Abstract
The accuracy, reliability and linearity in two hand-portable lactate analyzers, the Accutrend Lactate® (AL) and the Lactate Pro™ (LP) versus the EBIO® plus analyzer (EP) were evaluated. For accuracy, duplicate samples recorded on both the AL and LP revealed an overall average difference versus EP (P < 0.05). The limits of agreement between AL and EP were −0.7 to +1.0, and −1.3 to +1.5 mM between LP and EP. Reliability of AL and LP was assessed at different lactate concentrations; coefficient of variation ranged between 1.8 and 3.3% for AL and between 2.8 and 5.0% for LP. AL and LP had a good reliability for intra-, inter-analyzers, and between test strips (ICC r = 0.999). The linearity was determined versus the EP as reference. The slope coefficient of AL (1.0394) was closer to 1 than that of LP (1.1053). On these bases, AL and LP can be individually considered suitable for the sports research field.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
It is well known that lactate concentration provides a useful source to get training information such as optimal training intensity calculated on the individual anaerobic threshold (IAT) (Baldari and Guidetti 2000). Moreover, incremental blood lactate exercise tests are accepted as valid and reliable estimators of endurance performance (Bishop 2001), and also as a good indicator of the anaerobic contribution to exercise from lactic acid formations. Trainers claimed for the need to collect lactate concentration directly on the field session, as incremental load exercise assessed in laboratory is not versatile and often unsuitable. Portable analyzers are, in fact, useful tools for the coach and the athlete to monitor steady-state exercise intensities as well as to evaluate metabolic responses to exercise and training adaptation. Hand-portable lactate analyzers have the advantage to make field-testing both simple and affordable, providing rapid lactate determination of a whole blood sample. The main limitation would depend on if portable analyzers can be used instead of laboratory analyzers for research and field-based assessment during clinical or performance testing of subjects and athletes. In this sense, it is imperative that portable analyzers demonstrate high levels of accuracy reliability and linearity. The most commercially available (and cited in literature) hand-portable lactate analyzers are the Accutrend® (Roche Diagnostics, Basel, Switzerland, formerly released as Accusport® by Boehringer Mannheim, Mannheim, Germany) and the Lactate Pro™ (KDK Corporation, Kyoto, Japan, Arkray factory inc., KDK corporation, Shiga, Japan). Both instruments are battery-driven pocket-size instrument that might be suitable for outdoor testing. We have used, as our reference method, a standard enzymatic photofluorometric method, the EBIO® plus (Eppendorf, Hamburg, Germany).
There are several studies concerning the evaluation of these portable analyzers, but most of them deal with just one of these instruments (Bishop 2001; Fell et al. 1998; Pinnington and Dawson 2001) or with more than one portable analyzer compared to a fixed reference method, but not considering at the same time accuracy, reliability and linearity (Mc Naughton et al. 2001; Medbø et al. 2000; Pyne et al. 2000; Van Someren et al. 2005). All measurements are subject to some random error even if minimized as much as possible. Systematic errors may also exist. One of the goals of the present study has been to compare the imprecision of different instruments and also to look for possible systematic errors assessing the accuracy. We have in addition verified the reliability of each model of portable analyzer by intra-assay, inter-analyzers and between test strips blood sample measurements. Finally, we examined the linearity measuring different known dilutions of a standard lactate concentration. Therefore the aim of this study was to evaluate the differences between the two most common portable lactate analyzers, comparing their accuracy, reliability and linearity versus a reference standard method.
Methods
Subjects
Twenty-nine healthy young trained men and women have served as subjects in this study. Most of the subjects were athletes of different levels, both regional soccer players and national elite triathletes or physically active university students. All subjects were told that they were serving as volunteers in our experiments. The subjects were also told that they were free to leave the experiments at any stage without giving any reason. Written informed consent was obtained from all volunteers in accordance with the Institutional Review Board protocol approved in accordance with the principles of the Declaration of Helsinki.
Sample collection
Blood samples were drawn in laboratory conditions (20 m altitude a.s.l., 20–23°C temperature, 40–50% relative humidity) from athlete’s fingertip or via venepuncture while they were performing different protocols on a Technogym® Runrace HC 1200 (Gambettola, Italy) motor-driven treadmill or on a Lode© Excalibur Sport with PFM (Groningen, The Netherlands) cycle ergometer. On cycle ergometer subjects performed: 30 s Wingate anaerobic power test and an incremental test starting with 1 min warm up without any added load, then workload increased 30 W every 3 min till exhaustion; on treadmill subjects performed an incremental exercise test consisting of a 3 min walking warm up at 6 km/h with 0% slope, followed by velocity increment of 2 km/h every 3 min up to the work rate subsequent to IAT (Baldari and Guidetti 2000). Blood samples were taken at the third minute of each step during incremental exercise and immediately at the end of exercise. Experimenters carefully cleaned subjects’ whole hands before sampling collection process; moreover, before every subsequent collection, the single finger was cleaned, disinfected and dried in order to avoid any possible interference due to both sweat and dirt. Then, subjects’ skin was punctured with a lancet and the first drop of blood was placed straight on the strip. All samples for the portable analyzers were analyzed within few seconds of the collection. Instead for the EBIO plus (reference system) the sample was aspired through a disposable capillary and, differently from the portable analyzers, it was eventually stored in a refrigerator (at ≈ 6°C) for a period varying between 2 and 24 h. Then, after having left samples at room temperature for 30 min, they were placed onto the analyzer and processed.
Lactate analyzers
In general, for all instruments all measurements were carried out in agreement with the instructions for each instrument. Experienced test leaders who had a great experience in blood lactate concentration analysis did all the measurements. Hand-portable analyzers, such as for the glycaemia or cholesterol control analyzers (Accu-Check, Accutrend GC, Accutrend Plus), do not have an instrument calibration procedure requiring standard solutions before starting measurements. Hand-portable lactate analyzers are instruments that must be used by people who have not necessarily a specific competence on clinical measurements, such as coaches and athletes in the sport science field, or even patients who need to monitor their haematic parameters and their general health status. Hand-portable analyzers are characterized by a specific response function which relates the instrument output signal to the analyte concentration. They automatically selects the appropriate calibration curve from a calibration strip; it is required to insert the code strip which has the same lot number of the corresponding testing strips, this procedure “calibrate” the analyzer for the strips of the corresponding lot number. Hand-portable analyzers are provided with their own expiring code strips and test strips, therefore it is important to pay attention to their correspondence and the expiring date to be fine with all the measurements.
Accutrend (AL)
The portable AL analyzer is a portable (115 × 62 × 18.5 mm), battery-driven (3 × 1.5 V batteries, type AAA) analyzer that weighs approximately 100 g. AL measures whole blood lactate values sampled from capillary blood, as suggested by the manufacturer, and it does not need any other settings to be performed. The measuring range is 0.8–22 mM. The sample is first applied to a coded yellow test strip with a reagent chemical substance. Then the strip must be inserted in the analyzer where the lactate concentration is determined by reflectance photometry via a colorimetric lactate-oxidase mediator reaction. Result is displayed in about 60 s. When whole blood is added to the strip, some penetrates the surface and thus only the plasma portion reaches chemicals that start the reactions processing the lactate. Then, built-in equations, according to the manufacturer, shall calculate the concentration in whole blood from the measured value in plasma.
We required that there should be liquid blood on the top of the strip’s pad after each analysis. Otherwise the result was rejected. We have thus used 25–50 μl of blood for each analysis, as recommended by the manufacturer. Blood was added to the strip by letting it drip from a finger; in accordance with the instrument’s instructions we never let the finger touch the strip’s pad in order to exclude any possible interference due to the sweat. We have used for our measurements strips from Roche Diagnostics.
Lactate Pro (LP)
The LP is a portable (55 × 83.8 × 14.5 mm), battery-driven (2 × 3 V lithium batteries, DL or CR2032) analyzer that weighs 50 g. The measuring range is 0.8–23 mM. A 5 μl sample of whole blood is automatically aspirated into a single use, enzyme-coated electrode test strip; the reagent strip fills by capillary action directly from the finger tip or earlobe site: lactate in the sample reacts with potassium ferrocyanide and pyruvate. Upon the application of a given voltage, ferrocyanide is oxidized, releasing electrons and creating a current. This current is determined via amperometric measurement and the result displayed after 60 s. The LP is supplied with a check strip (to be sure that it is working correctly) and a calibration strip.
EBIO plus
We used as reference method the EBIO plus (EP) analyzer, which is not a hand-portable system but a laboratory method (dimensions 440 × 440 × 600 mm; weight 29 kg; power consumption approximately 80 W by cable power; measuring range 0.5–30 mM) and differently from the previous two it uses an automatic calibration procedure (a zero point, three standard measurements, and four quality control sera) before starting measurement and then every 30 min. Also, the EBIO plus uses the capillary blood method based on the enzyme electrode according to the enzymatic amperometric principle of measurement: sample is aspired through a disposable capillary and then placed into the 2 ml Safe-Lock Microcentrifuge Tube filled with 1 ml system solution. A sample of 20 μl is analyzed over a range between 0.5 and 30.0 mM and the measuring time is about 22 s.
Accuracy
The accuracy was measured on 240 blood samples within a large physiological range which went from 0.8 to 19.9 mM. For each sample, three fractions were drawn and assayed on the hand-portable analyzers (AL and LP) and the EBIO plus analyzer (EP) which was used as reference method.
The value measured by the three analyzers was examined with a repeated measure ANOVA followed by a post hoc analysis (Scheffe’s). Then, intra-class coefficient correlation (ICC) among the three analyzers was calculated. The results of each of two portable analyzers were compared to the EP reference analyzer using the regression analysis. Moreover, we have also carried out the comparison of each portable analyzer with the EP through Pearson product–moment correlation analysis. We have also determined the limits of agreement between the analyzers with a largely used technique, which measures agreement between two methods of clinical measurement (Bland and Altman 1986).
Reliability
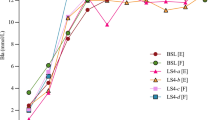
Reliability was assessed measuring three samples nominally called low, medium, and high. The venous blood samples were taken from the same subject during a cycle ergometer incremental test. We assessed for each portable analyzer, the coefficient of variation (CV) for intra-assay (7 repetitive determinations from the same analyzer with same test-strip function number), inter-analyzers (7 repetitive determinations from three different analyzer with the same test strip function number), and also between test strips (3 repetitive determinations from the same analyzer with four different test-strip function numbers) (Table 1). Reliability was also calculated through intra-class correlation coefficient (ICC). Low concentration mean value was 2.5 and 1.8 mM for AL and for LP respectively; medium was 6 and 6.7 mM for AL and LP, respectively, while the high was 14 and 13.7 mM for AL and LP, respectively.
Linearity
We also carried out the evaluation of the linearity of the two portable analyzers versus the EP as reference. Linearity was verified up to 14 mM measuring 13 different known solutions of a standard lactate concentration using the EP reference analyzer. Then, for each portable analyzer (AL and LP), we calculated the linear regression with equation and coefficient of determination (R 2), as indicator of the strength of the linear association between reference lactate concentrations [La] and the portable analyzer measurement (Fig. 3). The standard error of the estimate (SEE) was also calculated to assess the accuracy of predictions made with the regression line.
Results
Accuracy
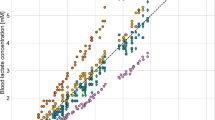
Repeated measurement ANOVA showed a significant main effect on blood lactate values due to the equipment type of lactate analyzer (F 2,239 10.387, P < 0.01). Post hoc analysis revealed that the three instruments were significantly different one versus each other, namely between AL and EP (P < 0.01), AL and EP (P < 0.05) and, LP and EP (P < 0.05). Instead the ICC among the three analyzers was 0.988. We also examined the two portable analyzers with a linear regression one versus the EP (Fig. 1). The linear relation between LP and EP was significant (R 2 = 0.973, SEE = 0.69 mM). However, a polynomial regression of third order (R 2 = 0.984, SEE = 0.52 mM) fitted slightly better. Instead for AL versus EP we found a linear best fitting (R 2 = 0.991, SEE = 0.39 mM).
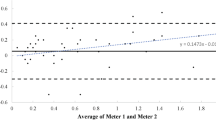
The overall level of agreement between AL and EP showed that in 95% of lactate concentrations, obtained with AL, ranged from less than 0.7 to greater than 1.0 mM of the values obtained with the EP (Fig. 2 panel a), whereas lactate concentrations obtained with LP ranged from less than 1.3 to greater than 1.5 mM of EP values (Fig. 2 panel b).
Bland-Altman plots showing limits of agreement between blood lactate concentrations expressed in mM ([La]) measured using the EBIO plus, Accutrend and Lactate Pro analyzers. Dashed lines are the limits of agreement for low, medium, high [La]. a Relationship of mean [La] determined by Accutrend and EBIO plus with the difference in La between analyzers (Δ [La] (AL-EP)). b Relationship of mean [La] determined by Lactate Pro and EBIO plus with the difference in La between analyzers (Δ [La] (LP-EP))
Reliability
The CV for AL ranged between 3.0 and 3.3% for the low concentration value, between 2.8 and 3.2% for medium concentration value, and between 1.8 and 3.0% for the high concentration value. For LP, instead, CV varied between 2.9 and 3.9% at low values, between 3.0 and 3.9% for the medium value and between 2.8 and 5.0% at the highest value (Table 1).
For each portable analyzer (AL and LP), a high test–retest intra-assay (ICC r = 0.999), inter-analyzers (ICC r = 0.999) and between test-strips (ICC r = 0.999) reliability was found.
Linearity
Linear regression curves for the reference instrument (EP) (y = 0.938x + 0.358; R 2 = 0.997; SEE = 0.18) showed the lowest error of estimates. The linear regression curves (Fig. 3) for AL (y = 1.0394x – 0.0485, R 2 = 0.990; SEE = 0.33 mM) and LP (y = 1.1053x − 0.3933; R 2 = 0.975; SEE = 0.55 mM) versus EP, showed that AL had the highest coefficient of determination and that the slope coefficient closest to 1.
Linear regression with equation, R 2, and SEE for: standard (open circle) lactate concentrations [La] versus the EBIO plus (filled triangle); Accutrend (open square), and Lactate Pro analyzer (open diamond) versus EBIO plus measured values up to 14 mM. Dashed lines are the hypothetical lines of identity
Discussion
The primary objective of our study was to evaluate AL and LP versus EP, used as reference method. These instruments were compared in accuracy, reliability and linearity across a wide range of measurement.
The results obtained in this evaluation support the use of both the AL and LP as accurate and reliable instruments that can be used easily and quite safely in the field.
Anyway, in some previous works, there was a lack of direct comparison between the two most common hand-portable analyzers, AL (Bishop 2001) and LP (Pyne et al. 2000). Other works, instead, have compared AL and LP to other fixed analyzers without taking into consideration the three validation parameters we have assessed (i.e. accuracy, reliability and linearity) at the same time (Mc Naughton et al. 2002; Nordström et al. 1998).
Accuracy
In our study, both AL and LP showed a good accuracy as demonstrated by level of agreement and the coefficient of correlation R which shows a strong association with the reference method, EP. However, repeated measures ANOVA revealed statistical differences among analyzers. In agreement with what was reported by Medbø et al. (2000), the absolute value of lactate concentration measured with AL could be little different from the values obtained with a different analyzer.
Both AL and LP seem to have a good accuracy up to 10 mM (Fig. 1), but at the highest values of the curve LP levelled off similar to that reported by Medbø et al. (2000); however, due to the few measurements at the highest values, this trend should be confirmed by a greater number of measurements. For these reasons, AL appears to be more suitable in those power athletes where high lactate concentrations are reached mainly due to the contribution of the lactic anaerobic metabolism, i.e. rowers, sprinters (e.g. 400 m). Instead, under the value of 2 mM, AL tends to overestimate (Fig. 2) and sometimes the very low values are not measured (between 0.8 and 1 mM from EP); in fact LP is largely used in obstetrical field where researchers usually deal with low and very low concentrations (Nordstrom et al. 1998, 2001; Westgren et al. 1999).
Results of our ANOVA analysis indicate that the two portable analyzers cannot be used interchangeably, and in a single study researchers must choose and keep the same instrument throughout all the measurements as it has been already suggested by Buckley et al. (2003) and Medbø et al. (2000). In addition, the statistically significant difference between values measured by different analyzer should be kept in mind when applying a protocol dealing with a fixed absolute value, such as 2 and 4 mM lactate thresholds (Heck et al. 1985), 0.5 mM increments’ method for lactate threshold (Baldari and Guidetti 2000), especially if the protocol was validated using one particular instrument. It should be recommended to use the same instrument proposed for that protocol, in order to avoid inconsistency between measured values and data reported by literature. On the other hand, Buckley et al. (2003) reported also that the lack of absolute agreement had little effect on blood lactate transition thresholds (BLTT) in relative terms. However, when BLTT were expressed as absolute lactate concentration the different analyzers gave results significantly different. Moreover, this absolute difference could be partly linked to whether or not different analyzers lysed the red blood cells during the measuring process; in fact [La] is higher in lysed red blood cells and becomes even greater as the concentration increases. Previous studies suggested that LP lyses blood samples during analysis (Medbø et al. 2000), while AL seems not to do the same, as it measures the lactate concentration in the plasma portion of whole blood sample.
Reliability
Both AL and LP analyzers were found to have high reliability as indicated by the low CV and high ICC over the range 2–14 mM (at low, medium and high concentration). The CVs ranging between 1.8 and 5.0% (intra-assays and between-strips, respectively) demonstrate the reliability of both AL and LP at different concentrations and also with different test strips. These values are consistent for the analyses of this type (Fell et al. 1998). Each hand-portable analyzer (AL and LP) revealed a high reproducibility (ICC r = 0.999) for intra-assay as previously reported for AL (Bishop 2001; Pinnington and Dawson 2001); also for inter-analyzers as previously reported for LP (Pyne et al. 2000), and also between-strips measurements. Reliability refers to the reproducibility of values of an assay in repeated trials. Better reliability implies better reproducibility of single measurements and better tracking of changes in measurements in research or practical settings. So it appears to be essential in the collection process for the sport research, which is often based on several repeated samples to detect any possible changes.
Linearity
The linearity of each analyzer between 2 and 14 mM was supported by the regression line equation as the coefficient of determination and the slope coefficient were nearest to 1, even though AL showed values closest to 1. A slope coefficient close to 1 indicates that the proportion between measured and standard values is kept constant from low until high concentrations. The closer a measurement system coefficient is to 1, the less error variance it reflects and the more the evaluated system can be considered reliable. In addition, we calculated SEE which revealed a less absolute standard error of estimate for AL (0.33 mM) than LP (0.55 mM), and the lowest value for our reference method (0.18 mM); these values were around that previously reported (0.39 mM) by Carvalho et al. (2005) for the YSI 1500 analyzer. As today trainers and sport scientist prefer to work and to talk about kinetic of lactate more than instantaneous lactate value (Baldari and Guidetti 2000; Baldari et al. 2004; Beneke et al. 2005; Bentley et al. 2001; Oosthuyse and Carter 1999; Pyne et al. 2001; Stegmann et al. 1981), measures repeated over the time with the same instruments must be consistent; thus linearity plays a fundamental role when an instrument is evaluated.
A potential limitation of the present study is the measurement range we assessed; we verified linearity and reliability up to 14 mM and accuracy up to 19.9 mM, even if the portable instrumentations (AL and LP) are able to measure up to 22–23 mM. It is worth noting that, during performance testing of athletes, lactate concentrations higher than 14 mM are rarely achieved and mainly in case of all-out test (e.g. Wingate anaerobic power test); the most observed and detected lactate concentrations in various sports are definitely lower (Goodwin et al. 2007).
Conclusion
In conclusion, in our study both the portable analyzers showed a good accuracy, even though the absolute measured lactate values showed some differences versus the reference analyzer. Therefore, for research purpose, a reference equation up to the value of interest would be necessary to generate corrected values in order to normalize data from different analyzers. Both AL and LP showed a high reliability. A good linearity between 2 and 14 mM was found for all analyzers. However, AL showed a better linearity than LP. On these bases AL and LP, considering their good accuracy and their high reliability and linearity, can be individually considered suitable for the research field across a wide range of sports.
References
Baldari C, Guidetti L (2000) A simple method for individual anaerobic threshold as predictor of max lactate steady state. Med Sci Sports Exerc 32:1798–1802. doi:10.1097/00005768-200010000-00022
Baldari C, Videira M, Madeira F, Sergio J, Guidetti L (2004) Lactate removal during active recovery related to the individual anaerobic and ventilatory thresholds in soccer players. Eur J Appl Physiol 93:224–230. doi:10.1007/s00421-004-1203-5
Beneke R, Hütler M, Jung M, Leithäuser RM (2005) Modeling the blood lactate kinetics at maximal short-term exercise conditions in children, adolescents, and adults. J Appl Physiol 99:499–504. doi:10.1152/japplphysiol.00062.2005
Bentley DJ, McNaughton LR, Thompson D, Vleck VE (2001) Peak power output, the lactate threshold, and time trial performance in cyclists. Med Sci Sports Exerc 33:2077–2081. doi:10.1097/00005768-200112000-00016
Bishop D (2001) Evaluation of the Accusport Lactate Analyser. Int J Sports Med 22:525–530. doi:10.1055/s-2001-17611
Bland JM, Altman DG (1986) Statistical methods for assess agreement between two methods of clinical measurement. Lancet 1:307–310
Buckley JD, Bourdon PC, Woolford SM (2003) Effect of measuring blood lactate concentrations using different automated lactate analyzers on blood lactate transition thresholds. J Sci Med Sport 6:408–421. doi:10.1016/S1440-2440(03)80267-0
Carvalho JF, Masako Masuda O, Pompeu AMS (2005) Methods for diagnosis and control of aerobic training in rats based on lactate threshold. Comp Biochem Physiol A 140:409–413. doi:10.1016/j.cbpb.2004.12.002
Fell JW, Rayfield JP, Gulbin PT, Gaffney PT (1998) Evaluation of the Accusport Lactate Analyser. Int J Sports Med 19:199–204. doi:10.1055/s-2007-971904
Goodwin ML, Harris JE, Hernandez A, Gladden LB (2007) Blood lactate measurements and analysis during exercise: a guide for clinicians. J Diabetes Sci Technol 1:558–569
Heck H, Mader A, Hess G, Mücke S, Müller R, Hollmann W (1985) Justification of the 4-mmol/l lactate threshold. Int J Sports Med 6:117–130. doi:10.1055/s-2008-1025824
Mc Naughton LR, Thompson D, Philips G, Backx K, Crickmore L (2002) A comparison of the Lactate Pro, Accusport, Analox GM7 and Kodak Ektachem Lactate Analysers in normal, hot and humid conditions. Int J Sports Med 23:130–135. doi:10.1055/s-2002-20133
Medbø JI, Mamen A, Holt Olsen O, Evertsen F (2000) Examination of four different instruments for measuring blood lactate concentration. Scand J Clin Lab Invest 60:367–380. doi:10.1080/003655100750019279
Nordstrom L, Chua S, Roy A, Arulkumaran S (1998) Quality assessment of two lactate test strip methods suitable for obstetric use. J Perinat Med 26:83–88
Nordstrom L, Achanna S, Naka K, Arulkumaran S (2001) Fetal and maternal lactate increase during active second stage of labour. BJOG 108:263–268
Oosthuyse T, Carter RN (1999) Plasma lactate decline during passive recovery from high-intensity exercise. Med Sci Sports Exerc 31:670–674. doi:10.1097/00005768-199905000-00008
Pinnington H, Dawson B (2001) Examination of the validity and reliability of the Accusport blood lactate analyser. J Sci Med Sport 4:129–138. doi:10.1016/S1440-2440(01)80014-1
Pyne DB, Martin DT, Logan A (2000) Evaluation of the Lactate Pro blood lactate analyser. Eur J Appl Physiol 82:112–116. doi:10.1007/s004210050659
Pyne DB, Lee H, Swanwick KM (2001) Monitoring the lactate threshold in world-ranked swimmers. Med Sci Sports Exerc 33:291–297. doi:10.1097/00005768-200102000-00019
Van Someren KA, Howatson G, Nunan D, Thatcher R, Shave R (2005) Comparison of the Lactate Pro and Analox GM7 blood lactate analysers. J Sports Med 26:657–661. doi:10.1055/s-2004-830337
Westgren M, Kublickas M, Kruger K (1999) Role of lactate measurements during labor. Obstet Gynecol Surv 54:43–48. doi:10.1097/00006254-199901000-00023
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Baldari, C., Bonavolontà, V., Emerenziani, G.P. et al. Accuracy, reliability, linearity of Accutrend and Lactate Pro versus EBIO plus analyzer. Eur J Appl Physiol 107, 105–111 (2009). https://doi.org/10.1007/s00421-009-1107-5
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00421-009-1107-5