Abstract
Background
Reports on quality of life (QOL) after minimally invasive esophagectomy (MIE) have been limited. This report compares perioperative outcomes, survival, and QOL after MIEs with open transthoracic esophagectomy (TTE) and open transhiatal esophagectomy (THE).
Methods
After institutional review board approval, retrospective review of a prospectively maintained database identified patients who underwent esophageal resection for esophageal cancer at Creighton University between August 2003 and August 2010. Patients with preoperative stage 4 disease, emergent procedures, laparoscopic transhiatal esophagectomies, or esophagojeujunostomies were excluded from the study. The study patients were categorized as having undergone open TTE, open THE, or MIE. Overall survival (OS) was the interval between diagnosis and death or follow-up assessment. Disease-free survival (DFS) was the interval between surgery and recurrence, death, or follow-up assessment. For the patients who survived at least 1 year after surgery, QOL was assessed using European Organization for Research and Treatment of Cancer (EORTC-QLQ, version 3.0) and esophageal module (EORTC-QLQ OES 18) questionnaires.
Results
The study criteria were satisfied by 104 patients. Lymph node harvest with MIE (median = 20) was similar to that with open TTEs (median = 19) and significantly higher (P < 0.001) than that with open THEs (median = 12). The percentage of patients requiring intraoperative blood transfusion in the MIE group (23.4%) was significantly lower (P < 0.001) than in the open TTE (73.1%) and THE (67.7%) groups. The volume of intraoperative blood product transfusion was significantly lower for the MIE patients (median = 0 ml) than for the open TTE (median = 700 ml) and THE (median = 700 ml) patients. The incidence of respiratory complications with MIEs (10.64%) was significantly lower than with open TTEs (34.61%) and THEs (32.26%). The groups did not differ significantly in terms of R0 resection rates, OS, DFS, or QOL.
Conclusions
MIEs offer a safe and viable alternative to open esophagectomies because they reduce the need and volume of intraoperative blood product transfusion and postoperative respiratory complications without compromising oncological clearance, survival, and QOL.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Esophageal resection is the primary method for the treatment of locoregional esophageal cancer, but it carries significant morbidity and mortality. Whereas perioperative mortality rates have fallen to below 5% at high-volume esophageal centers [1, 2], postoperative morbidity rates remain high, at approximately 50% [3].
Minimally invasive techniques were first incorporated into the surgical armamentarium in 1990s with laparoscopic cholecystectomy to reduce perioperative morbidity. De Paula et al. [4] were the first to describe a minimally invasive esophagectomy (MIE) via the transhiatal approach. Subsequently, Luketich et al [2] described a minimally invasive alternative to an open transthoracic esophagectomy (TTE). As with open esophagectomy, for which the optimal approach remains an issue of debate, no consensus has been reached on the optimal minimally invasive approach [5].
Although MIE was first described in the mid-1990s, its acceptance and incorporation has lagged behind that of other minimally invasive esophageal surgeries, such as antireflux surgery. The reason for this is that MIE, although a proven safe alternative to open esophagectomy with comparable perioperative outcomes, is a complex procedure with a steep learning curve, which may extend to more than 50 esophageal resections [5, 6].
Most studies have shown that MIEs are comparable with open procedures in terms of perioperative morbidity and have the potential to afford some benefit of reduced respiratory complications and hospital stay [7, 8]. Additionally, we have previously shown that MIEs can provide an oncologic clearance comparable with that of open TTEs in terms of R0 resections and lymph node harvest, both of which are important prognostic markers [9]. However, there have been limited reports in the literature on whether this translates into comparable long-term survival.
Increasing attention is being paid to quality of life (QOL) after surgery, and QOL should be taken into consideration, especially for high-morbidity procedures. It is reported that QOL returns to baseline within a year after open esophageal resection [10, 11]. Similarly, a single-center longitudinal study on QOL after MIE reported that QOL returns to baseline levels within 6 months of an MIE and that this is maintained at 1 year [12].
The current study aimed to extend our experience with MIEs by comparing them with open TTEs and open transhiatal esophagectomies (THEs), with a focus on survival and QOL.
Methods
A prospective database with information on pre-, intra-, and postoperative variables of patients undergoing esophageal resection is maintained at Creighton University Medical Center (CUMC). The program coordinator updates this database with survival and recurrence information at regular intervals. After institutional review board approval, this database was queried to identify and obtain information on patients who underwent an esophageal resection for esophageal cancer at CUMC between August 2003 and August 2010.
The exclusion criteria ruled out patients with emergent esophagectomy, preoperative stage 4 disease, laparoscopic transhiatal esophagectomy, or esophagojejunostomy. Patients with MIEs converted to hybrid or complete open procedures were analyzed as part of the MIE group.
Preoperative workup
All the patients were staged preoperatively using esophagogastroduodenoscopy with biopsies, computed tomography (CT) scans, and positron emission tomography (PET) scans. Some patients also underwent endoscopic ultrasound as part of their preoperative workup. The need for neoadjuvant therapy was based on preoperative staging, and the decision was made through discussion between the surgeon, oncologist, and patient.
In general, patients with stages 2 and 3 disease received neoadjuvant therapy. Neoadjuvant regimens largely involved two to four cycles of cisplatin and 5-fluorouracil with concurrent radiation (5,040 cGy). After neoadjuvant therapy, the patients were restaged with CT scan, PET scan, or both to ensure curative resectability.
Surgery
The type of surgery was dictated by tumor location and patient comorbidities. To ensure a valid comparison, we evaluated only esophageal resections with a gastric pull-up in this study. A feeding jejunostomy was placed intraoperatively if not before surgery.
Earlier in the series, a pyloroplasty or pyloromyotomy was performed as part of the operation. Since June 2010, we have injected botulinum toxin into the pylorus instead.
We have previously described our approaches for TTE, THE, and MIE [9]. More recently, we have started to perform minimally invasive Ivor Lewis esophagectomies by incorporating a minimally invasive intrathoracic anastomosis.
QOL
Since QOL returns to baseline within 1 year after open esophageal resection [10, 11], we surveyed patients more than 1 year after their surgery to determine whether the groups differed. Quality of life was assessed using the European Organization for Research and Treatment of Cancer QOL (EORTC-QLQ version 3.0) and esophageal module (EORTC-QLQ OES 18) questionnaires [13, 14]. These surveys have been extensively described elsewhere [13, 14].
The EORTC-QLQ version 3.0 collects information on global health status (GHS) using 5 functional scales (physical, role, emotional, cognitive, social) and 9 symptoms (fatigue, nausea and vomiting, pain, dyspnea, insomnia, appetite loss, constipation, diarrhea, financial difficulties) [13]. The EORTC-QLQ OES 18 collects information on 10 variables through 4 symptom scales (dysphagia, eating, reflux, pain) and 6 single items (trouble swallowing saliva, choking at swallowing, dry mouth, trouble with taste, trouble with coughing, trouble talking) [14]. The global health scale (GHS) is scored from 1 (very poor) to 7 (excellent), whereas the functional scales and symptoms in the two questionnaires are graded from 1 to 4 as follows: 1 (not at all), 2 (a little), 3 (quite a bit), 4 (very much) [15].
For this study, functional scales and symptoms were dichotomized in a manner previously described [15]. If a patient indicated even one response of 3 (quite a bit) or 4 (very much) for any item in a functional scale, the patient was considered to have poor function, whereas the remaining patients were considered to have good function [15].
For the symptom scales and items, the patients were considered to have “minor or no symptoms” or were considered to be “symptomatic” [15]. Patients with even one response of 3 (quite a bit) or 4 (very much) for any item within a symptom scale or for a single item were considered to be symptomatic, whereas the remaining patients were considered to have minor or no symptoms. For the GHS, patients with a response of 4 or less to either of the two questions in the scale were considered to have a poor GHS, whereas the remaining patients were considered to have a good GHS [15].
Survival
Overall survival (OS) was defined as the interval between diagnosis and death or follow-up assessment. Disease-free survival (DFS) was defined as the interval between surgery and recurrence, death, or follow-up assessment. The MIEs were further subcategorized into A (first group of 15 MIEs), B (second group of 15 MIEs) and C (third group of 17 MIEs) to evaluate the impact of the learning curve on lymph node harvest and operative time.
Statistical analysis
All statistical analyses were performed using SPSS version 17 (SPSS Inc., Chicago, IL, USA). Continuous variables were compared using the Kruskal–Wallis test or analysis of variance (ANOVA). If the Kruskal–Wallis test showed any significant differences, post hoc analysis was performed using the Mann–Whitney U test. Categorical variables were compared using chi-square and Fisher’s exact test. Kaplan–Meier curves were used to analyze survival. The log-rank test was used to compare survival between groups.
Results
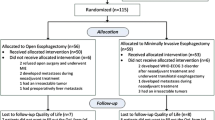
During the study period, 127 patients underwent esophageal resections for esophageal cancer at CUMC. All the procedures were performed by the senior author (S.K.M.). The study excluded patients who underwent laparoscopic THE (n = 7), had an emergent resection (n = 2), had preoperative stage 4 disease (n = 2), or had an esophagojejunostomy (n = 12) (Fig. 1). A total of 104 patients (26 TTEs, 31 THEs, 47 MIEs) satisfied the study criteria (Fig. 1). MIE was converted to a hybrid procedure in 7 patients (14.89%). Forty two patients either died within 1 year after surgery or had their last follow-up assessment less than 1 year after surgery. Of the remaining 62 patients eligible for assessment of QOL, we were able to survey 37 patients (59.67%) (10 TTEs, 11 THEs, 16 MIEs) using EORTC QLQ-30 and QLQ-OES18 questionnaires.
Preoperative variables
Preoperative variables were similar between the groups with the exception of age (Table 1). Patients in the open THE group (mean age = 67.3 years) were significantly older than the patients in the MIE (mean age = 61.7 years) and open TTE (mean age = 59.7 years) groups.
Intraoperative variables
Intraoperative variables are listed in Table 2. Open THEs (median = 300 min) were shorter in duration (P < 0.001) than MIEs (median = 420 min). In turn, MIEs were shorter in duration (P < 0.001) than open TTEs (median = 480 min). As seen in Fig. 2, the operative time for MIE showed a nonsignificant decrease with experience (P = 0.215).
The groups differed significantly in estimated blood loss (EBL). The EBL was lowest for the MIE group (median = 500 ml) and highest for the open TTE group (median = 800 ml). Consequently, both the number of patients who required intraoperative blood transfusion and the volume of intraoperative blood product transfusion were significantly lower with MIE than with open TTEs or THEs (Table 2).
The lymph node harvest was comparable between open TTEs (median = 19) and MIEs (median = 20). However, the lymph node harvest with open THEs (median = 12) was significantly lower than with either open TTEs or MIEs. There was a significant increase (P = 0.007) in lymph node harvest with MIEs as experience was gained (Fig. 3). The groups did not differ in terms of R0 resection rates or intraoperative morbidity.
Postoperative variables
Postoperative variables are listed in Table 3. The incidence of respiratory complications in the MIE group (10.64%) was significantly lower than in either the open TTE (34.61%) or open THE (32.26%) group. The postoperative morbidity rate in the open THE group (87.1%) was significantly higher than in the open TTE (53.85%) or MIE (59.5%) groups (Table 3). The perioperative in-hospital and 30-day mortality rate for the entire series was 2.88%. The groups had comparable perioperative mortality rates as follows: TTE group (0%, n = 0), THE group (3.23%, n = 1), and MIE group (4.26%, n = 2) (P = 0.577).
Survival
The median OS (open TTE = 51.367 months; open THE = 40.8 months; MIE = 51.433 months) did not differ between the groups (P = 0.602) (Fig. 4). Neither did the groups differ in terms of median DFS (open TTE = 45.33 months, open THE and MIE, median survival not reached to date; P = 0.693) (Fig. 5).
Quality of life (EORTC QLQ-30)
The EORTC QLQ-30 results are listed in Table 4. The groups did not differ significantly in any of the response categories. Approximately 81% of the MIE patients surveyed felt they had a good GHS. This response was not different (P = 0.4) from that for the open THE (81.82%) or the open TTE (60%) patients.
Quality of life (EORTC QLQ-OES 18)
The EORTC QLQ-OES 18 results are listed in Table 5. The groups did not differ significantly in any of the response categories. Approximately 94.6% of the surveyed patients had minor or no dysphagia (TTE = 90%; THE = 90.91%; MIE = 100%). For 81% of the surveyed patients, reflux was minor or did not exist (TTE = 100%; THE = 63.64%; MIE = 81.25%).
Discussion
Minimally invasive approaches were first described in the 1990s for esophageal surgeries, particularly gastroesophageal reflux disease. However, although the volumes of laparoscopic antireflux surgery have increased and replaced open approaches as the standard of care, acceptance and incorporation of MIEs have been more sedate [5]. In this retrospective review of a single-surgeon experience, MIEs were associated with a significant reduction in operative time, intraoperative transfusion, and postoperative respiratory complications compared with open TTEs. The MIEs allowed for a significantly more extensive lymph node resection than open THEs without increasing perioperative morbidity. Both survival and QOL after MIE were comparable with those after open TTE and THE.
In the current study 10.64% of the MIE patients had respiratory complications. This was significantly lower than the rates for the open TTE and THE groups. It is hypothesized that the decreased respiratory complications with MIEs are due to reduced postoperative pain and analgesic requirements [16]. Although not evident in every study [7, 16], most studies have found that MIEs are associated with a reduction in respiratory complications [8, 17, 18].
In the current study, respiratory complications encompassed a wide spectrum, ranging from the mild pneumonia and persistent pleural effusion to life-threatening adult respiratory distress syndrome (ARDS). Respiratory complications are important because they have been identified as responsible for more than 50% of in-hospital mortalities after esophagectomies [19]. In this series, ARDS was the immediate cause of death in two (67%) of the three perioperative in-hospital mortalities, with aortic hemorrhage secondary to an anastomotic leak accounting for the third in-hospital death.
Our results are consistent with the literature in showing that MIEs are safe and do not increase the risk of perioperative mortality [7, 8, 20, 21]. The perioperative in-hospital mortality for our study was 2.88%, with no difference between the groups (TTE = 0%; THE = 3.23%; MIE = 4.25%; P = 0.577).
The operative time for MIEs (mean = 420 min) was shorter than for open TTEs (mean = 480 min). An associated decrease in operative blood loss and intraoperative transfusion also has been reported previously [7, 17, 21, 22]. Although most studies report that MIEs require more time to complete than open transthoracic procedures, this could be secondary to a learning curve [7, 8, 18, 21]. When we compartmentalized MIEs into sequential groups, we found a nonsignificant decrease in operative time with increasing experience (Fig. 2). Open THEs (median = 280 min) required less time than either TTEs or MIEs.
The postoperative morbidity rate was significantly higher in the open THE group (87.1%). Although the groups did not differ significantly in comorbidities overall, an inherent selection bias favored THE over TTE for older and “fragile patients” early on based on the surgeon’s perception. This could account for the higher morbidity rate noted in the THE group. Currently, we routinely perform MIEs for these patients.
The oncologic clearance provided by MIEs was equivalent to that of open TTEs, as evidenced by the comparable lymph node harvest, R0 resection rates, overall survival, and disease-free survival. This has been validated elsewhere [17, 21]. However, in one study, the investigators reported a lower lymph node yield with MIE [8]. This could be attributable to a learning curve because, conceptually, a thoracoscopic laparoscopic MIE should provide access similar to that of an open TTE. Our lymph node harvest increased significantly with experience (Fig. 3).
An open TTE allows for a more extensive mediastinal lymph node dissection than an open THE while providing adequate exposure for hemostasis [20]. The MIE procedure affords the opportunity to perform a nodal dissection comparable with that of an open TTE. This was apparent in our study, in which the lymph node harvest with MIEs was similar to that with open TTEs and greater than that with open THEs.
Although the role of extended lymphadenectomy in esophageal resection has been debated, there are reports of a survival benefit afforded by extended lymphadenectomy [23, 24]. This could be due either to stage migration or eradication of occult micro-metastatic disease [24]. Peyre et al. [24] reported a survival benefit for patients (no neoadjuvant or adjuvant therapy) with 23 lymph nodes or more resected compared with patients who had fewer than 23 lymph nodes resected [24]. We also have previously reported a similar benefit of extended lymph node resection for patients undergoing surgery after neoadjuvant therapy [25].
However, extended lymphadenectomy did not translate into a survival benefit for open TTE patients in a randomized controlled clinical trial (RCT) that compared open TTEs with open THEs, although the open TTEs had a significantly greater nodal harvest [26]. In this trial, the overall and progression-free survival benefit with open TTEs was limited to patients with one to eight positive lymph nodes. [26]. Unfortunately, preoperative estimation to determine the number of affected lymph nodes based on endoscopic ultrasound and CT scans is highly subjective and unreliable. Hence, we advocate an extensive lymph node dissection for all patients undergoing esophageal resection for malignant disease.
To the best of our knowledge, this is the first review to compare QOL after MIE with that after TTE and THE. For this study, patients who were alive more than 1 year after surgery were surveyed because QOL returns to baseline within 1 year after open esophagectomy [10, 11]. Our response rate was approximately 60%. More than 75% of the patients surveyed had a good GHS. In the open THE and MIE groups, 80% of the patients had a good GHS compared with 60% of the patients in the open TTE group. This difference was not statistically significant.
About 95% of the patients had minor or no dysphagia, whereas 81% of those surveyed had minor or no reflux. By and large, the patients in all the groups scored well on the functional scales and had good control of symptoms, with no differences between the groups. We did not compare QOL in the immediate postoperative period (<1 year), limiting assessment of the potential impact of MIE during this interval.
The weaknesses of this study included its retrospective nature, small sample size, and short follow-up period. The study had some selection bias, as is evident by older patients in the THE group. Older patients were offered an open THE earlier in this series to reduce the morbidity associated with an open TTE. Toward the latter part of the series, we performed more MIEs.
The strengths of this study included balanced distribution of important prognostic variables such as comorbidities, pathology, positive lymph nodes, and performance of all the procedures by a single surgeon. There have been no published results from RCTs to date. To the best of our knowledge, this is the first study to review perioperative outcomes, survival, and QOL after thoracosopic laparoscopic MIEs by a single surgeon. This is important because it standardizes postoperative management across the various groups and minimizes its role as a source of bias.
Conclusions
Lymph node harvest with MIEs increases with experience, whereas operative duration decreases with experience. In experienced hands, MIEs offer a safe and viable alternative to open transthoracic esophagectomies without compromising oncological efficacy, survival, or QOL.
References
Orringer MB, Marshall B, Chang AC, Lee J, Pickens A, Lau CL (2007) Two thousand transhiatal esophagectomies changing trends, lessons learned. Ann Surg 246:363–374
Luketich JD, Alvelo-Rivera M, Buenaventura PO, Christie NA, McCaughan JS, Litle VR, Schauer PR, Close JM, Fernando HC (2003) Minimally invasive esophagectomy outcomes in 222 patients. Ann Surg 238:486–495
Bailey SH, Bull DA, Harpole DH, Rentz JJ, Neumayer LA, Pappas TN, Daley J, Henderson WG, Krasnicka B, Khuri SF (2003) Outcomes after esophagectomy: a ten-year prospective cohort. Ann Thorac Surg 75:217–222
De Paula AL, Hashiba K, Ferreira EA, De Paula RA, Grecco E (1995) Laparoscopic transhiatal esophagectomy with esophagogastroplasty. Surg Laparosc Endosc 5:1–5
Dunst CM, Swanstrom LL (2010) Minimally invasive esophagectomy. J Gastrointest Surg 14(Suppl 1):S108–S114
Decker G, Coosemans W, De Leyn P, Decaluwé H, Nafteux P, Van Raemdonck D, Lerut T (2009) Minimally invasive esophagectomy for cancer. Eur J Cardiothorac Surg 35:13–21
Gao Y, Wang YY, Chen L, Zhao Y (2011) Comparison of open three-field and minimally invasive esophagectomy for esophageal cancer. Interact CardioVasc Thorac Surg 12:366–369
Lee JM, Cheng JW, Lin MT, Huang PM, Chen JS, Lee YC (2011) Is there any benefit to incorporating a laparoscopic procedure into minimally invasive esophagectomy? The impact on perioperative results in patients with esophageal cancer. World J Surg 35:790–797
Willer BL, Mittal SK, Worrell SG, Mumtaz S, Lee TH (2010) Applicability and feasibility of incorporating minimally invasive esophagectomy at a high-volume center. J Gastrointest Surg 14:1201–1206
Blazeby JM, Farndon JR, Donovan J, Alderson D (2000) A prospective longitudinal study examining the quality of life of patients with esophageal carcinoma. Cancer 88:1781–1787
Lagergren P, Avery KNL, Hughes R, Barham CP, Alderson D, Falk SJ, Blazeby JM (2007) Health-related quality of life among patients cured by surgery for esophageal cancer. Cancer 110:686–693
Parameswaran R, Blazeby JM, Hughes R, Mitchell K, Berrisford RG, Wajed SA (2010) Health-related quality of life after minimally invasive oesophagectomy. Br J Surg 97:525–531
Aaronson NK, Ahmedzai S, Bergman B, Bullinger M, Cull A, Duez NJ, Filiberti A, Flechtner H, Fleishman SB, De Haes JCJM, Kaasa S, Klee M, Osoba D, Razavi D, Rofe PB, Schraub S, Sneeuw K, Sullivan M, Takeda F (1993) The European Organization for Research and Treatment of Cancer QLQ-C30: a quality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst 85:365–376
Blazeby JM, Conroy T, Hammerlid E, Fayers P, Sezere O, Kollerf M, Arrarasg J, Bottomleyh A, Vickerya CW, Etiennei PL, Aldersona D, On behalf of the European Organisation for research, treatment of cancer gastrointestinal, quality of life groups (2003) Clinical and psychometric validation of an EORTC questionnaire module, the EORTC QLQ-OES18, to assess quality of life in patients with oesophageal cancer. Eur J Cancer 39:1384–1394
Djarv T, Blazeby JM, Lagergren P. Predictors of postoperative quality of life after esophagectomy for cancer. J Clin Oncol 27:1963–1968
Safranek PM, Cubitt J, Booth MI, Dehn TCB (2010) Review of open and minimal access approaches to oesophagectomy for cancer. Br J Surg 97:1845–1853
Schoppmann SF, Prager G, Langer FB, Riegler FM, Kabon B, Fleischmann E, Zacherl J (2010) Open versus minimally invasive esophagectomy: a single-center case-controlled study. Surg Endosc 24:3044–3053
Parameswaran R, Veeramootoo D, Krishnadas R, Cooper M, Berrisford R, Wajed S (2009) Comparative experience of open and minimally invasive esophagogastric resection. World J Surg 33:1868–1875
Law S, Wong KH, Kwok KF, Chu KM, Wong J (2004) Predictive factors for postoperative pulmonary complications and mortality after esophagectomy for cancer. Ann Surg 240:791–800
Nguyen NT, Follette DM, Wolfe BM, Schneider PD, Roberts P, Goodnight JE (2000) Comparison of minimally invasive esophagectomy with transthoracic and transhiatal esophagectomy. Arch Surg 135:920–925
Smithers BM, Gotley DC, Martin I, Thomas JM (2007) Comparison of the outcomes between open and minimally invasive esophagectomy. Ann Surg 245:232–240
Hamouda AH, Forshaw MJ, Tsigritis K, Jones GE, Noorani AS, Rohatgi A, Botha AJ (2010) Perioperative outcomes after transition from conventional to minimally invasive Ivor-Lewis esophagectomy in a specialized center. Surg Endosc 24:865–869
Altorki NK, Zhou XK, Stiles B, Port JL, Paul S, Lee PC, Mazumdar M (2008) Total number of resected lymph nodes predicts survival in esophageal cancer. Ann Surg 248:221–226
Peyre CG, Hagen JA, DeMeester SR, Altorki NK, Ancona E, Griffin SM, Hölscher A, Lerut T, Law S, Rice TW, Ruol A, van Lanschot JJB, Wong J, DeMeester TR (2008) The number of lymph nodes removed predicts survival in esophageal cancer: an international study on the impact of extent of surgical resection. Ann Surg 248:549–556
Torgersen Z, Sundaram A, Hoshino M, Willer BL, Fang X, Tashi T, Lee TH, Mittal SK (2011) Prognostic implications of lymphadenectomy in esophageal cancer after neo-adjuvant therapy: a single-center experience. J Gastrointest Surg (in press)
Omloo JMT, Lagarde SM, Hulscher JBF, Reitsma JB, Fockens P, Van Dekken H, Kate FJWT, Obertop H, Tilanus HW, Van Lanschot JJB (2007) Extended transthoracic resection compared with limited transhiatal resection for adenocarcinoma of the mid/distal esophagus: five-year survival of a randomized clinical trial. Ann Surg 246:992–1001
Disclosures
Abhishek Sundaram, Juan C Geronimo, Brittany L. Willer, Masato Hoshino, Zachary Torgersen, Arpad Juhasz, Tommy H. Lee, and Sumeet K. Mittal have no conflicts of interest or financial ties to disclose.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sundaram, A., Geronimo, J.C., Willer, B.L. et al. Survival and quality of life after minimally invasive esophagectomy: a single-surgeon experience. Surg Endosc 26, 168–176 (2012). https://doi.org/10.1007/s00464-011-1850-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00464-011-1850-7