Abstract
Intense exercise is known to cause temporary impairments in immune function. Few studies, however, have investigated the effects of intense competitive exercise on immunoendocrine variables in elite team sport athletes. The aim of this study was to evaluate the time course of changes in selected immunoendocrine and inflammatory markers following an international rugby union game. Blood samples were taken from players (n = 10) on camp entry, the morning of the game (pre), immediately after (post) and 14 and 38 h into a passive recovery period. Players lost 1.4 ± 0.2 kg of body mass during the game (ambient conditions, 11°C, 45% RH). An acute phase inflammatory response was observed as reflected through immediate increases in serum cortisol and IL-6 (post) followed by delayed increases in serum creatine kinase (CK; 14 h) activity and C-reactive protein (CRP; 38 h); P < 0.05. Decreases in the number of circulating T lympocytes, NK cells and bacteria-stimulated neutrophil degranulation were also observed post-exercise (P < 0.05), indicative of decreased host immune protection. Following a large decrease in serum testosterone to cortisol (T/C) ratio immediately post and 14 h after exercise, T/C values then increased above those observed at camp entry 38 h into recovery (P < 0.05). This rebound anabolic stimulus may represent a physiological requirement for recovery following intense tissue damage resulting from game collisions. The findings also suggest that a game of international rugby elicits disturbances in host immunity, which last up 38 h into the recovery period.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Rugby union is considered to be one of the most intense and physically demanding field games in the world (Mashiko et al. 2004). Since its emergence as a professional sport (1995), the game appears to have become a faster, ruck dominated game that contains more phases of play (Eaves and Hughes 2003). Furthermore, players have become bigger and faster and are involved in more physical contact and collisions during game play (Duthie et al. 2003; Quarrie and Hopkins 2007). Despite its growing popularity throughout the world, few scientific data exist to help us understand the competitive stresses inherent within the game.
Traditionally, the physical demands of rugby union have been assessed through analysis of game activities (Roberts et al. 2008; Deutsch et al. 2007; Cunniffe et al. 2009), injury analysis (Brooks et al. 2008; Fuller et al. 2007) or from questionnaire analysis for signs of player burnout (Cresswell and Eklund 2006). Whilst notational analysis type studies do provide valuable insight into the various game activities undertaken by players, difficulties in the direct assessment of stresses arising from tackling and game contact instances inevitably occur. Despite the fact that competitive play is thought to elicit considerable physiological strain on players, few studies have investigated such through detailed biochemical assessment (Mashiko et al. 2004; Takarada 2003). This is particularly evident at the elite level. In other combative sports such as American football, structural tissue damage and associated biochemical disturbances have been associated with reductions in force production and athletic performance (Hoffman et al. 2002). In professional rugby union, it is feasible that high-impact collisions received by players during tackling, in addition to eccentric damage from locomotion, elicits extensive tissue trauma and associated inflammation. Indeed, a significant link between the level of tissue trauma and number of game collisions has been previously established (Smart et al. 2008; Takarada 2003), and that injury potential in rugby union is largely dependent on these contact phases of play (Fuller et al. 2007). However, it is not known if the degree of tissue disruption and biochemical disturbances is greater with higher levels of play or if these stress effects are capable of modifying host immune function.
Intense exercise is known to alter many aspects of the immune system, and in some instances depress host protection (see Gleeson 2007 for a review). Additionally, tissue trauma has been shown to influence immune cell recruitment into damaged muscle (Fielding et al. 1993; Paulsen et al. 2005). In particular, infiltration of blood neutrophils into injured tissue has been suggested as a key characteristic of the inflammatory process (Tidball 2005). Rugby union is a game played over 80 min, during which time players are required to run intermittently whilst participating in many game-related impacts such as tackling. It is possible that through investigation of biochemical and immunological status, a greater understanding of the game demands may be achieved. Knowledge of this at the highest level of play (international) may help in understanding recovery aspects of the game and potential adverse effects on host immune protection. To the authors’ knowledge, no studies have previously attempted to investigate physiological responses before and after an elite rugby game using detailed biochemical analysis. With the above in mind, the purpose of this study was to investigate the effects of acute game stress on immunological and hormonal responses, with specific reference to recovery duration.
Methods
Subjects
Ten international rugby union players [mean (±SEM), age 26.4 (0.7) years, height 186.5 (2.5) cm, body mass 103.1 (3.9) kg, \( \dot{V}{{\text{O}}_{\rm 2peak}} \) 53.2 (1.1) mL kg−1 min−1] agreed to participate in the current study. Players were informed of the purpose and risks of the study through visual presentation and player information sheets on entry to the international training base. All players provided written informed consent before volunteering for the study. Experimental procedures to be undertaken were approved by the University Research Ethics Committee and also by the medical committee of the Wales Rugby Union. Any player who wished to withdraw from the study could do so at any time.
Sample collection
Peripheral venous blood samples were taken from players on entry to the camp, the morning of the game (pre), within 15 min of the conclusion of the game (post) and again the following two mornings (14 h and 38 h respectively). Starting time of the game was 17.00 hours. Blood samples were obtained by venepuncture from an antecubital vein and collected into three Vacutainer tubes (Becton Dickinson, UK). All samples were obtained whilst players were in a supine position and this was standardised throughout. Blood samples collected in K3EDTA vacutainers (4 mL) were kept at room temperature and used for standard haematological analysis and assessment of changes in plasma volume (Dill and Costill 1974). Analysis was carried out within 3 h of collection. In addition, whole blood (7 mL) was collected into sterile lithium-heparin vacutainer tubes (Becton Dickinson, Oxford, UK) for measurement of neutrophil degranulation (see below). For other measurements (high-sensitivity C-reactive protein, hs-CRP, creatine kinase, CK, cortisol, C and testosterone T), blood (7 mL) was collected into tubes containing a clot activator (SST; Becton Dickinson, Oxford, UK) and subsequently centrifuged (1,500g × 10 min). Serum was then aspirated into appropriate aliquots and stored at −80°C until future analysis. The remaining blood (K3EDTA and heparin tubes) was also centrifuged (1,800g × 10 min) at 4°C for collection of plasma, and aliquots were stored as above for serum measurements.
Analytical methods
Determination of immune cell concentrations
Analysis of haemoglobin, haematocrit and total and differential leukocytes was performed using an automated cell counter (Coulter Electronics, USA). Blood leukocyte phenotypes were analysed by standard flow cytometry (Beckman-Coulter FC500 MPL). Specific staining of cells was performed by incubating the test sample (100 μl whole blood) with a test reagent. The staining procedure involved adding combinations of anti-human monoclonal antibodies, conjugated with fluorescein isothiocyanate (FITC), phycoerythrin (PE) or phycoerythrin-cyanin (PE-Cy). Cells were mixed for 1 s to reduce cell aggregation and incubated for 30 min (dark; room temp) before the red cells were removed by erythrocyte lysis with OptiLyse C (Beckman-Coulter, UK Ltd.) and subsequently analysed. Non-specific staining of monoclonal antibodies with isotypic controls was also employed to determine the existence of non-specific fixation/binding of specific conjugated monoclonal antibodies. Lymphocyte subsets were classified as total T cells (CD3+), Thelper cells (CD3+CD4+) and Tcytotoxic cells (CD3+CD8+). NK cell (CD3−CD16+CD56+) number was assessed via the co-expression of cell surface markers CD16 and CD56 and lack of expression of CD3 (Cooper et al. 2001).
In vitro bacteria-stimulated neutrophil degranulation
The neutrophil degranulation response to bacterial stimulant was determined according to methods previously outlined (Robson et al. 1999). Briefly, on collection of heparinised blood, 1 mL of heparinised blood was added to an eppendorf microcentrifuge tube containing 50 μl of bacterial stimulant (840-15, Sigma, Poole, UK). The blood and stimulant were then mixed by gentle inversion and incubated for 60 min in a water bath (37°C). After 30 min of incubation, the contents of the tube were gently mixed again via inversion. When the incubation period had elapsed, eppendorfs were centrifuged (10,000g × 2 min) immediately and the supernatant was stored at −80°C prior to analysis of elastase concentration (ELISA kit; Merck Chemicals, UK). Neutrophil degranulation was expressed as the amount of stimulated elastase release per neutrophil. The intra-assay coefficient of variation (CV) for this assay was 6.0%.
Biochemical and hormonal variables
Analysis of serum cortisol and testosterone was performed using two commercially available ELISA kits (DRG Diagnostics, Germany). Concentrations of high-sensitive C-reactive protein (hs-CRP) were determined in serum by means of an immunoturbidimetric assay (Randox Ltd, Antrim, UK; sensitivity <0.08 mg L−1) using an automated clinical chemistry system (RX DaytonaTM). Plasma IL-6 concentrations were analysed from K3EDTA-treated venous blood using a commercially available high-sensitivity ELISA (Diaclone Research, Besancon, France; sensitivity <0.8 pg mL−1). Finally, total creatine kinase activity was quantitatively measured using VITROS CK slides (Ortho-Clinical Diagnostics, Buckinghamshire UK) in serum and analysed on a VITROS 950 Chemistry System. The intra-assay CV was 3.2, 3.4, 4, 6.6 and 2.5% for cortisol, testosterone, hs-CRP, IL-6 and CK, respectively.
Dietary control
Players were provided with dietary plans by the affiliated nutritionist in the lead up to international games and this was not altered for the current investigation. Meals generally included a variation of low fat yogurts, fruit, pastas/rice, cereal bars, vegetables, cooked meat and pancakes. Additionally, players were asked to consume their ‘typical’ diet on the day before, day of, and day(s) after both sampling points (games). All morning blood sample points were taken from players following an overnight fast. The post-game sample was taken 3–4 h after the players last main meal. Players were asked to refrain from any post-game fuel/fluid consumption until blood samples (and measurement of body mass) were taken for that time point. All players were asked to refrain from alcoholic beverages for the duration of sampling.
Evaluation of fluid loss during exercise
Players were weighed (in shorts only) 3 h before the start of the game and again immediately after, using an electronic weighing scales (Seca Ltd, Birmingham, UK). Sweat loss was not estimated due to logistical difficulties. During the course of the game, all players were allowed to drink water ad libitum whenever breaks in play permitted. The volume of fluids consumed was not noted. Whole body fluid loss was calculated from the reduction in body mass that occurred over the course of the game. Mean temperature and relative humidity values were recorded from four readings taken over the course of the game using an electronic wireless climate gauge (Thermo-Hygro).
Game characteristics and analysis
The game (international rugby union) involved players from teams ranked number six and two in the world at that time (IRB World Rankings, November 2005). Game analysis and investigation of biochemical disturbances was performed on players from the home team only. Before the game, players participated in a standardised warmup, which included ball work, light tackling and pad work, stretching, calisthenics and position-specific drills. This lasted approximately for 30 min. To assess the contribution of direct muscle trauma on markers of muscle damage/inflammation, game analysis was performed via use of video analysis software (Sports code, Co™) and from video recordings following the event. The identified game statistics used in this study were contact events per player, player tackle number and game work:rest ratio. Player tackle number included the number of tackles received and made by each individual player, whilst game work:rest ratios were defined as the period from the beginning of a play to the interruption of that play period by the referee, and the period from the interruption of a play by the referee to the start of the next play (Takarada 2003). The number of contact events included any element of play that included physical player–player contact (i.e. tackles, scrums, mauls, rucks). These specific game statistics were deemed important in determining the relationship between the contact element of rugby union and its possible effect on immunological and biochemical variables.
Data analysis
Normality of distribution was initially checked on variables using the Shapiro–Wilk test. Upon assumption of normality, data was analysed using a one-way repeated measures analysis of variance (ANOVA) with Bonferroni post hoc correction. In the cases of CK, IL-6 and hs-CRP data, analysis revealed skewed distributions and these data sets were subsequently log transformed prior to statistical procedures. Pearson product moment correlations were used to establish possible relationships between variables. Levels of significance were set at the P < 0.05 level. Data were evaluated using an SPSS for Windows version 14.0 software package (Chicago, USA) and presented as means ± SEM. For neutrophil degranulation, a sample size of 10 was deemed sufficient to detect a significant difference from analyses of power and effect size calculations used previously (Bishop et al. 2003).
Results
Game data
Mean ambient temperature and relative humidity was 11.2°C and 45% RH, respectively. Players were involved in 69 ± 9.0 min of game time and lost 1.4 ± 0.2 kg of body mass over the course of the game.
Biochemical data
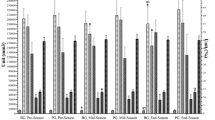
Main effects of time were observed for both cortisol and testosterone (P < 0.05) as shown in Table 1. Compared to baseline levels (camp entry), C decreased on the morning of the game (~18%; P < 0.05) before increasing considerably at the end of the game (post 40%; P < 0.05). Cortisol concentrations then decreased throughout the recovery period and the values 14 h post-exercise approached those at camp entry. However, C decreased further during recovery such that concentrations 38 h post-exercise were significantly lower (~33%) than those observed at camp entry (P < 0.05). Unlike C, concentrations of T decreased immediately after exercise such that values were significantly lower (~43%) than those observed on the morning of the game (P < 0.05). Like C, serum concentrations of T 14 h post-exercise were similar to those observed at camp entry. Notably, concentrations continued to increase further into the recovery period with T values 38 h post-exercise higher than those observed at camp entry (P > 0.05). T/C values decreased immediately post-exercise (P < 0.05) before rising steadily throughout the recovery period. T/C values 38 h post-exercise were significantly higher than those at camp entry (Fig. 1; P < 0.05).
The activity of serum creatine kinase (CK) is displayed in Table 1. Increases in CK were observed 14 and 38 h after the game with values 14 h post-exercise significantly higher than those observed immediately post-exercise (P < 0.05). Unlike CK activity, peak concentrations of CRP were observed 38 h post-exercise (Fig. 2). Pre-game CRP concentrations, as well as those observed immediately post-exercise, were significantly lower than those recorded at camp entry. Concentrations then increased 14 and 38 h following exercise such that values at 14 h post-exercise were significantly higher than both pre- and post-game samples. Although the highest CRP values were observed 38 h post, this increase did not reach statistical significance.
Highest concentrations of serum IL-6 were observed immediately post-exercise, after which values decreased progressively during recovery. IL-6 concentrations immediately post-exercise were significantly higher than those at 14 and 38 h post-game (Table 1; P < 0.05). Significant correlations were observed between the number of contact events (r = 0.78; P < 0.05) and tackles (r = 0.86; P < 0.05) completed by players with 38 h of CK activity. Weaker, but significant, correlations between contact events (r = 0.65) and tackle number (r = 0.63) were observed with 14 h of CK activity (P < 0.05). Significant correlations were also observed between the number of contact events (r = 0.82) and tackles (r = 0.64) with post-game neutrophil count (both P < 0.05).
Immune data
Exercise-induced changes in circulating number of blood leukocytes and leukocyte subsets are shown in Table 2. Numbers of leuckocytes and neutrophils significantly increased post-exercise, with concentrations decreasing gradually throughout the recovery period. Cell concentrations were still elevated 14 h into the recovery period (P < 0.05). In the case of blood neutrophils, cell numbers were elevated 38 h into recovery, although data did not reach significance (P = 0.08). Similar findings were observed for blood monocytes. A significant effect of time (P < 0.05) was observed for numbers of total lymphocytes, CD3+CD4+ lymphocytes, CD3+CD8+ lymphocytes and NK [CD3−CD16+CD56+] cells. Following an initial decrease in total lymphocytes immediately post-exercise (P = 0.08), a delayed increase was observed 14 h into the recovery period, concentrations of which were significantly higher than at 38 h post (P < 0.05; Table 2). Similar trends were observed for lymphocyte subsets. A decrease (P = 0.08) in the number of circulating CD4+ cells was observed immediately post-exercise and was followed by a significant increase in concentrations 14 h post-exercise, the values of which were higher than all other sampling points (P < 0.05). A greater decrease in the number of CD8+ cells was observed immediately post-exercise such that the values were lower than all other sample points (P < 0.05). Concentrations of CD8+ cells on the morning of the game (pre-game) were also lower than those observed at camp entry (P < 0.05). Similar to CD4+ and CD8+ cells, concentrations of NK cells decreased immediately following the game, with values significantly lower than all other sample points. Concentrations of NK cells then increased progressively throughout the recovery period (Table 2).
Neutrophil function
As mentioned above, a marked blood neutrophilia was observed. Following exercise, an initial increase (P = 0.08) in plasma elastase concentration was observed after which values decreased significantly below pre-game values (P < 0.05; Table 3). Concentrations remained low throughout the remainder of the recovery period. Total bacteria-stimulated elastase release followed a similar pattern with an initial increase in plasma elastase concentration observed immediately post-exercise. This increase was subsequently followed by decreases 14 and 38 h post-exercise (P < 0.05). Although total stimulated elastase concentrations remained lower than pre-game values, differences were non-significant. Adjusting data to take into account the number of circulating neutrophils revealed a 65% fall in bacteria-stimulated elastase release per cell immediately post-exercise (vs. pre-game values). Values began to increase gradually during recovery, with bacteria-stimulated elastase release (per cell) 45 and 28% lower than pre-game values 14 and 38 h post-exercise, respectively (Fig. 3).
Discussion
A few studies have examined the biochemical responses to game play in rugby union (Smart et al. 2008; Takarada 2003; Mashiko et al. 2004; Elloumi et al. 2003). However a paucity of data exists on the physiological stresses imposed on players within the elite competitive environment. With the above in mind, the current study represents a unique body of data, given that it is the first to examine detailed biochemical and immunological responses to competitive rugby play at an international level.
Decreased levels of testosterone and increased levels of cortisol are thought to represent disturbances in the overall anabolic–catabolic balance of the athlete (Banfi et al. 1993; Hoogeveen and Zonderland 1996). In the current study, significant increases in C (~40%) and corresponding decreases in T levels (~43%) were observed immediately after the game. These changes resulted in a large decrease in T/C ratio, with values still not recovered after 14 h of recovery. Such findings corroborate those of a previous study in top-level rugby union (Elloumi et al. 2003), although increases in C level were observed longer into the recovery period in the present study. It is possible that differences in playing standard (higher in this study) and playing environment contributed to this greater stress response. Interestingly, present findings also show that C decreased below resting values 38 h into recovery. This was matched by corresponding increased levels of T, such that a significant increase in T/C ratio was observed for this time point when compared to camp entry. These findings agree with those observed previously in rugby (Elloumi et al. 2003) and wrestling (Passelergue and Lac 1999) and may represent a rebound anabolic stimulus during the recovery period. Future studies should attempt to investigate the performance-related effects of this T/C rebound in athletes using physical performance tests and whether or not additional exercise during this period exacerbates the muscle repair processes required following heavy exercise.
Interestingly, significantly lower resting C concentrations were observed on the morning of the game (pre-game) than corresponding C levels at camp entry. This is in contrast to previous observations in American football where higher cortisol concentrations were observed in players closer to kick-off time (Hoffman et al. 2002). However, it is possible that these higher C values were a consequence of heavy training and competition prior to players entering the international camp in the current study. Although samples were taken on the morning of camp entry and players had refrained from previous exercise (minimum of 48 h), the time point (camp entry) occurred at the end of an intensified period in club competition (Heineken Cup). Furthermore, pre-game samples were taken 18 days into the international camp during which time training volume was considered ‘low’ relative to the previous phase of club competition. Consequently, these lower pre-game C levels may have been a product of decreased residual training stress when players were present at international squad headquarters. Higher resting levels of CK and CRP, along with lower T concentrations on camp entry compared to pre-game values would seem to substantiate this point.
In accordance with previous studies (Ispirlidis et al. 2008), a delayed increase in CK levels was observed after exercise. Peak levels occurred 14 h following the game, although values were still elevated (2.3-fold) above pre-game values 38 h into the recovery period. This suggests that significantly more time may be required to obtain adequate recovery from preceding tissue damage. Increases in the serum activity of this enzyme are considered a result of increased permeability of skeletal muscle membrane, suggestive of skeletal tissue damage (Clarkson and Hubal 2002). As expected, resting CK levels were higher in athletes within the present study (rugby union) compared to those observed in other team sports such as American football (Hoffman et al. 2002; 2005) and soccer (Ispirlidis et al. 2008). It is possible that differences in protein efflux, clearance rate or loss of membrane integrity (Baker et al. 2004; Sjödin et al. 1990) contributed to these findings. However, cumulative tissue damage arising from rugby-related physical trauma represents the most plausible explanation for this finding. Indeed, despite a small subject number, significant correlations were observed between serum CK activity (both 14 and 38 h) and player involvement in tackles and game contact events. Such correlations have been observed previously in other collision-type sports such as boxing (Zuliani et al. 1985), rugby union (Smart et al. 2008; Takarada 2003) and American football (Hoffman et al. 2002). Together, these findings are suggestive of a blunt trauma-like effect on muscle damage, which occurs during game contact events and resulting plasma CK leakage. However, the effect of collisions and tissue trauma on immunological indices was not investigated in the latter studies.
In the current study, a significant leukocytosis was observed after the rugby game. This was dominated by a corresponding increase in blood neutrophils (>3.5-fold) and, to a lesser extent, blood monocytes (2-fold increase). As observed previously, such findings are indicative of an exercise-induced acute phase response (Malm et al. 2000; Fielding et al. 1993) needed for initiation of tissue repair processes following exercise. Infusion of IL-6 has been shown to stimulate the release of neutrophils, hepatocyte-derived acute phase proteins such as hs-CRP, and cortisol production (Steensberg et al. 2003). In agreement with the latter study, increases in serum cortisol and blood neutrophils were found in conjunction with corresponding increases in IL-6 post-exercise. Furthermore, a delayed increase in CRP level was also found (peak levels 38 h post-exercise). This CRP rise has been implicated in monocyte activation and adhesion molecule synthesis that recruit leukocytes (Torzewski et al. 2000). It is possible that this prolonged anti-inflammatory response to tissue damage may be essential in further stimulation of necessary growth and repair processes following intense tissue damaging exercise like rugby. However, future research into the effects of this inflammatory response and subsequent repair processes is required.
In contrast to increases in markers of inflammation, decreases in the circulating number of certain immune cell types were observed immediately post-exercise. In particular, a large drop in the number of circulating NK cells was found as previously observed in cycling (Scharhag et al. 2006). This, together with decreases in the number of total lymphocytes and T cell lymphocytes (CD4+ and CD8+ cells) may be indicative of reduced host protection, in particular to viruses (Faabri et al. 2003). However decreases in CD4/8 ratio were not observed following the game. With respect to CD8+ T cytotoxic cells, a greater decline from resting values was observed compared to CD4+ helper cells as shown previously (Gray et al. 1993). This indicates that CD8+ cells are preferentially recruited and removed from the circulation with rugby exercise. T lymphocytes play a fundamental role in the orchestration and regulation of the cell-mediated immune response (Gleeson and Bishop 2005), whilst defects in T cell function are linked to increased viral infections (Faabri et al. 2003). It is not known whether decreases in T cell number (this study) and function (Bruunsgaard et al. 1997; Ronsen et al. 2001) are due to exercise-induced apoptosis (Mars et al. 1998) or redistribution of cells to other compartments (Gleeson and Bishop 2005). It should be noted that lymphocyte functional capacity was not measured in the current study and requires further investigation.
A large neutrophilia was observed post-exercise and contributed to ~70% of the observed leukocytosis. Neutrophils are the most abundant blood leukocyte and form part of the body’s innate immune defence against pathogen entry into the body (Bishop et al. 2003). Despite an observed increase in cell number, when bacterially challenged, a significant decrease (−65%) in the functional capacity of these cells (neutrophil degranulation) was observed. Although decreases in neutrophil degranulation has been previously shown following intense exercise (Laing et al. 2008; Bishop et al. 2003; Blannin et al. 1996), current findings suggest that this apparent suppression may last longer than previously thought. Indeed, findings revealed that decreases in neutrophil responsiveness were not fully resolved 38 h after the game. Such data point to the intense nature of competitive rugby union. Decreased functional capacity of this cell group has been suggested to result, in part, from an increased proportion of immature neutrophils in the blood (Blannin et al. 1996; Bishop et al. 2003). Increased mobilisation of these immature neutrophils (from bone marrow) is in turn thought to be glucocorticoid induced (Peake 2002; Blalock 1989). Given the high cortisol levels observed post-exercise, this represents the most plausible explanation in the present study. However, it should be noted that other hormones (epinephrine, growth hormone) have been recently implicated in this decreased degranulation response (Laing et al. 2008). Interestingly, although an increase in plasma elastase content was evident after exercise, possibly as a result of increased number of circulating neutrophils, a significant decrease in plasma elastase was also noted 14 and 38 h into recovery. This observation may also suggest that blood neutrophils enter a refractory state following intense rugby play.
It has been previously shown that repeated rugby exercise, without adequate recovery, causes depression in other indices of neutrophil function (Takahashi et al. 2007). These studies, together with current findings, suggest that rugby exercise is capable of significantly depressing the functional capacity of this cell group. Such findings are important in light of the large amount of tissue trauma, resulting inflammation and subsequent neutrophilia that is observed following game participation. It has been previously suggested that there is a limited pool of circulating mature neutrophils at any given time (Pyne 1994). Given observations to date, it is feasible that with repeated tissue injury and inflammation, contact sports such as rugby may leave this cell group in a chronic refractory state and ultimately limit players defence capacity to foreign agents and infection. Future studies should attempt to investigate the effects of repeated game involvement and ultimately tissue damage on neutrophil responsiveness. Such data may help determine the possible effects of heavy competition periods on host immunity and aid in management of training periodisation.
The limited amount of scientific data on acute physiological responses in team sports athletes is perhaps not surprising given the inaccessibility to players and logistical considerations of data collection. In the current study, data was collected on an international group of players in a game that was extremely competitive, with both teams within the top six nations of world rugby at the time. Indeed, the game (which ended in a home team defeat) was played in front of over 74,000 spectators and so it would be expected that environmental conditions would have posed a high degree of psychophysiological strain. Although every effort was made to control for time of sampling, it should be conceded that samples taken immediately post-game were representative of a different point on the diurnal cycle (game kick-off: 17.00 hours). This was in contrast to all other sample points (morning 8–9 a.m.). However, since many of the biomarkers measured in the current study are purportedly linked to the effects of C, known to decrease throughout the day; changes in these biomarkers immediately after the game would therefore be suggestive of a real exercise-induced effect. In conclusion, the current study demonstrates a marked inflammatory response to tissue damage resulting from playing rugby union. Such intense physical trauma is capable of resulting in transient but notable, decreases in host-immunity; a factor which poses important considerations in terms of exercise recovery time and illness potential in the elite rugby player. Perturbations in measured biomarkers were suggestive that a recovery period of at least 38 h was necessary following elite rugby union game play. Future studies should attempt to investigate the repeated effect of muscle damaging exercise, such as rugby, on inflammation and associated immune responses.
References
Baker JS, Bailey DM, Hullin D, Young I, Davies B (2004) Metabolic implications of resistive force selection for oxidative stress and markers of muscle damage during 30 s of high-intensity exercise. Eur J Appl Physiol 92:321–327
Banfi G, Marinelli M, Roi GS, Agape V (1993) Usefulness of free testosterone/cortisol ratio during a season of elite speed skating athletes. Int J Sports Med 14:373–379
Bishop NC, Walsh NP, Scanlon GA (2003) Effect of prolonged exercise and carbohydrate on total neutrophil elastase content. Med Sci Sports Exerc 35:1326–1332
Blalock JE (1989) A molecular basis for bidirectional communication between the immune and neuroendocrine systems. Physiol Rev 69:1–32
Blannin AK, Gleeson M, Brooks B, Cave R (1996) Acute effects of exercise on human neutrophil degranulation. J Physiol 485P:140P
Brooks JHM, Fuller CW, Kemp SPT, Reddin DB (2008) An assessment of training volume in professional rugby union and its impact on the incidence, severity, and nature of match and training injuries. J Sports Sci 26:863–873
Bruunsgaard H, Hartkopp A, Mohr T, Konradsen H, Heron I, Mordhorst CH, Pedersen BK (1997) In vivo cell-mediated immunity and vaccination response following prolonged, intense exercise. Med Sci Sports Exerc 29:1176–1181
Clarkson PM, Hubal MJ (2002) Exercise-induced muscle damage in humans. Am J Phys Med Rehabil 81:S52–S69
Cooper MA, Fehniger TA, Caligiuri MA (2001) The biology of human natural killer-cell subsets. Trends Immunol 22:633–640
Cresswell SL, Eklund RC (2006) Changes in athlete burnout over a thirty-week rugby year. J Sci Med Sport 9:125–134
Cunniffe B, Proctor W, Baker JS, Davies B (2009) An evaluation of the physiological demands of elite rugby union using Global Positioning System tracking software. J Strength Cond Res 23:1195–1203
Deutsch MU, Kearney GA, Rehrer NJ (2007) Time–motion analysis of professional rugby union players during match play. J Sports Sci 25:461–472
Dill DB, Costill DL (1974) Calculation of percentage changes in volumes of blood, plasma and red cells in dehydration. J Appl Phyiol 37:247–248
Duthie G, Pyne D, Hooper S (2003) Applied physiology and game analysis of rugby union. Sports Med 33:973–991
Eaves S, Hughes M (2003) Patterns of play of international rugby union teams before and after the introduction of professional status. Int J Perform Anal 3:103–111
Elloumi M, Maso F, Michaux O, Robert A, Lac G (2003) Behaviour of saliva cortisol [C], testosterone [T] and the T/C ratio during a rugby match and during the post-competition recovery days. Eur J Appl Physiol 90:23–28
Faabri M, Smart C, Pardi R (2003) T lymphocytes. Int J Biochem Cell Biol 35:1004–1008
Fielding RA, Manfredi TJ, Ding W, Fiatarone MA, Evans WJ, Cannon JG (1993) Acute phase response in exercise. III. Neutrophil and IL-1 beta accumulation in skeletal muscle. Am J Physiol 265:R166–R172
Fuller CW, Brooks JH, Cancea RJ, Hall J, Kemp SP (2007) Contact events in rugby union and their propensity to cause injury. Br J Sports Med 4:862–867
Gleeson M (2007) Immune function in sport and exercise. J Appl Physiol 103:693–699
Gleeson M, Bishop NC (2005) The T cell and NK cell immune response to exercise. Ann Transplant 10:43–48
Gray AB, Telford RD, Weidemann MJ (1993) The effect of intense interval exercise on iron status parameters in trained men. Med Sci Sports Exerc 25:778–782
Hoffman JR, Maresh CM, Newton RU, Rubin MR, French DN, Volek JS, Sutherland J, Robertson M, Gómez AL, Ratamess NA, Kang J, Kraemer WJ (2002) Performance, biochemical, and endocrine changes during a competitive football game. Med Sci Sports Exerc 34:1845–1853
Hoffman JR, Kang J, Ratamess NA, Faigenbaum AD (2005) Biochemical and hormonal responses during an intercollegiate football season. Med Sci Sports Exerc 37:1237–1241
Hoogeveen AR, Zonderland ML (1996) Relationships between testosterone, cortisol and performance in professional cyclists. Int J Sports Med 17:423–428
Ispirlidis I, Fatouros IG, Jamurtas AZ, Nikolaidis MG, Michailidis I, Douroudos I, Margonis K, Chatzinikolaou A, Kalistratos E, Katrabasas I, Alexiou V, Taxildaris K (2008) Time course of changes in inflammatory and performance responses following a soccer game. Clin J Sport Med 18:423–431
Laing SJ, Jackson AR, Walters R, Lloyd-Jones E, Whitham M, Maassen N, Walsh NP (2008) Human blood neutrophil responses to prolonged exercise with and without a thermal clamp. J Appl Physiol 104:20–26
Malm C, Nyberg P, Engstrom M, Sjodin B, Lenkei R, Ekblom B, Lundberg I (2000) Immunological changes in human skeletal muscle and blood after eccentric exercise and multiple biopsies. J Physiol. 15:243–262
Mars M, Govender S, Weston A, Naicker V, Chuturgoon A (1998) High intensity exercise: a cause of lymphocyte apoptosis? Biochem Biophys Res Commun 249:366–370
Mashiko T, Umeda T, Nakaji S, Sugawara K (2004) Position-related analysis of the appearance of and relationship between post-match physical and mental fatigue in university rugby football players. Br J Sports Med 8:617–621
Passelergue P, Lac G (1999) Saliva cortisol, testosterone and T/C ratio variations during a wrestling competition and during the post-competitive recovery period. Int J Sports Med 20:109–113
Paulsen G, Benestad HB, Strøm-Gundersen I, Mørkrid L, Lappegård KT, Raastad T (2005) Delayed leukocytosis and cytokine response to high-force eccentric exercise. Med Sci Sports Exerc 37:1877–1883
Peake JM (2002) Exercise-induced alterations in neutrophil degranulation and respiratory burst activity: possible mechanisms of action. Exerc Immunol Rev 8:49–100
Pyne DB (1994) Regulation of neutrophil function during exercise. Sports Med 17:245–258
Quarrie KL, Hopkins WG (2007) Changes in player characteristics and match activities in Bledisloe Cup rugby union from 1972 to 2004. J Sports Sci 25:895–903
Roberts SP, Trewartha G, Higgitt RJ, El-Abd J, Stokes KA (2008) The physical demands of elite English rugby union. J Sports Sci 26:825–833
Robson PJ, Blannin AK, Walsh NP, Castell LM, Gleeson M (1999) Effects of exercise intensity, duration and recovery on in vitro neutrophil function in male athletes. Int J Sports Med 20:128–135
Ronsen O, Pedersen BK, Øritsland TR, Bahr R, Kjeldsen-Kragh J (2001) Leukocyte counts and lymphocyte responsiveness associated with repeated bouts of strenuous endurance exercise. J Appl Physiol 91:425–434
Scharhag J, Meyer T, Auracher M, Gabriel HH, Kindermann W (2006) Effects of graded carbohydrate supplementation on the immune response in cycling. Med Sci Sports Exerc 38:286–292
Sjödin B, Hellsten Westing Y, Apple FS (1990) Biochemical mechanisms for oxygen free radical formation during exercise. Sports Med 10:236–254
Smart DJ, Gill ND, Beaven CM, Cook CJ, Blazevich AJ (2008) The relationship between changes in interstitial creatine kinase and game-related impacts in rugby union. Br J Sports Med 42:198–201
Steensberg A, Fischer CP, Keller C, Møller K, Pedersen BK (2003) IL-6 enhances plasma IL-1ra, IL-10, and cortisol in humans. Am J Physiol Endocrinol Metab 285:E433–E437
Takahashi I, Umeda T, Mashiko T, Chinda D, Oyama T, Sugawara K, Nakaji S (2007) Effects of rugby sevens matches on human neutrophil-related non-specific immunity. Br J Sports Med 41:13–18
Takarada Y (2003) Evaluation of muscle damage after a rugby match with special reference to tackle plays. Br J Sports Med 37:416–419
Tidball JG (2005) Inflammatory processes in muscle injury and repair. Am J Physiol Regul Integr Comp Physiol 288:R345–R353
Torzewski M, Rist C, Mortensen RF, Zwaka TP, Bienek M, Waltenberger J, Koenig W, Schmitz G, Hombach V, Torzewski J (2000) C-reactive protein in the arterial intima: role of C-reactive protein receptor-dependent monocyte recruitment in atherogenesis. Arterioscler Thromb Vasc Biol 20:2094–2099
Zuliani U, Bonetti A, Franchini D, Serventi G, Ugolotti G, Varacca A (1985) Effect of boxing on some metabolic indices of muscular contraction. Int J Sports Med 6:234–236
Acknowledgments
We would like to thank Mr Lewis Fall, Mr John Woodside and Mr Kevin Evans of the University of Glamorgan for their help with data collection. Special thanks also to Mr Paul Jones and Mr Gareth Walters at University of Wales Institute Cardiff for assistance with data analysis and Dr Glen Davison (Aberystwyth University) for sound laboratory advice. Finally, we are very grateful to the players who kindly participated in this study and coordination of staff at the Wales Rugby Union. The authors declare that the experiments comply with the current laws of the country in which they were performed.
Conflict of interest statement
The authors of this manuscript declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Cunniffe, B., Hore, A.J., Whitcombe, D.M. et al. Time course of changes in immuneoendocrine markers following an international rugby game. Eur J Appl Physiol 108, 113–122 (2010). https://doi.org/10.1007/s00421-009-1200-9
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00421-009-1200-9