Abstract
Purpose
To clarify the usefulness of optical coherence tomography (OCT) for the objective and quantitative evaluation of retinal nerve fiber layer (RNFL) thickness around the optic disc in a rodent model of nonarteritic ischemic optic neuropathy (rNAION).
Methods
Inner retinal thickness was measured using OCT before and after rNAION induction. The thicknesses of the RNFL and the inner plexiform layer (IPL) were measured using a histologic preparation before and 56 days after induction. We compared the inner retinal thickness measured by OCT with that measured by the histologic preparation.
Results
The mean inner retinal thickness around the optic disc of normal rats measured using OCT was similar to that measured using a histologic preparation (73.50 ± 4.94 vs. 75.94 ± 5.90 μm). The mean inner retinal thickness of rNAION significantly increased until the 7th day, returned to baseline on the 14th day, and decreased until the 90th day after induction. On the 56th day after rNAION induction, histologic measurements indicated that the mean RNFL thickness had decreased but that the IPL thickness was similar to that at baseline.
Conclusion
The mean inner retinal thickness measured by OCT correlated with the RNFL thickness of rNAION. OCT is useful for the objective and quantitative evaluation of RNFL thickness around the optic disc in a model of rNAION.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Nonarteritic ischemic optic neuropathy (NAION) is an optic nerve disease caused by an ischemic insult to the optic nerve that evokes a structural change and results in optic nerve dysfunction. Unfortunately, no effective treatment for NAION has been established [1]. The importance of confirming the effectiveness of a treatment using an animal model and then evaluating the effectiveness of the treatment through clinical trials has been stated. Recently, a rat model of NAION (rNAION) was developed [2], and we have succeeded in establishing the model in our laboratory [3]. The anatomic alterations and funduscopic appearance of rNAION are similar to those of human NAION in that disc swelling develops during the acute phase and results in optic disc atrophy [2, 3]. However, objective, quantitative, and noninvasive methods for evaluating these changes have not been reported, even though they are absolutely necessary to evaluate treatment effectiveness.
We used optical coherence tomography (OCT) to objectively and quantitatively evaluate the anatomic changes that occur in rNAION. However, some methodological alterations were required to eliminate the difference between rats and humans in refractive power, axial length, and maximum dilated pupil size [4, 5]. To confirm the accuracy of OCT, we compared measurements of the inner retinal thickness in normal rats using OCT with those measured by a histologic preparation. We then quantitatively evaluated a time course of changes in the retinal nerve fiber layer (RNFL) thickness of rNAION.
Materials and methods
rNAION induction
The animal protocols were approved by the University of Miyazaki Institutional Animal Care Committee and adhered to the guidelines recommended by the ARVO statement for the Use of Animals in Ophthalmic and Vision Research. Male Sprague–Dawley rats (200–220 g; Kyudou, Kumamoto, Japan) were anesthetized with intramuscular ketamine and xylazine (80 and 5 mg/kg, respectively). For the control group, the left optic nerve was directly treated with an argon 514 nm/500 μm spot laser (Ultima 2000 SE argon laser; Lumenis, Santa Clara, CA, USA) for 12 s. To induce rNAION, rose bengal (RB) (2.5 mM, 1 mL/kg) was injected into the tail vein. After administration of RB, the capillaries of the left optic nerve were photoactivated using a 514 nm argon green laser with an approximately 500 μm spot size for 12 s. Thus, in the rNAION-induced rats, the right eye was the RB-only eye and the left eye was the rNAION-induced (via RB and laser treatment) eye.
OCT measurement
We measured the inner retinal thickness around the disc using spectral domain OCT (iVUE-100; Optovue, Fremont, CA, USA). Some methodological alterations were required for the measurements owing to differences between rats and humans in refractive power, axial length, and maximum dilated pupil size. The retinal image at best focus in the rat requires a diopter compensation of approximately +20 D because of the typical strong, negative spherical aberration. Therefore, we fixed an achromatic +20 D doublet lens with a locking device in front of the OCT instrument. The high-power positive lens also helped reduce the required pupil diameter. After the maximum pupil dilation was obtained, rats under anesthesia were fixed in front of the OCT. The rat was considered qualified for evaluation and one scan was counted when the scan quality index (SQI) was over 45 in each scan (Fig. 1a). The examiner recorded three scans, and the mean value was analyzed. The iVue-100 covers a 6 × 6 mm2 scan including 20 horizontal lines. The scan interval is measured within the area of a 1.5 mm diameter circle at 250 μm and outside the area at 500 μm. The measurement area can be a 1, 3, or 5 mm diameter circle in the iVue-100. In this experiment, we measured the thickness between the 3 and 1 mm circles, the center of which was the optic disc (Fig. 1b, c). Segmentation was performed automatically by the OCT apparatus, and measurements were taken from the inner limiting membrane (ILM) to the bottom of the inner plexiform layer (IPL). However, because each retinal layer was slightly obscured when the swelling developed in the area near the optic disc edge, we segmented the layers from the lines by ourselves.
a OCT cross-sectional image of the optic disc. Each retinal layer in the normal rats runs horizontally to the optic nerve head. Bar 250 μm. b Fundus photograph (normal eye). Arrow 500 μm. c Measurement area of the inner retinal layer. The measured area was within the 3 mm diameter circle (large arrow), the center of which was the center of the optic disc; a 1 mm diameter circle within the center (small arrow) was excluded. The measurement was obtained by averaging the space that had been divided into four areas
We evaluated the change in RNFL thickness as the change in the inner retina thickness, defined as the distance from the ILM to the bottom of the IPL (Fig. 2). Scans were performed before induction and on days 1, 3, 5, 7, 14, 28, 56, and 90 after rNAION induction. Changes in the appearance and thickness of the inner retina were compared before and after rNAION induction.
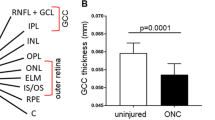
a Cross-sectional image obtained using OCT of the retina around the optic disc of the normal rat. The arrow shows the area of inner retinal thickness measured. b Histologic preparation of the retina around the optic disc in the area corresponding to that of a normal rat. All retinal layers were detectable in the OCT image and in the histologic preparation. RNFL retinal nerve fiber layer, GCL ganglion cell layer, IPL inner plexiform layer, INL inner nuclear layer, OPL outer plexiform layer, ONL outer nuclear layer, IS/OS junction line between the inner and outer segments of the photoreceptor cells. Inner retinal thickness is indicated by the arrow within the image
Histologic preparation and evaluation
Rats were killed using a sodium pentobarbital overdose after rNAION induction. The rat eyes were enucleated, and retinal tissue was removed for histologic analysis, fixed in 8 % paraformaldehyde/phosphate-buffered saline mixed with 70 % methanol (Super Fix; Kurabo, Osaka, Japan), and embedded in paraffin. The embedded tissue was sectioned at a 6 μm thickness along the vertical meridian through the optic nerve and stained with hematoxylin–eosin for microscopy. The thickness from the ILM to the IPL of each retina was measured (×100) at two points from the edge of the optic disc on both sides of the 500 μm area. We also compared the thickness of the RNFL/ganglion cell layer (GCL) and the IPL before rNAION induction with the thickness on the 56th day after induction (n = 10).
Statistical analysis
Values were expressed as the means ± SDs. Differences in inner retinal thickness were analyzed with the t test for comparisons between the control and rNAION eyes or by a one-way repeated-measures ANOVA for comparisons between different time points in the same eyes. Probability values of less than 0.01 were considered statistically significant.
Results
The OCT imaging of rats using our method was similar to that of humans (Fig. 2). Each of the six retinal layers was visibly distinctive. The mean inner retinal thickness of normal rats within the measurement area was 73.50 ± 4.94 μm (mean ± SD; n = 50). The thickness was similar to that measured using the histologic preparation (mean ± SD; 75.94 ± 5.90 μm; n = 10). These results show that the inner retinal thickness measurements obtained using OCT are accurate.
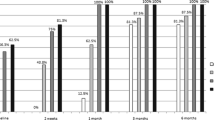
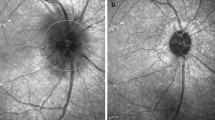
The mean inner retinal thickness of rNAION eyes within the measuring area was significantly increased in normal rats on the 1st, 3rd, 5th, and 7th days after rNAION induction (mean ± SD; 126.37 ± 6.93, 116.25 ± 4.42, 103.19 ± 4.73, 93.06 ± 5.34 μm, P < 0.001, respectively), fell to baseline on the 14th day (mean ± SD, 71.43 ± 6.52 μm), and then decreased on the 28th, 56th, and 90th days after induction (mean ± SD; 65.16 ± 2.52, 56.11 ± 4.98, and 51.90 ± 7.64 μm, respectively) (Fig. 3). These changes were similar to evaluations made using funduscopy (Fig. 4). The mean inner retinal thickness of the RB-only eyes within the measuring area did not change (Fig. 3). These results indicate that RB did not affect the anatomic damage to the inner retina. The mean inner retinal thickness of the laser-only eyes within the measurement area did not change significantly (Fig. 3), demonstrating that the laser did not affect the anatomic damage to the inner retina. Therefore, rNAION was induced by the combination of RB and the laser.
The mean inner retinal thicknesses of rNAION-induced eyes, RB-only eyes, and laser-only eyes measured by OCT scans 1, 3, 5, 7, 14, 28, 56, and 90 days after each intervention. The dark gray bar represents the rNAION-induced group (n = 10 eyes, each day). The light gray bar represents the RB-only group (n = 10 eyes, each day). The intermediate gray bar represents the laser-only group (n = 8 eyes, each day). Data are presented as the mean ± SD. *P < 0.01. The mean inner retinal thicknesses of the RB-only eyes and the laser-only eyes did not change significantly
The OCT appearance of the retinal swelling present in the acute stage was similar to that seen in the pathologic evaluation (Fig. 5). Measured using the histologic preparation, the mean inner retinal thickness before induction was 75.94 ± 5.90 μm, the mean RNFL/ganglion cell layer (GCL) was 39.06 ± 3.38 μm, and the mean IPL was 36.56 ± 3.30 μm. On the 56th day after rNAION induction, the mean inner retinal rNAION thickness had decreased to 59.64 ± 6.92 μm, the RNFL/GCL to 21.51 ± 6.02 μm, and the IPL to 33.26 ± 5.66 μm. The decrease in the RNFL thickness was significant (P < 0.001, n = 10), but the decrease in the IPL thickness was not (P > 0.05, n = 10). Therefore, the decrease in the inner retinal thickness can be attributed to the decrease in the RNFL thickness.
a Cross-sectional OCT image of the optic disc of rNAION on the third day after induction. Optic nerve edema with peripapillary retinal (especially the retinal pigment layer) displacement (arrows). b Retina and optic nerve histologic changes followed rNAION. H&E staining; bar = 200 μm. The figure shows that the change matched the OCT findings (double arrows)
Discussion
This study shows that OCT is useful for the objective and quantitative evaluation of the optic disc and the area surrounding the retina in rats. Each of the retinal layers from the normal rats was clearly detectable (Fig. 2). The inner retinal thickness was measurable, and the value was similar to that measured in the histologic preparation. The OCT image taken during the acute stage of rNAION was also similar to that observed in the histologic preparation (Fig. 5). In addition, the decrease in the inner retinal thickness during the chronic stage of rNAION correlated with that of the histologic specimen. Using OCT, we observed changes in the optic disc appearance over a time course, successfully measured the speed of those changes, and quantitatively evaluated the inner retinal thickness (Fig. 3). The inner retinal thickness increased from the first day to the seventh day and then gradually decreased. The success of the quantitative evaluation of these changes demonstrates that the effectiveness of various treatments can be evaluated using OCT.
Several reports have scanned rodents using OCT [5–8]. We used a compact spectral-domain OCT system, the iVue-100, which is a device that can perform 25,000 A-scans each second at a spatial resolution of 5 μm. Additionally, it is compatible with the ganglion cell complex scan program, which is designed to evaluate the combined thickness of the RNFL, retinal ganglion cells (RGCs), and IPL. The camera on the iVue-100 is small and easy to control, so the position of the camera can be easily adjusted, and it possesses high spatial resolution, which is useful for detecting each retinal layer under normal conditions. Unfortunately, the iVue-100 does not have sufficient spatial resolution to detect the RNFL when disc swelling is present. Therefore, as an alternative method of evaluating RNFL thickness using the ganglion cell complex scan program, we measured the thickness from the ILM to the IPL, which was detectable when disc swelling was present. In addition, reproducible values were obtained by increasing the scan times and measuring the 1 mm width band area around the optic disc 0.25 mm from the disc margin, in which it is easier to detect the layer difference when swelling is present.
Our data indicate that the mean inner retinal thickness of normal rats (73.50 ± 4.94 μm; mean ± SD; 50 eyes) was thinner than the previously reported mean thicknesses of the NFL and the IPL (36.65 ± 1.22 and 48.22 ± 2.51 μm, respectively) [5]. However, the previous measurement was obtained at the point next to the disc margin, whereas our data were obtained at a point 0.25 mm away from the optic disc margin. In addition, our OCT data were similar to those obtained using the histologic preparation. Therefore, we believe our data are accurate.
Wang et al. [9] previously observed the optic disc and retina of rNAION using OCT. However, they evaluated anatomic changes and did not include quantitative evaluations. They reported an elevation of the retina around the optic nerve during the acute stage and following the atrophic change in the chronic stage. Our observations included similar anatomic changes and the time course of the quantitative changes in the inner retinal thickness. To our knowledge, this is the first report with a detailed quantitative evaluation of rNAION. We believe that this is an important baseline for the evaluation of treatment effects in this model.
Contreras et al. [10] reported that in human NAION, the initial mean RNFL thickness in the affected eye was 200.9 μm, which represented a mean increase in RNFL thickness of 96.4 % compared with the RNFL thickness of the unaffected eye. The RNFL thickness decreased to values within normal limits at the 1.5 month visit. The percentages of RNFL loss 3, 6, and 12 months after onset were 38.9, 42.3, and 38.2 %, respectively. The maximum increase in rNAION in our study was 73.8 % at day 1, and decreased to values within normal limits by day 14; the percentages of RNFL loss two and three months after onset were 22.8 and 28.6 %, respectively. These results indicate that the speed of change in the RNFL thickness in rNAION is faster than that in human NAION.
We induced rNAION and found that nerve swelling could be attributed to the slowness of axial flow but not to increased permeability or inflammation. As such, each swollen nerve compresses the others and leads to dysfunction and obstruction of the main optic nerve vessels. Therefore, early improvement in swelling could result in a better visual prognosis. Additionally, it is important to assess the degree and speed of disc edema when evaluating new treatment options for rNAION. Our quantitative methods using OCT will be useful for the objective evaluation of NAION.
In this study, we determined that the decrease in the inner retinal layer thickness can be attributed to the decrease in the RNFL layer. In addition, this decrease can be attributed to the degree of RGC apoptosis [11]. As such, the evaluation of inner retinal thickness using OCT during the chronic stage of rNAION will be useful for evaluating the neuroprotective effects of various treatments.
In summary, scanning the optic disc and the area around the retina in rNAION using the iVue-100 OCT allows the quantitative and noninvasive evaluation of changes in thickness from early swelling to late atrophy. OCT is therefore a useful method for evaluating the effectiveness of treatment aimed at improving swelling and neuroprotection. We will use this method to evaluate potential rNAION treatments in future studies.
References
The Ischemic Optic Neuropathy Decompression Trial Research Trial Research Group. Optic nerve decompression surgery for nonarteritic anterior ischemic optic neuropathy (NAION) is not effective and may be harmful. JAMA. 1995;273:625–32.
Bernstein SL, Guo Y, Kelman SE, Flower RW, Johnson MA. Functional and cellular responses in a novel rodent model of anterior ischemic optic neuropathy. Invest Ophthalmol Vis Sci. 2003;44:4153–63.
Chuman H, Maekubo T, Oosako T, Kodama Y, Ishiai M, Nao-i N. Rodent model of nonarteritic ischemic optic neuropathy and its electrophysiological evaluation. Jpn J Ophthalmol. 2012;56:518–27. doi:10.1007/s10384-012-0167-y.
Campbell MC, Hughes A. An analytic, gradient index schematic lens and eye for the rat which predicts aberrations for finite pupils. Vision Res. 1981;21:1129–48.
Guo L, Normando M, Nizari S, Lara D, Cordeiro M. Tracking longitudinal retinal changes in experimental ocular hypertension using the cSLO and spectral domain-OCT. Invest Ophthalmol Vis Sci. 2010;51:6503–12.
Nagata A, Higashide T, Ohkubo S, Takeda H, Sugiyama K. In vivo quantitative evaluation of the rat retinal nerve fiber layer with optical coherence tomography. Invest Ophthalmol Vis Sci. 2009;50:2809–15.
Ruggeri M, Wehbe H, Jiao S, Gregori G, Jockovich ME, Hackam A, et al. In vivo three-dimensional high-resolution imaging of rodent retina with spectral-domain optical coherence tomography. Invest Ophthalmol Vis Sci. 2007;48:1808–14.
Xu J, Molday LL, Molday RS, Sarunic MV. In vivo imaging of the mouse model of X-linked juvenile retinoschisis with Fourier domain optical coherence tomography. Invest Ophthalmol Vis Sci. 2009;50:2989–93.
Wang RS, Lv PL, Wang WJ, Wang XD, Zhang XJ, Li SN, et al. Establishing an experimental model of photodynamically induced anterior ischemic optic neuropathy. Vis Neurosci. 2011;28:155–62.
Contreras I, Noval S, Rebolleda G, Murioz-Negrete F. Follow-up of nonarteritic anterior ischemic optic neuropathy with optical coherence tomography. Ophthalmology. 2007;114:2338–44.
Slater BJ, Mehrabian Z, Guo Y, Hunter A, Bernstein SL. Rodent anterior ischemic optic neuropathy (rAION) induces regional retinal ganglion cell apoptosis with a unique temporal pattern. Invest Ophthalmol Vis Sci. 2008;49:3671–6.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Maekubo, T., Chuman, H., Kodama, Y. et al. Evaluation of inner retinal thickness around the optic disc using optical coherence tomography of a rodent model of nonarteritic ischemic optic neuropathy. Jpn J Ophthalmol 57, 327–332 (2013). https://doi.org/10.1007/s10384-012-0195-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10384-012-0195-7