Abstract
Proteinase inhibitors (PIs) play an important role in plant responses to biotic and environmental stimuli, but little is known about the role of PIs in mediating plant immune responses to microbial infection. In this study, a gene named proteinase inhibitor I4 (MtPiI4) was isolated from Medicago truncatula and characterized as a serpin family gene with a typically conserved DUF716 domain. MtPiI4 was differentially expressed in seed, root, leaf, stem and flower tissues. Expression of MtPiI4 was induced by inoculation with a typical bacterial pathogen Pseudomonas syringae pv. tomato DC3000 strain (Pst DC3000). It was also up-regulated by methyl jasmonate (MeJA) treatment. To identify its function in regulating plant immunity against Pst DC3000, we constructed transgenic Arabidopsis plants over-expressing MtPiI4. Compared to wild type, 35S::MtPiI4 plants showed enhanced resistance to Pst DC3000. Expression of JA biosynthetic and responsive genes such as LOX2, PDF1.2, and VSP1 was depressed in 35S::MtPiI4 plants as compared to wild type, suggesting that the JA signaling response was attenuated in 35S::MtPiI4 plants upon Pst DC3000 exposure. Furthermore, over-expression of MtPiI4 led to up-regulation of NPR1 (nonexpressor of pathogenesis-related gene 1—a negative regulator of JA signaling) and down-regulation of MAPK4 (mitogen-activated protein kinase4—a positive regulator of JA signaling). These results indicate that MtPiI4 regulation of plant resistance to Pst DC3000 is involved in the JA signaling transduction pathway.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Proteinase inhibitors (PIs) are a group of plant polypeptides (or proteins) acting against biotic and abiotic stresses (Jongsma and Beekwilder 2011; Kim et al. 2009; Zhang et al. 2008). Based on the active amino acids in their reaction centers, four types of proteases are categorized: serine, cysteine, aspartic and metallo-proteinases (Koiwa et al. 1997). The serine proteinase inhibitors (PiI4 or serpin) are the PIs most frequently identified owning to their diverse biological functions (Fluhr et al. 2012; Roberts and Hejgaard 2008). In practice, plant PiI4 can be further classified into seven subfamilies, namely soybean trypsin inhibitor (Kunitz), soybean proteinase inhibitor (Bowman-Birk), Potato I, potato II, barley trypsin inhibitor, squash inhibitor and steptomyces subtilisin inhibitor (SSI) (Ryan 1989). PIs have been characterized with two basic functions: (1) to prevent uncontrolled proteolysis in cells to ensure the normal function of limited proteolysis, and (2) to protect proteins from foreign proteolytic enzymes (Ryan 1989). While PIs participate in nutrient accumulation due to their high contribution (usually 1–10 %, even 50 %) to total proteins in seeds and vegetative organs (Pearce et al. 1988; Ryan 1989), the most important characteristic of PIs is their contribution to protection of plants against pathogen and insect attack (John 2011; Schlüter et al. 2010).
Plant PIs have been reported to induce high mortality of insects by binding to insect digestive proteinases to block protein digestion (Dunse et al. 2010; Mosolov and Valueva 2008). In a field trial, co-expression of two types of serpin (potato type I and II proteinase inhibitors) in cotton conferred resistance to insect attack (Dunse et al. 2010). By contrast, Solanum nigrum plants with silenced serpin activity displayed a significantly higher degree of damage caused by generalist herbivores than the wild type; however, plant growth and development were not affected by silencing serpins, which suggests that serpins play a major role in mediating insect proteases but not endogenous proteases in the plant itself (Hartl et al. 2010). There are some cases indicating that plant serpins have the potential to block the growth of a variety of pathogenic bacterial and fungal strains (Hu et al. 2007, 2009; Kim et al. 2009). While serpins working in animals play a role in regulating the host immune response rather than interacting directly with pathogens (Law et al. 2006), those in plants work by way of a competitive interaction between plant immunity and pathogen infection (McDowell 2011; Spoel and Dong 2012). To date, despite studies focusing on manipulation of serpins for plant tolerance to exogenous pathogens or insects (Alvarez-Alfageme et al. 2011; Kim et al. 2009), little is known about whether and how plant serpins regulate plants’ own intrinsic immune responses against pathogen infection.
Jasmonic acid (JA) and its derivative methyl jasmonate (MeJA) are natural plant signaling molecules playing crucial roles in plant responses to various biotic stresses (Avanci et al. 2010). The positive regulation of PIs by JA signaling has been demonstrated as an effective plant strategy against insect attack (Hartl et al. 2010; Lomate and Hivrale 2012). JA can play a negative role in the plant response to microbes in an antagonistic way via salicylic acid (SA), which makes plant resistance to pathogen infection (Pieterse et al. 2009; Vlot et al. 2009). For instance, Pseudomonas syringae pv. tomato DC3000 (Pst DC3000) utilizes JA signaling to depress SA-mediated defense system to allow its invasion and development in host plants (Brooks et al. 2005; Laurie-Berry et al. 2006; Zheng et al. 2012). The antagonism between SA and JA is differentially regulated by intracellular factors and signaling molecules such as NPR1and MAPK4 in plants (Pieterse et al. 2009). Acting as a major positive regulator in SA signaling, NPR1 is required for repression of JA signaling in the plant response to Pst DC3000 (Spoel et al. 2003). As a repressor of SA-induced defensive signaling pathways, MAPK4 is necessary for JA-responsive gene expression in the plant response to Pst DC3000 (Petersen et al. 2000). In maize (Zea mays), a cysteine proteinase inhibitor has been reported to signal defense against Ustilago maydis in a negative way involving SA (van der Linde et al. 2012). Both AtSepin1 and AtSRP4 belong to the serpin family of genes identified in Arabidopsis thaliana; interestingly, expression of AtSepin1 was significantly induced by both Pst inoculation and cold stress (Fluhr et al. 2012; Roberts and Hejgaard 2008). Similarly, expression of AtSRP4 was markedly induced after salinity stress (Fluhr et al. 2012). These results indicate that plant serpins play potential roles in regulating the plant response to biotic and abiotic stresses. We recently isolated a serpin gene (here designed MtPiI4) from Medicago truncatula in response to heavy metal mercury exposure (Zhou et al. 2013). Examination of MtPiI4 revealed that it could be regulated by pathogen exposure. Here, we describe the functional characterization of MtPiI4 in the plant response to Pst DC3000. Our study revealed that MtPiI4 was induced by MeJA and inoculation by Pst DC3000. Transgenic Arabidopsis over-expressing MtPiI4 showed enhanced resistance to Pst DC3000 and reduced sensitivity to MeJA. This biological response is likely involved in the JA signaling pathway because both NPR1 and MAPK4 were regulated in 35S::MtPiI4 plants upon Pst DC3000 exposure.
Materials and Methods
Plant Materials and Cultivation
Seeds of M. truncatula (cv. Jemlog) were surface-sterilized and rinsed thoroughly with sterile water. The seeds were germinated in a growth chamber at 22 °C. The germinating seeds were transferred to ½ Hoagland nutrient solution for hydroponic cultivation for 4 weeks. The growth condition was set at 22 °C with 100 μE m−2 s−1 photosynthetically active radiation and a 16 h light/8 h dark cycle (Zhou et al. (2008). For Arabidopsis thaliana, sterilized seeds (ecotype Col-0) were germinated on Murashige and Skoog (MS) solid medium in a growth chamber under the same conditions described above. After 7 days, seedlings were transferred to half-strength Hoagland nutrient solution and grown for 3 weeks (Song et al. 2012). The prepared seedlings indicated above were used for treatment and analysis.
Plant Inoculation with Pst DC3000
Pst DC3000 strain was cultured with KB medium containing 100 mg L−1 rifampicin in a shaker at 28 °C. Pst DC3000 in the exponential phase was collected and re-suspended in 10 mM MgCl2 for inoculation. Bacterial inoculation on plant leaves was performed using syringe injection as described by Zipfel et al (2004). MgCl2 solution (10 mM) was injected to plants as a negative control. For Arabidopsis, a bacterial suspension containing 0.02 % silwet L-77 was sprayed on leaves until the surface was uniformly wetted (Keith et al. 2003). MgCl2(10 mM) solution containing 0.02 % silwet L-77 was sprayed on plants as a negative control. Plants inoculated with bacteria were kept in the dark for 1 h with plastic wrap to allow bacteria to grow, and were then transferred to normal growth conditions as mentioned above. For dose-dependent experiments, plants were inoculated with microbial suspensions with different concentrations (OD600 = 0–2). In time-course experiments, plant samples were harvested after inoculation with bacterial suspension (OD600 = 0.2) for different times.
MeJA Treatment
Plants were treated with MeJA according to the method described by Brown et al. (2003). MeJA (TCI, Shanghai, China) was dissolved into sterile water with the aid of 5 % ethanol. Before treatment, plants were transferred into a vessel with a fixed volume of 1.5 L that was sealed with plastic wrap. MeJA solution at different concentrations (0–500 μM) was sprayed onto plant leaves. Sterile water containing 5 % ethanol was sprayed on plants as a control.
Plant Transformation
MtPiI4 was PCR-amplified using primers with restriction enzyme sites at the 5′-end of forward and reverse primers by high-fidelity enzymes (fast pfu, TransGen). The initial amplified segment was cloned into a T vector (pEASY blunt, TransGen, Beijing, China), sequenced, and digested. The recycled segment was cloned into pCAMBIA1304 under the control of the cauliflower mosaic virus (CaMV) 35S promoter and transformed into Agrobacterium tumefaciens strain EHA105. Transformation to Arabidopsis was via the flower dipping method (Clough and Bent 1998). In this study, all lines used were homozygous transgenic lines (T3 generation).
Determination of Bacterial Population in Plant Leaves
Bacterial growth in planta was measured according to the method described by He et al. (2006). Leaf disks were cut with a leaf punch from whole plant leaves inoculated with bacteria. The collected leaf disks were soaked in sterile water for 1 min followed by washing twice. The leaf disks were then ground in 100 μL H2O and serial dilutions were plated on KB medium containing 25 mg/L rifampicin. The number of bacterial colony forming units (CFU) was counted after 2 days of growth at 28 °C.
Callose Staining
Callose in plant tissue was stained with aniline blue as described previously (Kim et al. 2005). Briefly, plant leaves were harvested after 12 h of bacterial infiltration, cleaned, and soaked into 95 % ethanol to remove the green background. The leaves were stained in 150 mmol L−1 K2HPO4 (pH 9.5) containing 0.01 % aniline blue for 30 min. Leaves were transferred to 50 % glycerol and examined under a fluorescence microscope (Olympus MVX10, Tokyo, Japan) with epifluorescent illumination (OLYMPUS MVX10). The views of the pictures were randomized. The number of callose deposits was counted and analyzed in triplicate.
Transcript Analysis
Total RNA was extracted from plant tissues using TRIZOL reagent (Invitrogen, Carlsbad, CA). After DNA digestion, 2.0 μg RNA was used as a template for cDNA synthesis (ThermoScript, Life Technologies, Carlsbad, CA). Reverse transcription was performed at 42 °C in a 25-μL reaction mixture including 2.0 μg RNA, 0.5 μg oligo (dT) primers, 12.5 nmol dNTPs, 20 units RiboLock RNase inhibitor and 200 units RevertAid Reverse Transcriptase. First strand cDNA was used as a template to analyze the expression of genes using real-time quantitative reverse transcription-polymerase chain reaction (qRT-PCR) using SYBR Green (Bio-Rad) with an Applied Biosystems 7500 Fast Real-Time PCR System (LifeTechnologiesTM). The relative expression level of each gene was calculated using the 2-∆∆CT method (Livak and Schmittgen 2001). In some cases, semi-quantitative RT-PCR was performed to detect expression of MtPiI4. Basically, the total 25 μL of PCR reaction mixture in Tris-HCl buffer (pH 8.3, 10 mM) comprised 1 μL normalized cDNA template, 10 pmol sense primer, 10 pmol antisense primer, 5 nmol dNTPs, 32.5 nmol Mg2+, and 0.5 U Taq DNA polymerase. PCR was performed as follows: 95 °C for 5 min, 30 cycles at 94 °C for 30 s, 58 °C for 30 s, 72 °C for 30 s, and a final extension step at 72 °C for 10 min. The relative abundance of MtEF1α was used as an internal standard for cDNA normalization in both RT-PCR and qRT-PCR analysis (Ding et al. 2008). All the primers used in these experiments were showed in Online Resource 1.
In Silico Analysis of Genes
Protein structure was predicted using SMART (http://smart.embl-heidelberg.de/). Protein subcellular location was predicted using WoLF PSORT server (http://wolfpsort.org/). The derived amino acid sequences were download from NCBI (http://www.ncbi.nlm.nih.gov/) and comparisons were analyzed using CLUSTALX 2.0 software. Phylogenetic trees were constructed using the maximum likelihood method in MEGA 5.2. Numbers on internal nodes are the percentage bootstrap support values (1,000 re-sampling). Only values exceeding 50 % are presented.
Statistical Analysis
Each result shown in the figures was the mean of at least three replicated treatments and each treatment contained at least 30 seedlings. Significant differences between treatments were evaluated statistically by standard deviation and one-way analysis of variance (ANOVA). The data between differently treated groups were compared statistically by ANOVA followed by the least significant difference (LSD) test if the ANOVA result was significant at P < 0.05. Statistical analyses were performed with SPSS 12.0. Unless indicated, equal amounts of mixed transgenic line (L1 and L2) seeds were used, and seedling samples (including all transgenic lines) were selected randomly for analysis.
Results
Isolation and Analysis of MtPiI4 from M. truncatula
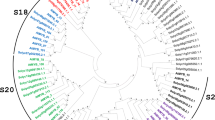
The full-length cDNA sequence of MtPiI4 was isolated from the M. truncatula genome using a RT-PCR-based strategy. The MtPiI4 cDNA sequence was 1,676 bp in length with an open reading frame (ORF) of 957 bp, a 5′-UTR of 120 bp, and a 3′-UTR of 496 bp (Fig. 1a; Online Resource 2). No intron was found in the DNA sequence of mtPiI4. The accession numbers of the full cDNA sequence of MtPiI4 deposited with NCBI and M. truncatula database (http://www.medicagohapmap.org/) are XM_003594077 and Medtr2g021690, respectively. The deduced protein of MtPiI4 consists of 318 amino acids, with a predicted molecular weight of 36 kDa and a pI of 6.73. MtPiI4 protein was predicted to contain a DUF716 domain (domain of unknown function 716) (Fig. 1b). This domain is conserved in plants, and clusters with its orthologues from other plant species (Fig. 1c). Further analysis revealed that MtPiI4 proteins contain two typical transmembrane domains (Fig. 1b; Online Resource 3). A phylogenetic analysis showed that MtPiI4 is related most closely to a PiI4 protein (XP_004486117) from Cicer arietinum, with 82 % identity (Fig. 1d). Thus, MtPiI4 most likely belongs to the serpin family.
Sequence analysis of Medicago truncatula proteinase inhibitor I4 (MtPiI4). a Schematic structure of MtPiI4 cDNA. UTR Untranslated region, CDS coding sequence. b Alignment of amino acid sequences of deduced MtPiI4 protein and comparison to orthologs from other plant species. The black box indicates a conserved DUF716 domain (domain of unknown function 716) and two transmembrane domains. c Phylogenetic relationship of the DUF716 domain of PiI4s from other plant species. d Phylogenetic relationship of PiI4s from other plant species. The phylogenetic trees were constructed using the maximum likelihood method in MEGA 5.2. Numbers on internal nodes are the percentage bootstrap support values (1,000 re-samplings). Only values exceeding 50 % are shown. At Arabidopsis thaliana, Tc Theobroma cacao, Ptr Populus trichocarpa, Mxr Malus x robusta, Fvs Fragaria vesca subsp. vesca, Ca Cicer arietinum, Mt Medicago truncatula, Gm Glycine max, Atr Amborella trichopoda, Os Oryza sativa, Si Setaria italica, Zm Zea mays
MtPiI4 is Expressed Constitutively in Various Tissues of M. truncatula
To determine the expression pattern of MtPiI4, the relative abundance of MtPiI4 transcripts in different plant tissues was analyzed using semi-quantitative RT-PCR and qRT-PCR. MtPiI4 was expressed ubiquitously in different tissues including roots, stems, leaves, flowers, and germinating seeds (Fig. 2). Strong expression was observed in root, stem, and leaf tissues, whereas expression in flowering tissues and germinating seeds was weak, indicating that transcripts of MtPiI4 were detectable throughout most of the lifecycle of M. truncatula, although its expression level varied in different tissues.
MtPiI4 expression in different tissues of M. truncatula. Total RNA was extracted from different tissues (root, stem, leaf, flower and seed) of plants cultured hydroponically for 4 weeks. The relative expression of MtPiI4 was analyzed using a semi-quantitative RT-PCR and b qRT-PCR. Error bars Standard deviation of the mean. Means with different lower case letters indicate significantly different gene expression (P <0.05). MtEF1α was used for cDNA normalization
MtPiI4 is Induced by Pst DC3000 Inoculation and MeJA in M. truncatula
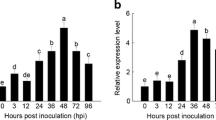
To investigate the response of MtPiI4 to pathogen infection, total RNA was extracted from shoots and roots of 4 week-old M. truncatula seedlings inoculated with Pst DC3000 and analyzed using RT-PCR. Compared to uninoculated plants (CK, control), Pst DC3000 infection induced a significant increase in MtPiI4 transcripts in shoots in a dose-responsive manner (Fig. 3a, c). In a time-course experiment, MtPiI4 was induced rapidly within 2 hpi (hours post inoculation) and the higher level remained up to 12 hpi (Fig. 3b, d). In roots, a similar pattern of MtPiI4 expression was observed after pathogen infection (Fig. 3e–h). The MtPiI4 transcripts increased initially at 4 hpi, peaked at 8–12 hpi, and then began to decline (Fig. 3h). At any level of Pst DC3000 inoculation, a basic level of MtPiI4 transcripts was detected. This was observed particularly in root tissues.
Expression pattern of MtPiI4 in wild type M. truncatula in response to Pst DC3000. Four-week-old seedlings were inoculated with Pst DC3000 at different concentrations (OD600 = 0–2) for 2 h or inoculated with Pst DC3000 at a specific concentration (OD600 = 0.3) for different times (0–24 h). Expression of MtPiI4 in shoots (a–d) and roots (e–h) was analyzed using semi-quantitative RT-PCR (a, b, e, f) and qRT-PCR (c, d, g, h). Asterisks indicate that mean values are significantly different between the control and treatment (P < 0.05). MtEF1α was used for cDNA normalization
MeJA has been suggested as an important regulator of plant PIs (Hartl et al. 2010; Lomate and Hivrale 2012). To investigate the possible effect of MeJA on expression of MtPiI4, shoots of M. truncatula plants were treated with MeJA at different concentrations and time intervals. As shown in Fig. 4, treatment with MeJA induced a marked increase in MtPiI4 transcripts in shoots and roots. Compared to the control, 50 μM MeJA was able to induce 2.5-fold higher expression of MtPiI4 than in the control (Fig. 4c). MeJA-induced MtPiI4 transcripts were also analyzed over the time of MeJA application (Fig. 4b, d, f, h). The pattern of MtPiI4 expression in the presence of MeJA was very similar to that of Pst DC3000 inoculation.
Expression pattern of MtPiI4 in wild type M. truncatula in response to methyl jasmonate (MeJA). Four-week-old seedlings were treated with MeJA at different concentrations (0–500 μM) for 4 h or treated with 500 μM of MeJA for different times (0–24 h). Expression of MtPiI4 in shoots (a–d) and roots (e–h) was analyzed using semi-quantitative RT-PCR (a, b, e, f) and qRT-PCR (c, d, g, h). Asterisks indicate that mean values are significantly different between the control and treatment (P < 0.05). MtEF1α was used for cDNA normalization
Ectopic Over-Expression of MtPiI4 in Arabidopsis Conferred Plant Resistance to Pst DC3000
To identify the role of MtPiI4 in regulating plant response to pathogen infection, we constructed transgenic Arabidopsis (Col-0) plants over-expressing MtPiI4 driven by the CaMV 35S promoter. The 35S::MtPiI4 transgenic lines were screened and identified from the T0 to the T3 generation. Two homozygous 35S::MtPiI4 transgenic lines were obtained. Transgenic plants carrying 35S::MtPiI4 have expression levels that are 105- to 351-fold MtPiI4 more than the wild-type (WT) (Fig. 5).
Analysis of MtPiI4 expression in 35S::MtPiI4 transgenic Arabidopsis lines. Transcripts of MtPiI4 in 3-week-old seedlings were analyzed using a semi-quantitative RT-PCR and b qRT-PCR. Asterisks indicate that mean values are significantly different between the wild type and 35S::MtPiI4 lines (P < 0.05). MtEF1α was used for cDNA normalization
The 35S::MtPiI4 plants inoculated with Pst DC3000 showed a phenotype of enhanced resistance to the disease (Fig. 6a) and reduced bacterial population relative to wild type (Fig. 6b). Callose formation in plants has been suggested as an important biomarker of defense priming in plants against pathogen attack (Luna et al. 2010). Aniline blue staining showed that more callose was deposited in the leaves of 35S::MtPiI4 plants with Pst DC3000 than in wild type leaves (Fig. 6c, d).
Effect of MtPiI4 over-expression on resistance of Arabidopsis leaves to Pst DC3000. Seven-day-old rosette leaves of plants were inoculated with Pst DC3000. a Leaf symptoms of WT (wild-type) and 35S::MtPiI4 Arabidopsis at 2 days post inoculation (dpi) with Pst DC3000. Symptoms of each treatment were examined on cotyledons (right) and the 1st euphylla (left). b In planta bacterial counting in the leaves of WT and 35S::MtPiI4 Arabidopsis at 0–4 days post inoculation (dpi). c, d Callose deposition in leaves of WT and 35S::MtPiI4 Arabidopsis at 2 dpi. White bar in c 1 mm
Over-Expression of MtPiI4 Alters Expression Patterns of JA Biosynthetic and Responsive Genes in Arabidopsis
LOX2 (LIPOXYGENASE2) is responsible for JA biosynthesis, while VSP1 (VEGETATIVE STORAGE PROTEIN1) and PDF1.2 (PLANT DEFENSIN1.2) were identified as typically JA-responsive genes (Avanci et al. 2010). Under normal conditions (without external MeJA provision), expression of LOX2 and PDF1.2 was very low and VSP1 was undetectable in WT and MtPiI4 transgenic plants (Fig. 7a–c). When exposed to 500 μM MeJA, wild-type plants showed a progressive increase in expression of LOX2, PDF1.2 and VSP1. However, 35S::MtPiI4 plants showed reduced transcripts of these genes compared to WT; significant depression of these genes was found 4 or 8 h after MeJA treatment.
Expression analysis of jasmonic acid (JA) biosynthetic and responsive genes in WT and 35S::MtPiI4 Arabidopsis treated with MeJA. Three-week-old seedlings were exposed to 500 μM MeJA for 0–8 h. Leaves were sampled at the indicated time. Total RNA was isolated from the samples and analyzed by qRT-PCR. The graphs indicate the fold-induction of the genes in MeJA-treated plants at the time point relative to the control (WT with 0 μM MeJA). Error bars Standard deviation of the mean of three treatments (n = 3). Asterisks indicate that mean values are significantly different between the 35S::MtPiI4 plants and WT (P < 0.05). MtEF1α was used for cDNA normalization
To identify further the impact of over-expression of MtPiI4 on JA signaling under pathogen infection, we analyzed the transcripts of LOX2, PDF1.2 and VSP1 in 35S::MtPiI4 plants inoculated with Pst DC3000. Compared to wild-type, expression of LOX2 was always lower in 35S::MtPiI4 plants before or after pathogen exposure (Fig. 8a). The lowest expression of LOX2 was determined at 2 hpi, and expression of LOX2 increased progressively thereafter.
qRT-PCR analysis of JA biosynthetic and responsive genes in 35S::MtPiI4 Arabidopsis plants exposed to Pst DC3000. Three-week-old seedlings of WT and 35S::MtPiI4 plants were inoculated with Pst DC3000 for 0–12 h. Leaves were sampled at different time points (0–12 hpi). Total RNA was isolated from the samples and analyzed by qRT-PCR. The graphs indicate the induction fold of the genes in Pst DC3000-exposed plants at the time point relative to the control (WT without Pst DC3000 exposure). Error bars Standard deviation of the mean three treatments (n = 3). Asterisks indicate that mean values are significantly different between the 35S::MtPiI4 plants and WT (P < 0.05). MtEF1α was used for cDNA normalization
Expression of PDF1.2 was higher in 35S::MtPiI4 plants than in wild-type before Pst DC3000 inoculation, but its expression pattern with Pst DC3000 was similar to that with MeJA treatment (Fig. 8b). Slightly higher expression of VSP1 was detected in 35S::MtPiI4 plants than in wild-type without Pst DC3000 exposure; however, expression of VSP1 at 2 hpi was drastically depressed compared to wild-type (Fig. 8c). During the following hpi, there was no difference in VSP1 expression between 35S::MtPiI4 and wild-type plants. We further examined transcripts of NPR1, a negative regulator and MAPK4, a positive regulator of JA signaling (Pieterse et al. 2009; Spoel et al. 2003). Our analysis showed that NPR1 expression was higher, whereas MAPK4 expression was lower in 35S::MtPiI4 plants compared to wild type (Fig. 8d, e). Taken together, these results indicate that manipulation of MtPiI4 was able to alter expression of genes involved in the JA synthetic and signaling pathway.
Discussion
Plant PIs serve as defensive molecules mainly against exogenous pathogens or insects (John 2011; Kim et al. 2009), but the regulatory role of PIs in plant immunity remains obscure. This study identified a new PI gene MtPiI4 from M. truncatula. Several lines of evidence indicate that MtPiI4 was able to resist Pst DC3000 infection by repressing JA signaling. First, the cDNA sequence of MtPiI4 showed similarity to the serine PI family genes that are well known for their anti-pathogen and anti-insect activities. Second, both Pst DC3000 inoculation and MeJA treatment induced expression of MtPiI4 in M. truncatula, which may be attributed to the existence of MeJA-responsive and defense-responsive elements in the promoter region of MtPiI4 (Online Resource 4). Third, ectopic over-expression of MtPiI4 in Arabidopsis improved plant resistance to Pst DC3000 and attenuated plant sensitivity to MeJA. Finally, over-expression of MtPiI4 in Arabidopsis resulted in repressed JA signaling through contrasting regulation of NPR1 and MAPK4 in plants exposed to Pst DC3000.
MtPiI4 contains the DUF716 domain in its predicted protein. This domain is a hallmark of a family with functionally uncharacterized membrane proteins restricted to eukaryotes (Okada et al. 2011). TMEM45A, which codes for a DUF716 protein in humans, is involved in anti-viral responses (Gerber et al. 2013; Justesen et al. 2000). Reduced expression of TMEM45A promoted a progression of ductal carcinoma to invasive breast cancer (Lee et al. 2012). Studies in mammals indicate that TMEM45A can stimulate host immunity, implicating it in defense against viral disease. The present study showed MtPiI4 involvement in regulation of plant immunity against bacterial invasion and possible association with suppression of the JA signaling pathway.
MtPiI4-mediated plant resistance to Pst DC3000 infection can be supported by the observation that over-expression of MtPiI4 in Arabidopsis attenuated disease symptoms, reduced bacterial populations, and increased callose deposition. Pst DC3000 can hijack the JA signaling pathway to weaken plant defenses to bacterial invasion (Katsir et al. 2008). When plants were treated with exogenous MeJA, wild-type Arabidopsis showed higher levels of LOX2, PDF1.2, and VSP1 transcripts, whereas expression of these genes were lower in 35S::MtPiI4 plants. Furthermore, transgenic Arabidopsis over-expressing MtPiI4 showed lower abundance of LOX2, PDF1.2, and VSP1 transcripts relative to wild-type under Pst DC3000 exposure. These results suggest that Pst DC3000 infection activated JA responsive genes, while MtPiI4 conferred plant resistance to Pst DC3000 infection via attenuation of JA-responsive genes.
In Arabidopsis, AtMC9 (Arabidopsis thaliana metacaspase 9) and RD21 (RESPONSIVE TO DESICCATION 21) were identified as targets of AtSerpin1 (Lampl et al. 2010; Vercammen et al. 2006). AtSerpin1 controls pathogen-induced programmed cell death (PCD) by directly connecting RD21 in vivo (Lampl et al. 2013). Interestingly, RD21 boosted immunity to the necrotrophic fungal pathogen Botrytis cinerea, but not to Pst DC3000 (Shindo et al. 2012). AtMC9, which is a type II metacaspase, acts as an important component for mediating the Pst DC3000-induced PCD process in plants (Bollhöner et al. 2013). Interaction between AtSerpin1 and AtMC9 occurs in apoplastic space, where priming of host innate immunity is initiated (Vercammen et al. 2006). AtSerpin1-mediated PCD was reported to link SA-dependent defense networks (Belenghi et al. 2007; Coll et al. 2011; Kim et al. 2013). Our results suggest that MtPiI4 appears to be a homologue of AtSerpin1 (Fig. 1d). Additionally, expression of NPR1 and MAPK4, two important regulators of SA-dependent systemic acquired resistance (SAR) and pathogen-induced PCD response (Taj et al. 2010; Yoshimoto et al. 2009), can be differentially regulated by MtPiI4 over-expression (Fig. 8). However, whether MtPiI4 functions in a similar way to AtSerpin1, or whether both MtPiI4 and AtSerpin1 coordinate to mediate their download genes and defense responses remains to be investigated. In conclusion, we have demonstrated here that expression of a new serpin gene MtPiI4 from M. truncatula enhanced plant resistance to Pst DC3000 infection by mediating some components in the JA and SA responsive signaling pathways. This gene could ultimately be applied to M. truncatula or other crop species to improve their pathogen resistance.
References
Alvarez-Alfageme F, Maharramov J, Carrillo L, Vandenabeele S, Vercammen D, Van Breusegem F, Smagghe G (2011) Potential use of a serpin from Arabidopsis for pest control. PLoS ONE 6:e20278
Avanci NC, Luche DD, Goldman GH, Goldman MHS (2010) Jasmonates are phytohormones with multiple functions, including plant defense and reproduction. Genet Mol Res 9:484–505
Belenghi B, Romero-Puertas MC, Vercammen D, Brackenier A, Inzé D, Delledonne M, Van Breusegem F (2007) Metacaspase activity of Arabidopsis thaliana is regulated by S-nitrosylation of a critical cysteine residue. J Biol Chem 282:1352–1358
Bollhöner B, Zhang B, Stael S, Denancé N, Overmyer K, Goffner D, Van Breusegem F, Tuominen H (2013) Post mortem function of AtMC9 in xylem vessel elements. New Phytol 200:498–510
Brooks DM, Bender CL, Kunkel BN (2005) The Pseudomonas syringae phytotoxin coronatine promotes virulence by overcoming salicylic acid-dependent defences in Arabidopsis thaliana. Mol Plant Pathol 6:629–639
Brown RL, Kazan K, McGrath KC, Maclean DJ, Manners JM (2003) A role for the GCC-box in jasmonate-mediated activation of the PDF1.2 gene of Arabidopsis. Plant Physiol 132:1020–1032
Clough SJ, Bent AF (1998) Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J 16:735–743
Coll NS, Epple P, Dangl JL (2011) Programmed cell death in the plant immune system. Cell Death Differ 18:1247–1256
Ding Y, Kalo P, Yendrek C, Sun J, Liang Y, Marsh JF, Harris JM, Oldroyd GED (2008) Abscisic acid coordinates nod factor and cytokinin signaling during the regulation of nodulation in Medicago truncatula. Plant Cell 20:2681–2695
Dunse KM, Stevens JA, Lay FT, Gaspar YM, Heath RL, Anderson MA (2010) Coexpression of potato type I and II proteinase inhibitors gives cotton plants protection against insect damage in the field. Proc Natl Acad Sci USA 107:15011–15015
Fluhr R, Lampl N, Roberts TH (2012) Serpin protease inhibitors in plant biology. Physiol Plant 145:95–102
Gerber PA, Hevezi P, Buhren BA, Martinez C, Schrumpf H, Gasis M, Grether-Beck S, Krutmann J, Homey B, Zlotnik A (2013) Systematic identification and characterization of novel human skin-associated genes encoding membrane and secreted proteins. PLoS ONE 8:e63949
Hartl M, Giri AP, Kaur H, Baldwin IT (2010) Serine protease inhibitors specifically defend Solanum nigrum against generalist herbivores but do not influence plant growth and development. Plant Cell 22:4158–4175
He P, Shan L, Lin NC, Martin GB, Kemmerling B, Nurnberger T, Sheen J (2006) Specific bacterial suppressors of MAMP signaling upstream of MAPKKK in Arabidopsis innate immunity. Cell 125:563–575
Hu LB, Shi ZQ, Zhang T, Yang ZM (2007) Fengycin antibiotics isolated from B-FS01 culture inhibit the growth of Fusarium moniliforme Sheldon ATCC 38932. FEMS Microbiol Lett 272:91–98
Hu LB, Zhang T, Yang ZM, Zhou W, Shi ZQ (2009) Inhibition of fengycins on the production of fumonisin B1 from Fusarium verticillioides. Lett Appl Microbiol 48:84–89
John AG (2011) Prospects for using proteinase inhibitors to protect transgenic plants against attack by herbivorous insects. Curr Protein Pept Sci 12:409–416
Jongsma MA, Beekwilder J (2011) Co-evolution of insect proteases and plant protease inhibitors. Curr Protein Pept Sci 12:437–447
Justesen J, Hartmann R, Kjeldgaard NO (2000) Gene structure and function of the 2'-5'-oligoadenylate synthetase family. Cell Mol Life Sci 57:1593–1612
Katsir L, Schilmiller AL, Staswick PE, He SY, Howe GA (2008) COI1 is a critical component of a receptor for jasmonate and the bacterial virulence factor coronatine. Proc Natl Acad Sci USA 105:7100–7105
Keith RC, Keith LM, Hernandez-Guzman G, Uppalapati SR, Bender CL (2003) Alginate gene expression by Pseudomonas syringae pv. tomato DC3000 in host and non-host plants. Microbiology 149:1127–1138
Kim JY, Park SC, Hwang I, Cheong H, Nah JW, Hahm KS, Park Y (2009) Protease inhibitors from plants with antimicrobial activity. Int J Mol Sci 10:2860–2872
Kim MG, da Cunha L, McFall AJ, Belkhadir Y, DebRoy S, Dangl JL, Mackey D (2005) Two Pseudomonas syringae type III effectors inhibit RIN4-regulated basal defense in Arabidopsis. Cell 121:749–759
Kim S-M, Bae C, Oh S-K, Choi D (2013) A pepper (Capsicum annuum L.) metacaspase 9 (Camc9) plays a role in pathogen-induced cell death in plants. Mol Plant Pathol 14:557–566
Koiwa H, Bressan RA, Hasegawa PM (1997) Regulation of protease inhibitors and plant defense. Trends Plant Sci 2:379–384
Lampl N, Alkan N, Davydov O, Fluhr R (2013) Set-point control of RD21 protease activity by AtSerpin1 controls cell death in Arabidopsis. Plant J 74:498–510
Lampl N, Budai-Hadrian O, Davydov O, Joss TV, Harrop SJ, Curmi PM, Roberts TH, Fluhr R (2010) Arabidopsis AtSerpin1, crystal structure and in vivo interaction with its target protease RESPONSIVE TO DESICCATION-21 (RD21). J Biol Chem 285:13550–13560
Laurie-Berry N, Joardar V, Street IH, Kunkel BN (2006) The Arabidopsis thaliana JASMONATE INSENSITIVE 1 gene is required for suppression of salicylic acid-dependent defenses during infection by Pseudomonas syringae. Mol Plant Microbe Interact 19:789–800
Law RH, Zhang Q, McGowan S, Buckle AM, Silverman GA, Wong W, Rosado CJ, Langendorf CG, Pike RN, Bird PI, Whisstock JC (2006) An overview of the serpin superfamily. Genome Biol 7:216
Lee S, Stewart S, Nagtegaal I, Luo J, Wu Y, Colditz G, Medina D, Allred DC (2012) Differentially expressed genes regulating the progression of ductal carcinoma in situ to invasive breast cancer. Cancer Res 72:4574–4586
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using Real-Time Quantitative PCR and the 2−ΔΔCT method. Methods 25:402–408
Lomate PR, Hivrale VK (2012) Wound and methyl jasmonate induced pigeon pea defensive proteinase inhibitor has potency to inhibit insect digestive proteinases. Plant Physiol Biochem 57:193–199
Luna E, Pastor V, Robert J, Flors V, Mauch-Mani B, Ton J (2010) Callose Deposition: A Multifaceted Plant Defense Response. Mol Plant-Microbe Interact 24:183–193
McDowell JM (2011) Plant science-Beleaguered immunity. Science 334:1354–1355
Mosolov VV, Valueva TA (2008) Proteinase inhibitors in plant biotechnology: a review. Appl Biochem Microbiol 44:233–240
Okada N, Yamamoto T, Watanabe M, Yoshimura Y, Obana E, Yamazaki N, Kawazoe K, Shinohara Y, Minakuchi K (2011) Identification of TMEM45B as a protein clearly showing thermal aggregation in SDS–PAGE gels and dissection of its amino acid sequence responsible for this aggregation. Protein Expr Purif 77:118–123
Pearce G, Ryan C, Liljegren D (1988) Proteinase inhibitors I and II in fruit of wild tomato species: transient components of a mechanism for defense and seed dispersal. Planta 175:527–531
Petersen M, Brodersen P, Naested H, Andreasson E, Lindhart U, Johansen B, Nielsen HB, Lacy M, Austin MJ, Parker JE, Sharma SB, Klessig DF, Martienssen R, Mattsson O, Jensen AB, Mundy J (2000) Arabidopsis MAP kinase 4 negatively regulates systemic acquired resistance. Cell 103:1111–1120
Pieterse CM, Leon-Reyes A, Van der Ent S, Van Wees SC (2009) Networking by small-molecule hormones in plant immunity. Nat Chem Biol 5:308–316
Roberts T, Hejgaard J (2008) Serpins in plants and green algae. Funct Integr Genomics 8:1–27
Ryan CA (1989) Proteinase inhibitor gene families: Strategies for transformation to improve plant defenses against herbivores. BioEssays 10:20–24
Schlüter U, Benchabane M, Munger A, Kiggundu A, Vorster J, Goulet M-C, Cloutier C, Michaud D (2010) Recombinant protease inhibitors for herbivore pest control: a multitrophic perspective. J Exp Bot 61:4169–4183
Shindo T, Misas-Villamil JC, Hörger AC, Song J, van der Hoorn RAL (2012) A role in immunity for Arabidopsis cysteine protease RD21, the ortholog of the tomato immune protease C14. PLoS ONE 7:e29317
Song JB, Huang SQ, Dalmay T, Yang ZM (2012) Regulation of leaf morphology by microRNA394 and its target LEAF CURLING RESPONSIVENESS. Plant Cell Physiol 53:1283–1294
Spoel SH, Dong X (2012) How do plants achieve immunity? Defence without specialized immune cells. Nat Rev Immunol 12:89–100
Spoel SH, Koornneef A, Claessens SMC, Korzelius JP, Van Pelt JA, Mueller MJ, Buchala AJ, Métraux J-P, Brown R, Kazan K, Van Loon LC, Dong X, Pieterse CMJ (2003) NPR1 modulates cross-talk between salicylate- and jasmonate-dependent defense pathways through a novel function in the cytosol. Plant Cell 15:760–770
Taj G, Agarwal P, Grant M, Kumar A (2010) MAPK machinery in plants: recognition and response to different stresses through multiple signal transduction pathways. Plant Signal Behav 5:1370–1378
van der Linde K, Hemetsberger C, Kastner C, Kaschani F, van der Hoorn RA, Kumlehn J, Doehlemann G (2012) A maize cystatin suppresses host immunity by inhibiting apoplastic cysteine proteases. Plant Cell 24:1285–1300
Vercammen D, Belenghi B, van de Cotte B, Beunens T, Gavigan J-A, De Rycke R, Brackenier A, Inzé D, Harris JL, Van Breusegem F (2006) Serpin1 of Arabidopsis thaliana is a suicide inhibitor for metacaspase 9. J Mol Biol 364:625–636
Vlot AC, Dempsey DMA, Klessig DF (2009) Salicylic acid, a multifaceted hormone to combat disease. Annu Rev Phytopathol 47:177–206
Yoshimoto K, Jikumaru Y, Kamiya Y, Kusano M, Consonni C, Panstruga R, Ohsumi Y, Shirasu K (2009) Autophagy negatively regulates cell death by controlling NPR1-dependent salicylic acid signaling during senescence and the innate immune response in Arabidopsis. Plant Cell 21:2914–2927
Zhang X, Liu S, Takano T (2008) Two cysteine proteinase inhibitors from Arabidopsis thaliana, AtCYSa and AtCYSb, increasing the salt, drought, oxidation and cold tolerance. Plant Mol Biol 68:131–143
Zheng XY, Spivey NW, Zeng W, Liu PP, Fu ZQ, Klessig DF, He SY, Dong X (2012) Coronatine promotes Pseudomonas syringae virulence in plants by activating a signaling cascade that inhibits salicylic acid accumulation. Cell Host Microbe 11:587–596
Zhou ZS, Huang SQ, Yang ZM (2008) Bioinformatic identification and expression analysis of new microRNAs from Medicago truncatula. Biochem Biophys Res Commun 374:538–542
Zhou ZS, Yang SN, Li H, Zhu CC, Liu ZP, Yang ZM (2013) Molecular dissection of mercury-responsive transcriptome and sense/antisense genes in Medicago truncatula. J Hazard Mater 252–253:123–131
Zipfel C, Robatzek S, Navarro L, Oakeley EJ, Jones JDG, Felix G, Boller T (2004) Bacterial disease resistance in Arabidopsis through flagellin perception. Nature 428:764–767
Acknowledgments
We appreciate Dr. De Yue Yu at College of Agriculture in Nanjing Agricultural University for providing M. Truncatula seeds. We thank Dr. Yun Peng Wang at Plant Protection College in Nanjing Agricultural University for providing Pst DC3000 strain.
Funding
This research was supported by the National Natural Science Foundation of China (31071343; 31200204), the China Postdoctoral Science Foundation (201003593) and the Priority Academic Program Development of Jiangsu Higher Education Institutions (200910).
Conflict of Interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
Supplementary data (Online Resource) are online available. (DOCX 128 kb)
Rights and permissions
About this article
Cite this article
Sun, D., Chen, J., Zhou, Z.S. et al. Ectopic Expression of a Proteinase Inhibitor I4 (MtPiI4) Gene from Medicago truncatula Confers Plant Resistance to Pseudomonas syringae pv. Tomato DC3000. Plant Mol Biol Rep 33, 1686–1696 (2015). https://doi.org/10.1007/s11105-015-0865-y
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11105-015-0865-y