Abstract
Resistance training is frequently performed with the goal of stimulating muscle hypertrophy. Due to the key roles motor unit recruitment and mechanical tension play to induce muscle growth, when programming, the manipulation of the training variables is oriented to provoke the correct stimulus. Although it is known that the nervous system is responsible for the control of motor units and active muscle force, muscle hypertrophy researchers and trainers tend to only focus on the adaptations of the musculotendinous unit and not in the nervous system behaviour. To better guide resistance exercise prescription for muscle hypertrophy and aiming to delve into the mechanisms that maximize this goal, this review provides evidence-based considerations for possible effects of neural behaviour on muscle growth when programming resistance training, and future neurophysiological measurement that should be tested when training to increase muscle mass. Combined information from the neural and muscular structures will allow to understand the exact adaptations of the muscle in response to a given input (neural drive to the muscle). Changes at different levels of the nervous system will affect the control of motor units and mechanical forces during resistance training, thus impacting the potential hypertrophic adaptations. Additionally, this article addresses how neural adaptations and fatigue accumulation that occur when resistance training may influence the hypertrophic response and propose neurophysiological assessments that may improve our understanding of resistance training variables that impact on muscular adaptations.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Skeletal muscle hypertrophy is targeted by bodybuilders (Hackett et al. 2013) but also by athletes of various sports such as weightlifting (Storey and Smith 2012) or judo (Callister et al. 1991; Franchini et al. 2011) to improve performance. In addition, greater muscle mass is associated with a lower risk of mortality due to multiple causes in different populations (Wannamethee et al. 2007; Srikanthan and Karlamangla 2014; Srikanthan et al. 2016; Sedlmeier et al. 2021). Skeletal muscle hypertrophy is defined as an increase in the size of skeletal muscle, which is accompanied by an increase in mineral, protein, or substrate abundance (Haun et al. 2019). When the size of the muscle is increased, there is not only myofibrillar hypertrophy, but also connective tissue and sarcoplasmic hypertrophy (Haun et al. 2019). The myofibrillar hypertrophy induced by resistance training contributes to the gains experienced by the subjects in muscle strength, increasing the capacity of the muscle to produce force (Taber et al. 2019). In this way, a recent study reported that for a given net neural drive to the muscles, chronically strength trained athletes were able to generate significantly higher absolute forces compared with the untrained subjects, what indirectly suggests that morphological factors (i.e., the muscle gain) play an important role for volitional force generation during submaximal force-modulating tasks after years of training practice (Casolo et al. 2021). The adaptations to the muscle are mediated by the recruitment and rate coding of motor units. This is not surprising as the muscle adapts to the neural stimulus in a stimulus-dependent fashion.
Resistance training, being more effective than aerobic training (Grgic et al. 2019), is commonly used to promote skeletal muscle hypertrophy. During resistance training, skeletal muscle fibers produce forces against the load used in each exercise. The correct manipulation of resistance training variables is needed to maximize the increases in muscle mass (Kraemer and Ratamess 2004). When optimizing the resistance training programming aimed to increase muscle hypertrophy, different variables such as volume (Schoenfeld et al. 2017a; Baz-Valle et al. 2018), load/intensity (i.e., % of 1RM) (Schoenfeld et al. 2017b), inter-set rest (Schoenfeld et al. 2016b; Grgic et al. 2017), and frequency (Schoenfeld et al. 2016a, 2019b) have been reported to produce the maximum hypertrophic response. In fact, resistance training is just a specific training based on programming external variables such as load or exercises selection to generate high mechanical forces by the neuromuscular system against resistance. The goal of strength and conditioning is to manipulate the resistance training variables to induce specific physiological responses that will trigger muscular gains. Thus, the stimulus caused by the resistance training performed is the key to the specific induced adaptations (Wackerhage et al. 2019). However, very little knowledge from both the technical and physiological perspective is given to an accurate quantification of the stimulus received by the muscle (e.g., the neural drive to the muscle). Neural drive to the muscle refers to the ensemble of action potential trains (reflecting the number of single motor units activated and their discharge rate) from the pool of α-motoneurons innervating a muscle. This neural signal is the ultimate code of a movement, containing the information on the motor task and modulating the forces produced by the muscles through motor unit recruitment and discharge rate adjustments (Farina et al. 2010). In fact, Desmedt and Godaux already demonstrated in 1977 that the force of ballistic contractions was graded by both the recruitment of additional motor units in stronger contractions and the increase in their firing rate, when the activity of the tibialis anterior motor units was measured during ankle dorsiflexion (Desmedt and Godaux 1977).
The behaviour of the human body is not static but dynamic. A proof of the dynamism of human body is the low reliability of the last repetition velocity of a set to failure (García-Ramos et al. 2020) or the nervous system plasticity, being the excitability of neurons modifiable in short periods of time due to reasons such as resistance training (Nuzzo et al. 2016; Colomer-Poveda et al. 2020). Because of these fluctuations caused by human physiology, not only the baseline of the subject but also the changes experienced by them during and after resistance training, will influence the responses and adaptations produced. In this regard, changes in the nervous system caused by physical exercise or other reasons could influence the results obtained by the resistance training. An example of the nervous system implications is the greater strength gains produced by resistance training when the muscle adaptations do not differ between groups, but the neural adaptations are greater (Jenkins et al. 2017; Nuzzo et al. 2017). Note that the central nervous system is responsible of human movement and it recruits motor units, and then, muscle fibers to contract producing forces (Kandel et al. 2000; Enoka 2015).
Muscle force is regulated by the central nervous system, which vary the activity of the motor units that comprise the muscle (Clamann 1993; Fuglevand et al. 1993). Thus, when movements such as lifting weights are performed, the central nervous system is responsible of its execution, causing the muscle contraction that trigger muscle force. The nervous system seems in general to compensate for the muscle mechanics when performing fast movements. In chronically strength trained athletes, it seems that they are able to generate higher explosive force by compressing the recruitment range (Del Vecchio et al. 2018); on the other hand, when the muscle show slow intrinsic force profiles such as in newborns, the only way for the central nervous system to increase the speed of contraction is to increase the synchrony between motor unit discharge times (Del Vecchio et al. 2020). Although muscle force has been extensively explored for its important role in skeletal muscle hypertrophy (Schoenfeld 2010; Wackerhage et al. 2019), the role of the central nervous system (which is responsible of active muscle force) has received less attention. Knowing that nervous system regulates and causes muscle force, the purpose of this review is to draw an overview of the importance of exploring the effects of the nervous system behaviour on muscle adaptations induced by resistance training. We also want to raise awareness of the possible importance of neurophysiological measurements in studies that explore the effect of different resistance training programs on skeletal muscle hypertrophy.
Resistance training and nervous system
Motor control in voluntary movements
During a voluntary movement such as lifting a weight during resistance training, the output from each motor neuron, that will determinate the force produced in its muscle fibers, depends on the input received from supraspinal levels, spinal cord circuitry, and afferent feedback (Enoka 2015; Taylor et al. 2016). In this sense, the cerebral cortex, excited by different areas such as thalamus or midbrain, has an important role to produce the muscle forces that allow human voluntary movement (Cheney 1985; Hanes and Schall 1996). From different areas of the motor cortex and brainstem pathways is sent the efferent output, commonly known as the central command, to the lower levels of the central nervous system to finally recruit the motor units (Graziano et al. 2002). In the cerebral cortex, not only the motor cortex has an important role in human movement but also other regions such as prefrontal cortex (Cheney 1985; Robertson et al. 2016). When performing high-intensity exercise (e.g., sets close to failure), cognitive functions play an important role (Hagger et al. 2010), being the prefrontal cortex involved in the inhibitory control (Diamond 2013), which is determinant for performance in these physical tasks (Hagger et al. 2010) (see Fig. 1). Non-invasive brain stimulation seems to enhance motor function targeting both motor (Angius et al. 2017; Alix-Fages et al. 2019) and prefrontal cortex (Vieira et al. 2020; Lattari et al. 2020; Alix-Fages et al. 2020). After stimulating their dorsolateral prefrontal cortex, subjects increased the total number of repetitions performed during resistance trainings sessions such as sets of bench press at 5 repetitions with 1 min of inter-set rest against the 75% of 1RM (i.e., one repetition maximum) till failing in a set (Alix-Fages et al. 2020) or 3 maximal sets of back squat at 80% of 1RM with 1 min of inter-set rest (Vieira et al. 2020). Prefrontal cortex does not only have a key role in cognitive tasks but also in motor function (Diamond 2000), being important for the integration of the afferent feedback from the periphery in combination with the motivational and emotional context provided by other areas (Robertson et al. 2016). In this regard, prefrontal cortex role is vital for making decisions during exercise, which will determinate performance (Robertson et al. 2016). In this line, the role of prefrontal cortex on self-regulation functions is also vital for attentional control when performing muscle endurance exercise at high levels of pain and effort (Wolff et al. 2018), being the cognitive resources of the prefrontal cortex key for strength endurance performance in dynamic resistance training involving moderate-to-high repetitions such as 10 (Barbosa-Netto et al. 2021) or 25–30 reps (Ribeiro et al. 2019) but not for shorter tasks such as maximum voluntary isometric contraction (MVIC), because after being depleted by mental fatigue, MVIC performance is not affected (Pageaux et al. 2013, 2015; Rozand et al. 2014; Martin et al. 2015). Then, prefrontal cortex roles could be determinant for reaching failure or getting close to it when performing resistance training sets at moderate–high repetitions (i.e., ≥ 8RM). Note that non-invasive brain stimulation over the prefrontal cortex seems to enhance physical performance at 75% (Alix-Fages et al. 2020) or 80% of 1RM resistance training (Vieira et al. 2020) but not at shorter physical tasks such as 1RM bench press (Alix-Fages et al. 2020), 30 m running sprint (Alix-Fages et al. 2021), or countermovement jumps (Romero-Arenas et al. 2019). Besides, prefrontal cortex and motor cortex excitabilities have been shown to be linked (Cao et al. 2018). Subcortical areas, such as basal ganglia, cerebellum, or different motor centers of the brain stem, are also deeply involved in human movement (Kandel et al. 2000; Enoka 2015). The complex cerebral circuitry along with spinal cord circuitry, being both affected by multitude of feedbacks, will finally determinate the neural drive from pools of motor neurons to the muscles (Enoka 2015; Taylor et al. 2016). Thus, any change given in some of these central nervous regions could influence the muscle forces produced. It means that resistance training performance could be affected and maybe also the induced adaptations.
Schematic illustration of the neural control of human voluntary movement during a resistance training exercise such as squatting. The example of a squat set going close to failure (e.g., 8 repetitions performed with a 10 RM load) is represented in the left side of the figure. Different structures involved in the motor control of the physical task are depicted in the right side of the figure. Not only efferent but also afferent activity is represented. Supraspinal and spinal circuitry are both affected by the afferent feedback and it influences the final efferent signalling affecting motor units’ recruitment, which will determinate the neuromuscular performance in the physical task
Once the action potential is propagated along the muscle fiber membrane and spreads through the T-tubules, it will provoke the release of the Ca2+ (calcium ions) from the sarcoplasmic reticulum after interacting with the dihydropyridine and ryanodine receptors (Dulhunty 2006; Calderón et al. 2014). With the transient increase of Ca2+ concentration in the muscle cell cytoplasm and its interaction with troponin, the crossbridge cycling is caused by the interaction between myosin and actin allowing the muscle contraction to produce force (Frontera and Ochala 2015). Other proteins such as titin and nebulin also contribute to the mechanical and physiological properties of muscle (Frontera and Ochala 2015). All these processes mentioned occur because of the nerve stimulation, being the action potential propagated by the axon of the motor neuron and initiated in the muscle fibers through the transmission produced in the neuromuscular junction by the release of acetylcholine neurotransmitters (Calderón et al. 2014). Thus, being the action potential the regulator of the excitation–contraction coupling (i.e., the series of events that happen from the generation of the action potential and its propagation through the membrane of the muscle fibers to the beginning of the muscle force after Ca2+ release), the nervous system controls muscle force. The amount of force produced by muscle fibers depends on the firing rate of their motor neuron (Duchateau and Baudry 2014; Enoka and Duchateau 2017; Del Vecchio et al. 2019b, c). Note that motor units are recruited in an orderly fashion, always from small motor units to large (Henneman et al. 1974), as Henneman’s Size Principle described after reporting in 1957 the relation between the motor neuron soma size and its susceptibility to generate action potentials at a given input current (Henneman 1957). In fact, a recent in vivo study that reported a very stable recruitment order between different sessions of fast contractions performed by monkeys showed that the neural drive to the muscle is highly structured in a hierarchical fashion, finding that the only component that predicted the rapid movements was constrained by the size principle (Del Vecchio et al. 2021b). In this sense, the membrane resistance of the motoneurons is essential for the orderly recruitment from low-threshold to high-threshold motor units (Powers and Binder 2001). As more motor units are recruited, higher mechanical forces will be produced by the muscles (Enoka and Duchateau 2017; Del Vecchio et al. 2019a). In this line, the central nervous system is able to modulate the mechanical forces produced by each muscle through sending them action potentials at different rates during simple (e.g., knee extension) or complex (e.g., squat) exercises. Because of that, even when synergistically activated muscles of the quadriceps seemed to be primarily controlled by a shared neural drive, sharing their motor neuron pools most of their synaptic input from both afferent feedback [measured signals at 6–12 Hertz (Hz)] and descending cortical inputs (∼20 Hz) (Laine et al. 2015), several motor neurons of the lateral and medial head did not share their synaptic input (Avrillon et al. 2021), reporting a large interindividual variability in the proportion of muscle-specific neural drive, that is, the drive unique to each muscle (range: 6–86%).
Neuromuscular adaptations to resistance training
Chronic adaptations induced by resistance training are not only produced at muscle levels but also at nervous system levels (Gabriel et al. 2006; Siddique et al. 2020). In 1981, Häkkinen, Komi, and Tesch performed a study where 14 trained men went through progressive 16-week dynamic resistance training of combined concentric and eccentric contractions for barbell squat at 80–120% of 1RM three times per week and a subsequent 8-week detraining period (Hakkinen et al. 1981). The training program improved the maximal isometric force and squat performance accompanied by fast-twitch and slow-twitch muscle fibers hypertrophy, but both, muscle force along with hypertrophy, decreased after the detraining period. Squat jump performance was also improved and related to relative fast-twitch muscle fibers hypertrophy (Hakkinen et al. 1981). Two years later, Häkkinen and Komi published a study using the same protocol but incorporated EMG measurements. They found that muscle force improvements were also accompanied by significant increases in maximum EMG of the trained muscles, being both reduced after the detraining period (Häkkinen and Komi 1983). After considering the results of both studies and the timing of the measured neural and hypertrophic adaptations, authors concluded that the early change in muscle strength may be related to neural factors with a gradually increasing contribution of later hypertrophic factors, although the magnitudes and the occurrence of these changes may vary depending on several variables (Häkkinen and Komi 1983). Besides, in a later study, after an explosive strength training program that included jumping exercise with and without load, authors observed specific changes to the training stimulus for the isometric force–time curve due to the greater improvements in the time of force production than in maximal force that were related to the changes of neural activation measured with EMG and the increase of fast-twitch:slow-twitch muscle fibers area ratio, being neural and selective muscle adaptations related to the specific training stimulus (Hakkinen et al. 1985b).
Neural adaptations induced by resistance training could be due to changes in different parts of the central nervous system, being involved supraspinal (Aagaard et al. 2002; Nuzzo et al. 2017) and spinal levels (Aagaard et al. 2002; Carroll et al. 2002; Del Vecchio et al. 2019a). A recent systematic review and meta-analysis (Siddique et al. 2020) found that although the excitability of corticospinal axons seems to not be affected by resistance training, and subcortical and cortical circuitry does, being the descending inhibition reduced, and also, the neural drive from the spinal cord increased. Besides, recent research showed that not only cortical but also reticulospinal adaptations contribute to strength gains in monkeys after 8–9 weeks of resistance training (5 days per week) pulling a loaded handle with progressively increased weights towards the body using their right hand (Glover and Baker 2020). These chronic responses to resistance training comprise a large type of changes such as modulations in agonist (Häkkinen et al. 1998; del Olmo et al. 2006; Gabriel et al. 2006) or antagonist muscle activity (Carolan and Cafarelli 1992; Häkkinen et al. 1998; Gabriel et al. 2006). Focusing on the adaptations produced to recruit a muscle, every change produced on the descending drive, the spinal circuitry, and the afferent feedback or in the motor neuron properties, could affect to the motor units activity (Duchateau et al. 2006). In this sense, finally, the output from the spinal cord to the muscles, which is expressed by the recruitment of motor units and their firing rate, will determinate the muscle force produced and then influence the motor performance (Desmedt and Godaux 1977; Duchateau et al. 2006).
Decoding the neural activity of the motor units recorded from the muscles of a joint using high-density surface electromyography (HDEMG), the real forces produced and measured by a dynamometer could be accurately estimated, which means that the neural information could be translated into the biomechanical function of the joint (Sartori et al. 2017). At the level of motor units, resistance training has demonstrated to increase its drive to the muscles (Carroll et al. 2002; Del Vecchio et al. 2019a). HDEMG allow scientists to explore changes in motor units’ behaviour longitudinally (Martinez-Valdes et al. 2016, 2017; Del Vecchio and Farina 2019). Thereby, it has been recently demonstrated by HDEMG that neural but not muscular adaptations are responsible of the absence in rate of force development (RFD) enhancement while increasing maximal muscle force production after 4 weeks of isometric strength training, consisting of ankle dorsiflexion MVICs and a combination of rapid (4 sets × 10 repetitions reaching 75% MVIC in 1 s) and sustained ramp (3 sets × 10 repetitions increasing force at 37.5% MVC/second till reaching 75% MVIC), which was supported by computer simulations using a model based on the neural and muscular determinants of maximal RFD (Del Vecchio et al. 2021a, b). Authors observed that even though the discharge rate of motoneurons increased during the plateau phase of the muscle contractions, the recruitment speed and discharge rate did not change during the initial phase (Del Vecchio et al. 2021a, b). These specific adaptations resulted in a similar rate of force development before and after training. In this sense, after 4 weeks of isometric resistance training in which maximal force of dorsiflexor muscles during a voluntary contraction was augmented by performing MVCs and 4 sets of 10 repetitions of 40 maximal ballistic contractions in 60 s with 1 min inter-set rest, significant increases in tibialis anterior motor units discharge rates and reductions in the recruitment threshold have been shown (Del Vecchio et al. 2019a). Despite this, the input–output gain of the motor neurons remained without changes and the adaptations in motor unit function could be due to changes in the synaptic input, but alterations at the motor neuron level cannot be excluded (Del Vecchio et al. 2019a). These results are in line with studies that found improvements in descending drive from the motor cortex to recruit motor units (Nuzzo et al. 2017; Siddique et al. 2020). Taken the actual evidence about the improvements in the activation of the muscles by the nervous system due to resistance training (del Olmo et al. 2006; Siddique et al. 2020), we hypothesize that this adaptations could not only affect strength but also muscle gains, influencing the hypertrophy obtained by resistance training because of the changes provoked in the motor units behaviour and then in the muscle fibers forces. It could be interesting to explore these relations in future research, measuring not only the muscle size but also voluntary activation and the activity of motor units using HDEMG. Besides, neurophysiological measurements such as neuronal excitability or inhibition using transcranial magnetic stimulation could also be interesting.
Several questions about neural adaptations to resistance training have still to be explored. As such, neural adaptations have been traditionally proposed to be experienced by the subjects during the first weeks of resistance training contributing strength gains, while structural adaptations occur later (Moritani and DeVries 1979; Goodall et al. 2014). Despite this, a work of Häkkinen, Alén, and Komi in 1985 already showed that during a 24-week dynamic resistance training program of combined concentric and eccentric squats at high loads (70–120% of 1RM) three times per week, although increases in neural activation measured by EMG accompanied improvements in strength during the course of all the very intense training (neural activation decreased when training with lower intensities), muscle adaptations only occurred during the first 12 weeks (Hakkinen et al. 1985a). In the same way, although some neural adaptations occur after 12 weeks of resistance training, in comparison, other are improved when 4-year resistance-trained subjects are tested (Balshaw et al. 2019), which could means that not all neural adaptations to resistance training occur in the first months of training, but they could be enhanced by years of resistance training. In this line, strength gains in resistance-trained individuals cannot always being explained by muscle growth (Dankel et al. 2017a), and while strength is highly associated to muscle hypertrophy, it could not be possible if the central nervous system does not recruit muscles effectively to produce force (Taber et al. 2019). Other factors including intrinsic changes on the myocytes (not hypertrophy) occur contributing to the neuromuscular performance (Chin et al. 1998; Dankel et al. 2019). Neural adaptations increasing the neural drive to the muscles have been shown in highly skilled athletes after 16 weeks of resistance training divided into four phases: (1) strength conditioning phase (~ 4 sets of 8–10 repetitions at 60% of 1RM for each exercise), weeks 1–4; (2) strength development phase (~ 1–4 sets of five repetitions at 85% of 1RM for each exercise), weeks 5–8; (3) strength–power phase (~ 1–4 sets of eight repetitions at 80% of 1RM for each exercise), weeks 9–12; and (4) peaking and maintenance phase, where exercises were limited to 3–5 repetitions of the classic power lifts and Olympic lifts at 80–90% of 1RM during the weeks 13–16 (Judge et al. 2003). In addition, in a case study, two active healthy subjects, one of them experienced in resistance-trained, increased their strength and voluntary activation after 8 weeks of a resistance training program consisting of high-intensity concentric and eccentric isokinetic exercise at 8–10 reps and three sets of traditional whole-body resistance training exercises at 8–10 RM (Brown et al. 2017). Taken the actual evidence together, it seems that neural adaptations could accompany structural adaptations and contribute to strength gains in resistance-trained subjects, because if not, hypertrophy could not be related to strength as it does because for muscles to produce forces, central nervous system has to recruit them.
Muscle hypertrophy adaptations induced by resistance training have been shown to be similar using high (> 60% of 1RM) or low (≤ 60% of 1RM) loads, but strength gains are greater training with high loads (Schoenfeld et al. 2017b; Lasevicius et al. 2018). In this sense, performing 3 set of leg extension to failure with 2 min of inter-set rest at 80% of 1RM during 6 weeks (3 times per week) has demonstrated to be more effective to increase 1RM and MVIC than performing the same training at 30% 1RM, while muscle thickness increased similarly in both groups (Jenkins et al. 2017). Resistance training at 80% 1RM also provoked greater improvements in voluntary activation during MVIC and was the only load that increased electromyographic amplitude, which means that greater neural adaptations were induced training at higher loads (Jenkins et al. 2017). In the same way, a classic study that evaluated the training adaptations of 13 elite weight-lifter during 1 year showed that during the 5–8 months of training, when they trained at the lowest average intensity (77.1%), the maximal neural activation measured by EMG decreased, while in the last 4 months, when they trained at slightly higher average intensities (79.1%), the maximum EMG and muscular strength increased (Häkkinen et al. 1987). Besides, another study that equated volume load reported lower relative increases of 1RM after training bench press during 10 weeks (2 days per week) at 12RM (18.7 ± 10.1%) compared to 8RM (29.5 ± 11.6%) or 4RM (28.4 ± 10.0%) (Kubo et al. 2021). Knowing that reaching failure during sets, motor units are fully recruited regardless of the load used (Morton et al. 2019) and central fatigue (i.e., fatigue attributed to processes within the nervous system) is not greater training at higher loads (Behm et al. 2002; Robbins et al. 2010; Farrow et al. 2021), mechanism of different neural adaptations between loads are still unknown. Despite this, motor units’ recruitment pattern differs between high and low loads. In this sense, an isometric-based model of fatiguing tasks at different percentages of MVIC force has been recently proposed (Potvin and Fuglevand 2017). This work showed that when applying forces against a low (i.e., 20% MVIC) or moderate load (i.e., 50% MVIC), all motor units will be recruited during more time, but higher threshold motor unit will not reach maximal firing rate, and therefore, they will not reach their maximal force production (Potvin and Fuglevand 2017). Instead, when applying forces against a high (i.e., 80% MVIC) or maximal load (i.e., 100% MVIC), all motor units reach their maximal firing rate, but they are activated during less time (Potvin and Fuglevand 2017). Knowing that an improvement of 16% have been shown for muscular endurance when following a low-load training, while no improvement was shown for high-load training (Schoenfeld et al. 2015), it is logical to think that not only muscle structural but also neural adaptations are specific of the resistance training type performed. In this sense, it is possible that while nervous system adapts to low-load training with the aim of being more efficient and energy saver modulating motor units recruitment pattern (De Luca and Contessa 2015) to increase long-duration task performance, high-load training adaptations could emphasize in the improvement of the maximal muscle force production in short time tasks such as higher voluntary activation when performing an MVIC (Jenkins et al. 2017). In this sense, a recent study found that in resistance-trained individuals, 3 weeks of a “strength training phase” (i.e., 4 sets of back squat and leg press 45° at 1–3 RM twice a week) prior to 5 weeks of a “hypertrophy oriented training” (i.e., 4 sets of back squat and leg press 45° at 8–12 RM twice a week), increased muscle thickness and maximum strength responses compared to 8 weeks of only hypertrophy oriented training (Carvalho et al. 2020). Despite this, not only the possible greater neural adaptations but also other reasons such as the stimulus variation or the lower fatigue period could have affected to the results. The role of neural adaptations increasing force production by maximal muscle activation in hypertrophy responses, is still unknown and more research is needed.
Stimulus and fatigue in hypertrophy training
What is the actual training stimulus for skeletal muscle hypertrophy?
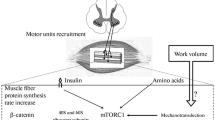
The main goal of hypertrophy-based resistance training programs is to induce an increase in muscle protein synthesis (MPS), to get a positive protein net balance (Damas et al. 2015; Figueiredo 2019). The skeletal muscle fiber is a mechanically sensitive cell and it has been proposed that muscle fiber growth is mainly dependent on intrinsic properties (Olsen et al. 2019; Wackerhage et al. 2019). Through different mechanotransducting proteins and complexes, the outside of the cell (extracellular matrix) and the inside (citoesqueleton) are linked, allowing signal transduction from inside to outside and vice versa and converting mechanical events into molecular and biochemical responses (Olsen et al. 2019). In summary, the force generated by each muscle fiber is transmitted laterally and longitudinally activating different mechanical sensors that triggers anabolic signalling events, increasing acutely MPS and generating chronic physiological adaptations that will finally favour muscle hypertrophy. Along with mechanical forces (stimuli proposed as the main muscle hypertrophy mechanism), metabolic stress and muscle damage have been proposed as hypertrophy mechanisms (Wackerhage et al. 2019). Despite this, studies finding metabolic stress or muscle damage as hypertrophy stimulus could be affected by confounding factors such as mechanical force. It has been proposed that the effect of metabolic stress triggering muscle hypertrophy is mainly related with the increased motor units recruitment provoked by muscle fatigue to maintain the mechanical forces when a set is close to failure (Dankel et al. 2017b). In fact, although low loads (i.e., ≤ 60% of 1RM) provoke higher levels of metabolic stress, they do not lead more muscle hypertrophy than higher loads (i.e., > 60% of 1RM) (Schoenfeld et al. 2017b). A recent study showed that when volume load was equated and sets were performed to failure, training at 40%, 60%, or 80% of 1RM was effective for maximizing muscle hypertrophy but not training at 20% of 1RM (Lasevicius et al. 2018). Besides, another recent study equating volume load reported no differences in the volume of pectoralis major muscle measured by 3 T magnetic resonance imaging after training bench press at 4RM, 8RM, or 12RM during 10 weeks (2 days per week) (Kubo et al. 2021). In the same way, although 1-min inter-set rest induces more metabolite accumulation, resting 3 min triggers greater muscle growth when there are not significant differences in volume load (Schoenfeld et al. 2016b) and when performing the same sets (3 sets) without equalized volume load (Longo et al. 2020). Also, a study that compared 6 weeks of blood flow restriction training at 30% of 1RM to increase metabolic stress with traditional resistance training at 70% of 1RM found no differences in long-term myofibrillar MPS, ribosomal biogenesis, or muscle remodelling (Sieljacks et al. 2019). However, a recent work found that the addition of blood flow restriction enhanced type 1 fibers myofiber hypertrophy and myonuclear addition after 6.5 weeks of resistance training in national-level powerlifters (BjØrnsen et al. 2019), what could suggest an independent way of metabolic stress inducing muscle growth. Although it is still not clear how important are acute increases in hormonal levels induced by resistance training in healthy subjects for the whole skeletal muscle hypertrophy stimulus (Gharahdaghi et al. 2021), the role of neuroendocrine signalling in the process of repair and remodelling of muscle fibers should be considered (Kraemer et al. 2020) and metabolic stress influences it (Schoenfeld 2013).
The immunological and satellite cells’ (SC) responses to resistance training have been shown to be determinant for muscle remodelling and hypertrophy (Fukada et al. 2020), which has been one of the main proposed mechanisms why muscle damage was thought to induce muscle hypertrophy. For example, larger hypertrophy seems to be experienced after eccentric damaging training compared to concentric (Schoenfeld et al. 2017c). However, this effect could be due to the higher mechanical forces caused by eccentric training. In this sense, a study published in 2014 discovered for the first time that there was a relationship between the acute temporal SC response to resistance training at 80% of 1RM and the accretion of lean mass as a result of exercise training (Bellamy et al. 2014). In the same way, a recent study confirmed these results for both males and females after 8 weeks of whole-body resistance training at 75% of 1RM (Sawan et al. 2021). As shown in studies cited above, SC responses occurs after the traditional resistance training and there is no need to accentuate muscle damage. In fact, a study where both trained and naïve subjects performed an 8 weeks lower body eccentric resistance training, although naïve subjects experienced higher muscle damage, both groups experienced the same quadriceps hypertrophy (Flann et al. 2011). Besides, a recent study reported that muscle damage measured directly by Z‐band streaming did not correlate with myofibrillar MPS or hypertrophy at any time point during 10 weeks of resistance training (3 sets of 45° leg press and 3 sets of leg extension exercises at 9–12 maximal repetitions) (Damas et al. 2016). Myofibrillar MPS was only correlated to muscle hypertrophy in the third and tenth weeks when muscle damage was attenuated but not in the first one when muscle damage was at the highest levels (Damas et al. 2016).
As muscle damage or metabolic stress protocols were not able to separate them from confounding stimuli such as mechanical forces, all studies investigating skeletal muscle hypertrophy induced by resistance training involve a common confounding factor, the neural stimulus. As explained before, the production of mechanical forces through muscle fibers contractions is modulated by the central nervous system through regulating the amount of motor units recruited and their firing rates (Duchateau and Baudry 2014; Enoka and Duchateau 2017). The neural stimulus is not only decisive for skeletal muscle hypertrophy, because it modulates muscle mechanical forces and then also metabolic stress and muscle damage but also because it could be a stimulus for muscle adaptations itself. In this sense, in an interesting study from 1960, Buller and colleagues cut motoneurons axons of fast-twitch motor units (that predominantly innervate type 2 muscle fibers) and of slow-twitch motor units (that predominantly innervate type 1 muscle fibers) from cat muscles and crossed them (Buller et al. 1960). Now, fast-twitch motoneurons innervated slow-twitch muscle fibers and slow-twitch motoneurons innervated fast-twitch muscle fibers. They found that slow-twitch muscle fibers were transformed into fast-twitch muscle fibers and vice versa (Buller et al. 1960). Considering that motoneurons characteristics did not change, it seemed that the muscle fiber type is dependent on how its motoneuron activates and can adapt accordingly. In 1998, after showing that treating the adult soleus muscle of rats with calcineurin inhibitor cyclosporin A partially transformed type 1 into type 2 muscle fibers, Chin and colleagues proposed that muscle fiber-type adaptations in response to motor nerve activity were controlled by a signalling mechanism that involves calcineurin, a cyclosporin-sensitive, calcium-regulated serine/threonine phosphatase (Chin et al. 1998). Frequent motor nerve activity, characteristic of slow-twitch motor units, would result in sustained elevations of intracellular Ca2+ and activate the calcineurin–NFAT (i.e., nuclear factor of activated t cells) pathway. MEF2 (i.e., myocyte enhance factor 2) require the collaboration between activated NFAT proteins and muscle-restricted transcription factors in slow-fiber-specific gene transcription, along with other proteins. When NFAT proteins are unavailable for DNA binding and protein–protein interactions at target promoters, the slow-fiber-specific program is down-regulated, and genes encoding fast-fiber-specific proteins are transcribed (Chin et al. 1998). Later studies support that calcineurin-NFAT signalling acts as a nerve activity sensor in skeletal muscle and controls nerve activity-dependent myosin switching (Serrano et al. 2001; McCullagh et al. 2004). Note that several studies reported that calcineurin–NFAT signalling is also key for skeletal muscle hypertrophy (Dunn et al. 1999; Sakuma et al. 2003; Miyazaki et al. 2006) and could work via MEF2, myostatin, and FOXO (i.e., forkhead box O) (Sakuma and Yamaguchi 2010; Mukund and Subramaniam 2020).
Calcineurin–NFAT signalling is not the only pathway that modulates nerve activity-dependent muscle growth. RasC40, a Ras mutant that selectively activates the phosphoinositide 3-kinase (PI3K) and its downstream target, the serine–threonine protein kinase B (PKB), also known as AKT, has been able to induce hypertrophy of transfected muscles (Murgia et al. 2000). In this study, authors denervated rat soleus muscles and found that when transfected with HA-tagged RasC40, the size of the fibers was comparable with those that were innervated and about three times larger than other denervated fibers (Murgia et al. 2000). Note that the major signalling pathway that regulates muscle growth is the PI3K–PKB signalling pathway via its downstream target mammalian target of rapamycin (mTOR) (Mukund and Subramaniam 2020). In this sense, Pallafacchina and colleagues found in 2002 that the increase in muscle fibers size induced by innervation and electrostimulation in regenerating muscles is partially inhibited by dominant negative inhibitor of PKB and rapamycin (Pallafacchina et al. 2002). Besides, authors discovered that RasC40 and PKB prevented the denervation atrophy in vivo in regenerating rat skeletal muscle. However, when muscle hypertrophy of denervated fibers was induced by RasC40 and constitutively active PKB, rapamycin completely blocked it, being mTOR the main PKB-dependent pathway that controls nerve activity-dependent muscle growth (Pallafacchina et al. 2002). In fact, when high-frequency (an intermittent 100 Hz protocol) and low-frequency (3 h at 10 Hz) electrical stimulations have been applied to rat isolated muscles to simulate resistance and endurance training respectively, high-frequency electrical increased myofibrillar and sarcoplasmic protein synthesis by activating PKB-tuberin-mTOR and its downstream translational regulators, while low-frequency electrical stimulation did not (Atherton et al. 2005).
Regardless of the mechanisms involved on nerve activity-dependent muscle growth, it is already clear that not only mechanical forces but also the excitation of membrane of the muscle fibers provoked by the neural action potentials inducing Ca2+-release is needed for an optimal the hypertrophy stimulus. In this sense, an interesting recent study explored the effects of the excitation–contraction coupling and mechanical force development on signals regulating transcription, translation, and protein synthesis in isolated rat extensor digitorum longus muscles (Rindom et al. 2021). Authors manipulated the elements of different steps of the excitation–contraction coupling sequence by combining (a) the excitation-induced Ca2+ release by electrical stimulation, (b) blocking muscle contractions by chemically inhibiting the myosin ATPase, and (c) passive stretch to achieve mechanical force at equal amounts compared to actively contracting muscles. They found that although signalling for translation initiation is only dependent on muscle force per se, acute increases in protein synthesis and signalling for transcriptional regulation of myofibrillar genes are dependent of both mechanical force and muscle excitation inducing Ca2+ release (Rindom et al. 2021). Thus, considering that not only mechanical forces but also muscle excitation by action potentials seem to be needed for increasing protein synthesis responsible for muscle hypertrophy, we propose that measuring the neural stimulus would represent the actual muscle hypertrophy stimulus. Note that in real resistance training, muscle mechanical forces are dependent on the neural stimulus and they could not be separated. Remember that decoding the neural activity of motor units using HDEMG, the mechanical forces could be accurately estimated (Sartori et al. 2017).
Relation between stimulus and fatigue
As explained above, for skeletal muscles to grow after performing resistance training, muscle fibers have to be recruited by the nervous system generating forces against a load. The mechanical forces produced along with the neural stimulus and other factors such as metabolic stress, which is also produced by the metabolite accumulation originated by the metabolism that support muscle contractions, will stimulate muscle fibers to grow. Although stimulus and fatigue are linked, they are not the same. In this sense, it is known that more fatigue produced by resistance training will not always induce more strength gains (Pareja-Blanco et al. 2017, 2020), which means that the same stimulus to get stronger was provoked when less fatigue occurred. About hypertrophy measurements, although higher training volume is associated with higher muscle gains (Krieger 2010; Schoenfeld et al. 2017a, 2019a), it has been shown that there is a volume threshold from which more training volume does not mean more muscle gains, or even less muscle gains (Amirthalingam et al. 2017; Heaselgrave et al. 2019). Knowing that more training volume provoke more fatigue (Bartolomei et al. 2017), these results demonstrate that more fatigue does not always cause more muscle growth. In this line, when training volume is equated, although reaching failure promotes greater fatigue than not (Fonseca et al. 2020), training to failure does not always cause more muscle gains (Terada et al. 2020). Thus, taking fatigue as a synonymous of stimulus is a great mistake.
How to measure the training stimulus for skeletal muscle hypertrophy?
Muscle adaptations are specific to the training stimulus (Chin et al. 1998; Tillin and Folland 2014; Pareja-Blanco et al. 2017). However, defining and measuring the training stimulus are a controversial and difficult topic. In this sense, aiming to measure the resistance training stimulus, variables such as volume or intensity use to be quantified (Marston et al. 2017). To quantify training volume, it is common to measure total repetitions, time under tension, or the volume load (sets*repetitions*load) performed (Mcbride et al. 2009; Marston et al. 2017). However, it has been proposed that displacement could be added to the volume load (sets*repetitions*load*displacement) for greater precision (Hornsby et al. 2018). In this line, it has been suggested that total work (force*displacement) is the most accurate measurement of resistance training stimulus (Mcbride et al. 2009). Despite this, other variables such as training intensity or density, are missed. In this sense, it has been proposed to measure exercise density in two different ways, one of them combining total work and time in seconds (total work/time) and the other one using volume load (volume load/time) (Marston et al. 2017). Exercise density has been shown to better correlate with markers of metabolic stress (Marston et al. 2017). However, the same problem is also present in these metrics, other training variables are missed. Training intensity is one of the most important variables for the resistance training stimulus (Schoenfeld et al. 2015). Training intensity has been measured in different ways such as load (Marston et al. 2017) or percentage of one repetition maximum (Thompson et al. 2020). Despite this, using repetition maximum targets or repetitions in reserve in the training sets would improve the training intensity prescription by a better precision of the level of effort (Helms et al. 2016; Zourdos et al. 2016b; Dos Santos et al. 2020). Note that strength performance change depending on when it is measured (Zourdos et al. 2016a; Gantois et al. 2021). Considering that each subject could perform different maximum repetitions against the same percentage of 1RM (Hoeger et al. 1990), if measuring the velocity of the repetitions is not practical or possible, monitoring the level of effort is a useful strategy due to its low intersubject and intrasubject variability among different strength levels, intensities, and exercises (Hernández-Belmonte et al. 2021).
Resistance training stimulus is affected by all training variable. Similarly, if an accurate method to quantify all training variables is ever reached, only the external load parameters would be monitored. Trying to link external and internal load parameters, promising methods, such as combining the repetitions performed with the session rating of perceived exertion (Martorelli et al. 2021), have being applied. Nevertheless, most of methods used to measure training stimulus only focus in kinetic and kinematic variables, focusing in external load parameters through indirect measurements far from the actual training stimulus, or physiological parameters such as acute hormonal responses. Evidence related to acute hormonal response is still not clear (Gharahdaghi et al. 2021), and even if it is relevant, it could be one of several responses to the application of a correct training stimulus. Neural data are missed in methods that try to measure resistance training stimulus. The measurement of motor units’ behaviour would imply an internal and direct approach of the stimulus that muscles fibers are receiving. It is known that the motor neurons behaviour will modulate the adaptations of the muscle fibers (Buller et al. 1960; Chin et al. 1998; McCullagh et al. 2004). Measuring data that represent the neural input to the muscle fibers (i.e., the amount of neural drive that the muscle fibers receive within a period of time) could be an interesting way of measuring the actual training stimulus. The neural input that muscle fibers receive will determinate the mechanical forces that modulate muscle hypertrophy (Olsen et al. 2019; Wackerhage et al. 2019) Also, the excitation of the muscle fibers by the nervous system will be a stimulus for increasing muscle protein synthesis itself (Rindom et al. 2021). In this sense, HDEMG could be an interesting method to use when trying to measure the actual resistance training stimulus. Despite this, a possible limitation of this method could be that the intrinsic proprieties of a muscle fiber, which could be modulated by phenomena such as fatigue (Allen et al. 2008), can modulate its responses to the neural input received.
Could fatigue improve or impair training adaptations?
Muscle fatigue is known as the reduction in the ability to produce force or power with a muscle or muscle group induced by exercise (Gandevia 2001; Taylor et al. 2016). Depending of the sites in which fatigue is produced, it can be divided as peripheral fatigue (i.e., fatigue attributed to processes at or distal to the neuromuscular junction) or central fatigue (i.e., fatigue due to processes within the central nervous system) (Taylor et al. 2016; Carroll et al. 2017). Despite this, central and peripheral fatigue are related and cannot be understood as separated levels, being nervous system behaviour affected by peripheral factors (Taylor et al. 2016). Due to peripheral fatigue, the ability of the muscle to produce force is declined because of many causes such as the deterioration of the action potential transmission through the membrane of the muscle fibers and its conduction through the T-tubules, the reduced Ca2+ release from the sarcoplasmic reticulum and its reuptake or the decrease of the Ca2+ sensitivity of myofibrillar proteins (Allen et al. 2008). Although several mechanisms promote peripheral fatigue, many of them are influenced by the accumulation of metabolites (Allen et al. 2008). On the other hand, central fatigue reduces the force produced by the muscles because of the reduction of the neural drive to them (Taylor et al. 2016) and it is due to processes within the central nervous system in both, spinal and supraspinal levels (Taylor et al. 2006, 2016; Tanaka and Watanabe 2012). Several neural changes such as disturbances in neurotransmitters homeostasis, changes in motor neuron excitability by its repetitive activation, reductions in the descending drive from the motor cortex or inhibitory afferent feedback, promote central fatigue (Taylor et al. 2016). Although Ib afferents group and Renshaw cell could not play an important role during physical fatigue (Gandevia 2001), the reduction of the motor neurons facilitation provoked by Ia afferents during fatiguing tasks seems to contribute to decline the motor units firing rate (Macefield et al. 1991). Besides, the increased firing from group III and IV muscle afferents caused by the muscle force and metabolite accumulation have documented to develop central fatigue affecting to both, spinal and supraspinal levels (Amann et al. 2011; Kennedy et al. 2014; Blain et al. 2016; Taylor et al. 2016; Sidhu et al. 2017, 2018). In this sense, these small-diameter muscle afferents could play an important role in central fatigue due to different causes such as inhibiting motor neurons directly and through the presynaptic inhibition of Ia afferents reducing or affecting supraspinal levels to reduce descending drive to the motor units (Taylor et al. 2016). In summary, because of several neural changes at different levels within the central nervous system, central fatigue will trigger in suboptimal muscle fiber recruitment to produce force.
Muscle fiber recruitment is not enough to produce hypertrophy. An example of this is that although a 1RM set fully recruits muscles, when it is performed by the elbow flexors of one arm during several training sessions along with a maximum voluntary contraction, no hypertrophy occurs (Dankel et al. 2017a). Despite this, when in addition, 3 sets were performed in the other arm, hypertrophy occurred (Dankel et al. 2017a). To provoke skeletal muscle hypertrophy, muscle fibers not only have to be recruited, but they also have to reach certain levels of force and fatigue (Dankel et al. 2017b), which is commonly reached going close to failure and performing multiple training sets. Thus, peripheral fatigue promoted partly by the metabolite accumulation, which is due to the metabolism that support muscle contractions to produce muscle force, could promote skeletal muscle hypertrophy (Dankel et al. 2017b). In contrast, knowing the role of the neural stimulus, muscle mechanical forces and peripheral fatigue in skeletal muscle hypertrophy, central fatigue could impair hypertrophic stimulus during training. Note that central fatigue reduces muscle force and attenuates peripheral fatigue by decreasing the neural drive to the muscles (Taylor et al. 2016). Related to this, it has been shown that mental fatigue induced by demanding cognitive tasks impairs the volume load performed in lower (de Queiros et al. 2021; Gantois et al. 2021) and upper body (Dorris et al. 2012; Graham et al. 2017) resistance training, being volume load related to hypertrophy gains (Krieger 2010; Schoenfeld et al. 2017a, 2019a). These effects of mental fatigue on physical performance have been reported regardless the duration of the cognitive task (Giboin and Wolff 2019) and even when the cognitive task was using social networks on a smartphone (Gantois et al. 2021). Although the mental fatigue induced by demanding cognitive tasks could be considered a distinct phenomenon than central fatigue and it involves different central nervous system functions and different brain areas (Pageaux et al. 2015), it could also impair the stimulus for muscle growth.
Central fatigue and resistance training programming variables
Central fatigue is affected by training variables. In this regard, longer duration’s endurance tasks have demonstrated to increase central fatigue more than shorter tasks (Behm and St-Pierre 1997; Eichelberger and Bilodeau 2007; Smith et al. 2007; Yoon et al. 2007; Pearcey et al. 2015; Goodall et al. 2015). About resistance training, different studies found no differences in central fatigue between different loads when performing a single set of biceps curl at 5RM, 10RM, or 20RM (Behm et al. 2002) or when comparing equal volume load protocols of multiple sets performing biceps curls at 5RM and 10RM (Robbins et al. 2010). Despite this, recent evidence is in line with endurance task studies, showing that although volume load was equated and every set was performed to failure, performing unilateral knee extensions at 40% of MVC reaching an average of induces greater central fatigue when compared to 80% of MVC (Farrow et al. 2021). Thus, although the lighter load group performed only one set to match volume load with heavier load group, which performed an average of 1.8 sets, longer set duration promoted greater central fatigue (Farrow et al. 2021). Note that the average total number of repetitions of the heavier load group was 14.8, performing more sets, while for the lighter load group was 27.2. The fatigue provoked by leg extension exercise was not only greater for the exercised leg in the lighter load group but also this load provoked fatigue in the other leg that was not exercised, while higher load group did not (Farrow et al. 2021). It is known that central fatigue can affect not only exercised muscles but also others that were resting (Halperin et al. 2014a, b). Also, greater perceived effort has been shown using light loads (Fisher and Steele 2017; Farrow et al. 2021), which is linked to central fatigue (Taylor and Gandevia 2008). In this sense, although mechanisms are still unknown, very light loads (i.e., 20% of 1RM) have shown to promote less muscle gains even when volume load is matched and sets are performed to failure (Lasevicius et al. 2018). Central fatigue, which can last for days and is linked to peripheral factors such as muscle damage (Carroll et al. 2017; Macgregor and Hunter 2018), and its affection to exercised and non-exercised muscles, could be interesting to consider when programming resistance training variables such as load, exercise selection, range of motion, or exercise order. Despite this, although longer sets tend to induce greater fatigue (Marshall et al. 2015; Bartolomei et al. 2017; Carroll et al. 2017; Farrow et al. 2021), it could be more difficult for resistance-trained subjects to experience central fatigue (Marshall et al. 2015). On the other hand, interference between different physical activities should be considered because of the performance impairment. For example, knowing that high-intensity running tasks could provoke a reduction in the voluntary activation of the knee extensors (Goodall et al. 2015), it is not interesting to perform a resistance training targeting knee extensors after that. For maximize adaptations, both trainings should be separated.
Conclusions
It would be a mistake to only focus in muscle tissue when resistance training is performed targeting skeletal muscle hypertrophy. Central nervous system is responsible of muscle force and controls human movement. In this regard, motor unit activity will greatly influence the stimulus promoted by resistance training. Thus, the modulation of the muscle fiber recruitment and the amount of force produced by them depends on central nervous system activity. In this way, future research should explore the role that neural activity has in the hypertrophic response to resistance training. Therefore, neurophysiological measurements such as voluntary activation, motor units activity measured by HDEMG or neuronal excitability/inhibition, should be tested to explore their role in the muscle growth stimulus promoted by training. This is important not only for professional athletes but also for patients aiming to achieve optimal results in their body composition. Besides, knowing that muscle fiber adaptations are influenced by the properties of their motor neurons, measuring neural activity to recruit muscles such as motor unit activity, could help to investigate skeletal muscle hypertrophy mechanisms.
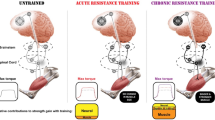
Based on the current literature, it is known that the human physiology is not static but dynamic. In this sense, changes in nervous system activity provoked by different reasons such as neural adaptations, central fatigue or mental fatigue, could affect to the stimulus induced by resistance training to promote skeletal muscle hypertrophy. Therefore, apart from the relation between neural changes and muscle hypertrophy, the optimal manipulation of training variables to promote the possible neural changes to advantage muscle hypertrophy should also be investigated. To facilitate the understanding of these applications, Fig. 2 provides examples to show possible changes in neural activity that could hypothetically influence muscle hypertrophy.
Overview of two different situations in which changes in nervous system behaviour could hypothetically influence the hypertrophy stimulus induced by resistance training. The baseline of the neuromuscular system [including not only musculotendinous but also central nervous system (CNS) and peripheral nervous system (PNS) levels] is represented at the top of the figure (1). In the left side, effects of impaired neural drive to the muscles from the motor neurons (MNs) of the spinal cord (SC) due to factors such as central fatigue reducing muscle force and then, possibly the hypertrophy stimulus induced by resistance training, are depicted (2). Conversely, the possible positive effects of the neural drive optimization originated by acute or chronic adaptations are also represented in the right side of the figure (3)
Neural science and hypertrophy training should be closer when trials are performed. Future research should link neural and muscle measurements.
Practical applications
It could be interesting to perform sets in different range of repetitions (i.e., 4–25), with set at ≥ 80% of 1RM being essential to ensure that the potential specific neuromuscular adaptations of the different ranges are beneficiating the muscle hypertrophy results from training. Besides, it is beneficial to avoid demanding cognitive tasks (e.g., studying or even using social media) regardless of their duration or strenuous physical activity of the targeted muscles before training them for muscle growth. Similarly, knowing that non-local fatigue (i.e., fatigue in non-exercised muscles) could occur, it is important to prioritize exercises that target the more important muscles to train when programming the exercises order, especially for long and strenuous resistance training sessions in which low-load (i.e., < 60% 1RM) maximal sets are performed.
When researching about muscle growth induced by resistance training, neurophysiological measurements should be performed to discover the potential effects of the nervous system behaviour on the muscle adaptations. Thus, knowing the key role of muscle force on muscle hypertrophy, variables such as motor units’ activity measured by HDEMG, voluntary activation, or neuronal excitability/inhibition should be tested and related to the manipulated variables of the resistance training and the induced muscle adaptations.
Abbreviations
- HDEMG:
-
High-density surface electromyography
- MPS:
-
Muscle protein synthesis
- MVIC:
-
Maximum voluntary isometric contraction
- RFD:
-
Rate of force development
- SC:
-
Satellite cells (SC)
- 1RM:
-
One repetition maximum
References
Aagaard P, Simonsen EB, Andersen JL et al (2002) Neural adaptation to resistance training: changes in evoked V-wave and H-reflex responses. J Appl Physiol 92:2309–2318. https://doi.org/10.1152/japplphysiol.01185.2001
Alix-Fages C, Romero-Arenas S et al (2019) Short-term effects of anodal transcranial direct current stimulation on endurance and maximal force production. A systematic review and meta-analysis. J Clin Med 8:536. https://doi.org/10.3390/jcm8040536
Alix-Fages C, García-Ramos A, Calderón-Nadal G et al (2020) Anodal transcranial direct current stimulation enhances strength training volume but not the force–velocity profile. Eur J Appl Physiol 120:1881–1891. https://doi.org/10.1007/s00421-020-04417-2
Alix-Fages C, Garcia-Ramos A, Romero-Arenas S et al (2021) Transcranial direct current stimulation does not affect sprint performance or the horizontal force-velocity profile. Res Q Exerc Sport. https://doi.org/10.1080/02701367.2021.1893260
Allen DG, Lamb GD, Westerblad H (2008) Skeletal muscle fatigue: cellular mechanisms. Physiol Rev 88:287–332. https://doi.org/10.1152/physrev.00015.2007
Amann M, Blain GM, Proctor LT et al (2011) Implications of group III and IV muscle afferents for high-intensity endurance exercise performance in humans. J Physiol 59:5299–5309. https://doi.org/10.1113/jphysiol.2011.213769
Amirthalingam T, Mavros Y, Wilson GC et al (2017) Effects of a modified German volume training program on muscular hypertrophy and strength. J Strength Cond Res 31:3109–3119. https://doi.org/10.1519/JSC.0000000000001747
Angius L, Hopker J, Mauger AR (2017) The ergogenic effects of transcranial direct current stimulation on exercise performance. Front Physiol 8:90. https://doi.org/10.3389/fphys.2017.00090
Atherton PJ, Babraj JA, Smith K et al (2005) Selective activation of AMPK-PGC-1α or PKB-TSC2-mTOR signaling can explain specific adaptive responses to endurance or resistance training-like electrical muscle stimulation. FASEB J 19:786–788. https://doi.org/10.1096/fj.04-2179fje
Avrillon S, Del Vecchio A, Farina D et al (2021) Individual differences in the neural strategies to control the lateral and medial head of the quadriceps during a mechanically constrained task. J Appl Physiol 130:269–281. https://doi.org/10.1152/JAPPLPHYSIOL.00653.2020
Balshaw TG, Massey GJ, Maden-Wilkinson TM et al (2019) Neural adaptations after 4 years vs 12 weeks of resistance training vs untrained. Scand J Med Sci Sport 29:348–359. https://doi.org/10.1111/sms.13331
Barbosa-Netto S, D’Acelino-E-Porto OS, Almeida MB (2021) Self-selected resistance exercise load: implications for research and prescription. J Strength Cond Res 35:S166–S172. https://doi.org/10.1519/JSC.0000000000002287
Bartolomei S, Sadres E, Church DD et al (2017) Comparison of the recovery response from high-intensity and high-volume resistance exercise in trained men. Eur J Appl Physiol 117:1287–1298. https://doi.org/10.1007/s00421-017-3598-9
Baz-Valle E, Fontes-Villalba M, Santos-Concejero J (2018) Total number of sets as a training volume quantification method for muscle hypertrophy. J Strength Cond Res. https://doi.org/10.1519/JSC.0000000000002776
Behm DG, St-Pierre DMM (1997) Effects of fatigue duration and muscle type on voluntary and evoked contractile properties. J Appl Physiol 82:1654–1661. https://doi.org/10.1152/jappl.1997.82.5.1654
Behm DG, Reardon G, Fitzgerald J, Drinkwater E (2002) The effect of 5, 10, and 20 repetition maximums on the recovery of voluntary and evoked contractile properties. J Strength Cond Res 16:209–218. https://doi.org/10.1519/1533-4287(2002)016%3c0209:TEOARM%3e2.0.CO;2
Bellamy LM, Joanisse S, Grubb A et al (2014) The acute satellite cell response and skeletal muscle hypertrophy following resistance training. PLoS ONE 9:e109739. https://doi.org/10.1371/journal.pone.0109739
BjØrnsen T, Wernbom M, Kirketeig A et al (2019) Type 1 muscle fiber hypertrophy after blood flow-restricted training in powerlifters. Med Sci Sports Exerc 51:288–298. https://doi.org/10.1249/MSS.0000000000001775
Blain GM, Mangum TS, Sidhu SK et al (2016) Group III/IV muscle afferents limit the intramuscular metabolic perturbation during whole body exercise in humans. J Physiol 594:5303–5315. https://doi.org/10.1113/JP272283
Brown N, Bubeck D, Haeufle DFB et al (2017) Weekly time course of neuro-muscular adaptation to intensive strength training. Front Physiol 8:329. https://doi.org/10.3389/fphys.2017.00329
Buller AJ, Eccles JC, Eccles RM (1960) Interactions between motoneurones and muscles in respect of the characteristic speeds of their responses. J Physiol 150:417–439. https://doi.org/10.1113/jphysiol.1960.sp006395
Calderón JC, Bolaños P, Caputo C (2014) The excitation-contraction coupling mechanism in skeletal muscle. Biophys Rev 6:133–160. https://doi.org/10.1007/s12551-013-0135-x
Callister R, Callister RJ, Staron RS et al (1991) Physiological characteristics of elite judo athletes. Int J Sports Med 12:196–203. https://doi.org/10.1055/s-2007-1024667
Cao N, Pi Y, Liu K et al (2018) Inhibitory and facilitatory connections from dorsolateral prefrontal to primary motor cortex in healthy humans at rest: an rTMS study. Neurosci Lett 687:82–87. https://doi.org/10.1016/j.neulet.2018.09.032
Carolan B, Cafarelli E (1992) Adaptations in coactivation after isometric resistance training. J Appl Physiol 73:911–917. https://doi.org/10.1152/jappl.1992.73.3.911
Carroll TJ, Riek S, Carson RG (2002) The sites of neural adaptation induced by resistance training in humans. J Physiol 544:641–652
Carroll TJ, Taylor JL, Gandevia SC (2017) Recovery of central and peripheral neuromuscular fatigue after exercise. J Appl Physiol 122:1068–1076. https://doi.org/10.1152/japplphysiol.00775.2016
Carvalho L, Junior RM, Truffi G et al (2020) Is stronger better? Influence of a strength phase followed by a hypertrophy phase on muscular adaptations in resistance-trained men. Res Sport Med. https://doi.org/10.1080/15438627.2020.1853546
Casolo A, Del Vecchio A, Balshaw TG et al (2021) Behavior of motor units during submaximal isometric contractions in chronically strength-trained individuals. J Appl Physiol 131:1584–1598. https://doi.org/10.1152/japplphysiol.00192.2021
Cheney PD (1985) Role of cerebral cortex in voluntary movements: a review. Phys Ther 65:624–635. https://doi.org/10.1093/ptj/65.5.624
Chin ER, Olson EN, Richardson JA et al (1998) A calcineurin-dependent transcriptional pathway controls skeletal muscle fiber type. Genes Dev 12:2499–2509. https://doi.org/10.1101/gad.12.16.2499
Clamann HP (1993) Motor unit recruitment and the gradation of muscle force. Phys Ther 73:830–843. https://doi.org/10.1093/ptj/73.12.830
Colomer-Poveda D, Hortobágyi T, Keller M et al (2020) Training intensity-dependent increases in corticospinal but not intracortical excitability after acute strength training. Scand J Med Sci Sport 30:652–661. https://doi.org/10.1111/sms.13608
Damas F, Phillips S, Vechin FC, Ugrinowitsch C (2015) A review of resistance training-induced changes in skeletal muscle protein synthesis and their contribution to hypertrophy. Sport Med 45:801–807. https://doi.org/10.1007/s40279-015-0320-0
Damas F, Phillips SM, Libardi CA et al (2016) Resistance training-induced changes in integrated myofibrillar protein synthesis are related to hypertrophy only after attenuation of muscle damage. J Physiol 594:5209–5222. https://doi.org/10.1113/JP272472
Dankel SJ, Counts BR, Barnett BE et al (2017a) Muscle adaptations following 21 consecutive days of strength test familiarization compared with traditional training. Muscle Nerve 56:307–314. https://doi.org/10.1002/mus.25488
Dankel SJ, Mattocks KT, Jessee MB et al (2017b) Do metabolites that are produced during resistance exercise enhance muscle hypertrophy? Eur J Appl Physiol 117:2125–2135. https://doi.org/10.1007/s00421-017-3690-1
Dankel SJ, Kang M, Abe T, Loenneke JP (2019) Resistance training induced changes in strength and specific force at the fiber and whole muscle level: a meta-analysis. Eur J Appl Physiol 119:265–278. https://doi.org/10.1007/s00421-018-4022-9
De Luca CJ, Contessa P (2015) Biomechanical benefits of the onion-skin motor unit control scheme. J Biomech 48:195–203. https://doi.org/10.1016/j.jbiomech.2014.12.003
de Queiros VS, Dantas M, de Fortes LS et al (2021) Mental fatigue reduces training volume in resistance exercise: a cross-over and randomized study. Percept Mot Skills 128:409–423. https://doi.org/10.1177/0031512520958935
del Olmo MF, Reimunde P, Viana O et al (2006) Chronic neural adaptation induced by long-term resistance training in humans. Eur J Appl Physiol 96:722–728. https://doi.org/10.1007/s00421-006-0153-5
Del Vecchio A, Farina D (2019) Interfacing the neural output of the spinal cord: robust and reliable longitudinal identification of motor neurons in humans. J Neural Eng 17:016003. https://doi.org/10.1088/1741-2552/ab4d05
Del Vecchio A, Negro F, Falla D et al (2018) Higher muscle fiber conduction velocity and early rate of torque development in chronically strength-trained individuals. J Appl Physiol 125:1218–1226. https://doi.org/10.1152/japplphysiol.00025.2018
Del Vecchio A, Casolo A, Negro F et al (2019a) The increase in muscle force after 4 weeks of strength training is mediated by adaptations in motor unit recruitment and rate coding. J Physiol 597:1873–1887. https://doi.org/10.1113/JP277250
Del Vecchio A, Falla D, Felici F et al (2019b) The relative strength of common synaptic input to motor neurons is not a determinant of the maximal rate of force development in humans. J Appl Physiol 127:205–214. https://doi.org/10.1152/japplphysiol.00139.2019
Del Vecchio A, Negro F, Holobar A et al (2019c) You are as fast as your motor neurons: speed of recruitment and maximal discharge of motor neurons determine the maximal rate of force development in humans. J Physiol 597:2445–2456. https://doi.org/10.1113/JP277396
Del Vecchio AD, Sylos-Labini F, Mondì V et al (2020) Spinal motoneurons of the human newborn are highly synchronized during leg movements. Sci Adv 6:eabc3916. https://doi.org/10.1126/sciadv.abc3916
Del Vecchio A, Casolo A, Dideriksen JL et al (2021a) Lack of increased rate of force development after strength training is explained by specific neural, not muscular, motor unit adaptations. J Appl Physiol. https://doi.org/10.1152/japplphysiol.00218.2021
Del Vecchio A, Jones RHA, Schofield IS et al (2021b) Interfacing spinal motor units in non-human primates identifies a principal neural component for force control constrained by the size principle. bioRxiv. https://doi.org/10.1101/2021.12.07.471592
Desmedt JE, Godaux E (1977) Ballistic contractions in man: characteristic recruitment pattern of single motor units of the tibialis anterior muscle. J Physiol 264:673–693. https://doi.org/10.1113/jphysiol.1977.sp011689
Diamond A (2000) Close interrelation of motor development and cognitive development and of the cerebellum and prefrontal cortex. Child Dev 71:44–56. https://doi.org/10.1111/1467-8624.00117
Diamond A (2013) Executive functions. Annu Rev Psychol 64:135–168. https://doi.org/10.1146/annurev-psych-113011-143750
Dorris DC, Power DA, Kenefick E (2012) Investigating the effects of ego depletion on physical exercise routines of athletes. Psychol Sport Exerc 13:118–125. https://doi.org/10.1016/j.psychsport.2011.10.004
Dos Santos WM, Junior ACT, Braz TV et al (2020) Resistance-trained individuals can underestimate the intensity of the resistance training session: an analysis among genders, training experience, and exercises. J Strength Cond Res. https://doi.org/10.1519/JSC.0000000000003412
Duchateau J, Baudry S (2014) Maximal discharge rate of motor units determines the maximal rate of force development during ballistic contractions in human. Front Hum Neurosci 8:234. https://doi.org/10.3389/fnhum.2014.00234
Duchateau J, Semmler JG, Enoka RM (2006) Training adaptations in the behavior of human motor units. J Appl Physiol 101:1766–1775. https://doi.org/10.1152/japplphysiol.00543.2006
Dulhunty AF (2006) Excitation-contraction coupling from the 1950s into the new millennium. Clin Exp Pharmacol Physiol 33:763–772. https://doi.org/10.1111/j.1440-1681.2006.04441.x
Dunn SE, Burns JL, Michel RN (1999) Calcineurin is required for skeletal muscle hypertrophy. J Biol Chem 274:21908–21912. https://doi.org/10.1074/jbc.274.31.21908
Eichelberger TD, Bilodeau M (2007) Central fatigue of the first dorsal interosseous muscle during low-force and high-force sustained submaximal contractions. Clin Physiol Funct Imaging 27:298–304. https://doi.org/10.1111/j.1475-097X.2007.00751.x
Enoka RM (2015) Neuromechanics of human movement, 5th edn. Human Kinetics, London
Enoka RM, Duchateau J (2017) Rate coding and the control of muscle force. Cold Spring Harb Perspect Med 7:a029702. https://doi.org/10.1101/cshperspect.a029702
Farina D, Holobar A, Merletti R, Enoka RM (2010) Decoding the neural drive to muscles from the surface electromyogram. Clin Neurophysiol 121:1616–1623. https://doi.org/10.1016/j.clinph.2009.10.040
Farrow J, Steele J, Behm DG et al (2021) Lighter-Load exercise produces greater acute- and prolonged-fatigue in exercised and non-exercised limbs. Res Q Exerc Sport 92:369–379. https://doi.org/10.1080/02701367.2020.1734521
Figueiredo VC (2019) Revisiting the roles of protein synthesis during skeletal muscle hypertrophy induced by exercise. Am J Physiol Regul Integr Comp Physiol 317:R709–R718. https://doi.org/10.1152/ajpregu.00162.2019
Fisher JP, Steele J (2017) Heavier and lighter load resistance training to momentary failure produce similar increases in strength with differing degrees of discomfort. Muscle Nerve 56:797–803. https://doi.org/10.1002/mus.25537
Flann KL, Lastayo PC, McClain DA et al (2011) Muscle damage and muscle remodeling: no pain, no gain? J Exp Biol 214:674–679. https://doi.org/10.1242/jeb.050112
Fonseca FS, de Costa BDV, Ferreira MEC et al (2020) Acute effects of equated volume-load resistance training leading to muscular failure versus non-failure on neuromuscular performance. J Exerc Sci Fit 18:94–100. https://doi.org/10.1016/j.jesf.2020.01.004
Franchini E, Del Vecchio FB, Matsushigue KA, Artioli GG (2011) Physiological profiles of elite judo athletes. Sport Med 41:147–166. https://doi.org/10.2165/11538580-000000000-00000
Frontera WR, Ochala J (2015) Skeletal muscle: a brief review of structure and function. Behav Genet 96:183–195. https://doi.org/10.1007/s00223-014-9915-y
Fuglevand AJ, Winter DA, Patla AE (1993) Models of recruitment and rate coding organization in motor-unit pools. J Neurophysiol 70:2470–2488. https://doi.org/10.1152/jn.1993.70.6.2470
Fukada S, Akimoto T, Sotiropoulos A (2020) Role of damage and management in muscle hypertrophy: different behaviors of muscle stem cells in regeneration and hypertrophy. Biochim Biophys Acta Mol Cell Res 1867:118742
Gabriel DA, Kamen G, Frost G (2006) Neural adaptations to resistive exercise: mechanisms and recommendations for training practices. Sport Med 36:133–149. https://doi.org/10.2165/00007256-200636020-00004
Gandevia SC (2001) Spinal and supraspinal factors in human muscle fatigue. Physiol Rev 81:1725–1789. https://doi.org/10.1152/physrev.2001.81.4.1725
Gantois P, de Lima-Júnior D, de Fortes LS et al (2021) Mental fatigue from smartphone use reduces volume-load in resistance training: a randomized, single-blinded cross-over study. Percept Mot Skills 128:1640–1659. https://doi.org/10.1177/00315125211016233
García-Ramos A, Janicijevic D, González-Hernández JM et al (2020) Reliability of the velocity achieved during the last repetition of sets to failure and its association with the velocity of the 1-repetition maximum. PeerJ 8:e8760. https://doi.org/10.7717/peerj.8760
Gharahdaghi N, Phillips BE, Szewczyk NJ et al (2021) Links between testosterone, oestrogen, and the growth hormone/insulin-like growth factor axis and resistance exercise muscle adaptations. Front Physiol 11:621226. https://doi.org/10.3389/fphys.2020.621226
Giboin LS, Wolff W (2019) The effect of ego depletion or mental fatigue on subsequent physical endurance performance: a meta-analysis. Perform Enhanc Heal 1:100150. https://doi.org/10.1016/j.peh.2019.100150
Glover IS, Baker SN (2020) Cortical, corticospinal, and reticulospinal contributions to strength training. J Neurosci 40:5820–5832. https://doi.org/10.1523/JNEUROSCI.1923-19.2020
Goodall S, Howatson G, Romer L, Ross E (2014). No Title. https://doi.org/10.1080/17461391.2012.704079
Goodall S, Charlton K, Howatson G, Thomas K (2015) Neuromuscular fatigability during repeated-sprint exercise in male athletes. Med Sci Sports Exerc 47:528–536. https://doi.org/10.1249/MSS.0000000000000443
Graham JD, Ginis KAM, Bray SR (2017) Exertion of self-control increases fatigue, reduces task self-efficacy, and impairs performance of resistance exercise. Sport Exerc Perform Psychol 6:70–88. https://doi.org/10.1037/spy0000074
Graziano MSA, Taylor CSR, Moore T, Cooke DF (2002) The cortical control of movement revisited. Neuron 36:349–362. https://doi.org/10.1016/S0896-6273(02)01003-6
Grgic J, Lazinica B, Mikulic P et al (2017) The effects of short versus long inter-set rest intervals in resistance training on measures of muscle hypertrophy: a systematic review. Eur J Sport Sci 17:983–993. https://doi.org/10.1080/17461391.2017.1340524
Grgic J, Mcllvenna LC, Fyfe JJ et al (2019) Does aerobic training promote the same skeletal muscle hypertrophy as resistance training? A systematic review and meta-analysis. Sport Med 49:233–254. https://doi.org/10.1007/s40279-018-1008-z
Hackett DA, Johnson NA, Chow CM (2013) Training practices and ergogenic aids used by male bodybuilders. J Strength Cond Res 27:1609–1617. https://doi.org/10.1519/JSC.0b013e318271272a
Hagger MS, Wood CW, Stiff C, Chatzisarantis NLD (2010) Self-regulation and self-control in exercise: the strength-energy model. Int Rev Sport Exerc Psychol 3:62–86. https://doi.org/10.1080/17509840903322815
Hakkinen K, Komi PV, Tesch PA (1981) Effect of combined concentric and eccentric strength training and detraining on force-time, muscle fiber and metabolic characteristics of leg extensor muscles. Scand J Sport Sci 3:50–58
Hakkinen K, Alen M, Komi PV (1985a) Changes in isometric force- and relaxation-time, electromyographic and muscle fibre characteristics of human skeletal muscle during strength training and detraining. Acta Physiol Scand 125:573–585. https://doi.org/10.1111/j.1748-1716.1985.tb07759.x
Hakkinen K, Komi PV, Alen M (1985b) Effect of explosive type strength training on isometric force- and relaxation-time, electromyographic and muscle fibre characteristics of leg extensor muscles. Acta Physiol Scand 125:587–600. https://doi.org/10.1111/j.1748-1716.1985.tb07760.x
Häkkinen K, Komi PV (1983) Electromyographic changes during strength training and detraining. Med Sci Sports Exerc 15:455–460. https://doi.org/10.1249/00005768-198315060-00003
Häkkinen K, Komi PV, Alén M, Kauhanen H (1987) EMG, muscle fibre and force production characteristics during a 1 year training period in elite weight-lifters. Eur J Appl Physiol Occup Physiol 56:419–427. https://doi.org/10.1007/BF00417769
Häkkinen K, Kallinen M, Izquierdo M et al (1998) Changes in agonist-antagonist EMG, muscle CSA, and force during strength training in middle-aged and older people. J Appl Physiol 84:1341–1349. https://doi.org/10.1093/geronj/36.6.648
Halperin I, Aboodarda SJ, Behm DG (2014a) Knee extension fatigue attenuates repeated force production of the elbow flexors. Eur J Sport Sci 14:823–829. https://doi.org/10.1080/17461391.2014.911355
Halperin I, Copithorne D, Behm DG (2014b) Unilateral isometric muscle fatigue decreases force production and activation of contralateral knee extensors but not elbow flexors. Appl Physiol Nutr Metab 39:1338–1344. https://doi.org/10.1139/apnm-2014-0109
Hanes DP, Schall JD (1996) Neural control of voluntary movement initiation. Science (80–) 274:427–430. https://doi.org/10.1126/science.274.5286.427
Haun CT, Vann CG, Roberts BM et al (2019) A critical evaluation of the biological construct skeletal muscle hypertrophy: size matters but so does the measurement. Front Physiol 10:247. https://doi.org/10.3389/fphys.2019.00247
Heaselgrave SR, Blacker J, Smeuninx B et al (2019) Dose-response relationship of weekly resistance-training volume and frequency on muscular adaptations in trained men. Int J Sports Physiol Perform 14:360–368. https://doi.org/10.1123/ijspp.2018-0427
Helms ER, Cronin J, Storey A, Zourdos MC (2016) Application of the repetitions in reserve-based rating of perceived exertion scale for resistance training. Strength Cond J 38:42. https://doi.org/10.1519/SSC.0000000000000218
Henneman E (1957) Relation between size of neurons and their susceptibility to discharge. Science (80–) 126:1345–1347. https://doi.org/10.1126/science.126.3287.1345
Henneman E, Clamann HP, Gillies JD, Skinner RD (1974) Rank order of motoneurons within a pool: law of combination. J Neurophysiol 37:1338–1349. https://doi.org/10.1152/jn.1974.37.6.1338
Hernández-Belmonte A, Courel-Ibáñez J, Conesa-Ros E et al (2021) Level of effort: a reliable and practical alternative to the velocity-based approach for monitoring resistance training. J Strength Cond Res. https://doi.org/10.1519/JSC.0000000000004060
Hoeger WWK, Hopkins DR, Barette SL, Hale DF (1990) Relationship between repetitions and selected percentages of one repetition maximum: a comparison between untrained and trained males and females. J Strength Cond Res 4:47–54. https://doi.org/10.1519/00124278-199005000-00004
Hornsby W, Gentles J, Comfort P et al (2018) Resistance training volume load with and without exercise displacement. Sports 6:137. https://doi.org/10.3390/sports6040137
Jenkins NDM, Miramonti AA, Hill EC et al (2017) Greater neural adaptations following high- vs. low-load resistance training. Front Physiol 8:331. https://doi.org/10.3389/fphys.2017.00331
Judge LW, Moreau C, Burke JR (2003) Neural adaptations with sport-specific resistance training in highly skilled athletes. J Sports Sci 21:419–427. https://doi.org/10.1080/0264041031000071173
Kandel E, Schwartz J, Jessell T (2000) Principles of neural science, 4th edn. McGraw-Hill, London
Kennedy DS, McNeil CJ, Gandevia SC, Taylor JL (2014) Fatigue-related firing of distal muscle nociceptors reduces voluntary activation of proximal muscles of the same limb. J Appl Physiol 116:385–394. https://doi.org/10.1152/japplphysiol.01166.2013
Kraemer WJ, Ratamess NA (2004) Fundamentals of resistance training: progression and exercise prescription. Med Sci Sports Exerc 36:674–688. https://doi.org/10.1249/01.MSS.0000121945.36635.61
Kraemer WJ, Ratamess NA, Hymer WC et al (2020) Growth Hormone(s), testosterone, insulin-like growth factors, and cortisol: roles and integration for cellular development and growth with exercise. Front Endocrinol (lausanne) 11:33. https://doi.org/10.3389/fendo.2020.00033
Krieger JW (2010) Single vs. multiple sets of resistance exercise for muscle hypertrophy: a meta-analysis. J Strength Cond Res 24:1150–1159
Kubo K, Ikebukuro T, Yata H (2021) Effects of 4, 8, and 12 repetition maximum resistance training protocols on muscle volume and strength. J Strength Cond Res 35:879–885. https://doi.org/10.1519/JSC.0000000000003575
Laine CM, Martinez-Valdes E, Falla D et al (2015) Motor neuron pools of synergistic thigh muscles share most of their synaptic input. J Neurosci 35:12207–12216. https://doi.org/10.1523/JNEUROSCI.0240-15.2015
Lasevicius T, Ugrinowitsch C, Schoenfeld BJ et al (2018) Effects of different intensities of resistance training with equated volume load on muscle strength and hypertrophy. Eur J Sport Sci 31:3508–3523. https://doi.org/10.1080/17461391.2018.1450898
Lattari E, Rosa Filho BJ, Fonseca Junior SJ et al (2020) Effects on volume load and ratings of perceived exertion in individuals advanced weight-training after transcranial direct current stimulation. J Strength Cond Res 34:89–96. https://doi.org/10.1519/JSC.0000000000002434
Longo AR, Silva-Batista C, Pedroso K et al (2020) Volume load rather than resting interval influences muscle hypertrophy during high-intensity resistance training. J Strength Cond Res. https://doi.org/10.1519/jsc.0000000000003668
Macefield G, Hagbarth KE, Gorman R et al (1991) Decline in spindle support to alpha-motoneurones during sustained voluntary contractions. J Physiol 440:497–512. https://doi.org/10.1113/jphysiol.1991.sp018721
Macgregor LJ, Hunter AM (2018) High-threshold motor unit firing reflects force recovery following a bout of damaging eccentric exercise. PLoS ONE 13:e0195051. https://doi.org/10.1371/journal.pone.0195051
Marshall PWM, Finn HT, Siegler JC (2015) The magnitude of peripheral muscle fatigue induced by high and low intensity single-joint exercise does not lead to central motor output reductions in resistance trained men. PLoS ONE 10:e0140108. https://doi.org/10.1371/journal.pone.0140108
Marston KJ, Peiffer JJ, Newton MJ, Scott BR (2017) A comparison of traditional and novel metrics to quantify resistance training. Sci Rep 7:1–8. https://doi.org/10.1038/s41598-017-05953-2
Martin K, Thompson KG, Keegan R et al (2015) Mental fatigue does not affect maximal anaerobic exercise performance. Eur J Appl Physiol 115:715–725. https://doi.org/10.1007/s00421-014-3052-1
Martinez-Valdes E, Laine CM, Falla D et al (2016) High-density surface electromyography provides reliable estimates of motor unit behavior. Clin Neurophysiol 127:2534–2541. https://doi.org/10.1016/j.clinph.2015.10.065
Martinez-Valdes E, Negro F, Laine CM et al (2017) Tracking motor units longitudinally across experimental sessions with high-density surface electromyography. J Physiol 595:1479–1496. https://doi.org/10.1113/JP273662
Martorelli AS, De Lima FD, Vieira A et al (2021) The interplay between internal and external load parameters during different strength training sessions in resistance-trained men. Eur J Sport Sci 21:16–25. https://doi.org/10.1080/17461391.2020.1725646
Mcbride JM, Mccaulley GO, Cormie P et al (2009) Comparison of methods to quantify volume during resistance exercise. J Strength Cond Res 23:106–110. https://doi.org/10.1519/JSC.0b013e31818efdfe
McCullagh KJA, Calabria E, Pallafacchina G et al (2004) NFAT is a nerve activity sensor in skeletal muscle and controls activity-dependent myosin switching. Proc Natl Acad Sci USA 101:10590–10595. https://doi.org/10.1073/pnas.0308035101
Miyazaki M, Hitomi Y, Kizaki T et al (2006) Calcineurin-mediated slow-type fiber expression and growth in reloading condition. Med Sci Sports Exerc 38:1065–1072. https://doi.org/10.1249/01.mss.0000222833.43520.6e
Moritani T, DeVries HA (1979) Neural factors versus hypertrophy in the time course of muscle strength gain. Am J Phys Med 58:115–130
Morton RW, Sonne MW, Farias Zuniga A et al (2019) Muscle fibre activation is unaffected by load and repetition duration when resistance exercise is performed to task failure. J Physiol 597:4601–4613. https://doi.org/10.1113/JP278056
Mukund K, Subramaniam S (2020) Skeletal muscle: a review of molecular structure and function, in health and disease. Wiley Interdiscip Rev Syst Biol Med 12:e1462. https://doi.org/10.1002/wsbm.1462
Murgia M, Serrano AL, Calabria E et al (2000) Ras is involved in nerve-activity-dependent regulation of muscle genes. Nat Cell Biol 2:142–147. https://doi.org/10.1038/35004013
Nuzzo JL, Barry BK, Gandevia SC, Taylor JL (2016) Acute strength training increases responses to stimulation of corticospinal axons. Med Sci Sports Exerc 48:139–150. https://doi.org/10.1249/MSS.0000000000000733
Nuzzo JL, Barry BK, Jones MD et al (2017) Effects of four weeks of strength training on the corticomotoneuronal pathway. Med Sci Sports Exerc 49:2286–2296. https://doi.org/10.1249/MSS.0000000000001367
Olsen LA, Nicoll JX, Andrew FC (2019) The skeletal muscle fiber: a mechanically sensitive cell mechanotransduction: a brief history. Eur J Appl Physiol 119:333–349. https://doi.org/10.1007/s00421-018-04061-x
Pageaux B, Marcora SM, Lepers R (2013) Prolonged mental exertion does not alter neuromuscular function of the knee extensors. Med Sci Sports Exerc 45:2254–2264. https://doi.org/10.1249/MSS.0b013e31829b504a
Pageaux B, Marcora SM, Rozand V, Lepers R (2015) Mental fatigue induced by prolonged self-regulation does not exacerbate central fatigue during subsequent whole-body endurance exercise. Front Hum Neurosci 9:67. https://doi.org/10.3389/fnhum.2015.00067
Pallafacchina G, Calabria E, Serrano AL et al (2002) A protein kinase B-dependent and rapamycin-sensitive pathway controls skeletal muscle growth but not fiber type specification. Proc Natl Acad Sci USA 99:9213–9218. https://doi.org/10.1073/pnas.142166599
Pareja-Blanco F, Rodríguez-Rosell D, Sánchez-Medina L et al (2017) Effects of velocity loss during resistance training on athletic performance, strength gains and muscle adaptations. Scand J Med Sci Sport 27:724–735. https://doi.org/10.1111/sms.12678
Pareja-Blanco F, Alcazar J, Sánchez-Valdepeñas J et al (2020) Velocity loss as a critical variable determining the adaptations to strength training. Med Sci Sport Exerc 52:1752–1762. https://doi.org/10.1249/mss.0000000000002295
Pearcey GEP, Murphy JR, Behm DG et al (2015) Neuromuscular fatigue of the knee extensors during repeated maximal intensity intermittent-sprints on a cycle ergometer. Muscle Nerve 51:569–579. https://doi.org/10.1002/mus.24342
Potvin JR, Fuglevand AJ (2017) A motor unit-based model of muscle fatigue. PLoS Comput Biol 13:e1005581. https://doi.org/10.1371/journal.pcbi.1005581
Powers RK, Binder MD (2001) Input-output functions of mammalian motoneurons. Rev Physiol Biochem Pharmacol 143:137–263. https://doi.org/10.1007/bfb0115594
Ribeiro AS, Dos Santos ED, Nunes JP, Schoenfeld BJ (2019) Acute effects of different training loads on affective responses in resistance-trained men. Int J Sports Med 40:850–855. https://doi.org/10.1055/a-0997-6680
Rindom E, Herskind J, Blaauw B et al (2021) Concomitant excitation and tension development are required for myocellular gene expression and protein synthesis in rat skeletal muscle. Acta Physiol 231:e13540. https://doi.org/10.1111/apha.13540
Robbins DW, Goodale TL, Docherty D et al (2010) The effects of load and training pattern on acute neuromuscular responses in the upper body. J Strength Cond Res 24:2996–3007. https://doi.org/10.1519/JSC.0b013e3181f67474
Robertson CV, Marino FE, Meeusen R et al (2016) A role for the prefrontal cortex in exercise tolerance and termination. J Appl Physiol 120:467–469. https://doi.org/10.1152/japplphysiol.00363.2015
Romero-Arenas S, Calderón-Nadal G, Alix-Fages C et al (2019) Transcranial direct current stimulation does not improve countermovement jump performance in young healthy men. J Strength Cond Res. https://doi.org/10.1519/jsc.0000000000003242
Rozand V, Pageaux B, Marcora SM et al (2014) Does mental exertion alter maximal muscle activation? Front Hum Neurosci 8:755. https://doi.org/10.3389/fnhum.2014.00755
Sakuma K, Yamaguchi A (2010) The functional role of calcineurin in hypertrophy, regeneration, and disorders of skeletal muscle. J Biomed Biotechnol. https://doi.org/10.1155/2010/721219
Sakuma K, Nishikawa J, Nakao R et al (2003) Calcineurin is a potent regulator for skeletal muscle regeneration by association with NFATc1 and GATA-2. Acta Neuropathol 105:271–280. https://doi.org/10.1007/s00401-002-0647-0
Sartori M, Yavuz U, Farina D (2017) In Vivo neuromechanics: decoding causal motor neuron behavior with resulting musculoskeletal function. Sci Rep 7:1–14. https://doi.org/10.1038/s41598-017-13766-6
Sawan SA, Hodson N, Babits P et al (2021) Satellite cell and myonuclear accretion is related to training-induced skeletal muscle fiber hypertrophy in young males and females. J Appl Physiol 131:871–880. https://doi.org/10.1152/japplphysiol.00424.2021
Schoenfeld BJ (2010) The mechanisms of muscle hypertrophy and their application to resistance training. J Strength Cond Res 24:2857–2872
Schoenfeld BJ (2013) Potential mechanisms for a role of metabolic stress in hypertrophic adaptations to resistance training. Sports Med 43:179–194. https://doi.org/10.1007/s40279-013-0017-1
Schoenfeld BJ, Peterson MD, Ogborn D et al (2015) Effects of low- vs. high-load resistance training on muscle strength and hypertrophy in well-trained men. J Strength Cond Res 29:2954–2963. https://doi.org/10.1519/JSC.0000000000000958
Schoenfeld BJ, Ogborn D, Krieger JW (2016a) Effects of resistance training frequency on measures of muscle hypertrophy: a systematic review and meta-analysis. Sport Med 46:1689–1697
Schoenfeld BJ, Pope ZK, Benik FM et al (2016b) Longer interset rest periods enhance muscle strength and hypertrophy in resistance-trained men. J Strength Cond Res 30:1805–1812. https://doi.org/10.1519/JSC.0000000000001272
Schoenfeld BBJ, Ogborn D, Krieger JJW (2017a) Dose-response relationship between weekly resistance training volume and increases in muscle mass: a systematic review and meta-analysis. J Sports Sci 35:1073–1082. https://doi.org/10.1080/02640414.2016.1210197
Schoenfeld BJ, Grgic J, Ogborn D, Krieger JW (2017b) Strength and hypertrophy adaptations between low- versus high-load resistance training. J Strength Cond Res 31:3508–3523. https://doi.org/10.1519/JSC.0000000000002200
Schoenfeld BJ, Ogborn DI, Vigotsky AD et al (2017c) Hypertrophic effects of concentric vs. eccentric muscle actions: a systematic review and meta-analysis. J Strength Cond Res 31:2599–2608. https://doi.org/10.1519/JSC.0000000000001983
Schoenfeld BJ, Contreras B, Krieger J et al (2019a) Resistance training volume enhances muscle hypertrophy. Med Sci Sport Exerc. https://doi.org/10.1249/mss.0000000000001764
Schoenfeld BJ, Grgic J, Krieger J (2019b) How many times per week should a muscle be trained to maximize muscle hypertrophy? A systematic review and meta-analysis of studies examining the effects of resistance training frequency. J Sports Sci 37:1286–1295. https://doi.org/10.1080/02640414.2018.1555906
Sedlmeier AM, Baumeister SE, Weber A et al (2021) Relation of body fat mass and fat-free mass to total mortality: results from 7 prospective cohort studies. Am J Clin Nutr 113:639–646. https://doi.org/10.1093/ajcn/nqaa339
Serrano AL, Murgia M, Pallafacchina G et al (2001) Calcineurin controls nerve activity-dependent specification of slow skeletal muscle fibers but not muscle growth. Proc Natl Acad Sci USA 98:13108–13113. https://doi.org/10.1073/pnas.231148598
Siddique U, Rahman S, Frazer AK et al (2020) Determining the sites of neural adaptations to resistance training: a systematic review and meta-analysis. Sport Med 50:1107–1128. https://doi.org/10.1007/s40279-020-01258-z
Sidhu SK, Weavil JC, Mangum TS et al (2017) Group III/IV locomotor muscle afferents alter motor cortical and corticospinal excitability and promote central fatigue during cycling exercise. Clin Neurophysiol 128:44–55. https://doi.org/10.1016/j.clinph.2016.10.008
Sidhu SK, Weavil JC, Thurston TS et al (2018) Fatigue-related group III/IV muscle afferent feedback facilitates intracortical inhibition during locomotor exercise. J Physiol 596:4789–4801. https://doi.org/10.1113/JP276460
Sieljacks P, Wang J, Groennebaek T et al (2019) Six weeks of low-load blood flow restricted and high-load resistance exercise training produce similar increases in cumulative myofibrillar protein synthesis and ribosomal biogenesis in healthy males. Front Physiol 10:649. https://doi.org/10.3389/fphys.2019.00649
Smith JL, Martin PG, Gandevia SC, Taylor JL (2007) Sustained contraction at very low forces produces prominent supraspinal fatigue in human elbow flexor muscles. J Appl Physiol 103:560–568. https://doi.org/10.1152/japplphysiol.00220.2007
Srikanthan P, Karlamangla AS (2014) Muscle mass index as a predictor of longevity in older adults. Am J Med 127:547–553. https://doi.org/10.1016/j.amjmed.2014.02.007
Srikanthan P, Horwich TB, Tseng CH (2016) Relation of muscle mass and fat mass to cardiovascular disease mortality. Am J Cardiol 117:1355–1360. https://doi.org/10.1016/j.amjcard.2016.01.033
Storey A, Smith HK (2012) Unique aspects of competitive weightlifting: Performance, training and physiology. Sport Med 42:769–790. https://doi.org/10.2165/11633000-000000000-00000
Taber CB, Vigotsky A, Nuckols G, Haun CT (2019) Exercise-induced myofibrillar hypertrophy is a contributory cause of gains in muscle strength. Sport Med 49:933–997. https://doi.org/10.1007/s40279-019-01107-8
Tanaka M, Watanabe Y (2012) Supraspinal regulation of physical fatigue. Neurosci Biobehav Rev 36:727–734. https://doi.org/10.1016/j.neubiorev.2011.10.004
Taylor JL, Gandevia SC (2008) A comparison of central aspects of fatigue in submaximal and maximal voluntary contractions. J Appl Physiol 104:542–550. https://doi.org/10.1152/japplphysiol.01053.2007
Taylor JL, Todd G, Gandevia SC (2006) Evidence for a supraspinal contribution to human muscle fatigue. Clin Exp Pharmacol Physiol 33:400–405. https://doi.org/10.1111/j.1440-1681.2006.04363.x
Taylor JL, Amann M, Duchateau J et al (2016) Neural contributions to muscle fatigue: from the brain to the muscle and back again. Med Sci Sports Exerc 48:2294–2306. https://doi.org/10.1249/MSS.0000000000000923
Terada K, Kikuchi N, Burt D et al (2020) Low-load resistance training to volitional failure induces muscle hypertrophy similar to volume-matched, velocity fatigue. J Strength Cond Res. https://doi.org/10.1519/JSC.0000000000003690
Thompson SW, Rogerson D, Ruddock A, Barnes A (2020) The effectiveness of two methods of prescribing load on maximal strength development: a systematic review. Sport Med 50:919–938. https://doi.org/10.1007/s40279-019-01241-3
Tillin NA, Folland JP (2014) Maximal and explosive strength training elicit distinct neuromuscular adaptations, specific to the training stimulus. Eur J Appl Physiol 114:365–374. https://doi.org/10.1007/s00421-013-2781-x
Vieira LAF, Lattari E, de Jesus Abreu MA et al (2020) Transcranial direct current stimulation (tDCS) improves back-squat performance in intermediate resistance-training men. Res Q Exerc Sport. https://doi.org/10.1080/02701367.2020.1815638
Wackerhage H, Schoenfeld BJ, Hamilton DL et al (2019) Stimuli and sensors that initiate skeletal muscle hypertrophy following resistance exercise. J Appl Physiol 126:30–43. https://doi.org/10.1152/japplphysiol.00685.2018
Wannamethee SG, Shaper AG, Lennon L, Whincup PH (2007) Decreased muscle mass and increased central adiposity are independently related to mortality in older men. Am J Clin Nutr 86:1339–1346. https://doi.org/10.1093/ajcn/86.5.1339
Wolff W, Bieleke M, Hirsch A et al (2018) Increase in prefrontal cortex oxygenation during static muscular endurance performance is modulated by self-regulation strategies. Sci Rep 8:1–10. https://doi.org/10.1038/s41598-018-34009-2
Yoon T, Delap BS, Griffith EE, Hunter SK (2007) Mechanisms of fatigue differ after low- and high-force fatiguing contractions in men and women. Muscle Nerve 36:515–524. https://doi.org/10.1002/mus.20844
Zourdos MC, Dolan C, Quiles JM et al (2016a) Efficacy of daily one-repetition maximum training in well-trained powerlifters and weightlifters: a case series. Nutr Hosp 33:437–443. https://doi.org/10.20960/nh.129
Zourdos MC, Klemp A, Dolan C et al (2016b) Novel resistance training-specific rating of perceived exertion scale measuring repetitions in reserve. J Strength Cond Res 30:267–275. https://doi.org/10.1519/JSC.0000000000001049
Funding
No funding was received for this study.
Author information
Authors and Affiliations
Contributions
CAF had the idea for this article. All authors contributed equally to this work. All authors read and approved the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Communicated by Michael Lindinger.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Alix-Fages, C., Del Vecchio, A., Baz-Valle, E. et al. The role of the neural stimulus in regulating skeletal muscle hypertrophy. Eur J Appl Physiol 122, 1111–1128 (2022). https://doi.org/10.1007/s00421-022-04906-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00421-022-04906-6