Abstract
Leaves, the collective organ produced by the shoot apical meristem (SAM), are polarized along their adaxial–abaxial axis. In this study, we characterized two rice (Oryza sativa) allelic rolled-leaf mutants, rolled leaf 9-1 (rl9-1) and rl9-2, which display very similar phenotypes with completely adaxialized leaves and malformed spikelets. We cloned the RL9 gene by way of a map-based cloning strategy. Molecular studies have revealed that RL9 encodes a GARP protein, an orthologue of Arabidopsis KANADIs. RL9 is mainly expressed in roots, leaves, and flowers. The transient expression of a RL9–GFP (green fluorescent protein) fusion protein has indicated that RL9 protein is localized in the nucleus, suggesting that RL9 acts as a putative transcription factor.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Plant leaves derive from primordia, which form in the peripheral zone of the shoot apical meristem (SAM). After emerging from the SAM flanks, the leaf primordia establishes polarity along three axes: the adaxial–abaxial, proximodistal, and mediolateral. Among these three axes, the adaxial–abaxial axis is fundamental for the subsequent asymmetric growth of the leaf and lamina expansion (Sussex 1955; Waites et al. 1995; McConnel and Barton 1998). The genetic mechanism behind leaf adaxial–abaxial polarity formation has been uncovered through a variety of mutants in Arabidopsis thaliana, Antirrhinum majus, and Zea mays, all of which exhibit abnormalities in leaf polarity. Morphological and molecular analyses of these mutants have led to the identification of genes that play important roles in leaf polarity establishment (for a review, see Xu et al. 2007).
In Arabidopsis, previous studies of abaxial–adaxial polarity establishment have indicated that transcription factors appear to be central, among all the components identified during leaf adaxial–abaxial polarity formation. Five functional categories of transcription factors were identified as being involved in the leaf adaxial–abaxial polarity formation. The first one is genes of the HD-ZIP III family, which are encoding members of a homeodomain/leucine-zipper (HD-ZIP) family of proteins, including PHABULOSA (PHB), PHAVOLUTA (PHV) and REVOLUTA (REV) genes. These genes have been shown to function in promoting leaf adaxial fate (Talbert et al. 1995; McConnell and Barton 1998; McConnell et al. 2001). Although carrying a recessive mutation in one of these three genes does not result in a plant having leaf defects in adaxial–abaxial patterning, plants lacking functional copies of all three genes exhibit a dramatic phenotype with an abaxialized, needle-like cotyledon distal to the hypocotyls (Emery et al. 2003; Prigge et al. 2005). Two miRNAs, miR165/166, were found to negatively regulate PHB/PHV/REV by mediating transcript cleavage and degradation (Emery et al. 2003; Reinhart et al. 2002; Rhoades et al. 2002; Juarez et al. 2004a, b; Bao et al. 2004).
The second category consists of ASYMETRIC LEAVES 1 and 2 (AS1 and AS2). AS1 encodes a putative R2–R3 MYB domain transcription factor (Bynre et al. 2000; Sun et al. 2002), while AS2 encodes a LATERAL ORGAN BOUNDARIES (LOB) domain protein containing a leucine-zipper motif (Lin et al. 2003; Xu et al. 2003; Iwakawa et al. 2002). AS1 and AS2 can bind each other to form a complex that functions within the adaxial leaf domain (Xu et al. 2003), suggesting that AS1 and AS2 participate in the determinant of adaxial cell fate. The results of overexpression of AS1 and AS2 indicate that the normally spatially restricted AS2 may be critical in determining the activity of the AS1–AS2 complex, and the role of AS1 in leaf polarity formation is dependent on its interaction with AS2.
The third category is KANADIs, which encodes putative GARP family transcription factors, including KAN1, KAN2, and KAN3. These three genes are expressed in a domain complementary to PHB/PHV/REV in multiple tissues (Emery et al. 2003; Eshed et al. 1999, 2001; Kerstetter et al. 2001; Hawker and Bowman 2004). Mutual suppression between KAN1/2/3 and PHB/PHV/REV was found, which might be very important to leaf adaxial–abaxial polarity formation.
The fourth group consists of AUXIN RESPONSE FACTORS 3 and 4 (ARF3 and ARF4), encoding a plant-specific transcription factors. ARF3 and ARF4 were shown to be required for specifying leaf abaxial identity (Pekker et al. 2005). ARF3/4 transcripts are targets of a TAS3-derived trans-acting short interfering RNA, tasiR-ARF, which guides the cleavage of ARF3/4 mRNAs (Allen et al. 2005; Williams et al. 2005).
The fifth and final group is FILAMENTOUS FLOWER (FIL) and YABBY3 (YAB3). FIL and YAB3 belong to the YABBY transcription factors family, and contain a conserved zinc-finger domain and an HMG-like YAB domain (Sawa et al. 1999; Siegfried et al. 1999). FIL and YAB3 are each expressed in the abaxial leaf domain, and thus promote abaxial cell fate (Siegfried et al. 1999).
In addition, 26S proteasome was found to target a regulator that promotes leaf abaxial identity through a characterization of an as1/as2 enhancer mutation, ae3 (Huang et al. 2006). Therefore, five groups of transcription factors, two types of small RNAs, and the 26S proteasome protein degradation machinery have been shown to modulate the establishment of adaxial–abaxial polarity in Arabidopsis. More recently, numerous genetic factors—including transcription factors and small RNAs—have been identified as being responsible for leaf adaxial–abaxial polarity formation; however, a great amount of evidence has shown that their functions vary in different species (for a review, see Kidner and Timmermans 2007).
Although many genes have been identified and characterized in eudicot Arabidopsis, little progress has been achieved on studies into leaf adaxial–abaxial polarity formation in rice, a model plant of cereal crops. Leaves, the lateral outgrowths from the stem, are the major collective organ where photosynthesis takes place, and they thus play an important role in natural and agricultural productivity. Therefore, the shape of the rice leaf has been considered a critical factor in rice plant-type breeding (Yuan 1997). Many studies have demonstrated that leaf-rolling, to some degree, would benefit the plant in keeping it erect, as it would consequently optimize canopy light transmission and increase the photosynthesis rate in crops (Shen 1983; Chen et al. 2001, 2002; Lang et al. 2004a, b). Recently, OsAGO7, an orthologue of Arabidopsis AGO7, was isolated and characterized as being involved in leaf-curling (Shi et al. 2007). Apart from that, several genes (e.g., RL1–RL9) responsible for the rice leaf-rolling characteristic have been identified in recent years, through conventional genetic approaches. Among them, RL7, RL8, and RL9 have been assigned to their corresponding chromosomes with molecular markers (Yan et al. 2006). However, to date, none of these rolled-leaf genes have been fully characterized in rice.
Previously, we isolated the rl9-1 mutant from the M2 generation of a japonica variety Zhonghua 11 via 60Co γ∼ray radiation and then anchored the gene within a 42-kb region on chromosome 9 (Yan et al. 2006). In this work, we further isolated the RL9 gene by way of a map-based cloning strategy. A sequence analysis revealed that the RL9 gene encodes a GARP domain protein, an orthologue of Arabidopsis KANADIs, acting as a putative transcription factor. The RL9 gene is mainly expressed in roots, leaves, and flowers, and the RL9-encoded protein is localized in nuclei.
Materials and methods
Transmission electron microscopy (TEM) analysis
Leaf samples of wild-type and rl9-1 mutant rice were harvested from two-month-old plants. Leaf sections were fixed in primary fixation solution (2% paraformaldehyde and 2% glutaraldehyde). After washing with 0.05 M sodium cacodylate buffer (pH 7.2), they were post-fixed with 1% osmium tetroxide. The samples were then bloc-stained and dehydrated in a gradient alcohol series before transitions and infiltrations were processed. The polymerization reaction was carried out overnight at 70°C. The sections were sliced to 60 nm with ultra-microtome (MT-X, RMC, USA), stained with 2% uranyl acetate and Reynolds’ lead citrate, and observed under transmission electron microscopy (JEM-1010, JEOL, Japan).
Fine mapping of the RL9 gene
The rl9-1 mutant was isolated in the M2 population of a japonica variety Zhonghua 11 radiated by 60Co γ∼ray, and the rolled-leaf phenotype was found to be inherently stable (Yan et al. 2006). The rl9-2 mutant was derived from the japonica variety Nipponbare during tissue culture. We crossed the rl9-1 mutant with an indica variety, Dular, and then generated F2 and F3 populations for fine-mapping and gene-cloning. All these plants, as well as the derived mapping populations, were grown in the Experimental Farm of Yangzhou University, China, in 2006. Field management followed essentially the normal agricultural practice described by Yan et al. (2007).
The RL9 gene has previously been restricted to the 42-kb region of AP005904 (Yan et al. 2006). A total of 1,267 rolled-leaf plants selected from F3 were used for further fine-mapping of RL9. Genomic DNA was extracted from rice leaves using the CTAB method. STS and CAPS markers were developed, based on the diversity between the genomic DNA sequence of Nipponebare and 93-11 within the 42-kb region, and the sequences of the STS and CAPS primers (Supplemental Table 1) were designed using Vector NTI 9.0 software. A contig was constructed according to the fine-mapping results.
Sequence analysis of candidate genomic region
Gene prediction was performed using FGENESH (Salamov and Solovyev 2000), and intron/exon structures were verified by RT-PCR. The genomic DNA fragments of candidate genes from mutants and corresponding wild-type plants were amplified and sequenced. The sequencing reaction was performed by Shanghai Sangon Inc. (Shanghai, China).
RT-PCR
Total RNA was extracted from seedling leaves of wild-type plants (Zhonghua 11) and different tissues of wild-type plants at the heading stage, using Trizol reagent according to the manufacturer’s instructions (Invitrogen, Carlsbad, CA, USA). For semi-quantitative RT-PCR analysis, total RNA (2 μg) was treated with RNase-free DNase, and first-strand cDNA was synthesized through reverse transcription by an oligo (dT) primer (TaKaRa). Subsequently, the first-strand cDNA was used as a template for semi-quantitative PCR analysis after being normalized with a rice Actin gene (Act). Amplification of the rice Act gene was performed with the forward primer 5′-GGAACTGGTATGGTCAAGGC-3′ and the reverse primer 5′-AGTCTCATGGATACCCGCAG-3′. The PCR reaction for RL9 was performed by using the specific primer r49, which defines a 341-bp fragment of the RL9 cDNA. The PCR procedure is as follows: 94°C for 4 min, followed by 30 cycles at 94°C for 30 s, 58°C for 30 s, and 72°C for 30 s, finishing with an elongation step at 72°C for 5 min. The PCR products were analyzed on 2% agarose gels.
In the quantitative RT-PCR experiments, total RNA was extracted from mature leaf tissue of the wild-type rl9-1 and rl9-2. A total of 1 μg of total RNA was used for cDNA synthesis with an oligo (dT) primer and PrimeScript RT Enzyme (TaKaRa). The reaction mixture was diluted to 50 μl, whereupon 1 μl was used for real-time PCR reaction. The primers r69f/r69r for RL9 were 5′-ATTCTTGCAACATGGACGCC-3′ and 5′-CATTAGCCTCTGTGATTGCC-3′, which were designed to cross an intron in order to eliminate the possibility of genomic DNA amplification. Formation of the expected PCR product was confirmed by agarose gel electrophoresis (2%). We used Act as an internal control, by using the following primers: 5′-CTTCATAGGAATGGAAGCTGCGGGTA-3′ and 5′-CGACCACCTTGATCTTCATGCTGCTA-3′. Amplification reactions were prepared with the SYBR PrimeScript RT-PCR Kit (TaKaRa), according to the manufacturer’s instructions, with 0.4 μM of primers and 1 μl of cDNA per reaction. Each reaction was performed in triplicate. The threshold cycle (Ct) was determined by using the maximum-second-derivative function of the software. Error bars represent the standard error calculated on experiment repetitions.
Identification of the RL9 coding sequence (CDS)
The CDS of RL9 was determined by RT-PCR, which was performed using gene-specific primers in a total volume of 20 μl, including 1.0 μl of the cDNA, 2 μl (0.25 μM) gene-specific primers, 3.2 μl (2.5 μM) dNTP, 10 μl 2 × GC I buffer, 0.1 μl LA Taq DNA Polymerase (Takara), and 3.7 μl ddH2O. PCR reactions were performed by denaturation at 94°C for 5 min, followed by 32 cycles at 94°C for 30 s, 60°C for 30 s, and 72°C for 1 min. PCR products were separated on 1% agarose gel and stained with ethidium bromide. Four overlapping RL9 transcript fragments, covering the entire coding sequence, were amplified after three rounds of PCR: in the first round, primers r62, r67, r49, and r59 were used on the first-strand cDNA; in the second round, r62f67r and r49f59r were used on the products of round I; and finally, in the third round, r62f59r was used on the products of round II (Fig. 1). PCR products were sequenced after ligation with pMD18-T Simple vector (Takara). An in-frame stop codon was present at the end of the obtained cDNA, indicating that the CDS was complete.
Flow chart of identification of RL9 coding sequences. Coding sequences were amplified after three rounds of PCR, as follows: first round, primers r62, r67, r49, and r59 were used on the first-strand cDNA; in the second round, r62f67r and r49f59r were used on the products of round I; and finally, in the third round, r62f59r was used on the products of round II
Complementation test
First, two primers—RLEcoF, 5′-GCCGAATTCTACATCTACGACACCTTG-3′ and RLXbaR, 5′-GCCTCTAGAAGTCTTCACCTTTTCTCT-3′—were used to amplify a 550-bp DNA fragment from –6644 to –5987 upstream of the start code containing one Apa I clone site (Fig. 4c). Then, the amplified fragment was inserted into the binary vector pCAMBIA1300, after digesting with EcoR I and Xba I to generate an intermediate vector pCF. At the same time, a 15,366-bp DNA fragment containing a full-length genomic RL9 gene was obtained by digesting the BAC clone, OSJNBa0083J10 (provided by National Center for Gene Research, CAS), by Apa I and Xba I. The digested fragment was then subcloned into plasmid pCF to form pCFRL, which was introduced into the rl9-1 mutant for complementation testing by an Agrobacterium tumefaciens-mediated transformation method.
Phylogenetic analysis
Amino acid sequences of RL9 homologues were obtained through a search of the National Center for Biotechnology Information (NCBI) database (http://www.ncbi.nlm.nih.gov/blast/Blast.cgi). For the GARP superfamily phylogenetic analysis, 10 KAN amino acid sequences from Physcomitrella patens subsp. patens, Arabidosis, Zea mays, Ipomoea nil, and Zinnia elegans; AtPHR1; AtAPL, AtMYR1 from Arabidosis; Psr1 from Chlamydomonas reinhardtii; eight type-B ARRs from Arabidosis; two type-B ARRs from Oryza sativa; six GLKs; and the RL9 were used. The alignment of amino acid sequences was carried out by using ClustalX 2.0 (Jeanmougin et al. 1998), and a neighbor-joining tree was generated by using MEGA 4.0 software (Tamura et al. 2007). The accession number of each amino acid sequence is shown in Supplemental Table 2.
Cellular localization assays with green fluorescent protein (GFP) fusion proteins
The green fluorescent protein (GFP) expression vector pJIT163-hGFP (gift of Prof. Zhukuang Cheng, IGDB, Beijing, China) is a recombinant derivative of pJIT163/hGFP. Two primers—RGHindf, 5′-TGAAGCTTGCTGATGGCGCCGATGATGC-3′ and RGBamr, 5′-CAGGATCCATCATGATCTGCACCGTGCCAGTC-3′—were used to amplify the 1.1-kb full-length coding sequence of RL9 not containing the stop codon from the aforementioned r62f59r PCR products. RL9–GFP fusion was then performed by in-frame fusion of the 1.1-kb sequence with pJIT163-hGFP. The RL9–GFP fusion and GFP alone (as a control) were introduced into onion skin epidermal cells with the Bio-Rad PDS-1000/He device (Bio-Rad, Hercules, CA, USA). After bombardment, tissues were incubated for 14 h at 25°C in the dark. Images were captured under the Olympus BX61 fluorescence microscope conjunct with a microCCD camera. Grayscale images were captured for each color channel and then merged using the software of IPlab.
Results
Characterization of the two rl9 mutants
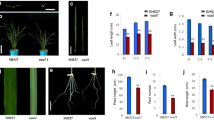
The leaves of both mutants rolled inward slightly at the seedling stage, and very much like a cylinder at the mature stage. The characteristics of the spikelets of the two mutants were distinct from those of the wild-type plants, with malformed spikelets resulting in low seed sets (Fig. 2a–c); furthermore, the rl9-2 spikelets were almost complete sterile. The heading date of rl9-2 was about 12 days later than that of the wild-type counterpart. In addition, previous studies into the morphology of leaves showed that in the rl9-1 mutant, the mesophyll cells covered the vascular bundles on the abaxial side of the leaf and were filled with sclerenchyma cells in wild-type (Yan et al. 2006). Further studies with transmission electron microscopic (TEM) observation revealed that the arrangement of chloroplast grana lamellae were disordered and irregular in rl9-1mutant leaves (Fig. 3b, d), whereas the grana lamellae were parallel to the long axis of the chloroplast in wild-type leaves (Fig. 3a, c).
Phenotypes of wild-type and rl9 mutants. (a) Wild-type, rl9-1, and rl9-2 plants. Bar = 10 cm. (b) Cross-sections of wild-type, rl9-1and rl9-2 mature leaves. Bar = 2 mm. (c) Seeds of wild-type and rl9-1 mutant. Bar = 2 mm. (d) Transgenic plant on the left; rl9-1 mutant on the right, as the control. Bar = 10 cm
Cloning of the RL9 gene
The RL9 gene was previously mapped onto a 42-kb region of chromosome 9 (Fig. 4a, b). In this study, by using 1267 F3 rl9-1 mutant plants, the gene was further narrowed down to a 22-kb genomic region between CAPS marker c42 and polymorphic insertion/deletion marker c28, and co-segregated with c26 on AP005904. Within the 22-kb region, there was only one annotated gene located (Fig. 4c). The identity of this annotated gene as RL9 was initially confirmed by the sequencing results with a 5-bp deletion in exon 1, which occurred in rl9-1, and a substitution of A (wild-type) with G at the splice site of intron 1/exon 2 occurred in rl9-2 (Fig. 4d).
Physical map of the RL9 locus and mutation sites in two allelic mutants. (a) Linkage map of the gene RL9 on chromosome 9. Vertical lines represent the positions of molecular markers; genetic distances (centimorgan, cM) between adjacent markers are shown below the linkage map. (b) Fine-mapping of the RL9 locus. The numbers beside the physical map indicate the number of recombinants identified from F2 and F3 plants. The physical map of the RL9 locus was constructed using six PAC clones; the RL9 locus was further narrowed to a 22-kb genome region between markers c42 and c28, and it was co-segregated with marker c26 based on 1,267 F3 recessive plants. (c) Only one candidate gene existed within the 22-kb restricted region. Three related enzymes used for functional complementation plasmid construction are marked. (d) The RL9 gene structure and mutation positions in two mutants. Black boxes indicate exons. The RL9 gene consists of six exons and five introns. The mutated DNA sequences of rl9-1 and rl9-2 are shown at the bottom. rl9-1 has a five-base deletion in exon 1. rl9-2 has a base substitution at the splicing site (framed), between intron 1 and exon 2. Numbers at right of the map indicate the size of the progeny mapping population
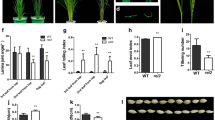
The candidate gene was further confirmed by a complementation test. A 15.5-kb wild-type genomic DNA fragment harboring the entire candidate gene was cloned into pCAMBIA1300 and introduced into rl9-1 mutant embryogenic callus via Agrobacterium tumefaciens-mediated transformation. Five independent transgenic lines were obtained, and they showed a complementation of the rl9-1 phenotype (Fig. 2d). The ratio of wild-type-looking and mutant plants in the T1 generation fitted 3:1 (Supplemental Table 3). Moreover, the Hygromycin Phosphotransferase (HPT) gene was detected in all wild-type-looking plants, but not in mutant plants, by PCR.
Identification of the RL9 CDS
The identification of CDSs is an important step in the functional analysis of genes. Unfortunately, we found no available EST/cDNA in the database that supported any predicted gene model for RL9. In addition, we searched recently published rice whole-genome transcription profiling microarray data, and found 13 probe sets that also could not cover any predicted gene model. We therefore redefined the RL9 CDS by means of PCR on the first strand cDNA of wild-type seedling leaves; however, the predicted RL9 gene structures differ in the TIGR database, the rice genome annotation database, and the FGENESH software, among others. Primers for RT-PCR were designed according to the CDS predicted by FGENESH, which was the longest of all predicted gene models. Due to the 3′ end structures being the same in all predicted gene models—which were validated by RT-PCR results—we tried a 5′ RACE (rapid amplification of cDNA ends) only, but failed for the GC-rich template of the 5′ end region (∼75%). Therefore, integration reactions were performed by three rounds of RT-PCR, to achieve the full-length CDS of RL9 (Fig. 1). The last integrated sequence was submitted to align with a genome DNA sequence with the Basic Local Alignment Search Tool (BLAST), to determine the introns; the reasonable genomic DNA sequence was selected for further gene structure prediction by FGENESH. As a result, we concluded that the RL9 gene consists of six exons and five introns—a 1,134-bp CDS, encoding 377 amino acids (Fig. 4d and Supplemental Fig. 1).
RL9 expression. (a) Semi-quantitative RT-PCR of RL9 in organs, including root, stem, seedling leaf, mature leaf, sheath, and flower; the transcript of Actin was used as a control. (b) Real-time quantitative PCR analysis in the mature leaves of wild-type (WT), and rl9-1 and rl9-2 mutants. The expression levels were normalized to Actin transcript levels and are shown relative to wild-type levels. Error bars represent standard deviations
RL9 encodes a GARP protein
A BLAST search with the RL9 protein and the predicated protein sequence revealed that the RL9 protein contains a 58-amino-acid domain (residues 167–224) that shares 50–100% amino-acid identity with more than 100 predicted genes in the rice genome, including many putative transcription factors. This domain has been named GARP (Riechmann et al. 2000) (Supplemental Fig. 1). However, the overall identity among these proteins is less than 33.5%.
RL9 gene is an orthologue of Arobidopsis KANADI
Orthologues of RL9 were found in the NCBI database, from Ipomoea nil, Arobidopsis, and Zea mays. RL9 has 31.6–40.8% amino acid sequence identity with the three KANADI proteins, which all matched very well within the GARP domain. Furthermore, the C-terminal region is conserved among RL9 and these KAN proteins (Fig. 6). Based on the role of KANs in Arabidopsis, we deduce that RL9 may also function in regulating abaxial identity during leaf development in rice, and the loss of function of RL9 is consistent with the rl9 mutant phenotypes.
Alignment of the RL9 with KAN proteins from Zea mays (ZmKAN1, accession number ABB89932), Arobidopsis thalina (AtKAN1, accession number NP_568334), and Ipomoea nil (InKAN, accession number BAE73188). The sequences were aligned by the AlignX program of Vector NTI 9.0 software. Dashes indicate gaps introduced for maximal alignment. Yellow-on-red letters represent identical amino-acids. Green-on-black letters represent conservative amino-acids
Phylogenetic analysis of RL9
To determine the evolutionary relationship between RL9 and GARP super-family members from the Arabidopsis, Zea mays, Oryza sativa, Chlamydomonas reinhardtii, and other representative identified GARP members, an unrooted phylogenetic tree was built using the neighbor-joining method, based on full-length protein sequences (Fig. 7). The result indicates that all the GARP protein sequences are divided into four subfamilies: subA [RL9, (Physcomitrella patens) PpKAN1, PpKAN2, PpKAN3, (Zea mays) ZmKAN, (Ipomoea nil) InKAN, (Zinnia elegans) ZeKAN, (Arabidopsis thaliana) AtKAN1, AtKAN2, AtKAN3, and AtKAN4], subB [(Chlamydomonas reinhardtii) CrPsr1, AtPHR1, AtAPL, and AtMYR1], subC [AtARR1, AtARR2, AtARR10, AtARR11, AtARR12, AtARR14, AtARR18, AtAPRR4, (Oryza sativa) OsARR10, and OsARR14], and subD (AtGLK1, AtGLK2, ZmGLK1, Zm GLK2, OsGLK1, and OsGLK2).
Phylogenic analysis of RL9. An unrooted phylogenetic tree was generated with the full-length amino-acid sequences of RL9 and GARP genes, from Arabidopsis and other eukaryotes. The subA family includes Oryza sativa RL9, Physcomitrella patens PpKAN1, PpKAN2, PpKAN3, Zea mays ZmKAN, Ipomoea nil InKAN, Zinnia elegans ZeKAN, Arabidopsis thaliana AtKAN1, AtKAN2, AtKAN3, and AtKAN4. The subB family includes Chlamydomonas reinhardtii CrPsr1, AtPHR1, AtAPL, and At MYR1. The subC family includes AtARR1, AtARR2, AtARR10, AtARR11, AtARR12, AtARR14, AtARR18, AtAPRR4, Oryza sativa OsARR10, and OsARR14. The subD family includes AtGLK1, AtGLK2, ZmGLK1, Zm GLK2, OsGLK1, and OsGLK2. The bar represents genetic distance in the phylogenetic tree. The accession numbers of each protein are provided in Table 2
RL9 is classified into subA; with 40.8% identity, it is the most homologous to maize KAN1 (ZmKAN1). The next most homologous protein of the 10 KAN proteins is AtKAN1, which is 32.7% identical to RL9. Of the 11 members of subA, three KANs (AtKAN1-3) in Arabidopsis were reported as having a function in regulating abaxial identity during leaf development (Eshed et al. 2001, 2004), and AtKAN4 has a role in the specification of polarity in Arabidopsis ovule integuments (McAbee et al. 2006). The other KAN members in subA have not been characterized yet. SubB includes four members; of those, Chlamydomonas reinhardtii Psr1 regulates phosphorus metabolism in vascular plants (Wykoff et al. 1999) and AtPHR1 has a role in repairing ultraviolet (UV)-damaged DNA (Sakamoto et al. 1998). The functions of the other two members, AtAPL and AtMYR1, remain to be elucidated. SubC consists of 10 type-B ARR proteins that are involved in His-to-Asp phospho-relay signal transduction systems in Arabidopsis, and function as a transcriptional activator for a type-A ARR, presumably in AHK-mediated cytokinin signaling (Hwang and Sheen 2001; Sakai et al. 2001). SubD comprises six GLK members from Arabidopsis, maize, and rice. GLK genes regulate chloroplast development in these plant species (Fitter et al. 2002).
It is therefore suggested that, with the exception of subB, GARP proteins in each subfamily are involved in similar processes within a plant. In this report, both of the rl9 mutations cause complete adaxialization of leaves; as RL9 belongs to subA, this indicates that RL9 has a similar function with AtKANs in specifying the abaxial fate of leaves.
RL9 expression pattern
To study the expression pattern of RL9, a semi-quantitative RT-PCR was performed with wild-type seedling leaves and different tissues in the heading stage. The results showed that RL9 is expressed in all organs of wild-type plants; is higher in roots, young leaves, mature leaves, and flowers; and is lower in stems and leaf sheaths (Fig. 5a). It is apparent that the expression of RL9 is correlated with the rolled-leaf trait, and the high level of RL9 expression in the flowers can explain the phenotype of abnormal seeds in the two rl9 mutants examined.
We further compared the levels of gene expression in the mature leaves of two mutants and wild-type Zhonghua 11, using quantitative real-time PCR. The RL9 transcripts of the two mutants were downregulated, but not significantly (Fig. 5b); this indicates that mutations in the coding sequence and the 3′-splicing site of the first intron of RL9 led to a slightly decreased level of rl9 mRNA.
RL9 is a nuclear protein
Previous studies indicated that the Arabidopsis KAN1 protein and GARP domain-containing proteins are localized in the nucleus (Kerstetter et al. 2001; Hosoda et al. 2002). We hypothesize that RL9 is a transcription factor in rice, for it contains a GARP domain that may possess a nuclear localization signal. In order to determine whether RL9 encodes a nuclear protein, a full-length RL9 CDS was introduced into an expression vector pJIT163-hGFP. Vectors expressing the RL9–GFP fusion protein or GFP alone were introduced into onion epidermal cells by particle bombardment. In the case of GFP alone, fluorescence was observed in both the nuclei and cytoplasm, whereas in the case of RL9–GFP, fluorescence was localized solely in the nucleus (Fig. 8). This result suggests that RL9 is a putative transcription factor.
Discussion
In this study, we cloned the RL9 gene by way of a map-based cloning strategy, using single-recessive mutants exhibiting rolled leaves. Nucleotide sequencing results revealed that rl9-1 had a 5-bp deletion in exon 1 and rl9-2 had a single nucleotide substitution at the splice site of intron 1/exon 2—both of which resulted in the failure to produce functional RL9 (Fig. 4d). The complementation of the rl9-1 phenotype indicated that the rolled-leaf mutant is due to the 5-bp deletion of the RL9 gene, and the results of a semi-quantitative RT-PCR were consistent with the phenotype of the two rl9 mutants.
Our previous studies found a cause of unambiguous adaxial to abaxial transformations, that the rl9-1 mutant produces mesophyll cells on the abaxial side of vascular bundles instead of sclerenchyma cells seen in the wild-type species (Yan et al. 2006). Further studies in this report demonstrated that the mutations in RL9 not only affect the biosynthesis of sclerenchyma cells of the vascular bundles on the abaxial side of leaves, but also affect chloroplast development—especially the arrangement of chloroplast grana lamellae (Fig. 3).
Sequence analysis revealed that the RL9 encodes a GARP DNA-binding protein. GARP was named after maize GOLDEN2, the ARR B-class proteins from Arabidopsis, and Chlamydomonas Psr1 (Riechmann et al. 2000). Phylogenetic and comparative genetic analyses indicated that the RL9 gene is an orthologue of AtKANs. In the phylogenetic tree, the most homologous gene to RL9 is ZmKAN1 (40.8% identity, at the protein level), which is publicized in the NCBI database but not yet characterized. The next most homologous gene to RL9 is AtKAN1 (32.7% identity, at the protein level) (Figs. 6 and 7). In addition, the collected GARP proteins are divided into four subfamilies; members of each subfamily, except those of subB, appear to be involved in similar processes within different plant species. The fact that RL9 belongs to subA indicates that the RL9 gene may function in a fashion similar to KANs.
In Arabidopsis, members of the KAN family of GARP transcriptional regulators have been found to play essential roles in the specification of abaxial fate in leaf development. A combined loss of function mutations in KAN family members leads to progressive loss of abaxial identity. Either kan1 or kan2 mutants alone have very limited or no morphological alterations, respectively, and double kan1kan2 mutants have dramatically reduced leaf expansion and form ectopic leaf-blade outgrowths on the abaxial leaf surface. Triple kan1kan2kan3 mutants further reduce blade expansion and display more complete adaxialization (Eshed et al. 1999, 2001, 2004; Kerstetter et al. 2001; Emery et al. 2003). However, in the present study, we found that the loss of function of RL9 in the two mutants results in not only the rolled-leaf phenotype, but also malformed spikelets, suggesting that RL9 functions similar to Arabidopsis KANs, with some differences. Single rl9 mutant leaves display complete adaxialization in rice, whereas triple mutants do in Arabidopsis, indicating that the KAN gene may exercise greater effects in rice than in dicots. However, it was found that, besides the role of KAN genes in specifying the abaxial fate in Arabidopsis, they also have some roles in ovule development (Eshed et al. 2001)—especially the Arabidopsis aberrant testa shape (ats) mutant, which produces a single integument instead of the two integuments seen in wild-type ovules; ATS (referred to as KAN4) encoding a member of the KAN family has a limited expression pattern in the ovules (McAbee et al. 2006). It is suggested that the seeds of rl9 mutants displaying aberrant hulls can also be explained by the loss of function in KAN.
In Arabidopsis asymmetric leaf development, KAN proteins have a mutually antagonistic relationship with HD-ZIP III proteins (Emery et al. 2003), and KAN genes are necessary for YABBY (YAB) expression (Eshed et al. 2004). However, no HD-ZP III genes have yet been characterized in rice, and four characterized YAB genes from rice, i.e. DROOPING LEAF, YAB1, YAB3, and OsYAB4, have been proved not to determine the abaxial cell fate, unlike their functions in dicot Arabidopsis (Jang et al. 2004; Yamaguchi et al. 2004; Dai et al. 2007a, b; Liu et al. 2007). The relationships among HD-ZP III, YAB genes, and RL9 in rice leaf development remain to be elucidated. The availability of knowledge vis-à-vis RL9 will facilitate an understanding of the mechanism of rice leaf polarity formation.
References
Allen E, Xie Z, Gustafson AM, Carrington JC (2005) MicroRNA-directed phasing during trans-acting siRNA biogenesis in plants. Cell 121:207–221. doi:10.1016/j.cell.2005.04.004
Bao N, Lye KW, Barton MK (2004) MicroRNA binding sites in Arabidopsis Class III HD-ZIP mRNAs are required for methylation of the template chromosome. Dev Cell 7:653–662. doi:10.1016/j.devcel.2004.10.003
Byrne ME, Barley R, Curtis M, Arroyo JM, Dunham M, Hudson A et al (2000) Asymmetric leaves1 mediates leaf patterning and stem cell function in Arabidopsis. Nature 408:967–971. doi:10.1038/35050091
Chen ZX, Pan XB, Hu J (2001) Relationship between rolled leaf and ideal plant type of rice (Oryza sativa L.). J Jiangsu Agric Res 22:88–91
Chen ZX, Chen G, Pan XB (2002) Genetic expression and effects of rolled leaf gene RL(t) in hybrid rice (Oryza sativa L.). Acta Agronomica Sin 6:847–851
Dai MQ, Hu YF, Zhao Y, Liu HF, Zhou DX (2007a) A WUSCHEL-LIKE HOMEOBOX gene represse a YABBY gene expression required for rice leaf development. Plant Physiol 144:380–390. doi:10.1104/pp.107.095737
Dai MQ, Zhao Y, Ma Q, Hu YF, Hedden P, Zhang QF (2007b) The rice YABBY1 gene is involved in the feedback regulation of Gibberellin metabolism. Plant Physiol 144:121–133. doi:10.1104/pp.107.096586
Emery JF, Floyd SK, Alvarez J, Eshed Y, Hawker NP, Izhaki A et al (2003) Radial patterning of Arabidopsis shoots by class III HD-ZIP and KANADI genes. Curr Biol 13:1768–1774. doi:10.1016/j.cub.2003.09.035
Eshed Y, Baum SF, Bowman JL (1999) Distinct mechanisms promote polarity establishment in carpels of Arabidopsis. Cell 99:199–209. doi:10.1016/S0092-8674(00)81651-7
Eshed Y, Baum SF, Perea JV, Bowman JL (2001) Establishment of polarity in lateral organs of plants. Curr Biol 11:1251–1260. doi:10.1016/S0960-9822(01)00392-X
Eshed Y, Izhaki A, Baum SF, Floyd SK, Bowman JL (2004) Asymmetric leaf development and blade expansion in Arabidopsis are mediated by KANADI and YABBY activities. Development 131:2997–3006. doi:10.1242/dev.01186
Fitter DW, Martin DJ, Copley MJ, Scotland RW, Langdale JA (2002) GLK gene pairs regulate chloroplast development in diverse plant species. Plant J 31:713–727. doi:10.1046/j.1365-313X.2002.01390.x
Hawker NP, Bowman JL (2004) Roles for Class III HD-Zip and KANADI genes in Arabidopsis root development. Plant Physiol 135:1–10. doi:10.1104/pp.104.040196
Hosoda K, Imamura A, Katoh E, Hatta T, Tachiki M, Yamada H et al (2002) Molecular structure of the GARP family of plant Myb-related DNA binding motifs of the Arabidopsis response regulators. Plant Cell 14:2015–2029. doi:10.1105/tpc.002733
Huang W, Pi L, Liang W, Xu B, Wang H, Cai R et al (2006) The proteolytic function of the Arabidopsis 26S proteasome is required for specifying leaf adaxial identity. Plant Cell 18:2479–2492. doi:10.1105/tpc.106.045013
Hwang I, Sheen J (2001) Two-component circuitry in Arabidopsis cytokinin signal transduction. Nature 413:383–389. doi:10.1038/35096500
Iwakawa H, Ueno Y, Semiarti E, Onouchi H, Kojima S, Tsukaya H et al (2002) The ASYMMETRIC LEAVES2 gene of Arabidopsis thaliana, required for formation of a symmetric flat leaf lamina, encodes a member of a novel family of proteins characterized by cysteine repeats and a leucine zipper. Plant Cell Physiol 43:5467–5478. doi:10.1093/pcp/pcf077
Jang S, Hur J, Kim SJ, Han MJ, Kim SR, An G (2004) Ectopic expression of OsYAB1 causes extra stamens and carpels in rice. Plant Mol Biol 56:133–143. doi:10.1007/s11103-004-2648-y
Jeanmougin F, Thompson JD, Gouy M, Higgins DG, Gibson TJ (1998) Multiple sequence alignment with Clustal X. Trends Biochem Sci 23:403–405. doi:10.1016/S0968-0004(98)01285-7
Juarez MT, Kui JS, Thomas J, Heller BA, Timmermans MC (2004a) MicroRNA-mediated repression of rolled leaf1 specifies maize leaf polarity. Nature 428:84–88. doi:10.1038/nature02363
Juarez MT, Twigg RW, Timmermans MC (2004b) Specification of adaxial cell fate during maize leaf development. Development 131:4533–4544. doi:10.1242/dev.01328
Kerstetter RA, Bollman K, Taylor RA, Bomblies K, Poethig RS (2001) KANADI regulates organ polarity in Arabidopsis. Nature 411:706–709. doi:10.1038/35079629
Kidner CA, Timmermans MCP (2007) Mixing and matching pathways in leaf polarity. Curr Opin Plant Biol 10:13–20. doi:10.1016/j.pbi.2006.11.013
Lang YZ, Zhang ZJ, Gu XY, Yang JC, Zhu QS (2004a) Physiological and ecological effects of crimpy leaf character in rice (Oryza sativa L.) I Leaf orientation, canopy structure and light distribution. Acta Agronomica Sin 30:806–810
Lang YZ, Zhang ZJ, Gu XY, Yang JC, Zhu QS (2004b) Physiological and ecological effects of crimpy leaf character in rice (Oryza sativa L.) II Photosynthetic character, dry mass production and yield forming. Acta Agronomica Sin 30:883–887
Lin WC, Shuai B, Springer PS (2003) The Arabidopsis LATERAL ORGAN BOUNDARIES-domain gene ASYMMETRIC LEAVES2 functions in the repression of KNOX gene expression and in adaxial–abaxial patterning. Plant Cell 15:2241–2252. doi:10.1105/tpc.014969
Liu HL, Xu YY, Xu ZH, Chong K (2007) A rice YABBY gene, OsYABBY4, preferentially expresses in developing vascular tissue. Dev Genes Evol 217:629–637. doi:10.1007/s00427-007-0173-0
McAbee JM, Hill TA, Skinner DJ, Izhaki A, Hauser BA, Meister RJ et al (2006) ABERRANT TESTA SHAPE encodes a KANADI family member, linking polarity determination to separation and growth of Arabidopsis ovule integuments. Plant J 46:522–531. doi:10.1111/j.1365-313X.2006.02717.x
McConnell JR, Barton MK (1998) Leaf polarity and meristem formation in Arabidopsis. Development 125:2935–2942
McConnell JR, Emery J, Eshed Y, Bao N, Bowman J, Barton MK (2001) Role of PHABULOSA and PHAVOLUTA in determining radial patterning in shoots. Nature 411:709–713. doi:10.1038/35079635
Pekker I, Alvarez JP, Eshed Y (2005) Auxin response factors mediate Arabidopsis organ asymmetry via modulation of KANADI activity. Plant Cell 17:2899–2910. doi:10.1105/tpc.105.034876
Prigge MJ, Otsuga D, Alonso JM, Ecker JR, Drews GN, Clark SE (2005) Class III homeodomain-leucine zipper gene family members have overlapping, antagonistic, and distinct roles in Arabidopsis development. Plant Cell 17:61–76. doi:10.1105/tpc.104.026161
Reinhart BJ, Weinstein EG, Rhoades MW, Bartel B, Bartel DP (2002) MicroRNAs in plants. Genes Dev 16:1616–1626. doi:10.1101/gad.1004402
Rhoades MW, Reinhart BJ, Lim LP, Burge CB, Bartel B, Bartel DP (2002) Prediction of plant microRNA targets. Cell 110:513–520. doi:10.1016/S0092-8674(02)00863-2
Riechmann JL, Heard J, Martin G, Reuber L, Jiang CZ, Keddie J et al (2000) Arabidopsis transcription factors: genome-wide comparative analysis among eukaryotes. Science 290:2105–2110. doi:10.1126/science.290.5499.2105
Sakai H, Honma T, Aoyama T, Sato S, Kato T, Tabata S et al (2001) ARR1, a transcription factor for genes immediately responsive to cytokinins. Science 294:1519–1521. doi:10.1126/science.1065201
Sakamoto A, Tanaka A, Watanabe H, Tano S (1998) Molecular cloning of Arabidopsis photolyase gene (PHR1) and characterization of its promoter region. DNA Seq 9:335–340. doi:10.3109/10425179809008473
Salamov AA, Solovyev VV (2000) Ab initio gene finding in Drosophila genomic DNA. Genome Res 10:516–522. doi:10.1101/gr.10.4.516
Sawa S, Watanabe K, Goto K, Liu YG, Shibata D, Kanaya E et al (1999) FILAMENTOUS FLOWER, a meristem and organ identity gene of Arabidopsis, encodes a protein with a zinc finger and HMG-related domains. Genes Dev 13:1079–1088. doi:10.1101/gad.13.9.1079
Shen FC (1983) Several opinions on how to use rolled leaf character of rice in breeding. Guizhou Agric Sci 5:6–8
Shi ZY, Wang J, Wan XS, Shen GZ, Wang XQ, Zhang JL (2007) Over-expression of rice OsAGO7 gene induces upward curling of the leaf blade that enhanced erect-leaf habit. Planta 226:99–108. doi:10.1007/s00425-006-0472-0
Siegfried KR, Eshed Y, Baum SF, Otsuga D, Drews GN, Bowman JL (1999) Members of the YABBY gene family specify abaxial cell fate in Arabidopsis. Development 126:4117–4128
Sun Y, Zhou Q, Zhang W, Fu Y, Huang H (2002) ASYMMETRIC LEAVES1, an Arabidopsis gene that is involved in the control of cell differentiation in leaves. Planta 214:694–702. doi:10.1007/s004250100673
Sussex IM (1955) Morphogenesis in Solanum tuberosum L.: experimental investigation of leaf dorsiventrality and orientation in the juvenile shoot. Phytomorphology 5:286–300
Talbert PB, Adler HT, Parks DW, Comai L (1995) The REVOLUTA gene is necessary for apical meristem development and for limiting cell divisions in the leaves and stems of Arabidopsis thaliana. Development 121:2723–2735
Tamura K, Dudley J, Nei M, Kumar S (2007) MEGA4: molecular evolutionary genetics analysis (MEGA) software version 4.0. Mol Biol Evol 24:1596–1599. doi:10.1093/molbev/msm092
Waites R, Hudson A (1995) phantastica: a gene required for dorsoventrality of leaves in Antirrhinum majus. Development 121:2143–2154
Williams L, Carles CC, Osmont KS, Fletcher JC (2005) A database analysis method identifies an endogenous trans-acting short-interfering RNA that targets the Arabidopsis ARF2, ARF3, and ARF4 genes. Proc Natl Acad Sci USA 102:9703–9708. doi:10.1073/pnas.0504029102
Wykoff DD, Grossman AR, Weeks DP, Usuda H, Shimogawara K (1999) Psr1, a nuclear localized protein that regulates phosphorus metabolism in Chlamydomonas. Proc Natl Acad Sci USA 96:15336–15341. doi:10.1073/pnas.96.26.15336
Xu L, Xu Y, Dong A, Sun Y, Pi L, Xu Y et al (2003) Novel as1 and as2 defects in leaf adaxial–abaxial polarity reveal the requirement for ASYMMETRIC LEAVES1 and 2 and ERECTA functions in specifying leaf adaxial identity. Development 130:4097–4107. doi:10.1242/dev.00622
Xu L, Yang L, Huang H (2007) Transcriptional, post-transcriptional and post-translational regulations of gene expression during leaf polarity formation. Cell Res 17:512–519. doi:10.1038/cr.2007.45
Yamaguchi T, Nagasawa N, Kawasaki S, Matsuoka M, Nagato Y, Hirano HY (2004) The YABBY gene DROOPING LEAF regulates carpel specification and midrib development in Oryza sativa. Plant Cell 16:500–509. doi:10.1105/tpc.018044
Yan CJ, Yan S, Zhang ZQ, Liang GH, Lu JF, Gu MH (2006) Genetic analysis and gene fine mapping for a rice novel mutant (rl9 (t)) with rolling leaf character. Chin Sci Bull 51:163–169
Yan CJ, Zhou JH, Yan S, Chen F, Yeboah M, Tang SZ et al (2007) Identification and characterization of a major QTL responsible for erect panicle trait in japonica rice (Oryza sativa L.). Theor Appl Genet 115:1093–1100. doi:10.1007/s00122-007-0635-9
Yuan LP (1997) Super-high yield hybrid rice breeding. Hybrid Rice 12:1–6
Acknowledgments
We are grateful to Dr. Liu Qiaoquan (Agricultural College of Yangzhou University) for kindly providing the rl9-2 mutant. This research was supported in part by grants from the Ministry of Science and Technology of China (No. 2005CB120804), the Jiangsu Innovation Talent Project (No. BK2006506) and the Postgraduate Science Innovation Plan of Jiangsu Province.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Song Yan and Chang-Jie Yan contributed equally to this work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Yan, S., Yan, CJ., Zeng, XH. et al. ROLLED LEAF 9, encoding a GARP protein, regulates the leaf abaxial cell fate in rice. Plant Mol Biol 68, 239–250 (2008). https://doi.org/10.1007/s11103-008-9365-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11103-008-9365-x