Abstract
Appropriate leaf shape has proved to be useful in improving photosynthesis and increasing grain yield. To understand the molecular mechanism of leaf morphogenesis, we identified a rice mutant nrl1, which was characterized by a phenotype of narrow and rolled leaves. Microscopic observation showed that the mutation significantly decreased the number of vascular bundles of leaf and stem. Genetic analysis revealed that the mutation was controlled by a single nuclear-encoded recessive gene. To isolate the nrl1 gene, 756 F2 and F3 mutant individuals from a cross of the nrl1 mutant with Longtepu were used and a high-resolution physical map of the chromosomal region around the nrl1 gene was made. Finally, the gene was mapped in 16.5 kb region between marker RL21 and marker RL36 within the BAC clone OSJNBa0027H05. Cloning and sequencing of the target region from the mutant showed that there was a 58 bp deletion within the second exon of the cellulose synthase-like D4 gene (TIGR locus Os12g36890). The nrl1 mutation was rescued by transformation with the wild-type cellulose synthase-like D4 gene. Accordingly, the cellulose synthase-like D4 gene was identified as the NRL1 gene. NRL1 was transcribed in various tissues and was mainly expressed in panicles and internodes. NAL7 and SLL1 were found to be upregulated, whereas OsAGO7 were downregulated in the nrl1 mutant. These findings suggested that there might be a functional association between these genes in regulating leaf development.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The leaf is the main photosynthetic organ in plants and its shape is closely associated with its photosynthetic function. Appropriate leaf shape improves photosynthetic efficiency, elevates plant biomass, and increases grain yield. Therefore, it is important to understand the molecular mechanism that controls leaf morphogenesis (Tsukaya 2006; Micol 2009).
Leaf development includes initiation of primordium, establishment of polarity, expansion and maturation. Molecular genetic studies indicate that MYB-domain ARP proteins, including PHANTASTICA (PHAN) and its orthologs ROUGH SHEATH2 (RS2) and ASYMMETRIC LEAVES1 (AS1), and meristem-promoting class-1 KNOX homeobox proteins are involved in leaf initiation. KNOX genes are expressed only in the shoot apical meristem, while ARP (AS1/RS2/PHAN) proteins are restricted to leaves. ARP and KNOX genes mutually repress their expression (Long et al. 1996; Waites et al. 1998; Tsiantis et al. 1999; Byrne et al. 2000).
The establishment of leaf polarity is central to leaf morphogenesis (Huang et al. 2006). HD-ZIP III transcription factors, including PHABULOSA (PHB), PHAVOLUTA (PHV), and REVOLUTA (REV), promote leaf adaxial identity (McConnell et al. 2001; Otsuga et al. 2001). The transcripts of the HD-ZIP III genes are regulated by microRNAs miR165 and miR166 (Zhou et al. 2007). ASYMMETRIC LEAVES ENHANCER3 (AE3), which encodes a putative 26S proteasome subunit RPN8a, and the AS1–AS2 complex are also required for specifying leaf adaxial identity (Xu et al. 2003; Huang et al. 2006).Conversely, the YABBY family proteins YAB2 and YAB3 and the KANADI family proteins play critical roles in establishing abaxial identity (Eshed et al. 2001; Kerstetter et al. 2001). In addition, AUXIN RESPONSE FACTORS 3 and 4 (ARF3 and ARF4) are involved in specifying leaf abaxial identity by regulating the activity of KANADI proteins (Pekker et al. 2005).
Leaf expansion is mainly controlled along the longitudinal and lateral axes. Leaf cell proliferation and elongation in the longitudinal and lateral directions determine the length and width of leaf blades accordingly. ROTUNDIFOLIA3 (ROT3) and ROTUNDIFOLIA4 (ROT4), respectively, regulate polarized growth and number of leaf cells in the longitudinal direction (Kim et al. 1998; Narita et al. 2004). The overexpression of ROT3, which encodes a plant-type cytochrome P450, causes specific cell elongation in leaf longitudinal axis. The ROT4 gene encodes a novel small peptide and its dominant mutant rot4-1D has shorter leaves due to the reduced cell proliferation in the longitudinal axis. On the other hand, ANGUSTIFOLIA (AN), a plant CtBP family gene, controls leaf width by regulating cell elongation in the leaf lateral axis. The mutation of the AN gene results in narrow leaves with normal leaf length (Kim et al. 2002). ANGUSTIFOLIA3 (AN3), a homolog of the human transcription coactivator SYT, influences leaf width by regulating cell proliferation in the leaf-width direction. Loss of function of AN3 results in narrow leaf phenotypes due to a decreased number of cells in the lateral axis (Horiguchi et al. 2005).
Although a large number of genes involved in leaf development have been identified and considerable progress has been made in understanding the regulatory mechanism of leaf morphogenesis in Arabidopsis, much less is known about the molecular mechanism that control leaf shape in rice. Recently, several genes for leaf development were identified in rice. ROLLED LEAF9 (RL9)/SHALLOT-LIKE1 (SLL1) encodes a KANADI family protein and is involved in leaf abaxial cell development (Yan et al. 2008; Zhang et al. 2009). CONSTITUTIVELY WILTED1 (COW1)/NARROW LEAF7 (NAL7) encodes a member of the YUCCA protein family. The nal7 mutant shows narrow and rolled leaves (Woo et al. 2007; Fujino et al. 2008). NARROW LEAF1 (NAL1) is a plant-specific protein with unknown biochemical function. Mutation in NAL1 results in reduced leaf width with a decreased number of longitudinal veins (Qi et al. 2008). ADAXIALIZED LEAF1 (ADL1) encodes a calpain-like cysteine protease that is associated with the maintenance of axis information in leaf development (Hibara et al. 2009). Rice OsAGO7 gene encodes an Argonaute (AGO) family protein. The overexpression of OsAGO7 leads to upward curling of rice leaves (Shi et al. 2007). In the present study, a novel narrow and rolled leaves mutant nrl1 (narrow and rolled leaves 1) was isolated and characterized. The genetic and molecular analyses indicated that loss of function of a cellulose synthase-like D4 protein gene was responsible for the mutant phenotype.
Materials and methods
Plant materials
The nrl1 mutant was isolated from a T1 transgenic rice line in the japonica rice cultivar Zhonghua11 (Zhu et al. 2001). The nrl1 mutant was crossed with Zhonghua11 to produce the F2 generation which was used for genetic analysis. nrl1 individuals from the F2 and F3 generations of a cross between the nrl1 mutant and Longtepu (Oryza sativa L. subsp. indica) were used for high-resolution mapping of DNA markers linked to the nrl1 region.
All rice plants used for genetic analysis and fine mapping were grown under natural conditions in the paddy field at China National Rice Research Institute (119°57′E, 30°03′N). The rice materials mentioned above were from State Key Laboratory of Rice Biology, China National Rice Research Institute. Student’s t tests were used for statistical significance in this study.
Hygromycin resistance assay
The nrl1 mutant was isolated from transgenic rice lines with T-DNA insertions carrying the hygromycin phosphotransferase gene. To determine whether the phenotype resulted from the T-DNA insertion, a hygromycin resistance assay was used for co-segregation analysis. The F2 generation from a cross between the nrl1 mutant and its parent cultivar Zhonghua11 was used in the assay. A segment of about 4 cm was excised from a healthy leaf blade of each plant and placed in petri dishes containing a solution with 50 mg/L hygromycin and 0.5 mg/L 6-BA. The cut ends of the segments were embedded to the solution for good contact. Plant materials were incubated at about 26°C for 5 days under 12 h light/12 h dark regime (Wang and Waterhouse 1997).
Microscopic analysis
Fresh hand-cut sections of rice leaf blade and stem were stained with Safranin O. The specimens were transferred to glass slides with 1–2 drops of water, examined and photographed under the microscope. The third leaf of rice plants was collected and fixed. The tissue samples were dehydrated in a graded ethanol series to 100%, embedded in paraffin, cut into sections with a microtome and adhered to glass slides. The sections were de-paraffinized using xylene, rehydrated using a graded ethanol series, and finally dried overnight before staining with Fast Green FCF.
Map-based cloning
A total of 118 InDel markers were selected from Temnykh et al. (2001), McCouch et al. (2002), and our laboratory collection for finding out the molecular markers linked to the nrl1 gene. To fine map the nrl1 gene, more InDel markers were developed based on the sequence differences between Nipponbare and 9311(Oryza sativa L. subsp. indica) according to our previous procedure (Liu et al. 2007). The primer sequences of these InDel markers used were listed in Supplemental Table S1.
PCR reactions for mapping were set-up as follows: denaturation at 94°C for 4 min, followed by 30 cycles of 94°C for 30 s, annealing for 30 s (annealing temperature determined by primer pair sequences), 72°C for 1 min, and with a final extension step at 72°C for 10 min. PCR products were separated on 6 or 8% polyacrylamide gels. After electrophoresis, the amplified DNA bands were detected with the silver staining method (Panaud et al. 1996).
Thirteen primer pairs were designed and synthesized within the 16.5 kb target region. Specific fragments from the nrl1 mutant and wild-type cultivar Zhonghua11 were amplified by PCR. After being purified by electrophoresis on an agarose gel, these fragments were cloned into the pMD18-T vector and then sequenced by Shanghai Sangon Biological Engineering Technology and Service Co. Ltd (Sangon, Shanghai, China).
Construction of complementary vector and rice transformation
A 6.8 kb genomic DNA fragment containing the entire NRL1 gene was excised from the BAC clone OSJNBa0027H05 (Fig. 3a) and cloned into the pCAMBIA1300 vector. The construct was transformed into the nrl1 mutants by Agrobacterium tumefaciens-mediated genetic transformation method (Zhu et al. 2001).
Phylogenetic analysis
The homologous proteins of NRL1 were searched with BLASTP using the NRL1 protein as query. Full-length amino acid sequences were aligned using the CLUSTAL program (Thompson et al. 1997). The neighbor-joining phylogenetic tree was constructed using MEGA version 4 (Tamura et al. 2007). The values for nodes in the phylogenetic tree were obtained from 1,000 bootstrap replications.
Quantitative real-time PCR analysis
Total RNA was extracted from various tissues using Qiagen RNeasy Plant Mini Kit (Qiagen, Hilden, Germany) and genomic DNA was removed by treatment with RNase-free DNase Set (Qiagen). First-strand cDNAs were synthesized using Transcriptor High Fidelity cDNA Synthesis Kit (Roche, Indianapolis, IN, USA) from 2 µg of total RNA primed with an oligo-dT primer. The cDNAs were assayed by real-time PCR using the 2× SYBR Green PCR Master Mix (Applied Biosystems, Warrington, UK) on the Applied Biosystems 7900HT Real-Time PCR System. The relative expression levels of each transcript were obtained by normalization to the OsACT1 gene using comparative C T method (Livak and Schmittgen 2001). PCR was carried out as follows: preheating at 95°C for 10 min, followed by 40 cycles of denaturation at 95°C for 15 s and annealing/extension at 60°C for 1 min. The primer sequences were listed in Supplemental Table S2.
Results
The nrl1 mutant showed a phenotype of narrow and rolled leaves
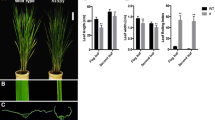
The narrow rolled leaves (nrl1) mutant was first found among T1 transgenic rice lines in the Zhonghua11 background (Zhu et al. 2001). The nrl1 mutant could be clearly distinguished from wild type 7 days after planting (Fig. 1a, b) and the mutant phenotype became even more obvious as rice grows (Fig. 1d). Wild-type leaves had a broad and flat blade (Fig. 1c, h), while the nrl1 leaves had significantly reduced blade width (Fig. 1h; Table 1) and were slightly curled inward along the longitudinal axis of leaves (Fig. 1d). In mature leaves, the length and width of two uppermost blades were measured (Table 1). The blade length and width of flag leaf of the nrl1 mutant were 69 and 64% those of wild type, respectively. However, there was no significant difference in the leaf index (the ratio of the leaf length to the leaf width) of flag leaf between nrl1 and wild type (Table 1). The blade length of the second leaf from top of the nrl1 mutant was similar to that of wild type, while the blade width was noticeably reduced in the nrl1 mutant and was 60% that of wild type. As a result, the leaf index of the second leaf from top was changed from 41.4 ± 4.1 in wild type to 66.7 ± 9.9 in the nrl1 mutant (Table 1).
The nrl1 mutant shows a phenotype of narrow and rolled leaves. Morphology of wild type (a) and the nrl1 mutant (b) at the seedling stage. Morphology of wild type (c) and the nrl1 mutant (d) at the milky stage. Transverse section of the middle part of the third leaf in wild type (e) and the nrl1 mutant (f) at the seedling stage. g Transverse section of the middle part of the second leaf from top in wild type (top) and the nrl1 mutant (bottom) at the mature stage. h Blade morphology of wild type (left) and the nrl1 mutant (right). Bars 0.1 mm (e–f), 0.5 mm (g), 1 mm (h)
To determine whether the reduced width of leaf blade in the nrl1 mutant was attributable to a reduction in cell number or cell size, we examined the transverse section of the mid point of the third leaf at 25 days after planting. There was no difference in cell shape and size between the nrl1 mutant and wild type (Fig. 1e, f). However, the number of vascular bundles was reduced in the nrl1 blades. Figure 1e showed that there are 12 vascular bundles on one side of the wild-type mid-vein, while only 10 vascular bundles were found on the same side of the nrl1 mid-vein (Fig. 1f). The finding prompted us to observe the leaf vascular system at the mature stage. We further found that the total numbers of both large vascular bundles and small vascular bundles were reduced in the nrl1 blade at the mature stage (Fig. 1g; Table 1). The results showed that the narrow leaf blade of the mutant mainly resulted from the defective formation of blade vascular bundles.
Pleiotropic phenotypes of the nrl1 mutant
Aside from narrow and rolled leaves, other defects in the nrl1 mutant were also observed. When compared with the wild type, the nrl1 mutant revealed a significant reduction in plant stature. In mature plants, the height of the mutant reached approximately 70% of the wild-type height (Table 1). We compared the length of the uppermost three internodes of the nrl1 mutant with that of wild type. As shown in Fig. 2a, the nrl1 gene inhibits the length of all the three internodes. The mutant also exhibited a higher tiller number (Table 1) and smaller culm diameter. Microscopic observation showed that there were fewer vascular bundles in the middle of the third internode from top in the nrl1 mutant (Fig. 2b, c). However, no obvious difference was found in parenchyma cell size between nrl1 and wild type (Fig. 2b, c).
Pleiotropic phenotypes of the nrl1 mutant. a Transverse section of the middle part of the third internode from top in wild type (left) and the nrl1 mutant (right). The sections in the blue boxes are highlighted in the bottom panel. b The appearance of the internodes of wild type and nrl1 I the first internode from top, II second internode, III third internode. c The nrl1 mutant (right) showing an increased leaf angle. d Roots of wild type (left) and the nrl1 mutant (right) after 25 days after planting. e Panicles of wild type (top) and the nrl1 mutant (bottom). f Seeds of wild type (left) and the nrl1 mutant (right). Bars 0.5 mm (a), 2 cm (b, e), 1 mm (c, f), 1 cm (d)
In addition, the angle between the blade and the stem to which it is attached is larger in the nrl1 mutant (Fig. 2d; Table 1). The nrl1 mutation affected root growth (Fig. 2e). The nrl1 mutation also has a negative effect on panicle size, fertility rate and seed width, but the seed length of the nrl1 mutant increased (Fig. 2f, g; Table 1). In comparison with wild type, the mature seeds of nrl1 also had a greater tendency to rupture (Fig. 2g).
Co-segregation analysis of the mutation and T-DNA insertion
The F1 progenies from a cross between the nrl1 mutant and its parent cultivar Zhonghua11 exhibited normal phenotype. The segregation of wild type and mutant individuals in the F2 generation showed a good fit to a 3:1 ratio (χ2 = 0.38 < χ 20.05 = 3.84), which suggested that the nrl1 phenotype was controlled by a single recessive gene. The nrl1 mutant was isolated from one of the transgenic lines with T-DNA insertion containing a hygromycin-resistant gene. To determine whether the nrl1 mutation resulted from the T-DNA insertion, 20 mutant individuals in F2 generation from a cross between nrl1 and its parent cultivar were used for co-segregation analysis based on the leaf response to hygromycin (Wang and Waterhouse 1997). Leaf sections of 6 individuals among 20 nrl1 mutant individuals became necrotic and the others remained green. Therefore, the mutation phenotype did not co-segregate with the T-DNA insertion. The results suggested that the mutation was not caused by T-DNA insertion.
Map-based cloning of the nrl1 gene
The chromosomal location of nrl1 was determined by observing the genotypes of 20 nrl1 individuals from the F2 population of a cross between the nrl1 mutant and indica cultivar Longtepu. The results showed that marker RM3331 (at 89.5 cM) was closely linked to the nrl1 gene. To fine map the nrl1 gene, we developed nine new InDel markers (Table 1). The nrl1 gene was further localized to an interval of 16.5 kb bracketed by RL21 and RL36 within the BAC clone OSJNBa0027H05 using the nine markers and 756 F2 and F3 mutant individuals (Fig. 3a). Three genes, including a hypothetical gene, a cellulose synthase-like D4 (CSLD4) gene, and an unknown gene encoding a protein similar to pathogenesis-related protein PR-10a, were predicted within the 16.5 kb interval by the rice genome automated annotation system (http://ricegaas.dna.affrc.go.jp/rgadb/). Sequence analysis of the candidate region revealed only a deletion difference between wild type and the nrl1 mutant. The nrl1 allele carried a 58 bp deletion within the coding sequence of the second exon of the CSLD4 gene (Fig. 3a). This deletion results in a frameshift, which changes the amino acid sequence from the site of the mutation (Fig. 3b). To verify this, the primer pair MD1 (Supplemental Table S1) flanking the mutation site was used to amplify the relevant fragments of wild type and nrl1. A shorter PCR product was amplified in the nrl1 mutant, confirming that nrl1 carried the mutation site (Fig. 4b). Real-time PCR analysis using gene-specific primers showed that there was a significant reduction in the transcript level of CSLD4 in the nrl1 mutant (Fig. 4c). The results suggested that the CSLD4 gene (TIGR locus Os12g36890) should be the NRL1 gene.
Fine mapping and mutation site analysis of the nrl1 gene. a Fine mapping of the NRL1 gene. The target gene was delimited to an interval of 16.5 kb between RL21 and RL36 within the BAC clone OSJNBa0027H05 on chromosome 12. The black rectangle indicates the NRL1 gene (TIGR locus Os12g36890), which encodes a cellulose synthase-like D4 protein. White and black boxes are exons; white boxes are untranslated region; black boxes are the coding region; the line between two black boxes is the only intron. Red box indicates the location of the 58-bp deletion in the nrl1 mutant. The fragment between S and B was used for the construction of complementary vector. b NRL1 encodes a cellulose synthase-like D4 protein. Conserved amino acids are shaded gray. The green underlining represents the active sites. The red arrowhead shows the start point of the mutations. The red markings indicate the mutated amino acid sequences of nrl1. Asterisks (*) indicate the translation termination sites
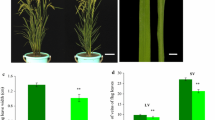
Transgenic complementation experiment rescued the nrl1 phenotype. a Second blade morphology from top of the nrl1 mutant (left), and transgenic nrl1 plant with the NRL1 gene (right). b PCR detection of transgenic nrl1 plants using the primer pair MD1 flanking the mutation site. WT wild type, C1, C2 transgenic nrl1 plants with the NRL1 gene. c Real-time PCR analysis of NRL1 transcript levels of transgenic nrl1 plant. Transverse section of the middle part of the second blade from top in wild type (d), the nrl1 mutant (e) and transgenic nrl1 plant (f). Bars 2 mm (a), 0.5 mm (d–f)
The NRL1 gene showed hits to the full-length cDNA clone J090014G03 (4,123 bp). Alignment between the cDNA and the genomic sequence of the NRL1 gene indicated that NRL1 consists of two exons and one intron. The NRL1 cDNA sequence contains a 3,648 bp open reading frame that encodes a CSLD4 protein of 1,215 amino acid residues. The subcellular location of the NRL1 protein was predicted by Predotar and PSORT (Nakai and Horton 1999; Small et al. 2004) and the results suggested that the NRL1 protein could be imported into endoplasmic reticulum.
Transgenic complementation rescued the nrl1 phenotype
To further confirm that the nrl1 phenotype was caused by the mutation of CSLD4, a construct was generated by insertion of the 6.8 kb wild-type genomic fragment containing the entire CSLD4 gene into pCambia1300 (Fig. 3a). The construct was introduced into the nrl1 mutant using Agrobacterium tumefaciens-mediated genetic transformation (Zhu et al. 2001). Transgenic plants were identified by PCR analysis using the primer pair MD1 (Supplemental Table S1; Fig. 4b). Quantitative real-time PCR analyses of transgenic plants indicated that NRL1 transcript level was similar to that of wild type, which implied that the NRL1 gene was expressed in the transgenic plants (Fig. 4c). Phenotypic observation showed that the leaf width of the transgenic plants (8.7 ± 0.5 cm) was significantly wider than that of the nrl1 mutants (5.7 ± 0.5 cm) (Fig. 4a). Examination of cross sections of the second blade from the top revealed that the transgenic nrl1 plants with the NRL1 gene restored the normal number of vascular bundles (Fig. 4f).
Phylogenetic analysis of NRL1 and its homologous proteins
BLASTP was used to search for homologous proteins using the NRL1 protein as query. We found that cellulose synthase-like D (CSLD) proteins were widely distributed in various land plants, including moss (Physcomitrella patens), which suggested a likely early origin of the CSLD family. Five CSLD proteins from rice, six from Arabidopsis and eight from moss (Physcomitrella patens) were identified and used for phylogenetic analysis along with the closest homologous proteins from sorghum (Sorghum bicolor), maize (Zea mays), grape (Vitis vinifera), black cottonwood (Populus trichocarpa) and castor (Ricinus communis). Phylogenetic analysis of NRL1 and its homologous proteins was conducted using MEGA version 4 software (Tamura et al. 2007) (Fig. 5). All moss CSLD proteins form a monophyletic group and are closely related to rice CSLD4 proteins. Only the AtCSLD5 protein among the Arabidopsis CSLD members was directly orthologous to NRL1 protein (Fig. 5). Loss of function of AtCSLD5 resulted in significantly reduced plant stature and xylan in stems, but did not affect leaf shape (Bernal et al. 2007).
Expression pattern of NRL1
Roots, inflorescences, leaf blades, leaf sheaths, internodes, nodes and root collars (the junction between stem and root) were excised from 75-day-old wild-type plants. Quantitative real-time RT-PCR analysis revealed the expression of NRL1 in various tissues with relatively high expression in panicles and internodes (Fig. 6).
NRL1 regulates the expression of leaf development-related genes
Previous studies showed that multiple genes, including OsAGO7, NAL1, NAL7/COW1, and SLL1/RL9, were involved in controlling leaf width and/or leaf rolling in rice. We analyzed OsAGO7, NAL1, NAL7 and SLL1 transcript levels in wild type and the nrl1 mutant at the milky stage by quantitative real-time PCR (Fig. 7). Loss of function of NRL1 resulted in significantly reduced expression of OsAGO7. Conversely, transcript levels of SLL1 and NAL7 were significantly increased in the nrl1 mutant. However, there was no obvious alteration in the expression of NAL1. The findings suggested that there might be a functional association between NRL1, OsAGO7, NAL7 and SLL1 in regulating leaf development.
The expression analysis of leaf shape-related genes in the nrl1 mutant and wild type. Total RNA was isolated from shoot at milky maturity stage. The transcript levels of OsAGO7, NAL1, NAL7 and SLL1 were normalized to the levels of OsACT1 gene expression. The transcript levels of all tested genes in wild type were arbitrarily set to 1. Values are the mean ± SD of three replicates
Discussion
In this study, we isolated a narrow and rolled leaves mutant nrl1 from one of T1 transgenic rice lines. Aside from altered leaf shape, the nrl1 mutant exhibited a significant reduction in plant height, stem diameter, inflorescence size, fertility rate and seed width. Map-based cloning and complementation experiments showed that the NRL1 gene encoded a cellulose synthase-like D4 protein, which belongs to the cellulose synthase superfamily. In rice, the cellulose synthase superfamily includes six cellulose synthase-like (CSL) families and one cellulose synthase (CesA) family (Yin et al. 2009). The CSL gene families play crucial roles in the formation of plant cell walls. The CSLA genes have been found to encode mannan synthases (Dhugga et al. 2004; Liepman et al. 2005) and the CSLC genes are involved in β-1,4 linked glucan biosynthesis, which is the backbone of xyloglucan (Cocuron et al. 2007). However, no CSLD genes have been biochemically characterized. In vitro GT (glycosyl transferase) assays and antibody and carbohydrate-binding module labeling showed that disruption of AtCSLD5 resulted in reduced xylan synthase activity and xylan occurrence in Arabidopsis (Bernal et al. 2007). Therefore, the AtCSLD5 gene could be involved in xylan synthase.
The CSLD gene subfamily is present in a wide range of plant species including the lower plant moss (Fig. 5). It suggests that the CSLD genes are conserved in terrestrial plants and might play important roles in plant growth. There are six and five CSLD genes in Arabidopsis and rice, respectively (Fig. 5). AtCSLD2 and AtCSLD3 are involved in root hair growth (Favery et al. 2001; Wang et al. 2001; Bernal et al. 2008). AtCSLD1 and AtCSLD4 are required for pollen tube development (Bernal et al. 2008). The rice CSLD1 gene, one of two direct orthologs of Arabidopsis AtCSLD2 and AtCSLD3 (Fig. 5), is also required for root hair morphogenesis (Kim et al. 2007). These findings suggest that there is a conserved function for CSLD genes in regulating root hair growth in dicots and monocots.
Arabidopsis AtCSLD5 is the directly orthologous protein of rice NRL1 in the phylogenetic tree (Fig. 5). Loss of function of AtCSLD5 results in reduced plant height, root hair elongation, rosette diameter and leaf size, but has no or little effect on leaf shape (Bernal et al. 2007; 2008). However, in rice, knockout ofNRL1 results in not only a significant reduction in plant height (Table 1), but also a phenotype of narrow and rolled leaves (Fig. 1a–d). In fact, the most pronounced characteristic of the nrl1 mutant was the phenotype of narrow and rolled leaves, which could be observed in the nrl1 mutant even though there was no height difference between the nrl1 mutant and wild type at the seedling stage. Loss of function of NRL1 significantly reduced the width of the second leaf blade from top, but not the length (Table 1). However, it is unclear whether the phenotypic difference between the two mutants is because that NRL1 and AtCSLD5 have different biochemical functions or because they play distinct roles in different species.
Leaf blade width is controlled by the regulation of cell expansion and cell proliferation in leaf lateral axis. The examination of transverse section of leaf blade showed that there was no significant difference in cell size between the nrl1 mutant and wild type (Fig. 1). In comparison with wild type, the nrl1 leaf blade had a significant reduction in the total number of vascular bundles at different developmental stages (Fig. 1). These findings suggested that the reduced leaf blade width in nrl1 was due to the decreased cell number in the lateral direction.
Rice NAL1 and NAL7/COW1 genes have also been identified in the regulation of the number of leaf vascular bundles (Woo et al. 2007; Fujino et al. 2008; Qi et al. 2008). NAL1 is involved in auxin biosynthesis, while NAL7 affects auxin polar transport. Loss of function of NAL7 not only decreases leaf width but also causes leaf rolling, whereas the NAL1 gene only affects leaf width. The nrl1 mutant is much more similar to nal7 than to nal1. Knockout of NRL1 resulted in upregulation of NAL7 transcription, but did not affect the expression levels of the NAL1 gene (Fig. 7), which was expected with the observed phenotypes. OsAGO7 and SLL1/RL9 were found to regulate leaf rolling. Overexpression of rice OsAGO7 gene induces leaf rolling (Shi et al. 2007) and loss of function of SLL1 conversely results in extremely rolled leaves due to the defective sclerenchyma formation in abaxial leaf surface (Yan et al. 2008; Zhang et al. 2009). The expression of OsAGO7 is suppressed in nrl1, while the expression of SLL1 is upregulated. These findings suggest that NRL1, NAL7, SLL1 and OsAGO7 might function in the same pathway to regulate leaf development.
Abbreviations
- CSL:
-
Cellulose synthase like
- InDel:
-
Insertion–deletion
- nrl1 :
-
Narrow and rolled leaves 1
References
Bernal AJ, Jensen JK, Harholt J, Sorensen S, Moller I, Blaukopf C, Johansen B, de Lotto R, Pauly M, Scheller HV, Willats WG (2007) Disruption of ATCSLD5 results in reduced growth, reduced xylan and homogalacturonan synthase activity and altered xylan occurrence in Arabidopsis. Plant J 52:791–802
Bernal AJ, Yoo CM, Mutwil M, Jensen JK, Hou G, Blaukopf C, Sorensen I, Blancaflor EB, Scheller HV, Willats WGT (2008) Functional analysis of the cellulose synthase like genes CSLD1, CSLD2 and CSLD4 in tip growing Arabidopsis cells. Plant Physiol 148:1238–1253
Byrne ME, Barley R, Curtis M, Arroyo JM, Dunham M, Hudson A, Martienssen RA (2000) Asymmetric leaves 1 mediates leaf patterning and stem cell function in Arabidopsis. Nature 408:967–971
Cocuron JC, Lerouxel O, Drakakaki G, Alonso AP, Liepman AH, Keegstra K, Raikhel N, Wilkerson C (2007) A gene from the cellulose synthase-like C family encodes β-1, 4 glucan synthase. Proc Natl Acad Sci USA 104:8550–8555
Dhugga KS, Barreiro R, Whitten B, Stecca K, Hazebroek J, Randhawa GS, Dolan M, Kinney AJ, Tomes D, Nichols S, Anderson P (2004) Guar seed β-mannan synthase is a member of the cellulose synthase super gene family. Science 303:363–366
Eshed Y, Baum SF, Perea JV, Bowman JL (2001) Establishment of polarity in lateral organs of plants. Curr Biol 11:1251–1260
Favery B, Ryan E, Foreman J, Linstead P, Boudonck K, Steer M, Shaw P, Dolan L (2001) KOJAK encodes a cellulose synthase-like protein required for root hair cell morphogenesis in Arabidopsis. Genes Dev 15:79–89
Fujino K, Matsuda Y, Ozawa K, Nishimura T, Koshiba T, Fraaije MW, Sekiguchi H (2008) NARROW LEAF 7 controls leaf shape mediated by auxin in rice. Mol Genet Genomics 279:499–507
Hibara KI, Obara M, Hayashida E, Abe M, Ishimaru T, Satoh H, Itoh JI, Nagato Y (2009) The ADAXIALIZED LEAF1 gene functions in leaf and embryonic pattern formation in rice. Dev Biol 334:345–354
Horiguchi G, Kim GT, Tsukaya H (2005) The transcription factor AtGRF5 and the transcription coactivator AN3 regulate cell proliferation in leaf primordia of Arabidopsis thaliana. Plant J 43:68–78
Huang W, Pi L, Liang W, Xu B, Wang H, Cai R, Huang H (2006) The proteolytic function of the Arabidopsis 26S proteasome is required for specifying leaf adaxial identity. Plant Cell 18:2479–2492
Kerstetter RA, Bollman K, Taylor RA, Bomblies K, Poethig RS (2001) KANADI regulates organ polarity in Arabidopsis. Nature 411:706–709
Kim GT, Tsukaya H, Uchimiya H (1998) The ROTUNDIFOLIA3 gene of Arabidopsis thaliana encodes a new member of the cytochrome P-450 family that is required for the regulated polar elongation of leaf cells. Genes Dev 12:2381–2391
Kim GT, Shoda K, Tsuge T, Cho KH, Uchimiya H, Yokoyama R, Nishitani K, Tsukaya H (2002) The ANGUSTIFOLIA gene of Arabidopsis, a plant CtBP gene, regulates leaf-cell expansion, the arrangement of cortical microtubules in leaf cells and expression of a gene involved in cell-wall formation. EMBO J 21:1267–1279
Kim CM, Park SH, Je BI, Park SH, Park SJ, Piao HL, Eun MY, Dolan L, Han C (2007) OsCSLD1, a cellulose synthase-like D1 gene, is required for root hair morphogenesis in rice. Plant Physiol 143:1220–1230
Liepman AH, Wilkerson CG, Keegstra K (2005) Expression of cellulose synthase-like (Csl) genes in insect cells reveals that CslA family members encode mannan synthases. Proc Natl Acad Sci USA 102:2221–2226
Liu W, Fu Y, Hu G, Si H, Zhu L, Wu C, Sun Z (2007) Identification and fine mapping of a thermo-sensitive chlorophyll deficient mutant in rice (Oryza sativa L.). Planta 226:785–795
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods 25:402–408
Long JA, Moan EI, Medford JI, Barton MK (1996) A member of the KNOTTED class of homeodomain proteins encoded by the STM gene of Arabidopsis. Nature 379:66–69
McConnell JR, Emery J, Eshed Y, Bao N, Bowman J, Barton MK (2001) Role of PHABULOSA and PHAVOLUTA in determining radial patterning in shoots. Nature 411:709–713
McCouch SR, Teytelman L, Xu YB, Lobos KB, Clare K, Walton M, Fu B, Maghirang R, Li Z, Xing Y, Zhang Q, Kono I, Yano M, Fjellstrom R, DeClerck G, Schneider D, Cartinhour S, Ware D, Stein L (2002) Development and mapping of 2240 new SSR markers for rice (Oryza sativa L.). DNA Res 9:199–207
Micol JL (2009) Leaf development: time to turn over a new leaf? Curr Opin Plant Biol 12:9–16
Nakai K, Horton P (1999) PSORT: a program for detecting sorting signals in proteins and predicting their subcellular localization. Trends Biochem Sci 24:34–36
Narita NN, Moore S, Horiguchi G, Kubo M, Demura T, Fukuda H, Goodrich J, Tsukaya H (2004) Overexpression of a novel small peptide ROTUNDIFOLIA4 decreases cell proliferation and alters leaf shape in Arabidopsis thaliana. Plant J 38:699–713
Otsuga D, DeGuzman B, Prigge MJ, Drews GN, Clark SE (2001) REVOLUTA regulates meristem initiation at lateral positions. Plant J 25:223–236
Panaud O, Chen X, McCouch SR (1996) Development of microsatellite markers and characterization of simple sequence length polymorphism (SSLP) in rice (Oryza sativa L.). Mol Genet Genomics 252:597–607
Pekker I, Alvarez JP, Eshed Y (2005) Auxin response factors mediate Arabidopsis organ asymmetry via modulation of KANADI activity. Plant Cell 17:2899–2910
Qi J, Qian Q, Bu Q, Li S, Chen Q, Sun J, Liang W, Zhou Y, Chu C, Li X, Ren F, Palme K, Zhao B, Chen J, Chen M, Li C (2008) Mutation of the rice Narrow leaf1 gene, which encodes a novel protein, affects vein patterning and polar auxin transport. Plant Physiol 147:1947–1959
Shi Z, Wang J, Wan X, Shen G, Wang X, Zhang J (2007) Over-expression of rice OsAGO7 gene induces upward curling of the leaf blade that enhanced erect-leaf habit. Planta 226:99–108
Small I, Peeters N, Legeai F, Lurin C (2004) Predotar: a tool for rapidly screening proteomes for N-terminal targeting sequences. Proteomics 4:1581–1590
Tamura K, Dudley J, Nei M, Kumar S (2007) MEGA4: Molecular Evolutionary Genetics Analysis (MEGA) software version 4.0. Mol Biol Evol 24:1596–1599
Temnykh S, DeClerck G, Lukashova A, Lipovich L, Cartinhour S, McCouch S (2001) Computational and experimental analysis of microsatellites in rice (Oryza sativa L.): frequency, length, variation, transposon associations, and genetic marker potential. Genome Res 11:1441–1452
Thompson JD, Gibson TJ, Plewniak F, Jeanmougin F, Higgins DG (1997) The CLUSTAL_X windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res 25:4876–4882
Tsiantis M, Schneeberger R, Golz JF, Freeling M, Langdale JA (1999) The maize rough sheath2 gene and leaf development programs in monocot and dicot plants. Science 284:154–156
Tsukaya H (2006) Mechanism of leaf-shape determination. Annu Rev Plant Biol 57:477–496
Waites R, Selvadurai HR, Oliver IR, Hudson A (1998) The PHANTASTICA gene encodes a MYB transcription factor involved in growth and dorsoventrality of lateral organs in Antirrhinum. Cell 93:779–789
Wang MB, Waterhouse PM (1997) A rapid and simple method of assaying plants transformed with hygromycin or PPT resistance genes. Plant Mol Biol Rep 15:209–215
Wang X, Cnops G, Vanderhaeghen R, De Block S, Van Montagu M, Van Lijsebettens M (2001) AtCSLD3, a cellulose synthase-like gene important for root hair growth in Arabidopsis. Plant Physiol 126:575–586
Woo YM, Park HJ, Su’udi M, Yang JI, Park JJ, Back K, Park YM, An G (2007) Constitutively wilted 1, a member of the rice YUCCA gene family, is required for maintaining water homeostasis and an appropriate root to shoot ratio. Plant Mol Biol 65:125–136
Xu L, Xu Y, Dong A, Sun Y, Pi L, Xu Y, Huang H (2003) Novel as1 and as2 defects in leaf adaxial-abaxial polarity reveal the requirement for ASYMMETRIC LEAVES1 and 2 and ERECTA functions in specifying leaf adaxial identity. Development 130:4097–4107
Yan S, Yan CJ, Zeng XH, Yang YC, Fang YW, Tian CY, Sun YW, Cheng ZK, Gu MH (2008) ROLLED LEAF 9, encoding a GARP protein, regulates the leaf abaxial cell fate in rice. Plant Mol Biol 68:239–250
Yin Y, Huang J, Xu Y (2009) The cellulose synthase superfamily in fully sequenced plants and algae. BMC Plant Biol 9:99
Zhang GH, Xu Q, Zhu XD, Qian Q, Xue HW (2009) SHALLOT-LIKE1 is a KANADI transcription factor that modulates rice leaf rolling by regulating leaf abaxial cell development. Plant Cell 21:719–735
Zhou GK, Kubo M, Zhong R, Demura T, Ye ZH (2007) Overexpression of miR165 affects apical meristem formation, organ polarity establishment and vascular development in Arabidopsis. Plant Cell Physiol 48:391–404
Zhu ZG, Xiao H, Fu YP, Hu GC, Yu YH, Si HM, Zhang JL, Sun ZX (2001) Construction of transgenic rice populations by inserting the maize transposon Ac/Ds and genetic analysis for several mutants. Chinese J Biotech 17:288–292 (in Chinese with English abstract)
Acknowledgments
This project was supported by National Natural Science Foundation of China (30900790), the National High Technology Research and Development Program of China (2006AA10Z1E8) and Zhejiang Natural Science Foundation (Y307070 and 2008C22077). We are grateful to Mrs Honglan Yan (China National Rice Research Institute, China) for taking pictures for the article.
Author information
Authors and Affiliations
Corresponding authors
Additional information
The sequences in this article can be found in NCBI under the following accession numbers: OsACT1 (NM_001057621), NRL1 (AK242601), SLL1 (FJ268748) NAL1 (EU093963), NAL7 (AB354302) and OsAGO7 (EF486281). The accession numbers of proteins used in the phylogeny are shown in Supplemental Data Set 1.
C. Wu, Y. Fu contributed equally to this work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Wu, C., Fu, Y., Hu, G. et al. Isolation and characterization of a rice mutant with narrow and rolled leaves. Planta 232, 313–324 (2010). https://doi.org/10.1007/s00425-010-1180-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00425-010-1180-3