Abstract
The molecular signaling pathways involved in regeneration after muscle damage have not been identified. In the present study, we tested the hypothesis that calcineurin, a calcium-regulated phosphatase recently implicated in the signaling of fiber-type conversion and muscle hypertrophy, is required to induce skeletal muscle remodeling. The amount of calcineurin and dephosphorylated nuclear factor of activated T cells c1 (NFATc1) proteins was markedly increased in the regenerating muscle of rats. The amount of calcineurin co-precipitating with NFATc1 and GATA-2, and NFATc1 co-precipitating with GATA-2 gradually increased in the tibialis anterior muscle after bupivacaine injection. Calcineurin protein was present in the proliferating satellite cells labeled with BrdU in the damaged muscle after 4 days. In contrast, calcineurin was not detected in the quiescent nonactivating satellite cells expressing Myf-5. At 4 days post injection, many macrophages detected in the damaged and regenerating area did not possess calcineurin protein. Calcineurin protein was abundant in many myoblasts and myotubes that expressed MyoD and myogenin at 4 and 6 days post injection. In the intact muscle, no immunoreactivity of calcineurin or BrdU was detected in the cell membrane, cytosol or the extracellular connective tissue. In mice, intraperitoneal injection of cyclosporin A, a potent inhibitor of calcineurin, induced extensive inflammation, marked fiber atrophy, the appearance of immature myotubes, and calcification in the regenerating muscle compared with phosphate-buffered saline-administered mice. Thus, calcineurin may have an important role in muscle regeneration in association with NFATc1 and GATA-2.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Skeletal muscle satellite cells are generally in a quiescent state in adult muscle, but when minor damage or injury occurs, signals are generated within the muscle that activate these satellite cells, stimulating them to migrate and enter the cell cycle. Activated satellite cells have been shown to migrate to the site of an injury where they proliferate, differentiate, and fuse with the damaged fibers or form new fibers [3, 18]. In vitro studies have documented many factors, primarily protein growth factors, that can modulate satellite cell activity [3, 18]. In particular, insulin-like growth factor I (IGF-I), known to be up-regulated both in hypertrophied and regenerating muscle in vivo [12, 20], positively regulated the proliferation and differentiation of satellite cells and myoblasts in vitro [15]. Indeed, Coleman et al. [8] found that over-expression (47-fold increase) of IGF-I in muscle leads to hypertrophy of muscles, but not of other tissues. In the proliferating satellite cells, IGF-I facilitated G1/S cell cycle progression by a down-regulation of p27 kip1 via activation of the phosphatidylinositol 3'-kinase/Akt pathway [5].
Recently, calcineurin, a cytoplasmic calcium-regulated phosphatase implicated in the pathogenesis of hypertrophic cardiomyopathy, has emerged as a possible candidate in the signaling of skeletal muscle cellular growth [13, 28, 38] and the fiber-type transformations of these cells [7, 11, 30]. For example, on subcutaneous injection of cyclosporin A (CsA) and FK506, a potent inhibitor of phosphate phosphatase activity of calcineurin, mechanical overloading-induced fiber hypertrophy was completely suppressed [13]. After stimulating by IGF-I and calmodulin, calcineurin promoted myotube growth in association with GATA-2 and NFAT1c [28]. Therefore, calcineurin may potently regulate the hypertrophy of muscle fibers in vivo and of myotubes in vitro possibly due to enhancing the differentiation of myoblasts.
Several studies [21, 23] suggested that rupture of the plasma membrane is an essential element for muscle hypertrophy, because of the resulting release of chemical mediators (growth factors) of remodeling stored in the cell cytosol [e.g., fibroblast growth factor (FGF)]. Thus, the muscle hypertrophy elicited by mechanical stress includes a damage-regeneration (remodeling) phase. The injection of bupivacaine hydrochloride into muscle induces extensive damage to muscle fibers. Muscle regeneration in vivo includes the extravagation of immune cells such as macrophages, the proliferation and differentiation of satellite cells and remodeling of neuromuscular connections. Since bupivacaine injection markedly increases Ca2+ and IGF-I in muscle, we hypothesized that calcineurin by potently regulating the hypertrophy of myotubes and myofibers, which is dependent on Ca2+ and IGF-I, has an important role in the remodeling of muscle fibers.
At the initial phase of muscle regeneration, satellite cells are activated and proliferated by the stimulation of several growth factors and cytokines such as IGF-I, basic FGF (bFGF), interleukin-6 and leukemia inhibitory factor (LIF) [12, 22, 36]. After the initial phase, some of the activated cells and/or progeny are suggested to differentiate into myoblast- like cells. In regenerating muscle, these myoblasts can fuse with each other to form new myofibers or become incorporated into existing myofibers [3]. Studies using developmental models, such as those involving embryonic muscle or muscle cell line in culture, revealed that MyoD and myogenin potently regulate the differentiation from myoblasts to myotubes. In myogenesis, calcineurin may promote the muscle differentiation in association with MyoD and myogenin. Indeed, calcineurin and NFATc3 markedly enhance the differentiation of 10T1/2 cells transfected with MyoD in vitro [11]. The transcription of myogenin gene was markedly elevated in L6 cells retrovirally transfected with a constitutively active form of calcineurin [17]. Thus, we hypothesized that calcineurin and a possible downstream mediator (NFATc1 and GATA-2) enhance the differentiation from activated satellite cells to myoblasts and/or from myoblasts to myotubes in the regenerating muscle.
We found that muscle regeneration after bupivacaine injection was markedly delayed by intraperitoneal injection of CsA. In the regenerating muscles, calcineurin and active NFATc1 proteins were markedly increased. The amount of calcineurin co-precipitating with NFATc1 and GATA-2, and NFATc1 co-precipitating with GATA-2 gradually increased in the tibialis anterior muscle after bupivacaine injection. Thus, calcineurin may play a crucial role in muscle regeneration in association with NFATc1 and GATA-2.
Materials and methods
Experimental animals
Male Wistar rats ( n =48,10 and 20 weeks of age) and male DWJ mice ( n =24, 12 weeks) were used in the experiments. The rats and mice were housed in a temperature- (22±2°C) and humidity- (60±5%) controlled room regulated to provide alternating 12-h periods of light and darkness. They were allowed to feed (commercial rat chow) and drink ad libitum. This experimental procedure was approved by the Committee for Animal Research, Kyoto Prefectural University of Medicine.
Normal adult organ and muscles
Three male Wistar rats (20 weeks of age) were used in this experiment. After excess pentobarbital administration, rats were killed, and brain, cerebellum, spinal cord, muscle, heart, liver, kidney, stomach, lung, testis and thymus were rapidly dissected.
Bupivacaine treatment
Adult male Wistar rats ( n =39, 10 weeks of age) with body weights of 270–360 g were used. Muscle regeneration of the tibialis anterior muscle was induced in one leg of each rat by an intramuscular injection of 0.5 ml of 0.5% bupivacaine hydrochloride prepared in 0.9% saline solution. Rats ( n =27) were killed after excess pentobarbital administration, and the tibialis anterior muscles of both legs dissected, in groups of three, at 1, 2, 3, 4, 6, 8 and 10 days and 2 and 4 weeks post surgery. In a separate experiment to examine whether calcineurin interacts with NFATc1 and GATA-2 in the regenerating muscle, rats ( n =12) were killed, after excess pentobarbital administration, in groups of two at 30 min and 1, 2, 4, 8, and 12 h post surgery.
CsA injection
Damage to the tibialis anterior muscle in the hind limb of male mice (20–27 g) was induced by bupivacaine injected with either CsA (25 mg/kg, intraperitoneally, n =12) or vehicle ( n =12) twice daily [27] for 1 or 2 weeks (each group, n =6). This dose of CsA was higher than that reported to inhibit 90% of total calcineurin phosphatase activity in the heart, to block Ca2+-induced NF-AT dephosphorylation in spleen cell lysates, and to inhibit calcineurin-mediated transcriptional activation in skeletal muscle [25, 39]. Injection of these chemical agents did not affect the health (from general observations or autopsy results), or growth (body weights were not significantly different among mice at any time during the treatment), and did not noticeably alter the daily amount of locomotor activity displayed by experimental animals.
Primary antibodies
The following antibodies were used: affinity-purified mouse monoclonal antibody to calcineurin [dilution 1:100–1:400, Transduction Laboratories (TDL)], acetylcholine receptor (AChR) alpha (1:100, TDL), MyoD (1:200, clone G106-647, Pharmingen, Becton Dickinson, USA) and bromodeoxyuridine (1:400, clone 3D4, Pharmingen), affinity-purified rat monoclonal antibody to Mac-1 (1:200, Boehringer Mannheim, Mannheim, Germany), affinity-purified goat polyclonal antibody to NFATc1 [1:400, N-20, Santa Cruz Biotechnology (SCB), Santa Cruz, Calif.] and GATA-2 (1:260, N-20, SCB) ; and affinity-purified rabbit polyclonal antibody to myogenin (1:100, M-225, SCB).
Tissue preparation, gel electrophoresis and immunoblots
Each tissue was homogenized in 10–20 vol of 50 mM TRIS-HCl pH 7.4, 5 mM EDTA, 10 μg/ml phenylmethylsulfonyl fluoride, 0.5 μg/ml leupeptin, 0.2 μg/ml aprotinin, 0.2% NP-40, 0.1% Triton X-100, 0.05% mercaptoethanol, and 1 mM Na3VO4 in a polytron (PCU-2, Kinematica, Steinhofhalde, Switzerland) for 30 s. The homogenized tissues were centrifuged for 25 min at 15,000 g at 4°C, and the protein concentration of the supernatant was determined colorimetrically (Bio-Rad protein determination kit, Bio-Rad Laboratories, Richmond, Calif.). Sodium dodecylsulfate (SDS)-polyacrylamide gel electrophoresis (8% acrylamide for NFATc1 and 10% for calcineurin and GATA-2) and Western analysis were performed as described previously [35].
Immunoprecipitation
Cell extracts (100–200 μg) were incubated with protein A-Sepharose beads (10 μl; Pharmacia Biotech, Uppsala, Sweden) and antibodies against NFATc1 and GATA-2 (anti-NFATc1, 1 μg, anti-GATA-2, 1 μg) in lysis buffer for 1 h at 4°C. The beads were washed four times with lysis buffer. Washed beads were resuspended in reducing sample buffer and boiled for 2 min before being size-fractionated on 10% SDS-polyacrylamide gels [37].
Immunohistochemistry
For the labeling of proliferating satellite cells, 5-bromo-2'-deoxy-uridine (BrdU, Boehringer Mannheim), a nonradioactive marker for DNA synthesis [10], was injected (100 mg/kg, intraperitoneally) 1 h before sampling according to the method of Tamaki et al. [40]. Six rats were used in this experiment. The animals were killed with an overdose of sodium pentobarbital (60 mg/kg, intraperitoneally), and the tibialis anterior muscle was dissected, in groups of two, at 4, 6 and 8 days post surgery ( n =6). Serial 8- to 10-μm transverse sections made with a cryostat (Bright 5030 Microtome, Bright Instrument Co., Huntingdon, UK) were mounted on silanized slides (Dako Japan, Tokyo). The sections were stained with hematoxylin-eosin (H&E) to observe the histological differences in tibialis anterior muscles between PBS- and CsA-injected mice. Frozen sections were fixed by cold acetone (7 min) and were incubated in 0.3% H2O2 diluted in methanol for 20 min to inhibit endogenous peroxidase. All subsequent steps were as described previously [33].
Morphometric analysis
The cross-sectional area was determined on at least 200 fibers per muscle, using tracings of microphotographs (×66) at different positions of the entire muscle and an image analysis computer program (NIH image software program). Using tracings of microphotographs at five different positions in the muscle, the percentage of the non-regenerating area was calculated from the percentage of the area occupied by the inflammation of mononuclear cells, connective tissues, calcium accumulation, very small fibers (myotubes?) possessing central nuclei and necrotic fibers against the total area in the muscle cross-section.
Statistical analysis
All values are expressed as means ± SEM. Paired t -tests were used to evaluate the significance of differences in the muscle weight, mean fiber area and non-regenerating area between intact and bupivacaine-injected muscles. P <0.05 was considered statistically significant.
Results
At 6, 8 and 10 days after bupivacaine injection, the tibialis anterior wet weight had decreased by 29.7%, 20.2% and 13.8% under the control value, respectively. Complete restoration of wet weight was achieved by 28 days in the tibialis anterior muscle subjected to bupivacaine. As shown in Fig. 1A, immunoblotting with antibodies to calcineurin revealed prominent bands consistent with a molecular size of 61 kDa (Fig. 1A). The calcineurin protein was abundant in the brain, cerebellum, spinal cord and lung, and was very low levels were detected in the skeletal muscle and heart (Fig. 1A). Calcineurin protein was weakly detected in all of the hind limb and diaphragm muscles examined in this study, and the protein level did not significantly differ between slow-type and fast-type muscles (Fig. 1B). Calcineurin was markedly increased in the regenerating muscles of rats up to 2 weeks post surgery (Fig. 1C). Both inactive (phosphorylated) and active (dephosphorylated) NFATc1 were detected weakly in the tibialis anterior muscle of normal rats (Fig. 1C). The amount of active NFATc1 was markedly increased in the regenerating muscle from 1 to 6 days after surgery compared with the control level, and then the level gradually decreased and reached the control level at 14 days post surgery (Fig. 1C).
A Western blot analysis of calcineurin and NFATc1 in various organs from normal adult rats. In normal adult rats, calcineurin protein is abundant in the brain, cerebellum, spinal cord and lung, and very low levels are detected in the skeletal muscle and heart. B Calcineurin protein is weakly detected in all of the hind limb and diaphragm muscles, although the protein level does not significantly differ between slow-type (soleus) and fast-type (EDL, TA, gastrocnemius and diaphragm) muscles. C The change in calcineurin and NFATc1 proteins in the regenerating TA muscles subjected to bupivacaine injection. Calcineurin is markedly increased in the regenerating muscle of rats for up to 2 weeks. Both inactive (phosphorylated) and active (dephosphorylated) NFATc1 are weakly detected in the TA muscles of normal rats. The amount of active NFATc1 is markedly increased in the regenerating muscle for 1–6 days following bupivacaine injection compared with the control level, and the level then gradually decreases and reaches the control level at 14 days post surgery. Immunoblotting with antibodies to calcineurin and NFATc1 shows prominent bands of 61 and 80–120 kDa, respectively ( EDL extensor digitorum longus, TA tibialis anterior, Gastroc. gastrocnemius)
Although interaction of NFATc1 and GATA-2 with calcineurin was not detected in the normal intact muscle, the amount of calcineurin co-precipitating with NFATc1 and GATA-2 increased at 8–12 h and 12 h after bupivacaine injection, respectively (Fig. 2). The amount of GATA-2 co-precipitating with NFATc1 markedly increased at 4 h after operation and thereafter (Fig. 2).
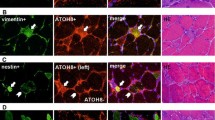
In the regenerating muscle, several proliferating satellite cells expressing BrdU (Fig. 3 A) contained calcineurin protein (Fig. 3B). In contrast, no expression of calcineurin protein was observed in the quiescent satellite cells possessing Myf-5 protein in the non-damaged fibers of the regenerating muscle (Fig. 3C, D). At 4 days post injection, many macrophages were detected in the damaged and regenerating area (Fig. 3E), but did not possess calcineurin protein (Fig. 3F). Immunoreactivity for calcineurin was observed in many myoblasts and myotubes that expressed MyoD (Fig. 3G) and myogenin (Fig. 3I) after 4 and 6 days following bupivacaine injection (Fig. 3H, J). In the intact muscle, no immunoreactivity for calcineurin or BrdU was detected in the cell membrane, cytosol or the extracellular connective tissue (Fig. 3K, L).
Serial cryosections of the regenerating ( A – J) and normal ( K – L) TA muscle of rats. Each cryosection was immunohistochemically stained with calcineurin ( B, D, F, H, J, L), BrdU ( A, K), Myf-5 ( C), Mac-1 ( E), MyoD ( G) and myogenin ( I) antibodies. ( empty circles, empty squares, filled squares) The same fiber in different successive cryosections. In the regenerating TA muscle, several proliferating satellite cells expressing BrdU ( A) show calcineurin protein ( B). In contrast, no expression of calcineurin protein is observed in the quiescent satellite cells possessing Myf-5 protein in the non-damaged fibers of the regenerating muscle ( C, D). At 4 days post injection, many macrophages are shown in the damaged and regenerating area ( E), but do not possess calcineurin protein ( F). The immunoreactivity of calcineurin is observed in many myoblasts and myotubes that express MyoD ( G) and myogenin ( I) at 4 and 6 days following bupivacaine injection ( H, J). In the intact muscle, no immunoreactivity of calcineurin or BrdU is seen in the cell membrane, cytosol or the extracellular connective tissue ( K, L). White and black arrows denote the same mononuclears (satellite cell, myoblast, macrophage, etc.) and myotubes of different successive cryosections. Bars 25 μm
After 1 week, the regenerating tibialis anterior muscle of the mouse receiving PBS intraperitoneally showed several necrotic fibers, infiltration of mononuclear cells and a wide range of the fiber area (Fig. 4A). After 2 weeks, the damaged muscle was completely regenerated in the PBS-administered group (Fig. 4C). In contrast, the CsA-administered mouse possessed many mononuclear cells (probably macrophages, myoblasts, satellite cells and/or endothelial cells) and some calcification in the mostly extracellular non-regenerating space after 1 week in the damaged muscle after bupivacaine injection (Fig. 4B). After 2 weeks, intraperitoneal CsA-injection had induced a wide range of inflammation, apparent fiber atrophy, the existence of very small myotubes and myofibers and marked calcification (Fig. 4D) in the regenerating muscle compared with PBS-administered mice.
Histological characteristics of the regenerating TA muscles of PBS-treated ( A, C) and CsA-treated ( B, D) mice. Each cryosection was stained with H&E. After 1 week, the regenerating muscle of the mouse receiving PBS intraperitoneally showed many necrotic fibers, inflammation of mononuclear cells and a wide range of the fiber area ( A). After 2 weeks, damaged muscle was completely regenerated in mice receiving PBS injection ( C). In contrast, in mice administered CsA many mononuclear cells (probably macrophages, myoblasts, satellite cells and/or endothelial cells) and some calcification in the mostly extracellular non-regenerating space are seen in the damaged muscle after intramuscular injection of bupivacaine after 1 week ( B). After 2 weeks, mice receiving an intraperitoneal injection of CsA show a wide range of inflammation, apparent fiber atrophy, the existence of non-regenerating very small myotubes and myofibers and marked calcification ( D) in the regenerating muscle compared with PBS-administered mice ( C). Bar 50 μm
The muscle weight (Fig. 5A), mean fiber area (Fig. 5B), non-regenerating area (Fig. 5C) and the distributing pattern of the muscle fiber area (Fig. 5D) were very similar after 2 weeks between intact and bupivaicaine-injected tibialis anterior muscles of the PBS-administered group. On the other hand, the mouse receiving CsA intraperitoneally revealed a significant decrease in muscle weight and cross-sectional area, and a marked increase in the non-regenerating area at 2 weeks after bupivacaine injection compared with intact muscle (Fig. 5A–C). The CsA-injected mice possessed small muscle fibers more frequently in the regenerating muscle than in the sham-operated muscle (Fig. 5E).
Comparison of muscle weight ( A), mean fiber area ( B), non-regenerating area ( C) and the distributing pattern of muscle fiber area ( D) in the TA muscle subjected to bupivacaine-injection between PBS- and CsA-administered mice. These parameters are very similar after 2 weeks between the intact and bupivacaine-injected TA muscles of the PBS-administered group. In contrast, the CsA-injected mice show significant decreases in muscle weight and cross-sectional area, and marked increases in the non-regenerating area compared with the intact muscle ( A – C). Small muscle fibers are seen more frequently in the regenerating TA muscle of CsA-injected mice than in sham-operated muscle ( E)
Discussion
Three main conclusions can be derived from the findings of the present study. First, calcineurin and active NFATc1 protein were markedly increased in the regenerating muscle. Secondly, CsA, a potent inhibitor of phosphate phosphatase activity of calcineurin, clearly blocked the regeneration of muscle fiber. Third, muscle regeneration is accomplished by calcineurin in association with NFATc1 and GATA-2.
Although a complex among calcineurin, NFATc1 and GATA-2 was not detected in the normal tibialis anterior muscle, a marked increase in calcineurin co-precipitating with NFATc1 and GATA-2, and NFATc1 with GATA-2 was observed in the regenerating muscle. These interactions may occur during hypertrophy of the myotube by the extensive fusion of myoblasts with each other, which occurs in the regenerating muscle of adult rodents. In myogenesis, myotube hypertrophy resulted from the interaction of calcineurin, NFATc1 and GATA-2. In addition, cardiac hypertrophy was induced by the interaction of the second zinc finger of GATA-4 and the C-terminal portion of the Rel homology domain of NFAT-c3 [27]. Thus, a similar mechanism may regulate certain muscle genes in response to regeneration. Nuclear translocation of NFATc1 appears to up-regulate the gene expression of troponin I [4] and T [6], myoglobin [6], the myosin heavy chain of slow and type IIA [6, 41]. These genes are required for complete regeneration of skeletal muscle.
MyoD and myogenin potently regulate muscle differentiation by up-regulating p21 and p300, creatin kinase, and the myosin heavy chain. For example, primary MyoD–/– muscle possesses a low potential of differentiation and a marked increase in satellite cells during self renewal [24, 32]. On the other hand, the null mutation of myogenin induces defects in myotube formation, because myogenin causes myoblasts to leave the cell cycle and fuse, forming multinucleated myotubes. In the present study, calcineurin immunoreactivity was observed in many myotubes possessing MyoD and myogenin. Calcineurin and NFATc3 markedly enhanced the differentiation of 10T1/2 cells transfected with MyoD in vitro [11]. The transcription of myogenin gene was markedly elevated in L6 cells retrovirally transfected with a constitutively active form of calcineurin [17]. Thus, calcineurin appears to enhance muscle differentiation from myoblasts to myotubes and/or from myotubes to myofibers in the regenerating muscle in vivo.
Intraperitoneal injection of CsA, a calcineurin inhibitor, induced marked fiber atrophy and the appearance of very small non-regenerating myotubes in the tibialis anterior muscle after bupivacaine injection. Myotube formation appeared to be markedly delayed in the regenerating muscle of mice injected with CsA. This finding is in agreement with the observations of Abott et al. [1] who demonstrated that CsA blocked differentiation by dose-dependent inhibition of creatin kinase and embryonic myosin heavy chain expression, and the formation of multinucleated myotubes. Calcineurin immunoreactivity was not detected in many satellite cells, although several satellite cells labeled with BrdU expressed calcineurin. Considering several sections were stained by H&E at 7 days after bupibacaine injection, many mononuclear cells including satellite cells, macrophages and immune cells must have existed in the damaged non-regenerating area in CsA-injected mice. Furthermore, Dunn et al. [13], found that, in mechanically overloaded muscle, CsA injection had no influence on the expression in PCNA, a marker of proliferating satellite cells. Thus, it is unlikely that calcineurin activates the proliferation of satellite cells and/or myoblasts.
The other source of calcineurin in the regenerating muscle may be macrophages. Damaged muscle is invaded by macrophages, neutrophils, T cells, platelets and MHC class II-positive dendritic cells [31]. In particular, macrophages have an important role in phagocytosis in the lesioned area. In a co-culture, macrophages enhanced satellite cell proliferation and delayed their differentiation by several chemokines such as bFGF, platelet-derived growth factor, transforming growth factor-β and LIF [26]. Calcineurin is present in macrophages and modulates the macrophage cytokine response effector functions [9]. However, in regenerating muscle, almost all macrophages that were labeled by anti-Mac-1 did not possess calcineurin protein. Thus, the concept that increased expression of calcineurin in the regenerating muscle was derived from macrophages is unlikely.
The majority of quiescent satellite cells express M-cadherin, Myf-5 and CD34 protein [2, 18]. In vitro, proliferating myoblasts enter one of two pathways and form multinucleated myotubes or become reserve, quiescent, or mononucleated cells. One recent study showed that in the reserve cell population of myotube cultures (quiescent satellite cells), calcineurin and the NFAT-dependent pathway regulated the expression of the Myf-5 gene [16]. However, calcineurin immunoreactivity was not observed in the quiescent satellite cells expressing Myf-5 protein in the tibialis anterior muscles of rats. It is unlikely that calcineurin up-regulates the Myf-5 gene in vivo in adult mature fibers of rats, because the distribution patterns clearly differ between calcineurin and Myf-5. Our previous study demonstrated that MyoD and Myf-5 proteins are preferentially localized in the fast-type muscle such as the extensor digitorum longus (EDL), tibialis anterior and gastrocnemius of rats [34]. In contrast, calcineurin protein was expressed similarly between the slow-type soleus muscle and the fast-type tibialis anterior muscle of rats in the present study by Western blot analysis (Fig. 1B). A recent study suggested that calcineurin is only activated in muscle possessing high Ca2+ ion levels within a concentration range of 100–300 nM by tonic continuous electrical activity [6]. In contrast, in fast myofibers, resting Ca2+ levels are maintained at 50 nM, and the high amplitude (~1 μM) calcium transients evoked by motor nerve activity [42]. Thus, the activity pattern of fast myofibers is of insufficient duration to evoke calcineurin-stimulated signaling.
The present observations strongly support the findings of Musaro et al. [29], who reported that in the EDL muscles of senescent mice, IGF-I transgene induced a potent regenerative possibility after cardiotoxin injection, probably due to GATA-2 up-regulation. Although the signal pathway between calcineurin and GATA-2 is important in myogenesis in vitro, this does not hold in muscle hypertrophy in vivo. Dunn [14] showed that in the mechanically overloaded muscle of the IGF-I transgenic mouse, the major downstream pathway of calcineurin was the NFATc1-myocyte enhancer factor 2D (MEF2D). Although CsA clearly delayed muscle regeneration, this drug nonselectively inhibited the calcineurin interacting with NFATc1, NFATc2, NFATc3 and NFATc4. In vitro, a calcineurin-NFATc3-dependent pathway regulates the differentiation from myoblasts to myotubes and the expression of the slow myosin heavy chain gene [11]. Moreover, mice with the null mutation of NFATc2 exhibited reduced muscle size due to a decrease in the myofiber cross-sectional area [19]. Thus, whether calcineurin interacts with the other subtypes of NFAT in the regenerating muscle remains to be determined.
In summary, calcineurin potently regulates muscle regeneration via NFATc1- and GATA-2-dependent pathways. In regenerating muscle, calcineurin protein is present in the myoblasts and myotubes leaving the cell cycle and entering a differentiation phase, but not in macrophages, quiescent satellite cells and the immature neuromuscular junction.
References
Abbott KL, Friday BB, Thaloor D, Murphy TJ, Pavlath GK (1998) Activation and cellular localization of the cyclosporine A-sensitive transcription factor NF-AT in skeletal muscle cells. Mol Biol Cell 9:2905–2916
Beauchamp JR, Heslop L, Yu DSW, Tajbakhsh S, Kelly RG, Wernig A, Buckingham ME, Partridge TA, Zammit PS (2000) Expression of CD34 and Myf5 defines the majority of quiescent adult skeletal muscle satellite cells. J Cell Biol 151:1221–1233
Bischoff R (1994) The satellite cell and muscle regeneration. In: Engel AG, Armstrong F (eds) Myology. McGraw-Hill, New York, pp 97–118
Calvo S, Venepally P, Cheng J, Buonanno A (1999) Fiber-type-specific transcription of the troponin I slow gene is regulated by multiple elements. Mol Cell Biol 19:515–525
Chakravarthy MV, Abraha TW, Schwartz RJ, Fiorotto ML, Booth FW (2000) Insulin- like growth factor-I extends in vitro replicative life span of skeletal muscle satellite cells by enhancing G1/S cell cycle progression via the activation of phosphatidylinositol 3'-kinase/Akt signaling pathway. J Biol Chem 275:35942–35952
Chin ER, Allen DG (1996) Changes in intracellular free Ca2+ concentration during constant 10 Hz stimulation of mouse single fibers. Physiologist 39:A75
Chin ER, Olson EN, Richardson JA, Yang Q, Humphries C, Shelton JM, Wu H, Zhu W, Bassel-Duby R, Williams RS (1998) A calcineurin-dependent transcriptional pathway controls skeletal muscle fiber type. Genes Dev 12:2499–2509
Coleman ME, Demayo F, Yin KC, Lee HM, Geske R, Montgomery C, Schwartz RJ (1995) Myogenic vector expression of insulin-like growth factor I stimulates muscle cell differentiation and myofiber hypertrophy in transgenic mice. J Biol Chem 270:12109–12116
Conboy IM, Manoli D, Mhaiskar V, Jones PP (1999) Calcineurin and vacuolar-type H+-ATPase modulate macrophage effector functions. Proc Natl Acad Sci USA 96:6324–6329
deFazio A, Leary JA, Hedley DW, Tattersal MH (1987) Immunohistochemical detection of proliferating cells in vivo. J Histochem Cytochem 35:571–577
Delling U, Tureckova J, Lim HW, De Windt LJ, Rotwein P, Molkentin JD (2000) A calcineurin-NFATc3-dependent pathway regulates skeletal muscle differentiation and slow myosin heavy-chain expression. Mol Cell Biol 20:6600–6611
DeVol DL, Rotwein P, Levis Sadow J, Npvakofski J, Bechtel PJ (1990) Activation of insulin-like growth factor gene expression during work-induced skeletal muscle growth. Am J Physiol 259:E89–E95
Dunn SE, Burns JL, Michel RN (1999) Calcineurin is required for skeletal muscle hypertrophy. J Biol Chem 274:21908–21912
Dunn SE, Chin ER, Michel RN (2000) Matching of calcineurin activity to upstream effectors is critical for skeletal muscle fiber growth. J Cell Biol 151:663–672
Engert JC, Berglund EB, Rosenthal N (1996) Proliferation precedes differentiation in IGF-I-stimulated myogenesis. J Cell Biol 135:431–440
Friday BB, Pavlath GK (2001) A calcineurin-and NFAT-dependent pathway regulates Myf-5 gene expression in skeletal muscle reserve cells. J Cell Sci 114:303–310
Friday BB, Horsley V, Pavlath GK (2000) Calcineurin activity is required for the initiation of skeletal muscle differentiation. J Cell Biol 149:657–665
Hawke TJ, Garry DJ (2001) Myogenic satellite cells: physiology to molecular biology. J Appl Physiol 91:534–551
Horsley V, Friday BB, Matteson S, Kegley KM, Gephart J, Pavlath GK (2001) Regulation of the growth of multinucleated muscle cells by an NFATC2-dependent pathway. J Cell Biol 153:329–338
Jennische E, Matejka GL (1992) IGF-I binding and IGF-I expression in regenerating skeletal muscle. Acta Physiol Scand 146:79–86
Kaye D, Pimental D, Prasad S, Maki T, Berger HJ, McNeil PL, Smith TW, Kelly RA (1996) Role of transiently altered sarcolemmal membrane permeability and basic fibroblast growth factor release in the hypertrophic response of adult rat ventricular myocytes to increased mechanical activity in vitro. J Clin Invest 97:281–291
Kurek JB, Nouri S, Kannourakis G, Murphy M, Lawrence A (1996) Leukemia inhibitory factor and interleukin-6 are produced by diseased and regenerating skeletal muscle. Muscle Nerve 19:1291–1301
McNeil PL, Steinhardt RA (1997) Loss, restoration, and maintenance of plasma membrane integrity. J Cell Biol 137:1–4
Megeney LA, Kablar B, Garrett K, Anderson JE, Rudnicki MA (1996) MyoD is required for myogenic stem cell function in adult skeletal muscle. Genes Dev 10:1173–1183
Mende U, Kagen A, Cohen A, Aramburu J, Schoen FJ, Neer EJ (1998) Transient cardiac expression of constitutively active Galphaq leads to hypertrophy and dilated cardiomyopathy by calcineurin-dependent and independent pathways. Proc Natl Acad Sci USA 95:13893–13898
Merly F, Lescaudron L, Rouaud T, Crossin F, Gardahaut MF (1999) Macrophages enhance muscle satellite cell proliferation and delay their differentiation. Muscle Nerve 22:724–732
Molkentin JD, Lu, J-R, Antos CL, Markham B, Richardson J, Robbins J, Grant SR, Olson EN (1998) A calcineurin-dependent transcriptional pathway for cardiac hypertrophy. Cell 93:215–228
Musaró A, McCullagh KJA, Naya FJ, Olson EN, Rosenthal N (1999) IGF-I induces skeletal myocyte hypertrophy through calcineurin in association with GATA-2 and NF-ATc1. Nature 40:581–585
Musaró A, McCullagh K, Paul A, Houghton L, Dobrowolony G, Molinaro M, Barton ER, Sweeney HL, Rosenthal N (2001) Localized Igf-1 transgene expression sustains hypertrophy and regeneration in senescent skeletal muscle. Nat Genet 27:195–200
Naya FJ, Mercer B, Shelton J, Richadson JA, Williams RS, Olson EN (2000) Stimulation of slow skeletal muscle fiber gene expression by calcineurin in vivo. J Biol Chem 275:4545–4548
Pimorady-Esfahani A, Grounds MD, McMenamin PG (1997) Macrophages and dendritic cells in normal and regenerating murine skeletal muscle. Muscle Nerve 20:158–166
Sabourin LA, Girgis-Gabardo A, Seale P, Asakura A, Rudnicki MA (1999) Reduced differentiation potential of primary MyoD-/-cells derived from adult skeletal muscle. J Cell Biol 144:631–643
Sakuma K, Watanabe K, Totsuka T, Uramoto I, Sakamoto K, Sano M (1998) Differential adaptations of insulin-like growth factor, basic fibroblast growth factor and leukemia inhibitory factor in the plantaris muscle of rats by mechanical overloading: an immunohistochemical study. Acta Neuropathol 95:123–130
Sakuma K, Watanabe K, Sano M, Sakamoto K, Uramoto I, Totsuka T (1999) The adaptive response of MyoD family protein in the overloaded, regenerating and denervated rat muscles. Biochim Biophys Acta 1428:284–292
Sakuma K, Watanabe K, Sano M, Sakamoto K, Uramoto I, Totsuka T (2000) The adaptive response of transforming growth factor-β2 and βRII in the overloaded, regenerating and denervated muscles of rats. Acta Neuropathol 99:177–185
Sakuma K, Watanabe K, Sano M, Uramoto I, Totsuka T (2000) Differential adaptation of growth and differentiation factor 8/myostatin, fibroblast growth factor 6 and leukemia inhibitory factor in overloaded, regenerating and denervated rat muscles. Biochim Biophys Acta 1497:77–88
Sakuma K, Watanabe K, Sano M, Uramoto I, Nakano H, Li Y-J, Kaneda S, Sorimachi Y, Yoshimoto K, Yasuhara M, Totsuka T (2001) A possible role for BDNF, NT-4 and TrkB in the spinal cord and muscle of rat subjected to mechanical overload, bupivacaine injection and axotomy. Brain Res 907:1–19
Semsarian C, Wu M-J, Ju Y-K, Marciniec T, Yeoh T, Allen DG, Harvey RP, Graham RM (1999) Skeletal muscle hypertrophy is mediated by a Ca2+-dependent calcineurin signalling pathway. Nature 40:576–581
Sussman MA, Lim HW, Gude N, Taigen T, Olson EN, Robbins J, Colbert MC, Gualberto A, Wieczorek DF, Molkentin JD (1998) Prevention of cardiac hypertrophy in mice by calcineurin inhibition. Science 281:1690–1693
Tamaki T, Akatsuka A, Tokunaga M, Ishige K, Uchiyama S, Shiraishi T (1997) Morphological and biochemical evidence of muscle hyperplasia following weight-lifting exercise in rats. Am J Physiol 273:C246–C256
Torgan CE, Daniels MP (2001) Regulation of myosin heavy chain expression during rat skeletal muscle development in vitro. Mol Biol Cell 12:1499–1508
Westerblad H, Allen DG (1991) Changes in myoplasmic calcium concentration during fatigue in single mouse muscle fibers. J Gen Physiol 98:615–635
Acknowledgements
This work was supported by research grant nos. 10670775, 11780059, 12470108 and 13780029 from the Ministry of Education, Science, Sports and Culture of Japan.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sakuma, K., Nishikawa, J., Nakao, R. et al. Calcineurin is a potent regulator for skeletal muscle regeneration by association with NFATc1 and GATA-2. Acta Neuropathol 105, 271–280 (2003). https://doi.org/10.1007/s00401-002-0647-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00401-002-0647-0