Abstract
Purpose
Minimally invasive esophagectomy (MIE) has been met with increased interest for the surgical treatment of esophageal cancer. One critical obstacle for the implementation of MIE has been the intrathoracic anastomosis. In this study, we describe a technique of thoracoscopic intrathoracic anastomosis using a linear stapler in prone position and present the short-term outcomes of this procedure.
Methods
This prospective pilot study included 46 consecutive patients with a cancer either of the gastroesophageal junction (GEJ) or the distal esophagus who underwent either total MIE or thoracoscopic-assisted esophagectomy followed by intrathoracic stapled side-to-side anastomosis. The short-term outcomes including postoperative complications were recorded and analyzed.
Results
This pilot study included 41 males (89 %) and 5 females (11 %) with a mean age of 65.7 years. The majority had adenocarcinoma (93 %). Before surgery, 4 patients (8.7 %) had an incomplete endoscopic submucosal resection, 5 patients (11 %) received chemotherapy alone, and 33 patients (71 %) had chemoradiotherapy. Mean operation time was 408 minutes. Postoperative complications classified as Clavien-Dindo Grade IIIa or more severe occurred in 7 patients (15 %), of whom 4 patients (8.7 %) developed anastomotic leakages without any need for intensive care. Another 2 patients (4.3 %) required intensive care due to aspiration pneumonia and acute renal failure. No in-hospital mortality was registered. Only one patient (2.2 %) with anastomotic leakage developed postoperative anastomotic stenosis requiring balloon dilatation.
Conclusions
The intrathoracic stapled side-to-side anastomosis technique seems to be feasible, safe, and easy to perform, associated with a limited postsurgical complication rate and a good functional outcome.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Esophageal cancer surgery has been one of the most demanding procedures for patients with a gastrointestinal malignancy burdened by complication rates and in-hospital mortality in the range of 55 and 6.0 %, respectively [1]. Along with recent advances in thoracoscopic and laparoscopic surgery, minimally invasive esophagectomy (MIE) has been promoted for the treatment of esophageal cancer. The potential advantages of MIE have been indicated by the favorable results in many cohort studies in the form of less pain, faster recovery, and even better quality of life when compared to conventional open esophagectomy [2–5].
The thoracoscopic intrathoracic anastomosis, on the other hand, remains technically demanding even for experienced surgeons skilled in minimal invasive techniques. This is partly due to the limited space in the thoracic cavity as well as the impaired mobility of the introduced instruments as a consequence of the narrow intercostal spaces. Therefore, several alternative solutions have been launched over the years to perform a safe intrathoracic anastomosis; the optimal procedure is still debatable [6–9].
When the MIE technique was introduced and implemented in our institution we were encouraged by the simplicity and attractiveness of the side-to-side anastomosis, which had previously been successfully practiced in the open setting in the construction of the esophagojejunostomy after total gastrectomy [10]. Hereby, we present our early experiences by the use of this technique in 46 consecutive patients operated on for cancer of the lower esophagus or the esophagogastric junction (GEJ).
Patients and methods
Study design and patients
This is a prospective, single institution cohort study. Clinical and pathological data of patients were collected from medical records and videos. In July 2014, we introduced a total MIE in our department, meaning laparoscopic surgery combined with thoracoscopy, on all eligible patients with esophageal cancer and performed an intrathoracic side-to-side stapled anastomosis. We did not have any exclusion criteria since we wanted to test the feasibility and safety of this method regardless of the tumor stage and the patient’s condition. Patients who underwent open thoracotomy due to intrathoracic firm adhesions or an anastomosis in the neck were excluded. Regarding preoperative treatment, neoadjuvant chemo- and chemoradiotherapy comprised three cycles of 5-fluorouracil and cisplatin with concomitant irradiation starting at the second cycle of chemotherapy up to a total radiation dose of 41.4 Gy.
Laparoscopic procedure
The laparoscopic procedure was performed with the patient in the supine French position. Capnoperitoneum was established and maintained with a pressure of 12 mmHg whereafter a 12-mm trocar was placed just below the umbilicus. Four additional trocars were inserted through the abdominal wall: two 12-mm trocars in the right lower and left upper quadrant, respectively, and two 5-mm trocars in the right upper and left lower quadrant, respectively (Fig. 1a).
Position of the ports in the laparoscopic and thoracosopic part. a Two 12-mm ports were placed in the left hypochondrium and right upper quadrant and two 5-mm ports were placed in the right hypochondrium and left upper quadrant. A 12-mm camera port was placed just below the umbilicus. b Marking was done after the patient was appropriately positioned. cTwo 12-mm ports and one 5-mm port were placed in the eighth intercostal space, the middle of the vertebral border of the scapula and the superior angle of the scapula, respectively. One scope trocar was placed under the inferior angle of the scapula.
The laparoscopic procedure included an abdominal lymphadenectomy removing lymph nodes along the suprapancreatic border and the coeliac trunk (Station 8a, 9, and 11p) as well as the root of the left gastric artery (Station 7). The lower esophagus was then mobilized transhiatally and circumferentially all the way up to the mid mediastinum preferably to the level of the inferior pulmonary vein, allowing for a complete lymphadenectomy in the lower mediastinum as well.
Before the conduit was created, the right gastric artery was identified and the first branch of the artery to the stomach was preserved in all patients. The stomach was then divided using Endo-GIATM Ultra Universal Stapler with several 60-mm purple cartridges (Covidien). The conduit was not completely divided from the specimen at this moment. Instead, a 2-cm-long proximal “bridge” remained undivided, acting as an anchor to the specimen, in order to facilitate the pull-up of the specimen into the chest during the thoracoscopic part of the procedure. No pyloroplasty was performed.
Thoracoscopic procedure
The patient was turned to the prone position. Artificial capnothorax with a pressure of 8 mmHg was induced after first 12-mm trocar was placed below the inferior angle of the scapula. Three additional trocars were inserted; two 12-mm trocars in the eighth intercostal space and the middle of the vertebral border of the scapula and a 5-mm trocar in the superior angle of the scapula. The operating surgeon was using the 12-mm middle scapula and the eighth intercostal trocars, while the camera was inserted through the 12-mm trocar at the tip of the scapula. The camera holder was also using the 5-mm trocar for assistance (Fig. 1b, c).
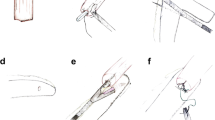
The thoracoscopic procedure involved a mediastinal lymphadenectomy removing lymph nodes under the carina, along the right and left main bronchus, along the entire aortic arch and descending aorta as well as the paraesophageal lymph nodes. After the completion of the lymphadenectomy and mobilization of the proximal esophagus, the gastric conduit was pulled up into the chest through the hiatus with the staple line facing towards us as a landmark for preventing rotation of the conduit. The bridge, remaining connection, between the conduit and the specimen was then divided (Fig. 2a, a′), following which the esophagus was transected at the level of the azygos arch with Endo-GIATM Ultra Universal Stapler with a 60-mm brown cartridge. The specimen was placed on the anterior side of the right lung not to obstruct the operating field.
Snapshots and sketches of minimally invasive esophagectomy followed by intrathoracic stapled anastomosis. a and a′ The “bridge” anchoring the specimen to the gastric conduit. b and b′ Small opening was made in the gastric conduit, located 5–6 cm away from the top. c and c′ A nasogastric tube was introduced intraluminally to accurately identify the opening. d and d′ A linear stapler was introduced into the esophageal stump and gastric conduit. e Two sutures were placed at both ends of the remaining defect. f The defect was closed using 3–0 V-LocTM with over-and-over suture from one end to the other. g and g′ The omentum was applied around the anastomosis as an extra reinforcement. h The specimen was taken out through a minithoracotomy, which was made by extending the hole of the leftmost trocar.
Intrathoracic side-to-side stapled anastomosis and closure of the defect
The esophageal stump and the conduit were brought close together to simulate the anastomosis. Redundant part of the top of the conduit was resected where necessary. A small gastrotomy was performed along the greater curvature side of the conduit, as far away as possible from the staple line, approximately 5 or 6 cm away from the top of the conduit (Fig. 2b, b′). A stay suture was placed at the proximal part of the gastrotomy which involved all the layers of the conduit wall. Then, a small esophagotomy was performed in the middle of the esophageal stump across the staple line. A nasogastric tube was then introduced intraluminally to accurately identify the opening (Fig. 2c, c′) and two stay sutures were placed to transfix all the layers of the esophageal stump wall, for safe introduction of the linear stapler. One of these stay sutures was positioned anteriorly and the other posteriorly.
The side-to-side anastomosis was then completed using Endo-GIATM Ultra Universal Stapler with a 45-mm brown cartridge by introducing the cartridge into the opening of the conduit and the anvil into the esophageal stump (Fig. 2d, d′). After firing, the tip of the nasogastric tube was placed in the conduit for decompression. Two interrupted sutures were placed at each end of the remaining defect using 3–0 Monocryl® (Ethicon, Cincinnati, OH) (Fig. 2e). The remaining defect was closed using 3–0 V-LocTM, an absorbable self-locking barbed suture, with over-and-over suture from one end to the other (Fig. 2f). Perioperative endoscopy was routinely performed to document the patency of the anastomosis. The omentum was wrapped around the anastomosis as an extra reinforcement in case of micro-leakage (Fig. 2g, g′). The specimen was taken out through a minithoracotomy, which was made by extending the hole of the leftmost trocar, with a 5-cm SurgiSleeveTM Would Protector (Covidien) (Fig. 2h). A 19-Fr BLAKE® drain (Ethicon, Cincinnati, OH) connected to AbdovacTM (Wellspect HealthCare, Pymble, Australia), a closed gentle suction drainage system, was inserted through the scope trocar.
Postoperative follow-up
All patients were following an enhanced recovery program (ERP) that was introduced by our team in April 2014. The patients were admitted to the high dependency unit (HDU) for the first three postoperative days and then allowed, if no complications occurred, to be transferred to the Upper-GI ward. All patients had a nasogastric tube placed which was kept until the postoperative day (POD) 3 and if it produced less than 300 ml/day. We allowed patients to start with sloppy diet on POD 6 and solid food on POD 8. Patients were followed-up in the outpatient clinic 1, 3, and 6 months after surgery. Postoperative endoscopy and barium swallow esophagogram were performed in the first 10 cases with intrathoracic stapled side-to-side anastomosis 3 months after surgery or later in order to assess the patency and function of the anastomosis [11]. During endoscopy, we used a 20-mm balloon to estimate the diameter of the anastomosis by moving it up and down through the anastomosis.
Results
Fifty-seven patients underwent esophagectomy for esophageal cancer between July 2014 and October 2015. We planned total MIE with intrathoracic stapled anastomosis in the prone position in 43 patients (75.4 %). In two cases, we converted from laparoscopy to laparotomy due to adhesions in the abdominal cavity. Scheduled thoracoscopic-assisted esophagectomy (meaning laparotomy followed by thoracoscopic surgery) was performed in three patients (5.3 %): two cases because of a history of previous abdominal surgery (abdominal aortic aneurysm repair, antireflux surgery) and one because of severe congenital abdominal deformity. As a result, 46 patients (80.7 %) were included in this study cohort (41 total MIE and five thoracoscopic-assisted esophagectomy). The remaining 11 patients (19.3 %) underwent either a scheduled 3-phase minimally invasive esophagectomy with cervical anastomosis (eight patients) or open transhiatal esophagectomy (two patients).
The demographic characteristics of the patients are shown in Table 1. There were 41 males (89.1 %) and 5 females with a mean age of 65.7 years (range, 38 to 83 years). Adenocarcinoma was found in 43 patients (93.0 %) and three (7.0 %) had squamous cell carcinoma. Regarding preoperative treatment, four patients (8.7 %) had undergone endoscopic submucosal resection, leaving unclear resection margins at the final histopathological examination, five patients (10.9 %) received neoadjuvant chemotherapy and 33 patients (71.7 %) received neoadjuvant chemoradiotherapy. No preoperative treatment was given in four patients (8.7 %) due to advanced age or past history of alcoholic liver injury. Table 2 and Table 3 present histopathological results and short-term postoperative outcomes, showing that mean operation time was 408 minutes with mean blood loss of 248 ml. Median number of lymph nodes retrieved was 23 nodes. Among 38 patients who received neoadjuvant chemo- or chemoradiotherapy, pathological complete response (pCR) was reported in 12 patients (31.6 %), all of whom received neoadjuvant chemoradiotherapy.
With regard to postoperative complications, 7 patients (15.2 %) developed postoperative complications that were classified as Clavien-Dindo Grade IIIa or more severe. Four patients (8.7 %) developed anastomotic leakage, two of whom were successfully treated with a self-expandable esophageal stent and the other two were treated conservatively (only fasting and intravenous antibiotics). None of them required admission to the intensive care unit (ICU). Two patients (4.3 %) were admitted to ICU, one due to aspiration pneumonia with respiratory failure requiring mechanical ventilator support and the other due to postoperative acute kidney failure requiring dialysis. One patient (2.2 %), who developed anastomotic leakage, developed subsequent anastomotic stricture that required one endoscopic dilatation. All the other patients who underwent postoperative endoscopy showed wide anastomosis which measured over 20 mm (Fig. 3a, b) in diameter and no signs of delayed esophageal emptying on timed barium esophagogram.
Discussion
In this study, we present our early experiences on intrathoracic side-to-side stapled anastomosis in 46 consecutive patients with cancer of the lower esophagus or GEJ and this is, to our knowledge, the first report of intrathoracic stapled anastomosis in the prone position on a Western population.
Minimally invasive approach to esophagectomy seems to contribute significantly to a smooth postoperative recovery by reducing complications [5]. However, the optimal design of the esophagogastrostomy is still been debated and the pivotal questions include whether to use a cervical or intrathoracic anastomosis and whether a circular-stapled or linear-stapled anastomosis is preferable [5, 12, 13]. Cervical anastomosis offers not only safer proximal surgical margins and less severe morbidity associated with an anastomotic leak but also technical simplicity when constructing the actual anastomosis. On the other hand, intrathoracic anastomosis offers reduced tension of the gastric conduit at the anastomotic site and a relatively well-nourished conduit tissue, which subsequently would lead to reduced incidence of anastomotic dehiscence. In fact, some studies including one small randomized controlled trial suggested no significant differences in major surgical complications between the two anastomoses [14, 15]. Nonetheless, huge amount of accumulated retrospective data, ending up with a meta-analysis of 5,483 esophagectomized patients, suggested that cervical anastomosis was associated with a higher risk of surgical complications, particularly anastomotic leakage and vocal cord paresis/paralysis [15–19]. Our cumulative experience supports this fact, leading us to apply minimally invasive Ivor-Lewis procedure for cardia cancers.
There are some alternative ways of making an intrathoracic anastomosis under minimally invasive surgical procedures where technical-access related difficulties prevail. The most common solution advocated in these situations has been to perform an end-to-side anastomosis using a circular stapler or a side-to-side anastomosis using a linear stapler. Circular-stapled anastomosis may well be faster to perform since most surgeons already harbor an experience in dealing with the device. However, the anastomosis has repeatedly been reported to be associated with a higher risk of postoperative stricture [12, 20, 21]. Linear-stapled anastomosis, on the other hand, seems to offer a wider anastomosis, potentially resulting in fewer postoperative strictures [22]. Moreover, the linear stapler can be introduced through a 12-mm trocar avoiding a minithoracotomy, which may compromise the capnothorax and obstruct the operating field, jeopardizing the surgical access at the crucial moment of completing the anastomosis. However, it requires a certain technical skill to close the remaining defect after stapling the linear anastomosis. The advantages mentioned above with the intrathoracic side-to-side anastomosis may therefore consequently outweigh the other options if we overcome some of the technical difficulties.
Our current practice using the linear intrathoracic anastomosis has enabled us to advocate some essential technical points that can facilitate the procedure. First, the patient is placed in a prone position during thoracoscopy, which allows a very good view of the operating field without having to collapse the right lung by using a double lumen endotracheal tube [23]. Second, the placement of two stitches securing the attachment between the mucosa and muscle layers of the respective organ. These stitches may play a crucial role for a safe introduction of the stapler, guaranteeing the inclusion of all wall layers into the closure and firing of the stapler device. Third, before performing the anastomosis, the insertion of the cartridge into the conduit and the anvil into the esophageal stump is recommended, which actually facilitates the insertion of the stapler into the small openings by enlarging them to an appropriate size. Fourth, when closing the defect, two stay sutures should be placed at both ends of the defect as landmarks followed by a continuous V-LocTM suture in-between, allowing a safe closure of the defect. Fifth, omentoplasty has currently been used to reinforce the anastomosis, which may be of value in case of anastomotic micro-leakage [24].
An advantage of the side-to-side stapled anastomosis seems to be that the technique can be widely applied to other procedures such as laparoscopic total gastrectomy followed by esophagojejunal anastomosis [25]. On the other hand, the tumor location is a major limitation in making the intrathoracic anastomosis. The technique is basically suitable for patients with a cancer of the GEJ or the distal esophagus in whom the esophagus can be divided at the level of or just above the azygos arch which offers a sufficient surgical margin. Technical difficulties, however, may arise when the anastomosis is located higher up in the thoracic aperture, particularly for patients with a cancer of the middle esophagus. In those situations the cervical anastomosis shall always be an alternative.
In conclusion, our prospective cohort study, where we assessed the feasibility of minimally invasive esophagectomy with intrathoracic stapled side-to-side anastomosis in the prone position, demonstrated that the technique is feasible and safe to perform with an acceptable postsurgical complication rate. Future clinical research has to document further whether the method can indeed minimize the risk of developing complications related to the anastomosis as well as postoperative anastomotic stricture formation.
References
Klevebro F, Johnsen G, Johnson E, Viste A, Myrnas T, Szabo E, Jacobsen AB, Friesland S, Tsai JA, Persson S, Lindblad M, Lundell L, Nilsson M (2015) Morbidity and mortality after surgery for cancer of the oesophagus and gastro-Oesophageal junction: a randomized clinical trial of neoadjuvant chemotherapy vs. Neoadjuvant chemoradiation. Eur J Surg Oncol 41(7):920–926. doi:10.1016/j.ejso.2015.03.226
Sundaram A, Geronimo JC, Willer BL, Hoshino M, Torgersen Z, Juhasz A, Lee TH, Mittal SK (2012) Survival and quality of life after minimally invasive esophagectomy: a single-surgeon experience. Surg Endosc 26(1):168–176. doi:10.1007/s00464-011-1850-7
Nagpal K, Ahmed K, Vats A, Yakoub D, James D, Ashrafian H, Darzi A, Moorthy K, Athanasiou T (2010) Is minimally invasive surgery beneficial in the management of esophageal cancer? A meta-analysis. Surg Endosc 24(7):1621–1629. doi:10.1007/s00464-009-0822-7
Dantoc MM, Cox MR, Eslick GD (2012) Does minimally invasive esophagectomy (MIE) provide for comparable oncologic outcomes to open techniques? A systematic review. J Gastrointest Surg : Off J Soc Surg Aliment Tract 16(3):486–494. doi:10.1007/s11605-011-1792-3
Biere SS, van Berge Henegouwen MI, Maas KW, Bonavina L, Rosman C, Garcia JR, Gisbertz SS, Klinkenbijl JH, Hollmann MW, de Lange ES, Bonjer HJ, van der Peet DL, Cuesta MA (2012) Minimally invasive versus open oesophagectomy for patients with oesophageal cancer: a multicentre, open-label, randomised controlled trial. Lancet 379(9829):1887–1892. doi:10.1016/S0140-6736(12)60516-9
Takeuchi H, Oyama T, Saikawa Y, Kitagawa Y (2012) Novel thoracoscopic intrathoracic esophagogastric anastomosis technique for patients with esophageal cancer. J Laparoendosc Adv Surg Tech A 22(1):88–92. doi:10.1089/lap.2011.0414
Campos GM, Jablons D, Brown LM, Ramirez RM, Rabl C, Theodore P (2010) A safe and reproducible anastomotic technique for minimally invasive Ivor Lewis oesophagectomy: the circular-stapled anastomosis with the trans-oral anvil. EurJ Cardiothorac Surg: Off J Eur Assoc Cardiothorac Surg 37(6):1421–1426. doi:10.1016/j.ejcts.2010.01.010
Okabe H, Tanaka E, Tsunoda S, Obama K, Sakai Y (2013) Intrathoracic esophagogastric anastomosis using a linear stapler following minimally invasive esophagectomy in the prone position. J Gastrointest Surg: Off J Society Surg Aliment Tract 17(2):397–402. doi:10.1007/s11605-012-2009-0
Gorenstein LA, Bessler M, Sonett JR (2011) Intrathoracic linear stapled esophagogastric anastomosis: an alternative to the end to end anastomosis. Ann Thorac Surg 91(1):314–316. doi:10.1016/j.athoracsur.2010.02.115
Walther BS, Zilling T, Johnsson F, Stael von Holstein C, Joelsson B (1989) Total gastrectomy and oesophagojejunostomy with linear stapling devices. Br J Surg 76(9):909–912
Kostic S, Andersson M, Hellstrom M, Lonroth H, Lundell L (2005) Timed barium esophagogram in the assessment of patients with achalasia: reproducibility and observer variation. Dis Esophagus 18(2):96–103. doi:10.1111/j.1442-2050.2005.00460.x
Zhou D, Liu QX, Deng XF, Min JX, Dai JG (2015) Comparison of two different mechanical esophagogastric anastomosis in esophageal cancer patients: a meta-analysis. J Cardiothorac Surg 10(1):67. doi:10.1186/s13019-015-0271-4
Maas KW, Biere SS, Scheepers JJ, Gisbertz SS, Turrado Rodriguez VT, van der Peet DL, Cuesta MA (2012) Minimally invasive intrathoracic anastomosis after Ivor Lewis esophagectomy for cancer: a review of transoral or transthoracic use of staplers. Surg Endosc 26(7):1795–1802. doi:10.1007/s00464-012-2149-z
Kayani B, Jarral OA, Athanasiou T, Zacharakis E (2012) Should oesophagectomy be performed with cervical or intrathoracic anastomosis? Interact Cardiovasc Thorac Surg 14(6):821–826. doi:10.1093/icvts/ivs036
Walther B, Johansson J, Johnsson F, Von Holstein CS, Zilling T (2003) Cervical or thoracic anastomosis after esophageal resection and gastric tube reconstruction: a prospective randomized trial comparing sutured neck anastomosis with stapled intrathoracic anastomosis. Ann Surg 238(6):803–812. doi:10.1097/01.sla.0000098624.04100.b1, discussion 812–804
Martin JT, Mahan A, Zwischenberger JB, McGrath PC, Tzeng CW (2015) Should gastric cardia cancers be treated with esophagectomy or total gastrectomy? A comprehensive analysis of 4,996 NSQIP/SEER patients. J Am Coll Surg 220(4):510–520. doi:10.1016/j.jamcollsurg.2014.12.024
Luketich JD, Pennathur A, Awais O, Levy RM, Keeley S, Shende M, Christie NA, Weksler B, Landreneau RJ, Abbas G, Schuchert MJ, Nason KS (2012) Outcomes after minimally invasive esophagectomy: review of over 1000 patients. Ann Surg 256(1):95–103. doi:10.1097/SLA.0b013e3182590603
Rindani R, Martin CJ, Cox MR (1999) Transhiatal versus Ivor-Lewis oesophagectomy: is there a difference? Aust and N Z J Surg 69(3):187–194
Kassis ES, Kosinski AS, Ross P Jr, Koppes KE, Donahue JM, Daniel VC (2013) Predictors of anastomotic leak after esophagectomy: an analysis of the society of thoracic surgeons general thoracic database. Ann Thorac Surg 96(6):1919–1926. doi:10.1016/j.athoracsur.2013.07.119
Price TN, Nichols FC, Harmsen WS, Allen MS, Cassivi SD, Wigle DA, Shen KR, Deschamps C (2013) A comprehensive review of anastomotic technique in 432 esophagectomies. Ann Thorac Surg 95(4):1154–1160. doi:10.1016/j.athoracsur.2012.11.045, discussion 1160–1151
Xu QR, Wang KN, Wang WP, Zhang K, Chen LQ (2011) Linear stapled esophagogastrostomy is more effective than hand-sewn or circular stapler in prevention of anastomotic stricture: a comparative clinical study. J Gastrointest Surg: Off J Soc Surg Aliment Tract 15(6):915–921. doi:10.1007/s11605-011-1490-1
Johansson J, Zilling T, von Holstein CS, Johnsson F, Oberg S, Walther B (2000) Anastomotic diameters and strictures following esophagectomy and total gastrectomy in 256 patients. World J Surg 24(1):78–84, discussion 84–75
Bonavina L, Laface L, Abate E, Punturieri M, Agosteo E, Nencioni M (2012) Comparison of ventilation and cardiovascular parameters between prone thoracoscopic and Ivor Lewis esophagectomy. Updat Surg 64(2):81–85. doi:10.1007/s13304-012-0156-1
Yuan Y, Zeng X, Hu Y, Xie T, Zhao Y (2014) Omentoplasty for oesophagogastrostomy after oesophagectomy. Cochrane Database Syst Rev 10:CD008446. doi:10.1002/14651858.CD008446.pub3
Nagai E, Ohuchida K, Nakata K, Miyasaka Y, Maeyama R, Toma H, Shimizu S, Tanaka M (2013) Feasibility and safety of intracorporeal esophagojejunostomy after laparoscopic total gastrectomy: inverted T-shaped anastomosis using linear staplers. Surgery 153(5):732–738. doi:10.1016/j.surg.2012.10.012
Acknowledgments
We would specifically like to acknowledge Dr. Alysha Shetye, Center for Digestive Diseases, Karolinska University Hospital Huddinge, Sweden for producing the drawings to elucidate the photos.
Authors’ contributions
TI and IR analyzed data and prepared the manuscript; IR and MN designed the study; IR, MN, JT, and LL included patients; and TI and JE acquired data. All authors (TI, JT, JE, MN, LL, and IR) participated in the critical revision of the manuscript and approved the final version.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
There is no research funding to disclose for the study.
Conflicts of interest
All of the authors declare no conflicts of interest.
Ethical approval
The protocol of the current study was approved by the hospital’s ethical review board.
Informed consent
Informed consent was obtained from all individual participants included in this study.
Rights and permissions
About this article
Cite this article
Irino, T., Tsai, J.A., Ericson, J. et al. Thoracoscopic side-to-side esophagogastrostomy by use of linear stapler—a simplified technique facilitating a minimally invasive Ivor-Lewis operation. Langenbecks Arch Surg 401, 315–322 (2016). https://doi.org/10.1007/s00423-016-1396-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00423-016-1396-1