Abstract
Purpose
We examined effects of a three-game, 1-week microcycle (G1, G2, G3) on recovery of performance and inflammatory responses in professional male footballers.
Methods
Players were randomized into an experimental (EXP; N = 20) and a control group (CON; N = 20). Blood was drawn and repeated sprint ability (RSA), muscle soreness and knee range of motion (KJRM) were determined pre- and post-games and during recovery.
Results
High-intensity running during G2 was 7–14 % less compared to G1 and G3. RSA declined in EXP by 2–9 % 3 days post-game with G2 causing the greatest performance impairment. In EXP, game play increased muscle soreness (~sevenfold) compared to CON with G2 inducing the greatest rise, while KJRM was attenuated post-game in EXP compared to CON (5–7 %) and recovered slower post G2 and G3 than G1. CK, CRP, sVCAM-1, sP-Selectin and cortisol peaked 48 h post-games with G2 eliciting the greatest increase. Leukocyte count, testosterone, IL-1β and IL6 responses, although altered 24 h post each game, were comparable among games. Plasma TBARS and protein carbonyls rose by ~50 % post-games with G2 eliciting the greatest increase 48 h of recovery. Reduced to oxidized glutathione ratio declined for 24 h post all games with G2 displaying the slowest recovery. Total antioxidant capacity and glutathione peroxidase activity increased (9–56 %) for 48 h in response to game play.
Conclusion
In summary, post-game performance recovery and inflammatory adaptations in response to a three-game weekly microcycle displayed a different response pattern, with strong indications of a largest physiological stress and fatigue after the middle game that was preceded by only a 3-day recovery.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Association football (soccer) is an intermittent sport with frequent activity changes (Bangsbo 1994), where 9–12 km are covered with 2–3 km at high intensities and ~0.5 km of sprinting (Mohr et al. 2003; Bradley et al. 2009). In addition, game demands include a high number of explosive movements such accelerations, decelerations, changes of direction (Varley and Aughey 2013) as well as jumps, impacts, shots and tackles (Andersson et al. 2008) having a powerful eccentric component with potential concomitant muscle damage (Nédélec et al. 2012). Players demonstrate a dramatically decline in high-intensity running towards the end of a game (Mohr et al. 2003) causing marked impairment in repeated sprint ability (RSA) (Mohr et al. 2004, 2012; Krustrup et al. 2006b), intense intermittent exercise (Krustrup et al. 2010), countermovement jump (Mohr et al. 2010; Mohr and Krustrup 2013) and strength performance (Krustrup et al. 2011; Marshall et al. 2014).
In football, players may participate in 50–80 games during a season and in most top leagues, it is a normal procedure for teams to compete in three games per week during several periods within a season. Thus, only 3–4 days of recovery are allowed between successive games which may be insufficient to restore normal homeostasis (Andersson et al. 2008; Ispirlidis et al. 2008; Fatouros et al. 2010). Repeated match play with short recovery time may therefore provoke acute and chronic fatigue potentially causing performance impairment and/or injuries, as indicated by studies of top-class players (Ekstand et al. 2004; Dupont et al. 2010).
Fatigue development after a football game has been linked to depleted muscle glycogen stores (Mohr et al. 2005), where for example a high number of muscle fibers have been shown to be depleted of glycogen (Krustrup et al. 2006b; Bendiksen et al. 2012) as well as different glycogen cell compartments (Nielsen et al. 2012). The recovery of both performance and muscle glycogen after a game have been shown to display slow recovery phase lasting between 48 and 72 h (Krustrup et al. 2011; Gunnarsson et al. 2013; Bendiksen et al. 2012; Nybo 2012) and may be associated with muscle damage or/and a severe inflammatory responses (Nédélec et al. 2012). This is supported by the observed elevation of plasma creatine kinase activity (CK), myoglobin (Nybo 2012; Thorpe and Sunderland 2012), and various inflammatory markers (Thorpe and Sunderland 2012; Heisterberg et al. 2013; Fatouros et al. 2010) during the same time-frame. For example, Heisterberg et al. (2013) showed that certain leucocyte subpopulations increased during in-season compared to a demanding pre-season, a finding that highlights the physiological stress of multiple match play in football.
High-intensity exercise up-regulates oxidative stress responses due to an increased production of reactive oxygen species (ROS) (Davies et al. 1982; Michailidis et al. 2013) that challenge the systems scavenging ROS. Disturbances in the equilibrium of free radicals such as ROS and the antioxidant defense in favor of the free radicals may lead to progressive change or degradation of biomolecules such as proteins and nucleic acids leading to exercise performance deterioration. To date, it is unclear to what extent a three-game weekly microcycle in football affects oxidative stress and antioxidant status in trained players.
The majority of studies investigating recovery aspects from football match-play have only followed the response a single game (Ispirlidis et al. 2008; Krustrup et al. 2011; Gunnarsson et al. 2013), while in practical reality there may be multiple game participation that allow only a brief recovery for players. Because of the large physical demands of football match-play, a game may always be affected by the preceding game. Andersson and colleges (2010a, b, c) performed a series of studies examining recovery from a football game in elite women players. In one study, two games separated by 3 days were studied and it was shown that the cytokine response was damped after the second game (Andersson et al. 2010a). However, no control group was used in that study and no preparatory period leading up to the study was applied. Moreover, gender differences may apply for recovery patterns since, for example, oestrogens have been shown to protect against ROS (Akova et al. 2001). Furthermore, no studies have quantified the training and game load in a week including three games in well-trained male football players.
Thus, the aims of this study were to examine the recovery kinetics, the degree of post-game fatigue, muscle damage, inflammatory and immune responses, as well as oxidative stress and antioxidant status during a microcycle consisting of three games. We hypothesize that recovery of performance and physiological stress variables are markedly affected by multiple match plays and that players do not fully recover during a three-game, 1-week microcycle.
Materials and methods
Participants
Forty competitive male football players (age 21.5 ± 0.3 years; height 1.77 ± 0.01 m; weight 73.4 ± 0.9 kg; body fat 6.7 ± 0.2 %; VO2max 58.5 ± 0.8 ml min−1 kg−1, Yo–Yo intermittent recovery level 1 and 2 test performances of 2672 ± 51 and 1371 ± 29 m, respectively), participated in the study. Selection criteria included: (1) participation at elite (top three division leagues) level of football competition for at least 5 years (average 4.6 years), (2) no recent history of febrile illness, muscle lesions, lower limb trauma, and metabolic diseases, (3) no consumption of performance-enhancing supplements and medications (for at least 6 months before the study), (4) none of the players were smokers and (5) all players participated in at least six 2-h training sessions per week and played at least one match per week. Participants represented all out-field playing positions. Participants signed an informed consent form after they were informed regarding all risks, discomforts, and benefits involved in the study. Procedures were in accordance with the 1975 Declaration of Helsinki, as revised in 2000, and approval was received from the institutional ethics committee.
Research design
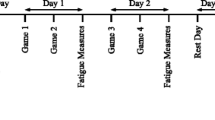
A two-group, repeated measures design was used to study performance and inflammatory responses of football players in response to three football games within a week. The study was performed during a time course of 11 days that included baseline testing, three 90-min games and 9 days of practice sessions and testing in between games as well after the third game. The study was conducted 1 week after the completion of the in-season period while players were still accustomed to intensive training and game load. Prior to the commencement of the study, players participated in recovery training for approximately 7 days from the last season game/practice to develop game tactics and team cohesion. Players from the same field position (defenders, mid-fielders, attackers) were randomly assigned to one of two groups: (1) a control group (CON, N = 20) to account for the daily variation of physiological measurements and biochemical assays; participants in this group participated only in daily practices and measurements, and (2) an experimental group (EXP, N = 20); participants in this group were randomly assigned to two different teams, of the same level, that played against each other in three football games organized according to official regulations. The second match was performed 3 days (Wednesday) after the first one (Sunday) and the third game was performed 4 days after the second one (Sunday) in an attempt to simulate an official in-season week consisting of three games. Game days did not include practices, but a normal football warm-up was performed before and a normal cool-down period after each game. In order to achieve the maximum competitiveness by the players and create the physical and psychological environment of a formal competition, scouts and coaches from various professional clubs were watching the three matches. Players participated in full games (there were no substitutions) and games took place between 1800 and 2000 h. On game day, athletes consumed a light standardized meal as previously described (Fatouros et al. 2010). During the game, participants were allowed to drink only water ad libitum. High time-resolution GPS (15 Hz; GPSport, Canberra, Australia) was utilized to monitor the activity profiles as previously described (Randers et al. 2010) during match play and training using similar speed threshold categories as presented in Randers et al. (2010). Heart rate during match play was continuously monitored (Team Polar, Polar Electro Oy, Kempele, Finland). Venous blood samples were collected, performance measurements were performed and muscle soreness ratings were taken at baseline and every morning until the third day after the final match. Additional blood samples were collected by veno-puncture before each game and 3–4 min after the completion of the first and second half of each game for the measurement of metabolites. Athletes participated in one practice session per day (in between games and for 3 days after the last game) in an attempt to simulate an in-season microcycle.
Training and dietary intake
In order to ensure that physical loading was comparable among groups, training activities were monitored by GPS recording as described by Randers et al. (2010). All practice sessions were conducted and supervised by the investigators and followed the normal procedures used by professional soccer teams during a period with multiple games. During training, players were instructed to follow a standardized meal plan. Dietary intake was monitored with daily diet recalls during the experimental period and was standardized for all participants by a nutritionist. Meal composition was analyzed by a Hellenic food database “Science Fit Diet 200A” (Science Technologies, Athens, Greece). Macronutrient intake (carbohydrate, fat, and protein) was calculated based on body mass of each participant to satisfy the recommendations for athletes engaged in daily training (Maughan 2004). The prescribed food choices (i.e., dairy products, bread, pasta, meat, fruits, vegetables, rice, cereals, etc.) were similar to athletes’ normal diet and contained physiological levels of antioxidants. In addition, daily diet recalls were completed by the participants during the experimental period to determine whether dietary intake impacted participants’ oxidative/antioxidant status. A dietician instructed participants how to complete diet recall questionnaires and estimate food serving and sizes. Diet recalls were analyzed with the nutritional analysis software “Science Fit Diet 200A” (Science Technologies, Athens, Greece).
Performance testing and measures of muscle damage
Participants visited the laboratory twice prior to the commencement of the preparatory phase: (a) 10 days before the first game, all players underwent a medical examination, completed a health history questionnaire, and had their body weight and height, as well as body fat assessed (Fatouros et al. 2010). A Harpenden caliper (John Bull, British Indicators, St. Albans, UK) was used to measure skinfolds and body fat (%) was estimated from measures of seven skinfold as previously described (Fatouros et al. 2010). In addition, VO2max was measured by open-circuit spirometry (averaged every 30 s) with the use of an automated online pulmonary gas exchange system via breath-by-breath analysis during a graded exercise test on a treadmill to exhaustion according to a standardized protocol as previously described (Malm et al. 2004). Also prior to the first game, the players completed the Yo–Yo intermittent recovery test, level 1 and 2 (Yo–Yo IR1 and IR2; see Krustrup et al. 2003; 2006a) on separate days according to procedures described by Bangsbo and Mohr (2012), as well as a repeated sprint test (RST; 5 × 30-m interspersed by 25 s active recovery; Krustrup et al. 2006b; Bangsbo and Mohr 2012). The RSA was performed on a natural grass surface. Each sprint time was recorded with two infrared photoelectric gates (NewTest Ltd, Kiviharjuntie, Finland) and a stopwatch was used to control the interval between sprints.
Muscle damage of knee flexors and extensors was evaluated by measuring the delayed onset of muscle soreness (DOMS) and knee joint range of motion (KJRM, as an index of muscle edema). After performing three repetitions of a full squat movement, participants rated their soreness level on a visual analog scale from 1 to 10 (VAS, with “no pain” at one end and “extremely sore” at the other) using palpation of the belly and the distal region of relaxed knee extensors and flexors in order to assess DOMS. During palpation, investigators applied pressure with the tips of fingers II, III, and IV briefly (~3 s) with the participant in the standing position (Nosaka et al. 2002). DOMS assessment was carried out by the same investigator in order to standardize the applied pressure on affected muscles. This method had a test–retest reliability of DOMS assessment of 0.95. Muscle (quadriceps) edema was determined by measuring KJRM following a 3-min warm-up on a Monark stationary bicycle by the use of a goniometer as previously described (Norkin and White 1985). The coefficient of variation for test–retest trials for knee flexion/extension was 2.3 %. Participants were familiarized with all testing procedures prior to the study.
Blood analysis and assays
Resting (at 7:00–8:00 a.m.) and post-game blood samples were collected via venipuncture (a 20-gauge disposable needle and a Vacutainer tube holder were used) from an antecubital arm vein and participants in a seated position after an overnight fast. Plasma or serum was obtained by centrifugation (at 1370g, 4 °C, 10 min for plasma separation and at 1500g, 4 °C, 15 min for serum separation) after collecting a blood portion into tubes containing ethylenediaminetetraacetic acid (EDTA) or SST-Gel/clot activator, respectively. For serum separation, blood was allowed to clot at room temperature first. Plasma was utilized for the measurement of protein carbonyls (PC), glucose, non-esterified fatty acids (NEFA), urea, ammonia, glycerol and adhesion molecules concentration as well as creatine kinase activity (CK). Plasma samples were lysed and packed erythrocytes were collected as described (Barbas et al. 2011). The resultant lysate was used for the assessment of reduced (GSH), oxidized glutathione (GSSG), thiobarbituric acid-reactive substances (TBARS) concentration and catalase activity (CAT). The concentration of cortisol, testosterone, cytokines, C-reactive protein (CRP) as well as total antioxidant capacity (TAC) was measured in serum samples. Plasma, erythrocyte lysates and serum samples were stored in multiple aliquots at −80 °C until measured. A small portion (1 mL) of whole blood was stored at −20 °C and was used for the measurement of glutathione peroxidase activity (GPX). Another small (2 mL) blood quantity was collected in EDTA tubes for the measurement of white blood cell count (WBC), hemoglobin and hematocrit, within 24 h, utilizing an automated hematology analyzer (Shenzhen Mindray, BC-5500, Shenzhen, China). Samples were protected from light and auto-oxidation and thawed once before measured. Assays were performed in duplicate. The post-game samples were corrected for plasma volume changes as previously described (Dill and Costill 1974).
Blood lactate concentration was assessed using a hand-portable analyzer (Accutrend Plus, Roche Diagnostics, Basel, Switzerland) as described (Baldari et al. 2009). Serum glucose, CK and urea were assayed by an enzymatic spectrophotometric method on a Cobas Integra Plus-400 chemistry analyzer (Roche Diagnostics, Mannheim, Germany) as described (Michailidis et al. 2013). Ammonia concentration was determined spectrophotometrically as previously described (Chatzinikolaou et al. 2014). Particle-enhanced immunonephelometry was used to measure CRP on a Dade-Behring BN Prospec nephelometer. Adhesion molecules in plasma (sVCAM-1 and sP-selectin) were determined on a Luminex-100 IS (Luminex, USA) using a multiplex assay kit (Linco, MO, USA) as previously described (Goussetis et al. 2009). Testosterone concentration was determined using a solid-phase, competitive chemiluminescent enzyme immunoassay (Immulite 2000, Siemens, Germany). Cortisol was measured using an electrochemiluminescence immunoassay (Roche, Basel, Switzerland). Concentrations of cytokines IL-1b and IL-6 were measured using two ELISA kits (Immunokontact, UK) as described (Ispirlidis et al. 2008), while the plasma IGF-1 was measured by the Diasorin using a LIAISON IGF-1 kit and analyzed by CLIA method (Lee et al. 2014). Inter- and intra-assay coefficients of variation ranged from 2.1 to 7.8 % and from 2.6 to 7.4 %, respectively.
GSH was analyzed as previously described (Theodorou et al. 2010). Briefly, erythrocyte lysates following a treatment with 5 % TCA were mixed with sodium–potassium phosphate (67 mM, pH 8.0) and 5,5′-dithiobis-2-nitrobenzoate (1 mM). Thereafter, samples were incubated in the dark for 45 min at room temperature and, subsequently, their absorbance was read at 412 nm. GSSG was measured as previously described (Theodorou et al. 2010). Briefly, erythrocyte lysates were mixed with 5 % TCA at a neutral pH (7.0–7.5). 2-vinyl pyridine (4 μL) was then added and the samples were incubated at room temperature for 2 h. Thereafter, sodium phosphate (143 mM, pH 7.5), NADPH (3 mM), 5,5′-dithiobis-2-nitrobenzoate (10 mM) and distilled water were added and the resultant solution was incubated at room temperature for 10 min. Glutathione reductase was then added and the change in sample’s absorbance was read at 412 nm for 3 min. TBARS were analyzed as described (Theodorou et al. 2010). Briefly, 35 % TCA and 200 mM, Tris–HCl (pH 7.4) were added to erythrocyte lysates and the solution was incubated at room temperature for 10 min. Thereafter, 2 M Na2SO4 and 55 mM thiobarbituric acid were added to the samples and the resultant mixture solution was incubated at 95 °C for 45 min. Samples were then cooled (5 min), mixed with 70 % TCA and centrifuged at 15,000g for 3 min. The supernatant’s absorbance was then read at 530 nm. PC was analyzed as previously described (Theodorou et al. 2010). Briefly, 20 % TCA was added to plasma samples and the solution was then incubated in an ice bath for 15 min and centrifuged at 15,000g and 4 °C for 5 min. 2,4-dinitrophenylhydrazine (10 mM in 2.5 N HCl) or 2.5 N HCl was then added in the pellet of the sample or blank solution, respectively. Thereafter, samples were incubated in the dark at room temperature for 1 h while mixed every 15 min and centrifuged again at 15,000g and 4 °C for 5 min. TCA (10 %) was then added to the pellet produced and samples were centrifuged again. Ethanol–ethyl acetate (1:1 v/v) was added to the new pellet and samples were centrifuged again at 15,000g and 4 °C for 5 min. This procedure was repeated two more times. Following the last centrifugation, 5 M urea (pH 2.3) was added to the pellet and samples were mixed and incubated at 37 °C for 15 min. Finally, samples were centrifuged at 15,000 g and 4 °C for 3 min and their absorbance was read at 375 nm. TAC was analyzed as previously described (Theodorou et al. 2010). Briefly, 10 mM sodium–potassium phosphate (pH 7.4) and 0.1 mM 2,2-diphenyl-1 picrylhydrazyl were added to serum. The solutions were then mixed, incubated in the dark at room temperature for 30 min and centrifuged at 20,000g for 3 min. Thereafter, samples’ absorbance was read at 520 nm. CAT was analyzed as previously described (Theodorou et al. 2010). Briefly, 67 mM sodium–potassium phosphate (pH 7.4) was added to erythrocyte lysates and samples were then incubated at 37 °C for 10 min, treated with 30 % hydrogen peroxide and finally the change in their absorbance was read at 240 nm for 1.5 min. A commercially available kit (Ransel RS 505; Randox, Crumlin, UK) was used to measure whole-blood GPX activity (Barbas et al. 2011). Inter- and intra-assay coeficients of variation for oxidative stress assays ranged from 2.2 to 7.1 % and from 3.2 to 7.8 %, respectively. A Hitachi 2001 UV/VIS (Hitachi Instruments Inc., US) was utilized for all spectrophotometric assays.
Statistical analyses
Data are presented as mean ± SE. A one-sample Kolmogorov–Smirnov test was used to determine data normality (since data normality was verified, a nonparametric test was not necessary). A two-way (time × group) for repeated measures analysis of variance (ANOVA) was used to analyze the data for dependent variables for all data where both groups (EXP and CON) had data points. A one-way ANOVA for repeated measures was used to analyze the game activity data where only EXP had data points. When a significant interaction was observed, a Tukey post hoc test with Bonferroni correction was used to identify the points of difference. Significance was accepted at P < 0.05. The SPSS was used for all analyses (SPSS for Windows, version 15.0, Chicago, IL, USA).
Results
Match and training activity profiles and heart rate loading
No difference was noted between games in total game and sprinting distance, as well as peak sprinting speed, frequency of accelerations and deceleration (Table 1). However, a distance deficit of 7 and 14 % (P < 0.05) in high intensity running was observed in G2 compared to G1 and G3, respectively (Table 1). Peak 5-min high intensity running distance was not different between game trials (Table 1). More (6 and 7 %; P < 0.05) impacts occured during G3 than G1 and G2, respectively. Average and peak HR reached during the game were higher (P < 0.05) in G1 (165 ± 3 and 191 ± 2 bpm, respectively), than in G2 (162 ± 2 and 188 ± 2 bpm, respectively), and G3 (158 ± 2 and 187 ± 1 bpm, respectively), corresponding to 83.3 ± 1.3 and 96.5 ± 1.9 (G1), 81.8 ± 1.7 and 94.9 ± 2.1 (G2) and 79.8 ± 1.5 and 94.4 ± 1.8 (G3) % of HRmax. No differences were noted between EXP and CON in total distance covered, high intensity running, sprinting or average and peak heart rates during the seven training sessions performed on post-game recovery days (Table 2).
Repeated sprint performance
A significant (P < 0.05) group × time interaction was present in RSA performance demonstrating no changes over the experimental period in CON compared to baseline (Fig. 1), but 2–9 % lower (P < 0.05) performance in EXP compared to baseline during the entire experimental period except on day 3 post G1 and day 4 post G2 (Fig. 1). RSA performance was lower (P < 0.05) in EXP compared to CON at day 1, 2 and 3 post G2 and G3, and at day 1 and two post G1 (Fig. 1). In addition, RSA performance was lower (P < 0.05) at day 2 and three post G2, and at day 2 post G3, compared to corresponding recovery days post G1 (Fig. 1).
Average sprint performance in a repeated sprint test (5 × 30 m with 25 s recovery) during the experimental period. (Start and end P is start and end of the preparatory period; G1, G2 and G3 is game 1, 2 and 3; D1, D2, D3 and D4 are day 1, 2, 3 and 4 post games). Data are mean ± SE. Hash significant difference from CON, currency sign significant difference from corresponding time-point after G1. P < 0.05
Muscle damage markers
A significant (P < 0.05) group × time interaction was present in DOMS scores for knee extensors and flexors, which were one- to sevenfold higher (P < 0.05) in EXP compared to baseline and CON over the entire experimental period (Fig. 2a). In EXP knee extensor DOMS peaked (P < 0.05) on the first day post G1 and G3, while the highest levels were reached on the first and second day of recovery after G2 (Fig. 2a). Moreover, DOMS values in EXP were higher (P < 0.05) on the second and third day of recovery post G2 compared to the corresponding time points post G1 and G3 (Fig. 2a). Average knee flexor DOMS were one- to sixfold higher (P < 0.05) in EXP than CON during the entire experimental period except at baseline (Fig. 2a). In EXP knee flexor DOMS levels peaked (P < 0.05) on the fist day post G1 and G3, while the highest level was reached on the first and second day of recovery after G2 (Fig. 2a). DOMS values in EXP were higher (P < 0.05) on the second and third day of recovery post G2 compared to the corresponding time points post G1 and G3 (Fig. 2a). A significant (P < 0.05) group × time interaction was found in average (dominant and non-dominant leg) knee joint range of motion (KJRM). KJRM did not change immediately post G1, G2 and G3 (Fig. 2b). However, in EXP KJRM was 5–7 % lower (P < 0.05) than baseline and corresponding time-points in CON 1 day into recovery after all three games as well as on the second day of recovery post G2 and G3 (Fig. 2b).
Knee extensor (KE) and flexor (KF) delayed onset of muscle soreness (DOMS) scores (a) and knee joint range of motion (KJRM) (b) during the experimental period. (Start and end P is start and end of the preparatory period; G1, G2 and G3 is game 1, 2 and 3; D1, D2, D3 and D4 are day 1, 2, 3 and 4 post games). Data are shown for dominat leg (DL) and now-dominat leg (NDL). Data are mean ± SE. Hash significant difference from CON, currency sign significant difference from corresponding time-point after G1. P < 0.05
Blood and plasma metabolites
A significant (P < 0.05) group × time interaction was in blood lactate concentrations, which increased (P < 0.05) fivefold in EXP compared to baseline and CON after the first half in G1 being 16 % higher (5.1 ± 0.3 mmol L−1; P < 0.05) than after the second half (4.4 ± 0.3 mmol L−1). Similar differences between halves were seen after G2 and G3 in EXP. A significant (P < 0.05) group × time interaction was in blood glucose values, which increased (P < 0.05) 14–25 % in EXP after both halves in G1-G3 peaking at 5.6 ± 0.1 mmol L−1 with no difference between games and halves. A significant (P < 0.05) group × time interaction was present in plasma NEFA concentrations, which rose (P < 0.05) by ~75 % in EXP compared to baseline after all three games reaching values between 0.68 and 0.72 mmol L−1 with no difference between game trials, while an approximate onefold game-induced increased (P < 0.05) was in plasma glycerol in EXP only reaching post game concentrations between 0.24 and 0.27 mmol L−1. Plasma urea concentration was 39.1 ± 0.9 and 37.8 ± 1.0 mg dL−1 in EXP and CON, respectively, at baseline and was unchanged after the three games in both conditions. A significant (P < 0.05) group × time interaction was present in plasma NH3 values, which was 52 ± 1 and 54 ± 2 µmol L−1 in EXP and CON, respectively, at baseline, but increased (P < 0.05) nearly threefold after G1 (144 ± 12 µmol L−1) in EXP compared to baseline and CON. Post G2 and G3 plasma NH3 peaked at 191 ± 20 and 181 ± 17 µmol L−1 being higher (P < 0.05) than after G1. A significant (P < 0.05) group × time interaction was present in plasma creatinine, which was 0.71 ± 0.01 and 0.76 ± 0.01 mg dL−1 in EXP and CON, respectively, at baseline and increased (P < 0.05) in EXP compared to baseline and CON after all three games (0.96 ± 0.03–1.01 ± 0.03 mg dL−1) with no differences between games. No change occurred in blood lactate and glucose or plasma NEFA, urea, ammonia and creatinine in CON over the experimental period.
Inflammatory blood markers
A significant (P < 0.05) group × time interaction was present in plasma CK concentrations, which increased (P < 0.05) in EXP compared to baseline and CON at all time points (Fig. 3). Peak plasma CK concentrations were reached on second day of recovery post G1, G2 and G3 (1043 ± 76, 1252 ± 130 and 1011 ± 114 U L−1, respectively), with the peak values after G2 being higher (P < 0.05) than after G1 and G3 (Fig. 3). CRP rose (P < 0.05) from prior to after the preparatory phase in EXP and CON (0.9 ± 0.1 and 0.9 ± 0.2 vs. 1.6 ± 0.3 and 1.5 ± 0.3 mg L−1). A significant (P < 0.05) group × time interaction was present in plasma CRP values, which were one- to threefold higher (P < 0.05) in EXP immediately after and 1 day into recovery after all three games in comparison to baseline and CON as well as on second day of recovery from G2 (Table 3). A significant (P < 0.05) group × time interaction was present, where the EXP WBC count was doubled (P < 0.05) compared to baseline and CON immediately post G1, G2 and G3 with no change in CON over the entire period (Table 3). WBC count remained elevated (P < 0.05) in comparison to baseline and corresponding CON levels on the first day of recovery after G1-3 (Table 3). A significant (P < 0.05) group × time interaction was present in plasma sVCAM-1 values, which rose (P < 0.05) by 22–34 % in EXP post-game and remained elevated on the first day of recovery after all three games compared to baseline and CON (Table 3). The plasma sVCAM-1 level was also elevated (41 %; P < 0.05) on the second day of recovery after G2. A significant (P < 0.05) group × time interaction was present in plasma sP-Selectin values, which increased (P < 0.05) in EXP post G1-3 and on the first recovery day after G2 in comparison to baseline and CON (Table 3).
Plasma creatine kinase (CK) during the experimental period. (Start and end P is start and end of the preparatory period; G1, G2 and G3 is game 1, 2 and 3; D1, D2, D3 and D4 are day 1, 2, 3 and 4 post games). Data are mean ± SE. Hash significant difference from CON, currency sign significant difference from corresponding time-point after G1, ampersand significant difference from recovery day 1 and 3. P < 0.05
Hormonal and cytokine responses
A significant (P < 0.05) group × time interaction was present in plasma cortisol, which in EXP increased (P < 0.05) by ~50 % immediately post G1 compared to baseline and CON, but returned to baseline after 1 day of recovery (Table 4). Plasma cortisol was also elevated (P < 0.05) immediately after G2 and 1 day into recovery in EXP compared to baseline and CON (Table 4). The cortisol levels at these time points after G2 were higher (P < 0.05) than corresponding time points after G1 and G2 (Table 4). After G3 plasma cortisol was also ~50 % higher (P < 0.05) in EXP than at baseline and CON, but recovered 1 day post G3 (Table 4). In contrast plasma testosterone did not change statistically in either group over the entire protocol (Table 4). Plasma IGF-1 ranged between 327 ± 90 and 528 ± 179 μg L−1 in EXP during the intervention period and did not change statistically in response to the gamesA significant (P < 0.05) group × time interaction was present for the plasma IL-1β data, which in EXP rose (P < 0.05) approximately threefold post G1-3 and was 15–21 % higher (P < 0.05) than baseline and CON one the first day post games with no difference between games (Table 4). A significant (P < 0.05) group × time interaction was also present in plasma IL-6 concentration, which doubled (P < 0.05) in EXP compared to baseline and CON post G1-3 and remained slightly elevated (P < 0.05) on the first recovery day, with no differences between game trials (Table 4).
Oxidative stress markers
A significant (P < 0.05) group × time interaction was in plasma TBARS values, which increased (P < 0.05) in EXP compared to baseline and CON post-game and at the first 3 days of recovery from G1-3 with peak concentrations (0.69–0.81 µmol g−1 Hb) reached at first day of recovery (Table 5). Peak plasma TBARS values after G2 were 13 and 17 % higher (P < 0.05) than after G1 and G3 (Table 5). A significant (P < 0.05) group × time interaction was present in plasma protein carbonyls (PC) concentrations, which increased (P < 0.05) 13–106 % in EXP compared to baseline and CON during the intervention period except on forth day of recovery from G2 and third day post G3 (Table 5). On second day of recovery from G2, plasma PC values were 31 and 42 % higher (P < 0.05) than corresponding values post G1 and G3 (Table 5). A significant (P < 0.05) group × time interaction was present in plasma GSH, which was lowered (P < 0.05) by 7–43 % in EXP in comparison to baseline and CON post G1-3, as well as at recovery day 1–2 post G1 and 3 and day 1–3 post G2 (Table 5). Plasma GSH levels on recovery day 1 were 32–36 % lower (P < 0.05) than the post game values in G1-3 (Table 5). A significant (P < 0.05) group × time interaction was present in plasma GSSG values, which was 11–24 % higher (P < 0.05) in EXP compared to baseline and CON at first day of recovery from G1, first and second day of recovery from G2 and 3 (Table 5). GSH/GSSG ratio was lower (P < 0.05) in EXP than baseline and CON post-game 1–3 and at recovery day 1 and 2 post G1 and 3 and on the first 3 days of recovery from G2 (see Table 5). Plasma GSH/GSSH ratio reach the lowest levels on 1 day of recovery from G1-3 being only around half of baseline and corresponding values in CON.
Antioxidant status markers
A significant (P < 0.05) group × time interaction was in plasma total antioxidant capacity (TAC), which was elevated (P < 0.05) by 9–57 % during the entire intervention period in EXP compared to baseline and CON except on day 4 of recovery post G2 (Table 6). Peak TAC level (0.85–0.88 mmol L−1 DPPH) was reached 2 days post-game and the TAC concentrations were higher (P < 0.05) during the first 2 days of recovery in comparison to the levels immediately post-game (Table 6). A significant (P < 0.05) group × time interaction was in plasma catalase activity (CAT) concentrations, which were 16–23 % higher (P < 0.05) in EXP compared to baseline and CON immediately post G1-3 (Table 6). A significant (P < 0.05) group × time interaction was also present in plasma glutathione peroxidase activity, which rose (P < 0.05) in EXP post G1 and remained elevated (P < 0.05) compared to baseline and CON at all time-points except on day 4 of recovery post G2 (Table 6). Peak plasma GPX activity (6333–6423 U L−1) was reached on day one of recovery from G1-3, and was higher (P < 0.05) at day one and two of recovery compared to values immediately post G1-3 (Table 6).
Discussion
The present study is the first to examine the effect of a normal three game weekly microcycle with three and four recovery days between game 1–2 and 2–3, respectively, on exercise performance, muscle damage, inflammatory response, as well as markers of oxidative stress and antioxidant status in professional male football players. The principal findings were that (1) repeated sprint performance is markedly impaired during a week with three games, with the largest impairment after game 2, (2) DOMS and decrement in KJRM also appear to be more pronounced after game 2, (3) markers of muscle damage and inflammation are also elevated during the recovery period, tending to be highest post-game 2, (4) repeated match play also results in an increased oxidative stress response being most pronounced after game-2, (5) the physiological stress response appears to be highly challenged when games are separated by 3 days, but seems to enable recovery when a fourth recovery day is included.
The present study applied experimental games which have been done previously to investigate fatigue patterns (Krustrup et al. 2006b) and effect of environmental factors (Mohr et al. 2012) in football. In relation to muscle damage, inflammation and oxidative stress this research approach may be beneficial, since simulated protocols may not provoke a response similar to real games due to the lack of football game-specific actions (Magalhaes et al. 2010). The experimental games in the present study displayed games distances of similar magnitude as in elite games using GPS technology (Randers et al. 2010; Varley and Aughey 2013), and provided a physiological response similar to reports from competitive high standard games (for review see Bangsbo et al. 2007).
Repeated sprint ability was impaired markedly during the first 2 days of recovery from G1, but also on the third recovery day from G2 and G3, demonstrating that 3 days of recovery is insufficient to recover repeated sprint ability with repeated match-play. Sprint performance was lowered by ~6 % 24 and 48 h post G1, which is of similar magnitude as post game findings on sprint ability in male players by others (Ascensao et al. 2008; Fatouros et al. 2010). However, in contrast to these two studies, the present study showed that performance was recovered 72 h post G1, which may be associated with the very high physical capacity of the players based on their Yo–Yo IR test scores (see Bangsbo and Mohr 2012). Interestingly repeated sprint performance was reduced to a greater degree on second and third days of recovery post G2 and on second day post G3 in comparison to G1. The impact of the game intensity could be argued to affect these responses, but most game activities were not different between game trials. Indeed the high-intensity running distance was slightly lower in G2 than G1 and G3 (see Table 1), despite that the recovery appeared slowest after G2, which may be caused by only 3 days separating G1 and G2, compared to 4 days of recovery between G2 and G3.
Post-game fatigue in football has for decades been attributed to low muscle glycogen levels (Jacobs et al. 1982; Bangsbo 1994) with more recent investigations demonstrating glycogen depletion in all fiber types (Krustrup et al. 2006b; Bendiksen et al. 2012), as well as in subcellular compartments (Nielsen et al. 2012). Muscle glycogen was not measured in the present study, but in all three games blood lactate levels were lower after the second half compared to the first and large increases were observed plasma FFA and glycerol indicating elevated fat oxidation and low muscle glycogen levels after the games. Also plasma creatinine was elevated after the games indicating a net protein catabolism further supporting low muscle glycogen stores. Recovery of muscle glycogen after football game play is slow compared to sports of similar duration and average intensity, but with a more continuous locomotion pattern. For example, it was shown by Krustrup et al. (2011) that muscle glycogen was not fully re-synthesized after 48 h after a game. In addition, to numerous performance inhibiting effects, such as impaired SR Ca2+ handling (Krustrup et al. 2011) and Na+-K+ ATPase function (Stephenson et al. 1999), lowered muscle glycogen has been linked to muscle damage and a concomitant elevated inflammatory response (Asp et al. 1998; Krustrup et al. 2011). In our study, we assessed subjective, functional and physiological markers of muscle damage, and large changes were seen in all variables. The DOMS scores increased markedly and the KJRM was lowered in the recovery period, but in support of the sprint data the largest responses were observed after G2. For example, knee extensor DOMS was not recovered on third day of recovery post G2 only, knee flexor DOMS were higher one and 2 days after G2 in comparison to G1 and G3 (Fig. 2a). Also KJRM is impaired longer post G2 and G3 compared to G1 (Fig. 2b). In the literature, as far as we know, there are no histological evidence of muscle damage and regeneration. However, the DOMS and KJRM data in the present study are strongly supported by plasma markers of muscle damage and inflammation, since both CK, CRP and adhesion molecules responses were higher during parts of the recovery period from G2 in comparison to G1 and G3. Thus, 3 days appear not sufficient to recover from game-induced muscle damage and concomitant inflammation during repeated match play in football.
WBC count is widely used a test to measure immune function by quantifying white blood cells (WBC) or leucocytes in the blood, which is covering the five major types of white blood cells (basophilocytes, eosinophilocytes, lymphocytes, monocytes and neutrophilocytes). In the present study, WBC nearly doubled after the games, which is supported by numerous studies (Gleeson 2007), and remained elevated 24 h after the game, but was normalized at baseline after 48 h post-game. The concomitant rise of adhesion molecules suggests that not only their number but also their adhesive capacity was also upregulated as also shown for other team sports during the same time frame after a game (Chatzinikolaou et al. 2014). Reports from others indicate that immune function may be impaired in professional footballers during periods with a high match frequency (Heisterberg et al. 2013). However, in the present leucocyte levels never dropped below baseline values, indicating that three game microcycle in a week does not compromise immune function despite a marked inflammatory response.
Alterations in systemic cortisol and testosterone concentrations have been applied as systemic indicators of over-reaching in scientific research (Silva et al. 2013). In this study, plasma cortisol concentrations were higher after G2 and on the first day of recovery compared to after G1 and G3. In contrast, plasma testosterone remained unaltered corroborating finding from previous studies (Ispirlidis et al. 2008; Malm et al. 2004; Thorpe and Sunderland 2012; Cunniffe et al. 2010) and the absence of changes in IGF-1 levels throughout the experimental period (Lagundžin et al. 2013). Thus, the post-game systemic stress response in trained football players appears higher when a game is completed after only 3 days of recovery from a preceding game, while four recovery days seem to be sufficient, which supports the performance, muscle soreness/damage indicators and inflammatory markers. The timeframe of cortisol rise coincided with that of ammonia elevation suggesting that cortisol may be associated with exercise-induced increase of ATP turnover rate (Krustrup et al. 2006b). Although cortisol was not measured during the game, its rise immediately post-game may be linked to increased gluconeogenesis and lipolysis during match play (Hoffman et al. 2002), a claim also supported by the post-match elevation of glucose and NEFA. However, cortisol has also been implicated in the regulation of the acute inflammatory response following exercise by increasing neutrophils and suppressing lymphocytes and natural killer cells (Pedersen 1997; Malm et al. 2004). In fact, cortisol increase following two football matches within a two-day frame was correlated with changes in neutrophil count (Malm et al. 2004). This cortisol action may be expressed through the up-regulation of immunoglobin M and adhesion molecule activity (Malm et al. 2004). In fact, football activity induced a simultaneous rise of both cortisol and adhesion molecules after the match. Cortisol rise is probably controlled by IL-6 (Steensberg et al. 2003) which coincides with the simultaneous post-game rise of IL-6 observed in this study. Interestingly, football practice left cortisol unaffected since no changes of cortisol were noted in the control group, supporting the notion that the exercise intensity and duration are important regulators of the cortisol homeostasis (Viru 1992). However, the fact that cortisol, cytokines and adhesion molecule concentrations demonstrated all a greater rise after G2 as compared to G1 and G3 suggests that cortisol response to football is related to muscle damage manifestations. Previous investigations have reported that a single football match elicits a marked rise of oxidative stress and antioxidant status markers for as long as 48 h (Ispirlidis et al. 2008; Fatouros et al. 2010; Silva et al. 2013; Andersson et al. 2010b). This is the first study to monitor such changes in response to three football games performed within a timeframe of 8 days. The main finding was that the rise in the circulating levels of these markers was more pronounced and more prolonged after G2 suggesting that a recovery time of less than 3 days may not be adequate for resolution of the inflammatory response, which again is supported by the inflammatory markers. During the post-game period, ROS are mainly produced by migrating leukocytes through the NADPH-oxidase mechanism and are used by these cells for the removal of damage tissue (Fielding et al. 1993). Specifically, immune cells produce the superoxide radical which is converted to H2O2, which then forms the highly reactive hydroxyl radical following the reaction of H2O2 with superoxide (Balakrishnan and Anuradha 1998). The hydroxyl radical then oxidizes lipids and proteins giving rise to various by-products such as TBARS and protein carbonyls (Balakrishnan and Anuradha 1998). TBARS elevation after football competition is most likely mainly attributed to oxidation of NEFA, lipoprotein phospholipids and cell membrane phospholipids (Robertson et al. 1991). The post-exercise rise of protein carbonyls is mainly the result of an increased oxidation of albumin and various circulating proteins (Miyamoto et al. 2003). GPX catalyzes the reaction of H2O2 removal by GSH that gives rise to GSSG (Sen and Packer 2000). The up-regulation of ROS during recovery is also supported by a parallel reduction of GSH and increase of GSSG suggesting a rise of GSH consumption after the games. This finding was accompanied by a simultaneous rise in GPX. As a result of GSH consumption and GSSG rise, their ratio declined markedly further confirming the onset of oxidative stress in response to repeated football games. Moreover, during recovery, ROS and RNS are actively involved in the mediation of the acute inflammatory response by regulating the translocation of various transcription factors (e.g. NF-κB) to the nuclei in a redox-sensitive manner (Aoi et al. 2004). ROS appear to regulate redox-dependent intracellular signaling pathways (e.g. NF-κB, MAPK) that control the synthesis and release of inflammatory mediators such as various cytokines (i.e. IL-1β, IL-6), adhesion molecules and immune cells (i.e., neutrophils, macrophages) and antioxidant enzymes (e.g., catalase, superoxide dismutase, GPX) (Michailidis et al. 2013) that were all elevated in this study. GSH reduction contributes to the alteration of post-exercise redox status (Michailidis et al. 2013). Interestingly, football practice in C did not increase oxidative stress and antioxidant status markers suggesting that games represent a more potent stimulus for ROS/RNS up-regulation as a part of the inflammatory response.
A similar timeframe of responses was observed for antioxidant status markers with G2 again eliciting a more prolonged and pronounced elevation of these markers. In any case, there was a parallel increase of pro- and anti-oxidant markers suggesting that the system was functioning properly. In cases of extreme exercise or overtraining, antioxidant capacity seems to be compromised thereby allowing pro-oxidation to escalate (Margonis et al. 2007; Tanskanen et al. 2010). Therefore, a congested football microcycle with three games and daily practices did not deregulate the overall antioxidant response. However, future research should explore this possibility over the course of a multi-month competitive season. Interestingly, Andersson et al. (2010c) reported that GSH/GSSH was similarly reduced after a football game but this response was normalized following a second game performed 3 days after the first one. It must be noted that participants in that study were female football players whereas in this study participants were male players. Although this discrepancy may be related to the higher levels of oestrogens in females that are known to have a protective role against oxidative stress, it may also be related to physical conditioning status since cardiovascular training upregulated antioxidant status (Fatouros et al. 2004). However, participants in this study demonstrated performance values similar to those observed at elite level. Elite players may be more familiarized with such congested game and training schedules during their competitive season and thus better adapted. This possibility warrants further investigation in the future.
Conclusions
In conclusion, post-game performance recovery and inflammatory adaptations in response to a three-game weekly microcycle displayed a different response pattern, with strong indications of a largest physiological stress and degree of post-game fatigue after the middle game that was preceded by only a 3-day recovery.
Abbreviations
- ANOVA:
-
Analysis of variance
- CAT:
-
Catalase activity
- CK:
-
Creatine kinase
- CON:
-
Control group
- CRP:
-
C-reactive protein
- D1, D2, D3, D4:
-
Day 1, day 2, day 3, day 4
- DL:
-
Dominant leg
- DOMS:
-
Delayed onset of muscle soreness
- EDTA:
-
Ethylenediaminetetraacetic acid
- EXP:
-
Experimental group
- IGF-1:
-
Insuline growth factor
- IL-1β, IL-6:
-
Interleukin
- G1, G2, G3:
-
Game 1, game 2, game 3
- GPS:
-
Global positioning system
- GPX:
-
Glutathione peroxidase activity
- GSH:
-
Reduced glutathinoe
- GSSG:
-
Oxidized glutathione
- HR:
-
Heart rate
- KJRM:
-
Knee range of motion
- NDL:
-
Non-dominant leg
- NEFA:
-
Non-esterified fatty acids
- PC:
-
Protein carbonyls
- RSA:
-
Repeated sprint ability
- sVCAM-1:
-
Soluble cell adhesion molecules
- TAC:
-
Total antioxidant capacity
- TBARS:
-
Thiobarbituric acid-reactive substances
- VO2max :
-
Maximal oxygen uptake
- WBC:
-
White blood cell count
- Yo–Yo IR1, IR2:
-
Yo–Yo intermittent recovery test, level 1 and 2
References
Akova B, Surmen-Gur E, Gur H, Dirican M, Sarandol E, Kucukoqlu S (2001) Exercise-induced oxidative stress and muscle performance in healthy women: role of vitamin E supplementation and endogenous oestradiol. Eur J Appl Physiol 84(1–2):141–147
Andersson H, Ekblom B, Krustrup P (2008) Elite football on artificial turf versus natural grass: movement patterns, technical standards, and player impressions. J Sports Sci 26(2):113–122
Andersson H, Bohn SK, Raastad T, Paulsen G, Blomhoff R, Kadi F (2010a) Differences in the inflammatory plasma cytokine response following two elite female soccer games separated by a 72-h recovery. Scand J Med Sci Sports 20(5):740–747
Andersson H, Karlsen A, Blomhoff R, Raastad T, Kadi F (2010b) Plasma antioxidant responses and oxidative stress following a soccer game in elite female players. Scand J Med Sci Sports 20(4):600–608
Andersson H, Karlsen A, Blomhoff R, Raastad T, Kadi F (2010c) Active recovery training does not affect the antioxidant response to soccer games in elite female players. Br J Nutr 104(10):1492–1499
Aoi W, Naito Y, Takanami Y, Sakuma K, Ichikawa H, Yoshida N, Yoshikawa T (2004) Oxidative stress and delayed onset muscle damage after exercise. Free Rad Biol Med 37:480–487
Ascensao A, Rebelo A, Oliveira E, Marques F, Pereira L, Magalhaes J (2008) Biochemical impact of a soccer match-analysis of oxidative stress and muscle damage markers throughout recovery. Clin Biochem 41:841–851
Asp S, Daugaard JR, Kristiansen S, Kiens B, Richter EA (1998) Exercise metabolism in human skeletal muscle exposed to prior eccentric exercise. J Physiol 509:305–313
Balakrishnan SD, Anuradha CV (1998) Exercise, depletion of antioxidants and antioxidant: manipulation. Cell Biochem Funct 16:269–275
Baldari C, Bonavolonta V, Emerenziani GP, Gallotta MC, Silva AJ, Guidetti L (2009) Accuracy, reliability, linearity of accutrend and lactate Pro versus EBIO plus analyzer. Eur J Appl Physiol 107:105–111
Bangsbo J (1994) The physiology of soccer-with special reference to intense intermittent exercise. Acta Physiol Scand Suppl 619:1–155
Bangsbo J, Mohr M (2012) Fitness testing in football. Fitness training in soccer II. Bangsbosport, Copenhagen
Bangsbo J, Iaia FM, Krustrup P (2007) Metabolic response and fatigue in soccer. Int J Sports Physiol Perform 2(2):111–127
Barbas I, Fatouros IG, Douroudos II et al (2011) Physiological and performance adaptations of elite Greco-Roman wrestlers during a one-day tournament. Eur J Appl Physiol 111(7):1421–1436
Bendiksen M, Bischoff R, Randers MB et al (2012) The Copenhagen soccer test: physiological responses and fatigue development. Med Sci Sports Exerc 44(8):1595–1603
Bradley SP, Sheldon W, Wooster B, Olsen P, Boanas P, Krustrup P (2009) High-intensity running in English FA Premier League soccer matches. J Sports Sci 27(2):159–168
Chatzinikolaou A, Draganidis D, Avloniti A et al (2014) The microcycle of inflammation and performance changes after a basketball match. J Sports Sci 32(9):870–882
Cunniffe B, Hore AJ, Whitcombe DM, Jones KP, Baker JS, Davies B (2010) Time course of changes in immuneoendocrine markers following an international rugby game. Eur J Appl Physiol 108(1):113–122
Davies KJ, Quintanilha AT, Brooks GA, Packer L (1982) Free radicals and tissue damage produced by exercise. Biochem Biophys Res Commun 107(4):1198–1205
Dill DB, Costill DL (1974) Calculation of percentage changes in volumes of blood, plasma, and red cells in dehydration. J Appl Physiol 37(2):247–248
Dupont G, Nedelec M, McCall A, McCormack D, Berthoin S, Wisloff U (2010) Effect of 2 soccer matches in a week on physical performance and injury rate. Am J Sports Med 38(9):1752–1758
Ekstand J, Walden M, Haqqlund M (2004) A congested football calendar and the wellbeing of players: correlation between match exposure of European footballers before the World Cup 2002 and their injuries and performances during that World Cup. Br J Sports Med 38(4):493–497
Fatouros IG, Jamurtas AZ, Villiotou V, Pouliopoulou S, Fotinakis P, Taxildaris K, Deliconstantinos G (2004) Oxidative stress responses in older men during endurance training and detraining. Med Sci Sports Exerc 36(12):2065–2072
Fatouros IG, Chatzinikolaou A, Douroudos II et al (2010) Time-course of changes in oxidative stress and antioxidant status responses following a soccer game. J Strength Cond Res 24(12):3278–3286
Fielding R, Manfredi T, Ding W, Fiatarone MA, Evans WJ, Cannon JG (1993) Acute phase response to exercise. III: Neutrophil and IL-1 accumulation in skeletal muscle. Am J Physiol 265:166–172
Gleeson M (2007) Immune function in sport and exercise. J Appl Physiol 103(2):693–699
Goussetis E, Spiropoulos A, Tsironi M et al (2009) Spartathlon, a246 kilometer foot race: effects of acute inflammation induced by prolonged exercise on circulating progenitor reparative cells. Blood Cells Mol Dis 42(3):294–299
Gunnarsson TP, Bendiksen M, Bischoff R et al (2013) Effect of whey protein- and carbohydrate-enriched diet on glycogen resynthesis during the first 48 h after a soccer game. Scand J Med Sci Sports 23(4):508–515
Heisterberg MF, Fahrenkruq J, Krustrup P, Storskov A, Kjaer M, Andersen JL (2013) Extensive monitoring through multiple blood samples in professional soccer players. J Strength Cond Res 27(5):1260–1271
Hoffman JR, Maresh CM, Newton RU et al (2002) Performance, biochemical, and endocrine changes during a competitive football game. Med Sci Sports Exerc 34(11):1845–1853
Ispirlidis I, Fatouros IG, Jamurtas AZ et al (2008) Time course of changes in inflammatory and performance responses following a soccer game. Clin J Sports Med 18:428–431
Jacobs I, Westlin N, Karlsson J, Rasmusson M, Houghton B (1982) Muscle glycogen and diet in elite soccer players. Eur J Appl Physiol Occup Physiol 48(3):297–302
Krustrup P, Mohr M, Amstrup T et al (2003) The yo-yo intermittent recovery test: physiological response, reliability, and validity. Med Sci Sports Exerc 35(4):697–705
Krustrup P, Mohr M, Nybo L, Jensen JM, Nielsen JJ, Bangsbo J (2006a) The Yo-Yo IR2 test: physiological response, reliability, and application to elite soccer. Med Sci Sports Exerc 38(9):1666–1673
Krustrup P, Mohr M, Steensberg A, Bencke J, Kjaer M, Bangsbo J (2006b) Muscle and blood metabolites during a soccer game: implications for sprint performance. Med Sci Sports Exerc 38(6):1165–1174
Krustrup P, Zebis M, Jensen JM, Mohr M (2010) Game-induced fatigue patterns in elite female soccer. J Strength Cond Res 24(2):437–441
Krustrup P, Ortenblad N, Nielsen J et al (2011) Maximal voluntary contraction force, SR function and glycogen resynthesis during the first 72 h after a high-level competitive soccer game. Eur J Appl Physiol 111(12):2987–2995
Lagundžin D, Vučić V, Glibetić M, Nedić O (2013) Alteration of IGFBP-1 in soccer players due to intensive training. Int J Sport Nutr Exerc Metab 23(5):449–457
Lee IH, Seo EJ, Lim IS (2014) Effects of aquatic exercise and CES treatment on the changes of cognitive function, BDNF, IGF-1, and VEGF of persons with intellectual disabilities. J Exerc Nutrition Biochem 18(1):19–24
Magalhaes J, Rebelo A, Oliveira E, Silva JR, Marques F, Ascensao A (2010) Impact of Loughborough intermittent shuttle test versus soccer match on physiological, biochemical and neuromuscular parameters. Eur J Appl Physiol 108(1):39–48
Malm C, Ekblom O, Ekblom B (2004) Immune system alteration in response to two consecutive soccer games. Acta Physiol Scand 180:143–155
Margonis K, Fatouros IG, Jamourtas AZ et al (2007) Oxidative stress biomarkers responses to physical overtraining: implications for diagnosis. Free Rad Biol Med 43:901–910
Marshall PW, Lovell R, Jeppesen GK, Andersen K, Siegler JC (2014) Hamstring muscle fatigue and central motor output during a simulated soccer match. PLoS One 9(7):e102753. doi:10.1371/journal.pone.0102753
Maughan R (2004) Contamination of supplements: an interview with professor Ron Maughan by Louise M. Burke. Int J Sport Nutr Exerc Metab 14(4):493
Michailidis Y, Karagounis LG, Terzis G et al (2013) Thiol-based antioxidant supplementation alters human skeletal muscle signaling and attenuates its inflammatory response and recovery after intense eccentric exercise. Am J Clin Nutr 98:233–245
Miyamoto Y, Koh YH, Park YS et al (2003) Oxidative stress caused by inactivation of glutathione peroxidase and adaptive responses. Biol Chem 384:567–574
Mohr M, Krustrup P (2013) Heat stress impairs repeated jump ability after competitive elite soccer games. J Strength Cond Res 27(3):683–689
Mohr M, Krustrup P, Bangsbo J (2003) Match performance of high-standard soccer players with special reference to development of fatigue. J Sports Sci 21(7):439–449
Mohr M, Krustrup P, Nybo L, Nielsen JJ, Bangsbo J (2004) Muscle temperature and sprint performance during soccer matches-beneficial effects of re-warm-up at half time. Scand J Med Sci Sports 14(3):156–162
Mohr M, Krustrup P, Bangsbo J (2005) Fatigue in soccer: a brief review. J Sports Sci 23(6):593–599
Mohr M, Mujika I, Santisteban J et al (2010) Examination of fatigue development in elite soccer in a hot environment: a multi-experimental approach. Scand J Med Sci Sports 20(3):125–132
Mohr M, Nybo L, Grantham J, Racinais S (2012) Physiological responses and physical performance during football in the heat. PLoS One 7(6):e39202. doi:10.1371/journal.pone.0039202
Nedelec M, McCall A, Carling C, Legall F, Berthoin S, Dupont G (2012) Recovery in soccer: part I—post-match fatigue and time course of recovery. Sports Med 42(12):997–1015
Nielsen J, Krustrup P, Nybo L et al (2012) Skeletal muscle glycogen content and particle size of distinct subcellular localizations in the recovery period after a high-level soccer match. Eur J Appl Physiol 112(10):3559–3567
Norkin CC, White DJ (1985) Measurement of joint motion: a guide to goniometry. Davis Company, Philadelphia
Nosaka K, Newton M, Sacco P (2002) Delayed-onset muscle soreness does not reflect the magnitude of eccentric exercise-induced muscle damage. Scand J Med Sci Sports 12(6):337–346
Nybo L (2012) Brain temperature and exercise performance. Exp Physiol 97(3):333–339
Pedersen BK (1997) Exercise Immunology. Steenberg, Georgetown
Randers MB, Mujika I, Hewitt A et al (2010) Application of four different football match analysis systems: a comparative study. J Sports Sci 28(2):171–182
Robertson JD, Maughan RJ, Duthie GG, Morricey PC (1991) Increased blood antioxidant systems of runners in response to training load. Clin Sci 80:611–618
Sen CK, Packer L (2000) Thiol homeostasis and supplements in physical exercise. Am J Clin Nutr 72:653S–669S
Silva JR, Ascensão A, Marques F, Seabra A, Rebelo A, Magalhães J (2013) Neuromuscular function, hormonal and redox status and muscle damage of professional soccer players after a high-level competitive match. Eur J Appl Physiol 113:2193–2201
Steensberg A, Fischer CP, Keller C, Moller K, Pedersen BK (2003) IL-6 enhances plasma IL-1ra, IL-10, and cortisol in humans. Am J Physiol Endocrinol Metab 285(2):433–437
Stephenson DG, Nguyen LT, Stephenson GM (1999) Glycogen content and excitation-contraction coupling in mechanically skinned muscle fibres of the cane toad. J Physiol 1:177–187
Tanskanen M, Atalay M, Uusitalo A (2010) Altered oxidative stress in overtrained athletes. J Sports Sci 28(3):309–317
Theodorou AA, Nikolaidis MG, Paschalis V, Sakellariou GK, Fatouros IG, Koutedakis Y, Jamurtas AZ (2010) Comparison between glucose-6-phosphate dehydrogenase-deficient and normal individuals after eccentric exercise. Med Sci Sports Exerc 42(6):1113–1121
Thorpe R, Sunderland C (2012) Muscle damage, endocrine, and immune marker response to a soccer match. J Strength Cond Res 26(10):2783–2790
Varley MC, Aughey RJ (2013) Acceleration profiles in elite Australian soccer. Int J Sports Med 34(1):34–39
Viru A (1992) Plasma hormones and physical exercise. Int J Sports Med 13:201–209
Acknowledgments
The authors thank the players and their coaches for their participation, as well as Dr. Sarah Jackman for technical assistance. The study was supported by departmental funding from the Democritus University of Thrace, Komotini, Greece, a donation by the Bodosakis Foundation and the Faroese Research Council.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflict of interest to disclose.
Additional information
Communicated by Fabio Fischetti.
Rights and permissions
About this article
Cite this article
Mohr, M., Draganidis, D., Chatzinikolaou, A. et al. Muscle damage, inflammatory, immune and performance responses to three football games in 1 week in competitive male players. Eur J Appl Physiol 116, 179–193 (2016). https://doi.org/10.1007/s00421-015-3245-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00421-015-3245-2