K, Taji T, Kobayashi M, Seki M, Shinozaki K, Yamaguchi-Shinozaki K (2006) Functional analysis of rice DREB1/CBF-type transcription factors involved in cold-responsive gene expression in transgenic rice. Plant Cell Physiol 47:141–153
Kaniuga Z (2008) Chilling response of plants: importance of galactolipase, free fatty acids and free radicals. Plant Biol 10:171–184
Kim SH, Kim JY, Kim SJ, An KS, An G, Kim SR (2007) Isolation of cold stress-responsive genes in the reproductive organs, and characterization of the OsLti6b gene from rice (Oryza sativa L.). Plant Cell Rep 26:1097–1110
Klimecka M, Muszyńska G (2007) Structure and functions of plant calcium-dependent protein kinases. Acta Biochim Pol 54:219–233
Lee BH, Henderson DA, Zhu JK (2005) The Arabidopsis cold-responsive transcriptome and its regulation by ICE1. Plant Cell 17:3155–3175
Lichtenthaler HK (1987) Chlorophylls and carotenoids: pigments of photosynthetic biomembranes. Methods Enzymol 148:350–382
Mahajan S, Tuteja N (2005) Cold, salinity and drought stresses: an overview. Arch Biochem Biophys 444:139–158
Mantiri FR, Kurdyukov S, Lohar DP, Sharopova N, Saeed NA, Wang XD, Vandenbosch KA, Rose RJ (2008) The transcription factor MtSERF1 of the ERF subfamily identified by transcriptional profiling is required for somatic embryogenesis induced by auxin plus cytokinin in Medicago truncatula. Plant Physiol 146:1622–1636
Morsy MR, Almutairi AM, Gibbons J, Yun SJ, de Los Reyes BG (2005) The OsLti6 genes encoding low-molecular-weight membrane proteins are differentially expressed in rice cultivars with contrasting sensitivity to low temperature. Gene 344:171–180
Nakano T, Suzuki K, Fujimura T, Shinshi H (2006) Genome-wide analysis of the ERF gene family in Arabidopsis and rice. Plant Physiol 149:88–95
Nuruzzaman M, Gupta M, Zhang C, Wang L, Xie W, Xiong L, Zhang Q, Lian X (2008) Sequence and expression analysis of the thioredoxin protein gene family in rice. Mol Genet Genomics 280:139–151
Orino K, Lehman L, Tsuji Y, Ayaki H, Torti SV, Torti FM (2001) Ferritin and the response to oxidative stress. Biochem J 357:241–247
Parvanova D, Ivanov S, Konstantinova T, Karanov E, Atanassov A, Tsvetkov T, Alexieva V, Djilianov D (2004) Transgenic tobacco plants accumulating osmolytes show reduced oxidative damage under freezing stress. Plant Physiol Biochem 42:57–63
Pirrello J, Jaimes-Miranda F, Sanchez-Ballesta MT, Tournier B, Khalil-Ahmad Q, Regad F, Latché A, Pech JC, Bouzayen M (2006) Sl-ERF2, a tomato ethylene response factor involved in ethylene response and seed germination. Plant Cell Physiol 47:1195–1205
Pré M, Atallah M, Champion A, De Vos M, Pieterse CM, Memelink J (2008) The AP2/ERF domain transcription factor ORA59 integrates jasmonic acid and ethylene signals in plant defense. Plant Physiol 147:1347–1357
Quan R, Hu S, Zhang Z, Zhang H, Zhang Z, Huang R (2010) Overexpression of an ERF transcription factor TSRF1 improves rice drought tolerance. Plant Biotechnol J 8:476–488
Saijo Y, Hata S, Kyozuka J, Shimamoto K, Izui K (2000) Overexpression of a single Ca2+-dependent protein kinase confers both cold and salt/drought tolerance on rice plants. Plant J 23:319–327
Saijo Y, Kinoshita N, Ishiyama K, Hata S, Kyozuka J, Hayakawa T, Nakamura T, Shimamoto K, Yamaya T, Izui K (2001) A Ca2+-dependent protein kinase that endows rice plants with cold- and salt-stress tolerance functions in vascular bundles. Plant Cell Physiol 42:1228–1233
Sharma P, Sharma N, Deswal R (2005) The molecular biology of the low-temperature response in plants. Bioessays 27:1048–1059
Su CF, Wang YC, Hsieh TH, Lu CA, Tseng TH, Yu SM (2010) A novel MYBS3-dependent pathway confers cold tolerance in rice. Plant Physiol 153:145–158
Theil EC (1987) Ferritin: structure, gene regulation, and cellular function in animals, plants, and microorganisms. Annu Rev Biochem 56:289–315
Troll W, Lindsley J (1955) A photometric method for the determination of proline. J Biol Chem 215:655–660
Vernié T, Moreau S, de Billy F, Plet J, Combier JP, Rogers C, Oldroyd G, Frugier F, Niebel A, Gamas P (2008) EFD is an ERF transcription factor involved in the control of nodule number and differentiation in Medicago truncatula. Plant Cell 20:2696–2713
Wang Q, Guan Y, Wu Y, Chen H, Chen F, Chu C (2008) Overexpression of a rice OsDREB1F gene increases salt, drought, and low temperature tolerance in both Arabidopsis and rice. Plant Mol Biol 67:589–602
Wu L, Chen X, Ren H, Zhang Z, Zhang H, Wang J, Wang XC, Huang R (2007) ERF protein JERF1 that transcriptionally modulates the expression of abscisic acid biosynthesis-related gene enhances the tolerance under salinity and cold in tobacco. Planta 226:815–825
Wu L, Zhang Z, Zhang H, Wang XC, Huang R (2008) Transcriptional modulation of ethylene response factor protein JERF3 in the oxidative stress response enhances tolerance of tobacco seedlings to salt, drought, and freezing. Plant Physiol 148:1953–1963
Xie G, Kato H, Sasaki K, Imai R (2009) A cold-induced thioredoxin h of rice, OsTrx23, negatively regulates kinase activities of OsMPK3 and OsMPK6 in vitro. FEBS Lett 583:2734–2738
Xu K, Xu X, Fukao T, Canlas P, Maghirang-Rodriguez R, Heuer S, Ismail AM, Bailey-Serres J, Ronald PC, Mackill DJ (2006) Sub1A is an ethylene-response-factor-like gene that confers submergence tolerance to rice. Nature 442:705–708
Yamaguchi-Shinozaki K, Shinozaki K (2006) Transcriptional regulatory networks in cellular responses and tolerance to dehydration and cold stresses. Annu Rev Plant Biol 57:781–803
Yi SY, Kim JH, Joung YH, Lee S, Kim WT, Yu SH, Choi D (2004) The pepper transcription factor CaPF1 confers pathogen and freezing tolerance in Arabidopsis. Plant Physiol 136:2862–2874
Zhang Z, Huang R (2010) Enhanced tolerance to freezing in tobacco and tomato overexpressing transcription factor TERF2/LeERF2 is modulated by ethylene biosynthesis. Plant Mol Biol 73:241–249
Zhang Z, Zhang H, Quan R, Wang XC, Huang R (2009) Transcriptional regulation of ethylene response factor LeERF2 in the expression of ethylene biosynthesis genes controls ethylene production in tomato and tobacco. Plant Physiol 150:365–377
Zhu J, Shi H, Lee BH, Damsz B, Cheng S, Stirm V, Zhu JK, Hasegawa PM, Bressan RA (2004) An Arabidopsis homeodomain transcription factor gene, HOS9, mediates cold tolerance through a CBF-independent pathway. Proc Natl Acad Sci USA 101:9873–9878
Zhu J, Dong CH, Zhu JK (2007) Interplay between cold-responsive gene regulation, metabolism and RNA processing during plant cold acclimation. Curr Opin Plant Biol 10:290–295
Acknowledgments
This work was supported by the National High Technology Research and Development Program of China (2008AA10Z110), the National Natural Science Foundation of China (30730060, 30671250, and 31000536), Grant Special Program of Transgenic Plants and Animals in China (2008ZX08001-003), and the Hunan Provincial Natural Science Foundation (09JJ3043).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Yun Tian, Haiwen Zhang, and Xiaowu Pan contributed equally to this article.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Tian, Y., Zhang, H., Pan, X. et al. Overexpression of ethylene response factor TERF2 confers cold tolerance in rice seedlings. Transgenic Res 20, 857–866 (2011). https://doi.org/10.1007/s11248-010-9463-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11248-010-9463-9