Abstract
Purpose
To investigate the ability of ganglion cell complex (GCC) analysis by optical coherence tomography (OCT) to detect early axonal damage in nonarteritic anterior ischaemic optic neuropathy (NAION), and to assess the relationship of GCC measurements with visual field defects and function parameters.
Methods
Twenty-two patients with NAION participated in this retrospective case-series study. Patients underwent spectral-domain OCT measurement of retinal nerve fibre layer (RNFL) and GCC average and minimum thicknesses, best-corrected visual acuity, Ishihara test and Humphrey visual field (SITA Standard 24–2). These measurements were recorded in the acute (2–6 weeks after the ischaemic episode) and chronic (≥6 months later) phases. Spearman’s coefficients were used to assess the relationship between GCC thickness and visual field defects.
Results
In the acute phase, none of the patients showed atrophy of the optic disc, while early damage was observed in the GCC average and minimum thickness in 54.54 % and 77.27 % of patients. At 6 months, the rate of patients with RNFL below normal limits increased to 90 % in the RNFL, and 92.85 % and 100 % in the GCC average and minimum GCC respectively. Spearman’s coefficients indicated significant relationships of GCC in the acute phase with visual field index and mean deviation in both acute and chronic phases. A significant correlation was also found with location of the defects.
Conclusions
GCC thickness measurement by OCT is capable of detecting early axonal damage in NAION eyes in the acute phase that cannot be detected by RNFL. GCC defects are significantly correlated with visual field globally and the defect location.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Nonarteritic anterior ischaemic optic neuropathy (NAION) constitutes 95 % of all anterior ischaemic optic neuropathies, and is the most common cause of acute optic neuropathy in people over the age of 50 [1]. It is characterized by a sudden onset of altitudinal visual loss resulting from hypoperfusion or non-perfusion of the posterior ciliary arteries that feed the optic nerve head. These arteries are divided into two main sectors, a superior one and an inferior one. Accordingly, the visual field (VF) defect primarily found is altitudinal, and its magnitude depends on the extension of the infarct of the posterior ciliary arteries.
When the patient is examined at onset, a relative afferent pupillary defect on the affected eye and optic nerve head swelling can be seen on fundoscopy. Over the first 3 months, the optic disc swelling resolves until degeneration of axons and apoptosis of ganglion cell bodies in the retina result in optic nerve head atrophy [1].
Spectral-domain optical coherence tomography (SD-OCT) is a non-invasive imaging technique producing high-resolution cross-sectional images of the retina that allows in-vivo measurements of retinal thickness in the macula and around the optic nerve. SD-OCT imaging has become a valuable tool for identifying and monitoring peripapillary retinal nerve fibre layer thickness in optic neuropathies [2–4] .
The appearance of the optic nerve head following NAION has been evaluated using OCT measurements, demonstrating a moderate-to-strong correlation between peripapillary retinal nerve fibre layer (RNFL) loss and corresponding VF sensitivity loss measured with automated perimetry [5–9]. However, the characteristics of optic disc oedema as shown by OCT at onset have limited prognostic value: specifically, initial RNFL thickness is not correlated with final RNFL thickness, visual acuity, or VF mean deviation (MD) [5]. On the other hand, some authors have measured the whole macular thickness (MT) in NAION at least 6 months after the acute event, and found correlations with VF [10, 11].
Recent advances in segmentation algorithms have made it possible to visualise and measure individual retinal layers with SD-OCT. Ganglion cell–inner plexiform layer (GCIPL) analysis in the macula has become an important examination in the assessment of various optic neuropathies [12, 13]. Recently, GCIPL losses have also been measured in a chronic phase of NAION (6 months after the acute event) by Fourier-domain OCT, authors reporting a correlation with both magnitude and location of VF losses [14, 15].
The primary purpose of our study was to assess the role of the GCIPL or ganglion cell complex (GCC) analysis by SD-OCT in detecting early axonal damage, avoiding the artefact caused by oedema in peripapillar RNFL measurements. The secondary aim was to analyse the relationship between GCC measurements and the magnitude and location of VF losses.
Methods
Design
This was an observational retrospective study. Patients with diagnosis of NAION admitted to the Department of Ophthalmology at Cruces University Hospital, Bilbao, Spain between October 2010 and June 2013 were revised for the study; a total of 28 participants were recruited. Four patients were excluded because of coexistence of ophthalmic or neurologic disease, and another two because they showed initial atrophy of optic disc at first OCT scan. The study was approved by the Clinical Research Ethics Committee, and conducted in accordance with the principles of the Declaration of Helsinki.
All participants underwent a comprehensive neuro-ophthalmic examination, which included measurement of the best-corrected visual acuity (BCVA) using a Snellen chart, an Ishihara test and a stereoscopic fundus examination. Automated VF testing was also performed (see below). RNFL, GCIPL, and macular thickness were measured with the Cirrus OCT system (Carl Zeiss Meditec, Dublin, CA, USA).
The following parameters were recorded in both acute (between 2 and 6 weeks from the NAION acute episode) and chronic (at least 6 months after the acute episode) phases: BVCA, number of correct plates on the Ishihara test (out of 17), VF (description of defects) mean deviation (MD), and visual field index (VFI), as well as SD-OCT measurements of peripapillary RNFL and macular GCC average and minimum thicknesses.
Diagnosis of NAION
NAION diagnosis was made on the basis of a sudden and painless visual loss, optic disc swelling and VF defects. Fluorescein angiography was only performed in 40 % because most patients met clear diagnostic criteria (age, ARPD, contralateral axonal crowding, hiperemic edema at onset, no demyelinating or infectious disease...) and showed weak leakage from the optic disc. Clinical and laboratory findings ruled out arteritic anterior ischaemic optic neuropathy. Further, all patients recruited to the study had resolution of the disc oedema within 8 weeks and followed a clinical course consistent with NAION. Follow-up VF tests and OCT were performed between 6 and 8 months after the acute event.
Exclusion criteria
Patients were excluded if they had media opacities that would preclude SD-OCT scanning, glaucoma, coexistence of ophthalmic or neurological disease, or any other retinal pathology, or if they had undergone previous eye surgery (other than uneventful cataract surgery).
Visual field testing
VF testing (Humphrey Visual Field Analyzer, 2010 (Carl Zeiss Meditec) was performed with the SITA-Fast 24–2 program with central fixation strategy. VFI and global MD were recorded. MD for the superior and inferior hemifields were calculated from the total deviation values. VF defects were categorised as superior or inferior if the scotoma did not cross the horizontal middle line and remained in one of the two hemifields or bihemispheric if both hemifields were affected.
Optical coherence tomography scanning
Subjects underwent ocular imaging with dilated pupils; scans only were included if they were high quality, had focused images with the circular ring of the scan centred around the optic disc and retinal layers were correctly identified. We performed Optic Disc Cube 200 × 200 scans covering an area of 6 x 6 mm around the optic disc in the OCT image (Carl Zeiss Meditec, Dublin, CA, USA) and used RNFL OU analysis to record the mean RNFL thickness, as well as thickness in superior, inferior, temporal, and nasal quadrants. The GCC analysis obtained from the Macular Cube 512 × 128 analysis was used to obtain the average GCC (GCCav) and minimum GCC (GCCmin) thicknesses, as well as the thickness in six sectors around the macular map provided. For each patient, the colour provided in the OCT report characterising the thickness relative to normal values was also recorded (red and yellow for atrophy, green for values within the normal range, and white for oedema).
Statistical analysis
The qualitative variables were described in percentages, and quantitative variables were described with means and standard deviations or median and maximum and minimum depending on their distributional characteristics. Pearson’s coefficient was used to assess correlations between OCT values and BCVA, RNFL, and correct Ishihara plates. Spearman´s coefficient was used to find correlations between OCT and visual field parameters. Wilcoxon’s test was used to detect significant changes between the acute and chronic phases within the same variable. Statistical analysis was performed with SPSS (vers. 21).
Results
Demographics and visual function
Overall, eight patients were female and 14 male, while 16 of the eyes included were right and six left. Mean age of participants was 63.4 ± 11.7 years ,and mean BCVA at onset was 0.55 ± 0.35 with a wide range between 0.05 and 1; the same variability was found in the Ishihara test, the mean number of correct plates being 10 ± 6, with a range of 1 to 17.
In the acute phase, the VF MD was −17.51 ± 9.58 and mean VFI was 50.57 % ± 31. VF defects were categorised as superior in four patients (19 %), inferior in six (28.5 %) and bihemispheric in 11 (52.4 %). BCVA and the number of correct Ishihara plates did not change significantly between the acute and chronic phases (+0.04, 95 % CI (−0.14 to 0.11) p = 0.82 and −1.1, 95 % CI (−1.7 to 3.9) p = 0.41 respectively), while MD and VFI decreased slightly (−0.77 95 % CI (−3.6 to 2.1) p = 0.55 and −7.84 95 % CI (−0.6 to 16.3), p = 0.122 respectively) (Table 1).
OCT measurements
Peripapillary RNFL
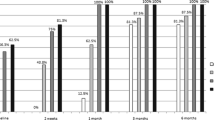
In the acute phase, OCT showed a mean peripapillary RNFL thickness of 150.35 ± 11.4 μm. OCT measurements indicated that 19 patients (86.4 %) showed values above normal limits (white), while three (13.6 %) had values within normal limits (green) and none showed values below normal limits (red or yellow). Correlations between GCC av at onset and final RNFL, BCVA, and correct Ishihara plates were not significant (Pearson’s coefficients: −0.020, 0.21, 0.41, p = 0.93, p = 0.43, p = 0.11 respectively). Table 2 reports the percentages of patients that showed values above, within, or below normal in the RNFL and GCC, both the mean and values for the four quadrants at acute and chronic phases. Figure 1 represents the number of patients that showed values below normal limits considering the mean RNFL, GCC av, and GCC min thicknesses in the two phases of the NAION. It is easy to appreciate that despite an apparent lack of damage at the onset in the RNFL measurements, the GCC av value shows that there was ganglion cell damage in more than half of the patients, and the GCC min value identifies damage in as many as 17 (77.3 %) of them. However, when NAION is followed by resolution of the optic disc oedema and optic nerve atrophy becomes visible, the damage is also reflected in the RNFL, with chronic phase measurements of this layer showing values below normal limits in 20 of the patients (90 %).
Ganglion cell complex (GCC)
With regard to the GCC analysis, the number of patients that showed red or yellow color at the same time point was 12 (54.5 %) considering GCC av thickness, and as high as 17 (77.3 %) considering the GCC min thickness.
The GCC measurements also showed statistically significant decrease in the average and minimum thicknesses between acute and chronic phases (p = 0.005 for GCC av and p = 0.008 for GCCmin) (Table 1). Overall, the percentage reductions in layer thickness from the acute to the chronic phase were RNFL, 56.9 %; GCCmin, 28.88 %; and GCC av, 19.7 %, that is, there was a greater percentage reduction in RNFL thickness due to the resolution of optic disc edema.
GCC and VF correlations
We found significant correlations between GCC av at onset and VF parameters (MD and VFI respectively) in both acute and chronic phases. Spearman’s coefficients (Fig. 2) were −0.50 (p = 0.021) and 0.54 (p = 0.016) for the acute phase and −0.59 (p = 0.005) and 0.57 (p = 0.008) for the chronic phase. Correlations between GCC thickness in the chronic phase and VF defect (MD) were −0.6 (p = 0.020) for GCC av and −0.54 (p = 0.05) for GCCmin).
Upper row: scatter plots showing correlations between mean values of ganglion cell complex thickness at acute phase (GCCac) and visual field index at acute (VFI acute), and chronic (VFI chron) phases and mean deviation at acute phase (MD acute). Lower row: Correlations between GCCac and mean meviation at chronic phase (MD chron) and between ganglion cell complex at chronic phase (GCCchron) and MDchron and VFIchron
With regard to the GCC analysis and the VF defect location, Fig. 3 summarizes the results of correlations between GCC thickness at onset with VF of the superior and inferior hemifields. Means of the three superior and inferior values provided by the GCC analysis map were correlated with means of total deviation values of the corresponding superior and inferior hemifields of the VF. Correlations were significant for both initial and final VFs for the inferior GCC thickness and the corresponding superior visual field [−0.57 (p = 0.008) and −0.59 (p = 0.006)] respectively and between superior GCC thickness and the corresponding inferior visual hemifield in the chronic phase [−0.52 (p = 0.02)], but not between the superior GCC thickness and the corresponding inferior visual field in the acute phase [−0.41 (p = 0.07)]. In contrast, Spearman’s correlations between location of GCC defects in the chronic phase and location of final VF defect were not significant (−0.083, p = 0.76 for inferior VF and −0.32, p = 0.23 for the superior one).
GCC defect at onset was not significantly correlated with location of the final optic nerve atrophy, but we did observe a trend: specifically, all patients that showed bihemispheric atrophy in the GCC measurements at onset also showed bihemispheric atrophy of the optic nerve at the final assessment.
Upper row: scatter plots showing correlations between mean values of upper ganglion cell complex thickness at acute phase (GCCac sup) and inferior visual field at acute (VF acute inf) and chronic (VF chron inf) phases; and between inferior thickness of GCC at acute phase (GCCac inf) and superior visual field at acute stage (VF acute sup). Lower row: correlations between inferior GCC at acute phase (GCC ac inf) and superior visual field at the chronic phase (VF chron sup) and between superior and inferior GCC at chronic stage (GCC chron sup, GCC chron inf) and the correspondig visual field at the chronic stage (VF chron inf, VF chron sup)
Figure 4 shows OCT reports for the right eye of one of the patients in the acute and chronic phases. At first, RNFL measurements indicate values above normal limits at the same point that the GCC map exhibits damage of the superior hemisphere corresponding to an inferior VF defect. Six months later, red color appears in the superior pole of the optic nerve and ganglion cell complex measurements show a slight increase in the defect. Note that VF defects are more strongly correlated with GCC defects in the acute phase than those observed in the chronic phase.
Discussion
The present study used SD-OCT to demonstrate the important role of ganglion cell–inner plexiform layer or GCC analysis in the macular region in detecting early axonal damage in the acute onset of NAION (6 weeks or less after the acute episode).
Some authors have measured the whole macular thickness in NAION in the chronic phase, showing correlations with VF and supporting the role of macular assessment in this neuropathy [10, 11]. However, recent advances in segmentation algorithms have made it possible to visualise and measure individual retinal layers with OCT in the macular region [16, 17, 19]. Approximately 50 % of the retinal ganglion cells are concentrated within 4.5 mm of the fovea [18]. SD-OCT can be used to measure the thickness of the ganglion cell layer or GCC, defined as the combination of retinal nerve fibre, ganglion cell and inner plexiform layers (GCL + IPL or GCIPL).
Some previous studies support the view that GCC measurements have predictive value: Marzoli SB et al. [20] have found damage in the GCC while the optic disc seemed normal and patients retained good visual acuity in idiopathic intracranial hypertension; and Tan et al. [21] found damage in the GCC in patients with glaucoma, but a preserved VF without scotomas. Choi et al. [22] suggests that, with time, GCC damage results in damage to the photoreceptors that produce an irreversible scotoma. In contrast, in inflammatory neuritis, in which the VF defect is reversible, the GCC is intact.
The RNFL measurements in the acute phase of NAION indicated values above normal limits in most patients, while they were within normal limits in a few cases but no patients demonstrated values below normal limits. We also expect peripapillary axonal damage at onset, but this is being masked by the peripapillary oedema, and so cannot be detected. In contrast, in the GCC analysis in the macular region, higher percentages of patients showed values below normal limits at the same time point: over 50 % with regard to the average GCC thickness and as many as 77 % with regard to the minimum GCC thickness.
The present results relating to early GCC damage detected by early SD-OCT measurements are in accordance with SD-OCT imaging in experimental NAION models and histological measurements of retinal ganglion cell loss and axonal thinning occurring early after the onset of the ischaemia, inducing a gradual thinning over the first few weeks [23–25]. Further, histological analysis showed a decrease in ganglion cell layer thickness over the first 4 weeks [26].
In addition, findings with RNFL measurements in the present sample were consistent with previous studies, that is, the characteristics of optic disc oedema as shown by OCT at onset have limited prognostic value; specifically, initial RNFL thickness is not correlated with final RNFL thickness, visual acuity, or VF MD [5, 27].
We found a significant correlation between GCC av at onset and VF parameters in both acute and chronic stages, suggesting that the GCC thickness in the acute stage may be a determining factor to predict final VF defects, while a correlation was also found between GCC thickness in the chronic stage and VF defects. Moreover, there were good correlations between the location of the damage in the GCC in the macular region and the location of the scotoma in the VF, with superior GCC defects being significantly correlated with inferior altitudinal defects in the chronic phase, and inferior GCC defects with superior altitudinal defects in the acute and chronic phases.
These findings agree with previous research evaluating the GCC in patients with NAION in a chronic phase that observed a correlation between GCC av and VF in magnitude and location [15], and add to previous evidence in that we found a correlation in the acute phase that, to our knowledge, has not previously been reported. On the other hand, in our sample, although the GCC in the chronic stage was significantly correlated with global VF parameters [MD, −0.59 (p = 0.005) and VFI, 0.57 (p = 0.008)], correlations between location of the GCC defect in the chronic stage and that of the final VF defect were not significant (−0.083, p = 0.76 for the inferior VF and −0.32, p = 0.23 for the superior VF), suggesting that GCC in the acute stage could be a better prognostic factor than GCC chronic measurements. This could be explained because as seen in Fig. 4, GCC damage becomes more severe and diffuse with time, and more internal plexus layer cells such as glyal cells may be damaged. Ganglion cell complex decrease detected by the OCT can be explained by damage of other structures of the internal layers but may partially preserve ganglion cell function, and so it has less repercussion on the visual field with the consequent minor correlation.
When NAION is followed by resolution of disc oedema and optic nerve atrophy becomes visible, it is also reflected in the RNFL, with values below normal limits being observed in 90 % of patients. There was a slight decrease in GCC thickness from the acute to the chronic phase 6 months later, but the difference is significantly larger in the RNFL changes, supporting the view that GCC analysis is a more useful tool for the early diagnosis of damage because the big changes observed in the RNFL thickness are mainly due to the resolution of optic disc edema and not to axonal damage.
Despite being a retrospective study with a limited number of patients, the current study demonstrates the role of the ganglion cell–inner plexiform layer or ganglion cell complex (GCC) analysis by SD-OCT in detecting early ganglion cell and inner plexiform layers damage in NAION, avoiding the artefact caused by oedema in peripapillar RNFL measurements. Early GCC measurements (within 6 weeks after the acute episode) were found to have a good correlation with quantitative measures of VF (VFI and MD), as well as being a useful tool in detecting hemisphere location of the damage in VF. Although more studies would be necessary to describe more precisely the time point at which the ganglion cell complex begins to show damage, this study indicates that early GCC damage does occur in patients with acute NAION, and this damage can be accurately measured with SD-OCT and correlated with final VF defect in the chronic phase.
References
Hattenhauer MG, Leavitt JA, Hodge DO et al (1997) Incidence of nonarteritic anterior ischemic optic neuropathy. Am J Ophthalmol 123(3):103–107
Kernstock C, Friebe K, Tonagel F (2013) Applications of optical coherence tomography (OCT) in neuro-ophthalmology. Klin Monatsbl Augenheilkd 230(11):1097–1105
Maekubo T, Chuman H, Kodama Y, Nao-I N (2013) Evaluation of inner retinal thickness around the optic disc using optical coherence tomography of a rodent model of nonarteritic ischemic optic neuropathy. Jpn J Ophthalmol 57(3):327–332
Savini G, Carbonelli M, Barboni P (2011) Spectral-domain optical coherence tomography for the diagnosis and follow-up of glaucoma. Curr Opin Ophthalmol 22(2):115–123
Contreras I, Noval S, Rebolleda G, Muñoz-Negrete FJ (2007) Follow-up of nonarteritic anterior ischemic optic neuropathy with optical coherence tomography. Ophthalmology 114:2338–2344
Contreras I, Rebolleda G, Noval S, Muñoz-Negrete FJ (2007) Optic disc evaluation by optical coherence tomography in nonarteritic anterior Ischemic optic neuropathy. Invest Ophthalmol Vis Sci 48:4087–4092
Deleón-Ortega J, Carroll KE, Arthur SN, Girkin CA (2007) Correlations between retinal nerve fiber layer and visual field in eyes with nonarteritic anterior ischemic optic neuropathy. Am J Ophthalmol 143:288–294
Hood DC, Anderson S, Rouleau J et al (2008) Retinal nerve fibre structure versus visual field function in patients with ischemic optic neuropathy a test of a linear model. Ophthalmology 115:904–910
Bellusci C, Savini G, Carboneli M, Carelli V, Sadun AA, Barboni P (2008) Retinal nerve fiber layer thickness in nonarteritic anterior ischemic optic neuropathy: OCT characterization of the acute and resolving phases. Graefes Arch Clin Exp Ophthalmol 246:641–647
Fernández-Buenaga R, Rebolleda G, Muñoz-Negrete FJ, Contreras I, Casas-Llera P (2009). Macular thickness. Ophthalmology 116(8):1587
Papchenko T, Grainger BT, Savino PJ, Gamble GD, Danesh-Meyer HV (2012) Macular thickness predictive of visual field sensitivity in ischaemic optic neuropathy. Acta Ophthalmol 90:e463–e469
Syc SB, Saidha S, Newsome SD, Ratchford JN, Levy M, Ford E, Crainiceanu CM, Durbin MK, Oakley JD, Meyer SA, Frohman EM, Calabresi PA (2012) Optical coherence tomography segmentation reveals ganglion cell layer pathology after optic neuritis. Brain 135(Pt 2):521–533
Renard JP, Fénolland JR, El Chehab H, Francoz M, Marill AM, Messaoudi R, Delbarre M, Maréchal M, Michel S, Giraud JM (2013) Analysis of macular ganglion cell complex (GCC) with spectral-domain optical coherence tomography (SD-OCT) in glaucoma. J Fr Ophtalmol 36(4):299–309
Aggarwal D, Tan O, Huang D, Sadun AA (2012) Patterns of ganglion cell complex and nerve fiber layer loss in nonarteritic ischemic optic neuropathy by Fourier-domain optical coherence tomography. Invest Ophthalmol Vis Sci 53(8):4539–4545
Gonul S, kokterik BE, Bakbak B, Gedik S (2013) Comparison of the ganglion cell complex and retinal nerve fibre layer measurements using Fourier domain optical coherence tomography to detect ganglion cell loss in non-arteritic anterior ischaemic optic neuropathy. Br J Ophthalmol 97:1045–1050
Wang M, Hood DC, Cho JS, Ghadiali Q, De Moraes CG, Zhang X, Ritch R, Liebmann JM (2009) Measurement of local retinal ganglion cell layer thickness in patients with glaucoma using frequency-domain optical coherence tomography. Arch Ophthalmol 127(7):875–881
Fabritius T, Makita S, Miura M, Myllylä R, Yasuno Y (2009) Automated segmentation of the macula by optical coherence tomography. Opt Express 17(18):15659–15669
Curcio CA, Allen KA (1990) Topography of ganglion cells in human retina. J Comp Neurol 300(1):5–25
Mwanza JC, Oakley JD, Budenz DL, Chang RT, Knight OJ, Feuer WJ (2011): Macular ganglion cell-inner plexiform layer: automated detection and thickness reproducibility with spectral domain-optical coherence tomography in glaucoma. Invest Ophthalmol Vis Sci 52(11):8323–8329
Marzoli SB, Ciasca P, Curone M, Cammarata G, Melzi L, Criscuoli A, Bussone G, D´Amico D (2013) Quantitative analysis of optic nerve damage in idiopathic intracranial hypertension (IIH) at diagnosis. Neurol Sci 34: S143–S145
Tan O, Li G, Lu AT, Varma R, Huang D (2008) Mapping of macular substructures with optical coherence tomography for glaucoma diagnosis. Ophthalmology 115:949–956
Choi SS, Zawadzki RJ, Keltner JL, Wern JS (2008) Changes in cellular structures revealed by ultra-high resolution retinal imaging in optic neuropathies. Invest Ophthalmol Vis Sci 49:2013–2119
Bernstein SL, Johnson MA, Miller NR (2011) Nonarteritic anterior ischemic optic neuropathy (NAION) and its experimental models. Prog Retin Eye Res 30(3):167–187
Goldenberg-Cohen N, Guo Y, Margolis F, Cohen Y, Miller NR, Bernstein SL (2005) Oligodendrocyte dysfunction after induction of experimental anterior optic nerve ischemia. Invest Ophthalmol Vis Sci 46(8):2716–2275
Zhang C, Guo Y, Slater BJ, Miller NR, Bernstein SL (2010) Axonal degeneration, regeneration and ganglion cell death in a rodent model of anterior ischemic optic neuropathy (rAION). Exp Eye Res 91:286–292
Ho JK, Stanford MP, Shariati MA, Dalal R, Liao YJ (2013) Optical coherence tomography study of experimental anterior ischemic optic neuropathy and histologic confirmation. Invest Ophthalmol Vis Sci 54:5981–5988
Kupersmith MJ, Anderson S, Durbin M, Kardon R (2013) Scanning laser polarimetry, but not optical coherence tomography predicts permanent visual field loss in acute nonarteritic anterior ischemic optic neuropathy. Invest Ophthalmol Vis Sci 54(8):5514–5519
Acknowledgments
We wish to thank Maialen Lopez Aricha, Jose María Losada Domingo, Bárbara Berasategui, and Ana Orive for collected data and care of study patients.
None of the authors have financial, commercial, or proprietary interest in any device mentioned. The authors have no conflicts of interest to declare.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Larrea, B.A., Iztueta, M.G., Indart, L.M. et al. Early axonal damage detection by ganglion cell complex analysis with optical coherence tomography in nonarteritic anterior ischaemic optic neuropathy. Graefes Arch Clin Exp Ophthalmol 252, 1839–1846 (2014). https://doi.org/10.1007/s00417-014-2697-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00417-014-2697-0