Abstract
The aim of this study was to determine the effects of a simulated one-day Greco-Roman wrestling tournament on selected performance and inflammatory status indices. Twelve competitive wrestlers (22.1 ± 1.3 years) completed five matches according to the official Olympic wrestling tournament regulations following a ~6% weight loss. Performance measurements, muscle damage assessment, and blood sampling were performed before and following each match. Performance and inflammatory markers were not affected by weight loss. Mean wrestling heart rate reached ~85% of maximal and lactate concentration exceeded 17 mM. Fatigue rating demonstrated a progressive rise (P < 0.05) throughout the tournament, peaking in match 4. Performance demonstrated a progressive deterioration (P < 0.05) throughout the tournament, especially in the last two matches (P < 0.05), with upper-body measures exhibiting a greater decline (P < 0.05) and remaining below baseline (P < 0.05) until the end of the tournament. Muscle damage markers increased during the course of the tournament with upper limbs affected more. Creatine kinase activity, CRP levels, IL-6 concentration, and leukocyte counts increased (P < 0.05) progressively throughout the tournament, peaking in the last two matches. Cortisol, epinephrine and norepinephrine increased (P < 0.05) after each match, but testosterone declined (P < 0.05) progressively, reaching a nadir before the last match. This inflammatory response was accompanied by a marked increase (p < 0.05) in lipid peroxidation, protein oxidation, and antioxidant status markers indicating the development of oxidative stress. These results suggest that a one-day wrestling tournament may induce significant physiological demands on wrestlers that may adversely affect their performance and inflammatory status especially during the later stages of the tournament.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Wrestling is a sport consisting of high-intensity efforts interspersed with brief periods of mild- to moderate-intensity work or resting characterized by the effort of the athlete to maintain physical control over his opponent (Horswill 1994). Recent rule changes have shortened match duration (from 5 to 2-min rounds) and encouraged power maneuvers that require both absolute whole-body strength and explosiveness integrating a large isometric component for technical performance (Horswill 1994). As a result, modern wrestling taxes more the anaerobic energy system (Sharratt et al. 1986) elevates heart rates to maximal levels (Kraemer et al. 2001) and accumulates moderate to high lactate concentrations (10–20 mM) following competition (Houston et al. 1983; Kraemer et al. 2001) that may disrupt acid–base balance and ultimately lead to performance deterioration (Aschenbach et al. 2000).
Although medical society warned of possible health hazards (ACSM 1996), wrestlers continue to reduce their body mass through a combination of dietary restriction practices (such as dehydration) and exercise in their effort to participate in a lower weight class during competition (Steen and Brownell 1992). Repetitive weight loss over a wrestling season is associated with a reduction of strength and anaerobic performance (Kraemer et al. 2001), mortality (MMWR 1998), and disturbance of endocrine environment (Hoffman et al. 1994; Kraemer et al. 2001; Webster et al. 1990). Nevertheless, it has been shown that elite wrestlers are physiologically resistant to a 5–6% weight loss prior to a wrestling tournament (Yankanich et al. 1998). Furthermore, Yankanich et al. suggested that the rate and of weight loss prior to wrestlers’ weigh-in does not affect their performance and health status prior to competition. It is unknown, however, whether a 5–6% weight loss affects physiological adaptations and performance of wrestlers during a one-day competition tournament that consists of five matches performed within only a few hours.
National, international, and Olympic wrestling events are organized in a tournament format thereby requiring athletes to compete in multiple matches over the course of hours or a few consecutive days. Consequently, wrestlers are subjected to various stresses (i.e. physiological and psychological) beyond that already caused by weight loss alone (Kraemer et al. 2001) while performance may demonstrate marked fluctuations during the course of a tournament (Horswill 1992; Kraemer et al. 2001). A well-designed study by Kraemer et al. (2001) examined whether a tournament competition would further exacerbate the already reduced physiological and performance capabilities of wrestlers previously exposed to a significant weight loss. That study concluded that a two-day tournament wrestling increased further the physiological stress and performance reductions due to weight loss in a progressive manner suggesting that the combination of these two stressful factors may impair athletes’ ability to maintain performance over the course of a wrestling event (Kraemer et al. 2001). In 2005, the International Wrestling Federation (FILA) decreased tournament duration from two- to one-day event. Although this reduction of tournament duration may further exacerbate the physiological stress and performance output, no data are yet available regarding this issue.
Wrestling incorporates a strong eccentric component during athletes’ effort to resist a fall, i.e. when they exert resistance to gravity. Eccentric contractions elicit a muscle damage response which is mainly induced by mechanical stress, disturbances of calcium homeostasis, and a sensation of discomfort within the muscle may be experienced by the athlete (Armstrong et al. 1983). The intensity of discomfort increases within the first 24 h after exercise, peaks between 1 and 3 days, subsides, and eventually disappears 5–7 days after exercise, a phenomenon that is referred to as delayed onset of muscle soreness (DOMS) (Clarkson and Tremblay 1988). Exercise-induced muscle damage is associated with an acute-phase inflammatory response characterized by phagocyte infiltration into muscle, free radical production, and elevation of cytokines and other inflammatory molecules (Malm et al. 2004). Furthermore, wrestling may create a pro-inflammatory environment by altering testosterone, cortisol, and immune responses due to rapid weight loss practices (Kraemer et al. 2001). The ability of wrestlers to fully recover before their next match during a tournament is crucial not only for their performance but for injury prevention as well. Therefore, it is crucial to understand how wrestlers’ physiological and performance responses fluctuate during the successive matches of a single tournament when compared with the first match of the tournament. In this study, we hypothesized that wrestlers’ ability to recover between matches, in respect to performance and inflammatory status, is hampered during a simulated one-day tournament. Therefore, the aim of the present investigation was to determine the physiological and performance responses of well-trained wrestlers to a simulated one-day tournament of Greco-Roman wrestling following a typical weight loss regimen.
Methods
Participants
Twelve injury-free, elite (almost all of them were medal winners in various international wrestling tournaments such as the European juniors championships) male wrestlers (training experience 12.1 ± 2.9 years; age 22.1 ± 1.3 years; height 174.3 ± 2.8 cm; weight 72.1 ± 3.6 kg; body fat 7.6 ± 0.9%; VO2max 56.8 ± 3.8 ml/kg/min; maximal heart rate 196.2 ± 7.3) from Division I clubs across Greece, competing (in the 66-kg weight class) in national and international competitions for approximately 12 years, volunteered to participate in the present study. Athletes were highly ranked (top five) in their weight division at least once during the 5-year period that preceded the study. Subjects were informed of the potential experimental risks and gave their written informed consent to participate in this study, which was consistent with the human subject policy of the American College of Sports Medicine and approved by the Institutional Review board for use of human subjects. Additionally, all subjects were screened by a physician before participating in the study.
Experimental design
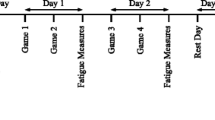
The present study intended to determine the inflammatory and performance responses during a single-day simulated Greco-Roman wrestling tournament that was preceded by a 1-week weight loss period. The initial hypothesis of the present study is that the accumulation of repetitive stress during a 1-day tournament consisting of five matches will produce a greater burden and different performance and inflammatory responses than a single wrestling match. To ensure athletes’ full recovery and peak wrestling condition, the experimental phase was performed during the off-season period (end of May), 4 weeks following the completion of the athletes’ in-season period (end of April). Participants followed a typical training routine consisting mainly of technical and tactical training as well as multiple wrestling matches during the period (3 weeks) that preceded the experimental wrestling tournament. Participants were asked to lose approximately 6% of body mass (with restriction of food and fluid intake, multiple 30–40 min sessions of cardiovascular exercise on a cycloergometer, rope jumping etc., sauna sessions) during the week that preceded the pre-competition weigh-in that preceded the first match (~12 h according to official regulations for Greco-Roman wrestling). Participants’ actual weight loss ranged from 5.1 to 6.5%. Before their first match, wrestlers regained 1.2% of their body weight but their weight remained below its baseline value throughout the tournament. The tournament consisted of five matches scheduled according to the official regulations of the FILA. Each match (three 2-min rounds, 30-s breaks between rounds) was formally refereed and scored. The second match was performed 80–90 min after the first, the third 60–70 min after the second, the fourth 30–40 min after the third, and the fifth 5–6 h after the fourth, with the first match always starting at 9.00 a.m. (Fig. 1). If a fall occurred, the wrestlers started again on the feet until the full match was wrestled. Each wrestler competed against an opponent of his own club and with similar weight, skills, and training background. Wresters were competing for a leading placement in their weight class (in their club) to create the physical and psychological environment of a formal competition. Similar to the study by Kraemer et al. (2001), this study also adapted the use of two baseline testing days (one performed in the a.m.: baseline 1) and another in the p.m. (baseline 2) to control for diurnal variations in the blood and performance variables examined. Baseline testing were performed 3 weeks following the completion of athletes’ in-season period (VO2max, height, and body fat were measured once during the a.m. baseline measurement). Testing was repeated immediately before and after each one of the five matches wrestled during the tournament (the test battery was completed in approximately 20 min). Wrestlers were allowed to rest for 10 min before warming up (10–15 min) for each match and following performance testing. Performance variables selected so that anaerobic (vertical jumping, hip-back strength) and wrestling-related (bear-hug testing, hand-grip testing) performance could be evaluated. Biochemical assessments were representative of the physiological function (hormonal, metabolite, and heart rate responses), muscle damage (CK activity, DOMS, and joints’ range of motion), and inflammatory response (C-reactive protein, leukocyte counts, IL-6, oxidative stress markers) associated with wrestling. The intensity of the wrestling matches was determined by measuring the participants’ heart rate response, the lactate and glucose concentrations, and the fatigue level. Measurement of heart rate, fatigue, muscle soreness, and joints’ (elbow and knee) range of motion as well as blood sampling always preceded the performance of the testing battery. Tests were performed always in the order described in the experimental procedures section below. Volunteers had access to water ad libitum throughout the tournament. Wrestlers were given breakfast at 07.00, a light snack in between matches, lunch at 14.00–14.45, and dinner at 19.30–20.00. The composition of the meals and the snacks was standardized for all participants. The diet consisted of nutritionally balanced foods providing total daily energy intake of 50% over resting energy expenditure (calculated by the Harris–Benedict formula). Total energy intake was provided as 18% protein, 27% fat, and 55% carbohydrate, and the distribution was 20, 35, 35, and 10% at breakfast, lunch, dinner, and snacks, respectively. No vegetarian wrestlers were included in the study. Subjects were allotted 30–45 min to complete their meals and 5 min to complete the snack. Wrestlers either rested or were confined to very light physical activity (i.e. walking, stretching, technical warm-up) between matches.
Anthropometric measurements and VO2max assessment
Body weight was measured according to FILA’s official regulations. Standing height was measured to the nearest 0.5 cm (Stadiometer 208, Seca). Percentage body fat was calculated from seven skinfold measures (Jackson and Pollock equation), using a Harpenden caliper (John Bull, UK). Percentage body fat was calculated from seven skinfold measures (average of two measurements of each site), using a Harpenden caliper (John Bull, UK). The Siri skinfold equation was used to calculate body fat. VO2max was assessed using a standard USOC cycling (Monark 834E, Sweden) protocol as described (Callan et al. 2000) with a pulmonary gas exchange system (Oxycon Mobile, Yorba Linda, CA, USA) via breath-by-breath analysis.
Diet records
To control for the effect of previous diet on the inflammatory/oxidative stress variables, 5-day diet recalls were completed 1 week prior to the study. A trained dietician taught the subjects how to complete diet recall questionnaires and determine food serving and sizes. Diet records were analyzed using the computerized nutritional analysis system Science Fit Diet 200A (Science Technologies, Athens, Greece).
Experimental procedures
Heart rate and fatigue scale
Heart rate was measured at rest and throughout the matches at 5-s intervals (Polar Vantage NV monitor; Polar Electro, Kempele, Finland). Fatigue rating was obtained as described (Kraemer et al. 2001).
Muscle damage markers
Delayed onset of muscle soreness for knee extensors (DOMSke) and elbow flexors (DOMSef) was determined by deep palpation of the muscle belly on the DOMSef and DOMSke (at the distal end of the vastus medialis, vastus lateralis, and rectus femoris) and evaluated by a visual analog scale (VAS) that had a 100-mm line with “no pain” on one end and “extremely sore” on the other. During evaluation, subjects were lying supine on an examination table with both extremities relaxed. During palpation, pressure was applied with the tips of three fingers (II, III, and IV) for approximately 3 s with the participant standing. Perceived soreness for both conditions was rated on a scale ranging from 1 (normal) to 10 (very, very sore). The test–retest reliability of DOMS measurement by palpation for biceps and quadriceps was 0.94 and 0.96. Knee (KJRM) and elbow (EJRM) joint range of motion was measured as an index of muscle edema. KJRM was measured in a prone position while active flexion was performed slowly from a full extended position. The knee angle at which subjects reported discomfort was recorded as the point of the end of the pain-free ROM. Likewise, EJRM was determined with subjects lying on their back while the elbow was moved from a fully extended position to a fully flexed position. The elbow angle at which subjects reported discomfort was recorded as the point of the end of the pain-free ROM. The coefficients of variation for test–retest trials for knee flexion and extension were 2.4 and 2.9%, respectively.
Strength and power tests
Grip strength (GS) was determined on a calibrated Harpenden (British Indicators, UK) handgrip dynamometer with visual feedback as described (Kraemer et al. 2001). Leg power was determined with a vertical jump (VJ) measured on an Ergojump contact platform (Newtest, Oulu, Finland) after three maximal efforts (the best jump was recorded). Subjects started from a standing position, allowed a preparatory counter-movement motion, and had their hands on their waist throughout the jump. Flight time was measured using a digital timer connected to the platform and was used to calculate jump height. Hip and back (HB) strength was determined using a Lafayette HB dynamometer (Lafayette Instruments, IN, USA) as described by Kraemer et al. (2001). Isometric upper body pull strength was measured with the “bear hug” test (BH) as previously described (Kraemer et al. 2001). The coefficient of variation for test–retest trials were was 2.5, 2.9, 2.2, and 3.2% for HG, VJ, HB, and BH testing, respectively. The test–retest reliability coefficients for performance measurements ranged from 0.82 to 0.92.
Blood collection and handling
Blood samples were drawn via venipuncture from an antecubital arm vein using a 20-gauge disposable needle equipped with a Vacutainer tube holder (Becton–Dickinson, Franklin Lakes, NJ, USA) with the subject always in a semirecumbent position. Blood was collected into Vacutainer tubes containing either SST-Gel and Clot Activator for serum separation, EDTA for plasma/erythrocyte separation or heparin (for catecholamine determination). Whole blood (500 μl) was used for glutathione peroxidase (GPX) measurement. Blood for serum separation was allowed to clot at room temperature and subsequently centrifuged (1,500 g, 4°C, 15 min). The resulting serum was used for the determination of the concentration of thiobarbituric acid reactive substances (TBARS), protein carbonyls (PC), cortisol, testosterone, IL-6, IL-1b, C-reactive protein (CRP), and creatine kinase (CK) activity. EDTA blood was centrifuged immediately (1,370 g, 4°C, 10 min) and plasma was collected. The packed erythrocytes were lysed with 1:1 (v/v) distilled water, inverted vigorously and centrifuged (4,000 g, 4°C, 15 min). Plasma was used for the determination of glucose, total antioxidant capacity (TAC) and hemoglobin, while erythrocyte lysates were used for the measurement of reduced (GSH) and oxidized (GSSG) as well as catalase activity (CAT). Serum and plasma samples were stored in multiple aliquots and frozen at −75°C for later analyses. Blood samples thawed only once before analysis. A small quantity of blood (200 mL) was immediately added to 400 mL of 5% TCA and centrifuged (2,500 g, 15 min). The supernatant was removed and frozen at −75°C until analysis for lactate by an enzymatic method with reagents purchased from Sigma Chemicals (St. Louis, MO, USA). A blood aliquot (1 mL) was immediately mixed with EDTA to prevent clotting for hematology. Complete blood count and hematocrit were determined within 24 h via matching duplicate counts using an automated hematology analyser (Sysmex K-1000 autoanalyzer; TOA Electronics, Japan).
Analytical methods
Hematocrit was measured in triplicate using standard microcapillary techniques and hemoglobin was determined by a cyanmethemoglobin method (Sigma Chemical Co., St. Louis, MO, USA). Plasma volume percent changes were computed based on hematocrit and hemoglobin as described (Dill and Costill 1974). Glucose was determined spectophotometrically by the glucose oxidase method (Sigma–Aldrich, St. Louis, MO, USA) with 1.8 and 4.2% inter- and intra-assay coefficients of variation, respectively. CK activity was determined spectrophotometrically using a commercially available kit (Spinreact, Sant Esteve, Spain) with 1.6 and 3.7% inter- and intra-assay coefficients of variation, respectively. CRP was measured by means of particle-enhanced immunonephelometry using the Dade-Behring BN Prospec nephelometer with 2.6 and 5.7% inter- and intra-assay coefficients of variation, respectively. Cortisol and testosterone were analyzed by using two commercially available ELISA kits (DRG Diagnostics, Germany). Plasma norepinephrine and epinephrine levels were measured by reverse-phase HPLC with electrochemical detection (Agilent HP 1100 Series HPLC system, Santa Clara, CA, USA). The intra-and inter-assay CV for norepinephrine were 4.0 and 5.4 and for epinephrine were 3.9 and 6.1%, respectively. Serum IL-6 concentration was measured using a commercially available ELISA kit (Immunokontact, UK) based on an immunoenzymatic method (the intra-and inter-assay CV for IL-6 were 4.2 and 5.9%, respectively). Total protein in serum was assayed using a Bradford reagent.
TBARS was assayed as described (Margonis et al. 2007). Serum (100 μL) was mixed with 500 μL of TCA 35% and 500 μL of Tris–HCl (200 mM, pH 7.4) and was incubated for 10 min at room temperature. One milliliter of 2 M Na2SO4 and 55 mM thiobarbituric acid solution was added, and the samples were incubated at 95°C for 45 min. The samples were cooled on ice for 5 min and were vortexed after adding 1 mL of TCA 70%. The samples were centrifuged at 15,000 g for 3 min, and the absorbance of the supernatant was read at 530 nm. A baseline shift in absorbance was taken into account by running a blank along with all samples during the measurement. A standard curve was constructed by using malondialdehyde as a standard at concentrations of 0, 1.25, 2.5, 5, and 10 KM.
For protein carbonyls (Margonis et al. 2007), 50 μL of 20% TCA was added to 50 μL of serum, and this mixture was incubated in an ice bath for 15 min and centrifuged at 15,000 g for 5 min at 4οC. The supernatant was discarded, and 500 μL of 10 mM 2,4-dinitrophenylhydrazine (in 2.5 N HCL) for the sample, or 500 μL of 2.5 N HCL for the blank, was added in the pellet. The samples were incubated in the dark at room temperature for 1 h, with intermittent vortexing every 15 min, and were centrifuged at 15,000 g for 5 min at 4οC. The supernatant was discarded and 1 mL of 10% TCA was added, vortexed, and centrifuged at 15,000 g for 5 min at 4οC. The supernatant was discarded, and 1 mL of ethanol–ethyl acetate (1:1 v/v) was added, vortexed, and centrifuged at 15,000 g for 5 min at 4οC. The washing step was repeated two more times. The supernatant was discarded, and 1 mL of 5 M urea (pH 2.3) was added, vortexed, and incubated at 37οC for 15 min. The samples were centrifuged at 15,000 g for 3 min at 4οC, and the absorbance was read at 375 nm.
For GSH determination (Margonis et al. 2007), 20 μL of erythrocyte lysate was treated with 5% TCA mixed with 660 μL of 67 mM sodium potassium phosphate (pH 8.0) and 330 μL of 1 mM 5,5′- dithio bis-2 nitrobenzoate (DTNB). Samples were incubated in the dark at room temperature for 45 min, and the absorbance was read at 412 nm. For GSSG determination (Margonis et al. 2007), 50 μL of erythrocyte lysate were treated with 5% TCA and neutralized up to pH 7.0–7.5 with NaOH. One microlitre of 2-vinyl pyridine was added and the samples were incubated for 2 h at room temperature. Five microliter of erythrocyte lysate treated with TCA were mixed with 600 μL of 143 mM sodium phosphate (6.3 mM EDTA, pH 7.5), 100 μL of 3 mM NADPH, 100 μL of 10 mM DTNB, and 194 μL of distilled water. The samples were incubated for 10 min at room temperature. After the addition of 1 μL of glutathione reductase the change in absorbance at 412 nm was read for 3 min. GSH and GSSG concentration was calculated on the basis of calibration curves constructed using commercial standards.
TAC was determined as described by Margonis et al. (2007). Briefly, 20 μL of plasma was added to 480 μl of 10 mM sodium potassium phosphate (pH 7.4) and 500 μl of 0.1 mM 2,2-diphenyl-1-picrylhydrazyl (DPPH·) free radical and the samples were incubated in the dark for 30 min at room temperature. The samples were centrifuged (20,000g, 3 min) and the absorbance was read at 520 nm. TAC is presented as millimole of 1,1-diphenyl2-picrylhydrazyl radical (DPPH·) reduced to 1,1-diphenyl-2-picrylhydrazine (DPPH:H) by the antioxidants of plasma. Catalase activity was measured adding 4 μl of erythrocyte lysate (diluted 1:10), respectively, to 2,955 μL of 67 mM sodium potassium phosphate (pH 7.4) and the samples were incubated (37°C, 10 min). Five microliters of 30% H2O2 was added to the samples and the change in absorbance was immediately read at 240 nm for 1.5 min. Calculation of catalase activity was based on the molar extinction coefficient of H2O2. Whole-blood GPX activity was measured spectophotometricaly, at 37°C, using cumene hydroperoxide as the oxidant of glutathione (Ransel RS 505, Randox, Crumlin, UK).
Inter- and intra-assay coefficients of variation for all oxidative stress markers assays ranged from 2.1 to 7.55% and from 3.2 to 7.9%, respectively. Spectrophotometric assays were performed on a Hitachi 2001 UV/VIS (Hitachi Instruments Inc., US) in triplicates. Concentrations of blood variables were corrected for hemoconcentration.
Statistical analysis
Data are presented as means ± SE. Normality was examined by the Shapiro–Wilk test and was found not to differ significantly from normal. Data were analyzed using a repeated measures multivariate analysis of variance (MANOVA). If a significant interaction was obtained, Tukey post hoc procedures were performed to determine pairwise differences. P < 0.05 was considered statistically significant.The SPSS was used for all analyses (SPSS Inc., Chicago, IL, USA). Power calculations were based on the effect sizes (computed as the difference between pre exercise and post exercise values at the point where the maximum exercise effect appeared divided by the standard deviation of pre exercise at the same time-point) using the software G*Power 3 (Faul et al. 2007). The statistical power of the present study to detect an average change of 20% (at a = 0.05) for all dependent parameters between rest and post exercise ranged from 0.73 to 0.91 for performance variables and from 0.69 to 0.96 for biochemical variables). The test–retest reliability of the physiological and biochemical measurements was determined by performing the intraclass reliability test. The pooled SD of the resting and exercise values and one tail were used for these calculations while the alpha level was set at 0.05.
Results
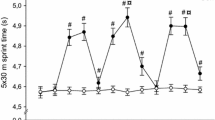
Wrestlers’ dietary intakes were comparable (data not shown). Pre-match heart rates (Table 1) were comparable with baseline values. No differences were detected for all dependent variables between baseline and pre-match 1 measurement. Post-match heart rates demonstrated similar elevations (P < 0.05) from baseline and corresponding pre-match values reaching the 92–96% of maximal heart rate. Mean and peak heart rate recorded during the five matches demonstrated similar elevations from baseline reaching the 83–86% and 96–98% of maximal heart rate. Pre-match lactate concentration (Table 1) was similar with baseline for matches 1, 2, and 5 and above (P < 0.05) baseline for matches 3 and 4. Post-match lactate levels demonstrated a marked increase (P < 0.05) compared with baseline and pre-match values. Pre-match glucose (Table 1) values were all similar to baseline values. Post-match glucose concentrations were all elevated (P < 0.05) compared with baseline and their corresponding pre-match values. Resting fatigue rating (Table 1) was higher (P < 0.05) than baseline values after all matches. Subjects also reported higher (P < 0.05) fatigue levels following the last three matches compared with the first one. Post-match fatigue ratings exhibited similar elevations (P < 0.05) from baseline and corresponding pre-match values. Fatigue rating before match 2 was higher (P < 0.05) than the respective values of the first match. Additionally, pre-match fatigue rating declined (P < 0.05) before the last match as compared with the resting value of the previous match.
Performance changes are shown in Table 1. VJ (effect sizes: −1,3 to 0.06) demonstrated a decline (P < 0.05) from baseline value only before match 4. Post-match VJ values did not differ from their respective pre-match values. Pre-match HB (effect sizes: −2.0 to −0.4) declined only before match 4 relatively to its corresponding baseline value. Post-match HB performance declined (P < 0.05) after matches 3–5 but not following the first two. Pre-match “bear hug” strength performance (effect sizes: −2.5 to −0.6) for matches 4 and 5 was lower (P < 0.05) than the respective baseline measures. Furthermore, post-match “bear hug” declined (P < 0.05) relatively to their respective pre-match values only following matches 4 and 5. Pre-March HG strength (effect sizes: −2.6 to −0.6) measures declined (P < 0.05) compared with baseline values prior to matches 3–5. Post-match HG values for matches 2–5 were lower (P < 0.05) than the corresponding pre-match values.
CK (Table 2) remained comparable to baseline before and following match 1. Nevertheless, pre- and post-match CK values (effect sizes: 1.0–3.6) progressively increased (P < 0.05) from match 2 through match 5 compared with the respective CK values at baseline and those of the first match. CK peaked (P < 0.05) at the end of the fifth match. CK response at each time point was greater (P < 0.05) than the corresponding time point of the previous match. DOMSef increased (P < 0.05) after each match compared with its pre-match value. Pre-match DOMSef value progressively increased (P < 0.05) in matches 2 and 3, plateaued in match 4 and declined (P < 0.05) before match 5. Post-match DOMSef value progressively increased (P < 0.05) in matches 2 and 3, peaked (P < 0.05) after match 4 and declined (P < 0.05) again before match 5. Post-match DOMSke increased (P < 0.05) following each match and peaked following match 4. Post-match DOMSke declined (P < 0.05) in match 5 compared with its respective value in match 4. Pre-match DOMSke values were comparable to baseline values with the exception (P < 0.05) of that prior to match 4. Both pre- and post-match EJRM remained unaltered in matches 1-3. However, EJRM declined (P < 0.05) compared with its baseline value before matches 4 and 5 and demonstrated its lowest value (P < 0.05) following the match 4. Post-match EJRM increased (P < 0.05) compared with its respective value in match 4 but remained below (P < 0.05) the corresponding value of the first match. KJRM did not demonstrate any variation throughout the tournament.
Norepinephrine and epinephrine (Fig. 2) pre-match values were all similar to those measured at baseline. Wrestling activity induced a marked elevation (P < 0.05) of both norepinephrine (effect sizes: −0.1 to 1.9) and epinephrine (effect sizes: −0.7 to 1.8) concentrations relative to their baseline and pre-match values. Norepinephrine post-match levels demonstrated an increase (P < 0.05) from match 3 to match 4 and peaked (P < 0.05) following the last match of the day. In contrast, epinephrine post-match values were all comparable.
Epinephrine (a), Norepinephrine (b), testosterone (c) and cortisol (d) concentrations at baseline and before and after each match (mean ± SE). AM morning baseline testing; PM afternoon baseline testing; *P < 0.05 from baseline; † P < 0.05 between pre- and post-match value; ‡ P < 0.05 from respective value of the first match; ¶from respective value of the previous match
Post-match testosterone concentrations (Fig. 2) were all similarly elevated (P < 0.05) compared with their corresponding pre-match values, whereas pre-match concentrations for matches 2–5 were lower (P < 0.05) than the corresponding baseline and match 1 measures (effect sizes: −1.1 to −0.9). Cortisol levels (Fig. 3) were decreased (P < 0.05) at PM compared with AM. Cortisol post-match concentration increased (P < 0.05) similarly following all matches, peaking after the fourth match (effect sizes: −0.6 to −1.7). Cortisol pre-match levels were all similar to baseline values.
C-reactive protein concentration (a), leukocyte counts (b), and IL-6 concentration (c) at baseline and before and after each match (mean ± SE). AM morning baseline testing; PM afternoon baseline testing; CRP C-reactive protein; IL-6, interleukin 6; *P < 0.05 from baseline; † P < 0.05 between pre- and post-match value; ‡ P < 0.05 from respective value of the first match; § P < 0.05 between AM and PM; ¶from respective value of the previous match
CRP levels (Fig. 3) were elevated (P < 0.05) PM relative to AM. CRP (effect sizes: 1.6 to 2.9) pre-match concentration remained similar to its baseline value only before the first match, whereas in matches 2–5 it demonstrated a gradual increase (P < 0.05) peaking following the last match of the day. CRP pre-match were higher (P < 0.05) in match 3 compared to match 2 and in match 4 compared to match 3 (P < 0.05). Wrestling activity induced a marked CRP elevation (P < 0.05) following each match. Leukocytosis (P < 0.05) was developed following all matches with the last two matches inducing the greatest elevation (P < 0.05) in leukocyte counts. Pre-match leukocyte counts (effect sizes: −0.4 to 1.9) were all similar to baseline values with the exception of that match 4 which was above (P < 0.05) its corresponding baseline value. IL-6 (effect sizes: 0.1 to 2.4) pre-match concentrations were similar to its baseline values for matches 1 and 2. However, pre-match IL-6 increased (P < 0.05) thereafter before matches 3 and 4, peaking (P < 0.05) before the fourth match, and declined (P < 0.05) prior to the last match without reaching baseline values. Post-match IL-6 concentration increased (P < 0.05) following all matches, peaking (P < 0.05) after the fourth match. IL-6 post-match was higher (P < 0.05) in match 3 compared with matches 1–2. IL-6 post-match value declined (P < 0.05) after the last match compared with the respective values for matches 2 and 3 without reaching baseline levels (P < 0.05).
TBARS and PC (Fig. 4) increased (P < 0.05) following each match compared with their pre-match value. TBARS (effect sizes: 0.5 to 2.1) post-match value exhibited a gradual increase (P < 0.05) for matches 2 through 5 with the fourth match demonstrating the greatest elevation. TBARS pre-match concentration was similar to its baseline value but increased (P < 0.05) thereafter, peaking (P < 0.05) after the last match. PC (effect sizes: 0.8 to 2.9) pre-match values were similar to the corresponding baseline values for matches 1 and 2 but increased (P < 0.05) thereafter, peaking (P < 0.05) before the fourth match. PC pre-match concentration declined (P < 0.05) in match 5 compared with match 4 without reaching (P < 0.05) baseline values. PC concentration increased following all wrestling matches relative to their pre-match value with matches 3 and 4 demonstrating the greatest elevation and peaking following the fourth match.
TBARS (a), protein carbonyls (b), reduced glutathione (c), oxidized glutathione (d), the GSH/GSSG ratio (e), total antioxidant capacity (f), catalase activity (g), glutathione peroxidase activity (h) at baseline and before and after each match (mean ± SE). AM morning baseline testing; PM afternoon baseline testing; TBARS thiobarbituric acid reactive substances; PC protein carbonyls; GSH reduced glutathione; GSSG oxidized glutathione; TAC total antioxidant capacity; GPX glutathione peroxidase activity; *P < 0.05 from baseline; † P < 0.05 between pre- and post-match values; ‡ P < 0.05 from respective value of the first match; ¶from respective value of the previous match
GSH (Fig. 4) was elevated (P < 0.05) at PM compared with AM. GSH (effect sizes: −1.6 to −0.2) declined (P < 0.05) following all wrestling matches except the first one. The greatest post-match decline (P < 0.05) was observed following the fourth match. GSH pre-match concentrations were all similar to the respective baseline values with the exception (P < 0.05) of those measured before the last two matches. GSSG concentration (Fig. 4) increased (P < 0.05) following all wrestling matches, peaking (P < 0.05) after the fourth one. GSSG post-match elevation was greater (P < 0.05) in match 3 compared with match 2 and lower in match 5 compared (P < 0.05) with match 4. GSSG (effect sizes: 0.1 to 1.2) pre-match concentration in matches 2–4 was greater than the respective baseline and match 1 values and greater in match 4 compared with matches 2 and 3. GSH/GSSG (Fig. 4) increased PM compared with AM. GSH/GSSG (effect sizes: 0.1 to 1.2) pre-match values demonstrated a decline (P < 0.05) in matches 2–4 reaching their nadir (P < 0.05) in match 4. In match 5, pre-match GSH/GSSG increased (P < 0.05) compared with match 4 without reaching the respective value in match 1. Post-match GSH/GSSG decreased (P < 0.05) following all matches reaching its lowest value (P < 0.05) in match 4 whereas in match 5 increased (P < 0.05) compared with match 4 without reaching baseline values (P < 0.05).
Pre-match TAC (Fig. 4) increased (P < 0.05) in matches 3 and 4 compared with baseline values with match 4 demonstrating the greatest elevation (P < 0.05). TAC (effect sizes: 0.1 to 1.4) increased (P < 0.05) similarly after all matches. All catalase (Fig. 4) pre-match values were similar to those measured at baseline. Catalase activity (effect sizes: −0.1 to 1.5) increased (P < 0.05) similarly following each match. GPX (Fig. 4) pre-match levels were similar to those measured at baseline but increased (P < 0.05) thereafter. Although post-match GPX (effect sizes: 0.1–1.8) declined (P < 0.05) in match 5 compared with match 4, it never reached baseline values. GPX increased (P < 0.05) following all matches and peaked (P < 0.05) in match 4. Post-match GPX elevation was greater (P < 0.05) in match 4 compared with matches 3 and 5.
Discussion
In this study, we evaluated the effects of a single-day Greco-Roman wrestling tournament on physiological responses as well as on performance and inflammatory status markers of elite wrestlers. The present investigation provides evidence that (1) a one-day Greco-Roman wrestling tournament induces significant physiological perturbation in elite wrestlers, (2) elite wrestlers demonstrate a remarkable ability to adapt and rebound from a weight loss period before the first match of a tournament (all performance and inflammatory markers examined remained unaltered by weight loss practices) as it has been also shown for free-style wrestling (Kraemer et al. 2001), and (3) performance and inflammatory status exhibit a progressive deterioration during the course of a one-day tournament that is mainly attributed to the simultaneous accumulations of multiple stressors such as physical competition, fatigue, muscle damage, fluctuation in dietary intake, and psychological stress. Our data indicate that this deterioration may be more pronounced during a single-day tournament as compared with a two-day tournament.
Participants’ physiological strain during the tournament approximated the values reported earlier for elite wrestling competition (Kraemer et al. 2001). Athletes reached a mean, post-match, and peak heart rate that corresponded to the 83–86, 92–96, and 96-98% of their maximal heart rate, respectively, suggesting that the matches were highly competitive. In agreement with previous findings, post-match lactate concentration reached maximal values (16–19 mM) illustrating wrestling’s anaerobic profile (Houston et al. 1983; Kraemer et al. 2001; Kraemer 1982). Due to increased lactate accumulation, body’s acid–base balance may be disrupted with each subsequent match in the tournament (Houston et al. 1983; Kraemer et al. 2001; Kraemer 1982) which in turn may hamper muscle’s contractile properties. However, elite wrestlers probably adapt to this type of stress by up-regulating various buffering reserves (Horswill 1992). Lactate rise may also be attributed to glycogen depletion due to athletes’ restricted food intake and insulin’s maintenance during a wrestling tournament as shown by Kraemer et al. (Kraemer et al. 2001). Glycogen depletion may also be supported by the observed hyperglycemia following each match (43–50% increase of glucose concentration) that coincides with earlier observations (Houston 1983). These metabolic stresses might have caused the progressive decline of physical performance during the tournament. The performance decline was paralleled by a rise in fatigue ratings during the course of this tournament. Resting fatigue increased consistently before all matches peaking before match 4 probably suggesting inadequate recovery between matches. Interestingly, 5–6 h of recovery between matches 4 and 5 was inadequate to induce a perceptual recovery. Similar findings have also been reported during a two-day wrestling tournament (Kraemer et al. 2001).
As previously shown, performance was more affected by the course of the tournament but not from weight loss confirming previous observations (Kraemer et al. 2001) that elite wrestlers have the ability to adapt and rebound from a 5–6% weight loss prior to their first match. Similarly, a 5% weight loss did not affect judo-related performance measures (Artioli et al. 2010) suggesting that elite athletes may have adaptive defense mechanisms. It appears that although blood and plasma volumes are considerably reduced by weight loss, pre-competition osmolality is not affected by either weight loss or the rate of weight decrements during a 1-week period prior to wrestlers’ weigh-in (Yankanich et al. 1998). It has been suggested that due to the fact that elite wrestlers are in a chronically dehydrated state, their fluid regulatory systems have been reset to a new “normal” indicating a compensatory response (Kraemer et al. 2001; Yankanich et al. 1998). Kraemer et al. (2001) reported that elite wrestlers although sustain a hyperosmotic status, they are still capable of competing at an elite level demonstrating a significant resiliency suggesting an adaptation of the hypothalamic control of osmolality regulation under such conditions. One limitation of the present study is that osmolality was not measured and hence comparisons with previous investigations cannot be made.
Most performance markers (VJ, HB, Bear Hug, HG) deteriorated (~13–16%) after the third game as compared with baseline and their values following the first match. However, only VJ recovered prior to the final match of the tournament. Furthermore, HG strength, which is a rather important element of wrestling performance, declined earlier (after match 2) and remained considerably below its baseline value throughout the tournament. This is in contrast to previous studies reporting no changes in GS under hypohydration conditions (Serfass et al. 1984) but coincides with that of Kraemer et al. (2001). These results underline that the recovery time between successive wrestling matches may be inadequate to allow optimal performance during the last two marches of a 1-day wrestling tournament which are most crucial for athletes’ success (medal-winning matches in the main international tournaments). The present findings also suggest that the upper body strength (HB and HG) and performance (bear hug test, mainly performed by the upper musculature) may be more susceptible to decline during the course of a 1-day wrestling tournament than those of the lower-body musculature as previously shown. (Kraemer et al. 2001).
In agreement with earlier observations (Kraemer et al. 2001), cortisol increased following each match and returned to its baseline values prior to the next match. The catabolic state that accompanies elevated cortisol levels may result in an attenuation of physical performance (i.e. force) due to reduced protein synthesis as well as losses of contractile proteins and neurotransmitters (Kraemer and Ratamess 2005). This cortisol elevation was accompanied by marked fluctuations of serum testosterone responses. Similar to earlier observations (Kraemer et al. 2001), serum testosterone increased following each match and declined progressively (below its corresponding baseline value by ~13%) at rest (between matches) during the course of the tournament. This decline may jeopardize the testosterone-mediated metabolic recovery between matches (Kraemer et al. 1998). Similar adaptations have been observed during periods characterized by excessive exercise volumes (Fry et al. 1992) and they are likely to be associated with either an inhibition of the hypothalamic-pituitary–gonadal axis due to sustained inflammation and increased catabolism and/or blood flow fluctuations at a testicular level that may hamper testosterone secretion (Kraemer et al. 2001). The combined cortisol elevation and testosterone decline may further exacerbate the catabolic environment at tissue level thereby reducing force production and overall performance. Other factors that may have contributed to this reduced testosterone response may be the limited recovery between matches and the increased match intensity. Prior weight loss is unlikely to have caused this testosterone response since the study took place during the recovery phase of the season and dehydration was avoided during the tournament (Wheeler et al. 1992).
Both norepinephrine and epinephrine increased following each match and returned to baseline prior to the next. However, norepinephrine increased at a greater extent compared with epinephrine. Both hormones reached a peak after match 1 and plateaued thereafter. In the study of Kraemer et al. (2001) epinephrine remained unaltered after the last match of each tournament day, a response attributed by the authors to possible adrenal insufficiency which might also explain the reduced performance. Impaired epinephrine secretion has been linked to a reduced short-term exercise performance (Fry et al. 1994). However, epinephrine results indicate that an adrenal insufficiency is unlikely to have occurred. In the present study, wrestling five matches in only one-day seems to produce a higher stress for the athletes. Elevated levels of stress hormones have been reported in exercise-induced muscle damage and accelerated reactive oxygen species (ROS) generation (Giraldo et al. 2009). A catecholamine involvement in exercise-induced immune changes has also been suggested (Giraldo et al. 2009). In fact, recent findings of altered cytokine release following catecholamine administration during the early phase after trauma in humans (Batistaki et al. 2008) and modulation of IL-6 (that was increased in the present study) release with β-adrenergic blockade in septic mice (Schmitz et al. 2007) provide further support for catecholamines’ involvement in inflammation. Furthermore, epinephrine has been shown to enhance platelet-neutrophil adhesion and adhesion molecule expression (Schmitz et al. 2007). Collectively, these observations suggest that a sustained post-exercise rise in catecholamines may be related to an exercise-induced inflammatory response.
An inflammatory response is usually associated with exercise-induced muscle damage that is also accompanied by a marked deterioration of performance as the one seen during the latter part of the tournament. In order to explore this possibility, we measured CK as well as other muscle damage indices. In agreement with previous findings (Kraemer et al. 2001), a CK rise was evident during the earlier stages of the tournament (after match 1) which progressively increased to 1,000 U L−1 prior to the last match. This CK rise was even more pronounced (~100%) when compared with its respective values during a two-day tournament (Kraemer et al. 2001). This CK rise was accompanied by a marked elevation of DOMS of both the upper- and lower-body musculature and a pronounced decline in elbow, but not knee joint range of motion. The CK protein efflux from muscle may be attributed to the increased permeability of plasma membrane and/or intramuscular vasculature due to muscle tissue disruption that was evident after the first match of the tournament. The pressure and swelling around the injured tissue contributes to the muscle soreness sensation and the decline of strength and joint’s range of motion leading to a deterioration of technical skills performance due to compensatory movements in an attempt to protect the injured tissue. Wrestling incorporates intermittent repetitions of intense eccentric activation during athletes’ effort to resist gravity. Eccentric actions produce higher tension per cross-sectional area of active muscle mass compared with concentric actions resulting in significant structural muscle damage (disruption of structural proteins such as Z-lines, troponin, tropomyosin) (Clarkson and Tremblay 1988). It was also evident that the upper body musculature demonstrated a greater deterioration of muscle damage markers (DOMSef and EJRM) suggesting that the upper body and limbs muscles may be more affected than those of the lower limbs during successive wrestling matches (also seen with upper body performance measures).
Each wrestling match induced an acute-phase inflammatory response characterized by leukocytosis due to leukocyte infiltration in the damaged tissue as well as a rise in cytokines, cortisol, and acute-phase proteins such as CRP (Malm et al. 2004; Margonis et al. 2007). Neutrophils demonstrate a transient increase immediately post-exercise followed by a delayed rise several hours later, a response that was also seen in this study following each match as well as at rest before the fourth match (Malm et al. 2004). The post-exercise immunological response has been partially attributed to the increased circulating cortisol which exerts complex action on leukocyte subpopulations (Smith et al. 2008). Cortisol is increased by IL-6, which is in agreement with our finding of a simultaneous post-match rise of IL-6 and cortisol (Smith et al. 2008). Muscle damage per se elicits a repair response, including entry of macrophages into the muscle resulting in a more pronounced IL-6 elevation. The small magnitude and the time frame (delayed) of the IL-6 rise suggest that its source was the cells involved in the inflammatory response and not the skeletal muscle. In the present study, IL-6 not only increased after each match but it was also elevated (by ~200–300%) at rest after the first match and throughout the tournament. During inflammation, IL-6 stimulates the synthesis of acute-phase proteins such as CRP in hepatocytes (Malm et al. 2004). CRP, an established inflammatory marker, increased both at rest and following each match, especially after match 3. Cortisol and cytokines act to degrade muscle protein, resulting in both an amino-acid release into the circulation and a stimulation of hepatocytes to absorb these amino-acids to synthesize new acute-phase proteins such as CRP (Malm et al. 2004; Smith et al. 2008). CRP rise has been implicated in monocyte activation and adhesion molecules’ synthesis that result in recruitment of leukocytes (Malm et al. 2004). In agreement with these observations, cortisol, IL-6, and CRP demonstrated a marked elevation reflecting an intensified inflammatory response throughout the tournament.
ROS are implicated in the regulation of exercise-induced inflammation associated with muscle micro-trauma by enhancing the nuclear translocation of redox-sensitive transcription factors and other molecules mediating inflammation (i.e. adhesion molecules, cytokines, chemokines) (Aoi et al. 2004). Exercise-induced ROS generation may be linked to DOMS through its association with phagocyte infiltration and the leukocytosis observed during recovery following exercise. Leukocytes produce ROS that up-regulate post-exercise inflammation, removal of traumatized tissue, and healing (Fielding et al. 1993). Superoxide produced by neutrophils and macrophages is converted to H2O2 which then reacts with superoxide in the presence of a transition metal to form the highly reactive hydroxyl radical that oxidizes lipids and proteins producing by-products such as the MDA and PC measured in this study (Balakrishnan and Anuradha 1998). Although exercise-induced oxidative stress is a well-known phenomenon, described by hundreds of research papers, limited information exists regarding oxidative stress and antioxidant status responses associated with wrestling. TBARS and PC increased both following each match and at rest prior to the last two (MDA) or three (PC) matches. The MDA rise in the circulation may be attributed to peroxidation of lipoprotein phospholipids and non-esterified fatty acids as well as oxygen-mediated injury of muscle cell membranes (Robertson et al. 1991). Increased serum CK and MDA levels may suggest a relationship between the exercise-induced ROS production and the protein outflow into circulation. PC rise may be attributed to increased oxidation of albumin and other serum proteins (Miyamoto et al. 2003). These results clearly suggest that wrestling enhances oxidative stress as a part of the inflammatory response to exercise-induced muscle injury.
Strenuous exercise reduces GSH concentration in liver and muscle probably due to a decline of cystine in the liver which is crucial for glutathione synthesis (Somani et al. 1995). In this study, GSH decreased following the last four matches and at rest prior to the last two matches, a finding that may be associated with the rise in MDA and PC at the same time points. MDA and PC have been negatively correlated with GSH and cysteine post-exercise (Liu et al. 2000). In contrast, GGSG increased throughout the tournament which is in agreement with previous reports of GSSG upregulation following intense exercise (Margonis et al. 2007). Consequently, GSH/GSSG ratio declined during recovery indicating the onset of oxidative stress.
A novel finding of the present study is the TAC rise with wrestling indicating that this type of activity stimulated body’s antioxidant defences in serum to counteract elevated ROS production. This TAC rise was accompanied by an increase of antioxidant enzyme activity, i.e. catalase and GPX. Mobilization of tissue antioxidant stores into the plasma is a widely accepted phenomenon that would help maintain antioxidant status in plasma in times of need (Margonis et al. 2007). Wrestling effects on antioxidant enzyme activity have not been studied so far. Increased GPX activity may be related to scavenger cell migration into damaged muscle tissue usually observed in damaged muscle fibers following exercise (Uchiyama et al. 2006). Intense exercise was shown to increase GPX activity in immune cells suggesting blood cell damage (Sureda et al. 2005).
Conclusion
This investigation adapted the experimental model developed by Kraemer et al. (2001) who initially examined the physiological responses to a two-day freestyle wrestling tournament organized according to the old wrestling regulations. Our intention was to determine whether a one-day Greco-roman wrestling tournament, organized according to the latest wrestling regulations, could produce any significant physiological perturbations that could affect the performance and inflammatory responses of elite wrestlers as compared with a two-day tournament. We observed slightly higher heart rates than those reported by Kraemer et al. (2001), suggesting a higher intensity during the matches as a result of the latest changes in the duration and structure of the rounds that each match is consisted of. As in the case of the two-day tournament (Kraemer et al. 2001), weight loss alone did not affect performance and/or inflammatory responses. However, muscle damage markers demonstrated a pronounced and sustained elevation throughout the tournament. This finding may not be surprising since athletes have to complete the same number of matches in just half of the time allowed by previous regulations. Interestingly, the upper body seems to be more vulnerable to performance deterioration and damage of muscle tissue. These results were accompanied by changes seen in the inflammatory markers, i.e. CRP, leukocyte counts, IL-6, stress hormones, and oxidative stress markers. Therefore, it appears that a one-day Greco-Roman wrestling tournament imposes significant physiological demands on wrestlers that may adversely affect their performance and inflammatory status, especially during the later stages of the tournament placing the athletes in a greater risk for injury. In light of this evidence, training and recovery strategies should be developed to offset the adverse effects on inflammatory status and performance of elite wrestlers competing in one-day tournaments.
References
American College of Sports Medicine (1996) American College of Sports Medicine position stand on weight loss in wrestlers. Med Sci Sports Exerc 28:9–12
Aoi W, Naito Y, Takanami Y, Sakuma K, Ichikawa H, Yoshida N, Yoshikawa T (2004) Oxidative stress and delayed onset muscle damage after exercise. Free Rad Biol Med 37:480–487
Armstrong RB, Oglivie RW, Schwane JA (1983) Eccentric exercise-induced injury to rat skeletal muscle. J Appl Physiol 54:80–93
Artioli GG, Iglesias RT, Franchini E, Gualano B, Kashiwagura DB, Solis MY, Benatti FB, Fuchs M, Lancha AH (2010) Rapid weight loss followed by recovery time does not affect judo-related performance. J Sports Sci 28:21–32
Aschenbach W, Ocel J, Craft L, Ward C, Spangenburg E, Williams J (2000) Effect of oral sodium loading on high-intensity arm ergometry in college wrestlers. Med Sci Sports Exerc 32:669–675
Balakrishnan SD, Anuradha CV (1998) Exercise, depletion of antioxidants and antioxidant: manipulation. Cell Biochem Funct 16:269–327
Batistaki C, Kostopanagiotou G, Myrianthefs P, Dimas C, Matsota P, Pandazi A, Baltopoulos G (2008) Effect of exogenous catecholamines on tumor necrosis factor alpha, interleukin-6, interleukin-10 and beta-endorphin levels following severe trauma. Vasc Pharmacol 48:85–91
Callan SD, Brunner DM, Devolve KL, Mulligan SE, Hesson J, Wilber RL, Kearney JT (2000) Physiological profiles of elite freestyle wrestlers. J Strength Cond Res 14:162–169
Clarkson PM, Tremblay I (1988) Exercise-induced muscle damage, repair, and adaptations in humans. J Appl Physiol 65:1–6
Dill DB, Costill DL (1974) Calculation of percentage changes in volumes of blood, plasma, and red cells in dehydration. J Appl Physiol 37:247–248
Faul F, Erdfelder E, Lang AG, Buchner A (2007) G*Power 3: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav Res Methods 39:175–191
Fielding R, Manfredi T, Ding W, Fiatarone MA, Evans WJ, Cannon JG (1993) Acute phase response to exercise. III: neutrophil and IL-1 accumulation in skeletal muscle. Am J Physiol 265:R166–R172
Fry AC, Kraemer WJ, Stone MH (1992) The effect of amino acid supplementation on testosterone, cortisol, and growth hormone responses to one week of intensive weightlifting. Int J Sport Nutr 3:306–322
Fry AC, Kraemer WJ, Van Borselen F, Lynch JM, Triplett NT, Koziris LP, Fleck SJ (1994) Catecholamine responses to short-term high-intensity resistance exercise overtraining. J Appl Physiol 77:941–946
Giraldo E, Garcia JJ, Hinchado MD, Ortega E (2009) Exercise entensity-dependent changes in the nnflammatory response in sedentary women: Role of neuroendocrine parameters in the neutrophil phagocytic process and the pro-/anti-inflammatory cytokine balance. Neuroimmunomodulation 16:237–244
Hoffman JR, Maresh CM, Armstrong LE, Gabaree CL, Bergeron MF, Kenefick RW, Castellani JW, Ahlquist LE, Ward A (1994) Effects of hydration state on plasma testosterone, cortisol, and catecholamine concentrations before and during mild exercise at elevated temperature. Eur J Appl Physiol 69:294–300
Horswill CA (1992) Applied physiology of amateur wrestling. Sports Med 14:114–143
Horswill CA (1994) Physiology and nutrition for wrestling. In: Lamb DR, Knuttgen HG, Murray R (eds) Physiology and nutrition for competitive sport. Cooper Publishing, Carmel, pp 131–180
Houston ME, Sharratt MT, Bruce RW (1983) Glycogen depletion and lactate responses in freestyle wrestling. Can J Appl Sports Sci 8:79–82
Kraemer WJ (1982) The physiological basis for conditioning in wrestling. Natl Str Cond Assoc J 4:24–25
Kraemer WJ, Ratamess NA (2005) Hormonal responses and adaptations to resistance exercise and training. Sports Med 35:339–361
Kraemer WJ, Volek JS, Bush JA, Putukian M, Sebastianelli WJ (1998) Hormonal responses to consecutive days of heavy resistance exercise with or without nutritional supplementation. J Appl Physiol 85:1544–1555
Kraemer WJ, Fry AC, Rubin MR, Triplett-McBride T, Gordon SE, Koziris LP, Lynch JM, Volek JS, Meuffels DE, Newton RU, Fleck SJ (2001) Physiological and performance responses to tournament wrestling. Med Sci Sports Exerc 33:1367–1378
Liu J, Yeo HC, Overnik-Douki E, Hagen T, Doniger SJ, Chyu DW, Brooks GA, Ames BN (2000) Chronically and acutely exercised rats: biomarkers of oxidative stress and endogenous antioxidants. J Appl Physiol 89:21–28
Malm C, Sjodin LB, Sjoberg B, Lenkei R, Renstrom P, Lundberg IE, Ekblom B (2004) Leukocytes, cytokines, growth factors, and hormones in human skeletal muscle and blood after uphill or downhill running. J Physiol 556:983–1000
Margonis K, Fatouros IG, Jamurtas AZ, Nikolaidis MG, Douroudos I, Chatzinikolaou A, Mitrakou A, Mastorakos G, Papassotiriou I, Taxildaris K, Kouretas D (2007) Oxidative stress biomarkers responses to physical overtraining: implications for diagnosis. Free Rad Biol Med 43:901–910
Miyamoto Y, Koh YH, Park YS, Fujiwara N, Sakiyama H, Misonou Y, Ookawara T, Suzuki K, Honke K, Taniguchi N (2003) Oxidative stress caused by inactivation of glutathione peroxidase and adaptive responses. Biol Chem 384:567–574
Robertson JD, Maughan RJ, Duthie GG, Morricey PC (1991) Increased blood antioxidant systems of runners in response to training load. Clin Sci 80:611–618
Schmitz D, Wilsenack K, Lendemanns S, Schedlowski M, Oberbeck R (2007) β-Adrenergic blockade during systemic inflammation: impact on cellular immune functions and survival in a murine model of sepsis. Resuscitation 72:286–294
Serfass RC, Strull GA, Ewing JL (1984) The effect of rapid weight loss and attempted rehydration on strength endurance of the hand gripping muscles in college wrestlers. Res Q Exerc Sport 55:46–52
Sharratt MT, Taylor AW, Song TMK (1986) A physiological profile of elite Canadian freestyle wrestlers. Can J Appl Sports Sci 11:100–115
Smith C, Kruger MJ, Smith RM, Myburgh KH (2008) The inflammatory response to skeletal muscle injury: illuminating complexities. Sports Med 38:947–969
Somani SM, Frank S, Rybak LP (1995) Responses of antioxidant system to acute and trained exercise in rat heart subcellular fractions. Pharmacol Biochem Behav 51:627–634
Steen SN, Brownell KD (1992) Patterns of weight loss and regain in wrestlers: has the tradition changed? Med Sci Sports Exerc 22:762–768
Sureda A, Tauler P, Aguilo A, Cases N, Fuentespina E, Cordova A, Tur JA, Pons A (2005) Relation between oxidative stress markers and antioxidant endogenous defences during exhaustive exercise. Free Rad Res 39:1317–1324
Uchiyama S, Tsukamoto H, Yoshimura S, Tamaki T (2006) Relationship between oxidative stress in muscle tissue and weight lifting-induced muscle damage. Eur J Appl Physiol 452:109–116
Webster S, Rutt R, Weltman A (1990) Physiological effects of a weight loss regimen practiced by college wrestlers. Med Sci Sports Exerc 22:229–234
Wheeler GD, Mcfadyen SG, Symbaluk D, Pierce WD, Cummings DC (1992) Effects of training on serum testosterone and cortisol levels in wrestlers. Clin J Sports Med 2:257–260
WR MM (1998) Hyperthermia and dehydration-related deaths associated with intentional rapid weight loss in three collegiate wrestlers: North Carolina, Wisconsin, and Michigan, November–December 1997. MMWR 47:105–108
Yankanich J, Kenney WL, Fleck SJ, Kraemer WJ (1998) Precompetition weight loss and changes in vascular fluid volume in NCAA Division I college wrestlers. J Strength Cond Res 12:138–145
Acknowledgments
The authors wish to thank all the subjects for their participation and commitment to the study and Mr Ioannis Galanis for his technical assistance with diet analysis. The work described in the present manuscript was entirely supported by funding received by the Democritus University of Thrace.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by William Kraemer.
Rights and permissions
About this article
Cite this article
Barbas, I., Fatouros, I.G., Douroudos, I.I. et al. Physiological and performance adaptations of elite Greco-Roman wrestlers during a one-day tournament. Eur J Appl Physiol 111, 1421–1436 (2011). https://doi.org/10.1007/s00421-010-1761-7
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00421-010-1761-7