Abstract
Background
To evaluate longitudinal changes in retinal nerve fiber layer (RNFL) thickness in patients with nonarteritic anterior ischemic optic neuropathy (NAION) using optical coherence tomography (OCT).
Methods
Prospective, observational case series study. Sixteen eyes from 15 consecutive patients affected with NAION were analyzed. The fellow unaffected eyes served as controls.
Patients were divided into three different study groups: (1) patients with visual field (VF) defect confined to the inferior hemifield (five eyes), (2) patients with diffuse VF loss (seven eyes), and (3) patients with central or centrocecal scotoma (four eyes). The main outcome was peripapillary RNFL thickness measurement by Stratus-OCT.
Results
In group 1, OCT demonstrated RNFL involvement limited to the temporal , superior and nasal optic disc quadrants, both in acute and athophic stages. Diffuse RNFL damage involving all quadrants around the disc was observed in group 2 patients. Group 3, by contrast, revealed RNFL atrophy limited to the superior and temporal sectors of the disc.
Conclusions
OCT can identify different patterns of RNFL involvement specific to different classic VF defects in eyes with NAION. Our results corroborate previous histologic findings in optic nerves affected with NAION.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Nonarteritic anterior ischemic optic neuropathy (NAION) typically occurs in middle-aged and older patients with vasculopathic risk factors [9]. The affected optic nerve head (ONH) is noted to be swollen, and the fellow optic disc appears “crowded” (small and with a small optic cup). This configuration is thought to predispose to a spreading watershed infarction of the ONH in patients with NAION [2, 3, 7]. The presence of ONH edema and related visual field defects form the classic presentation of NAION. Moreover, symptomless ONH edema may precede visual loss in NAION, and could constitute the earliest sign of the disease [8].
Histologic studies on optic nerves affected with NAION have shown that ischemic optic neuropathy can have a devastating effect on the integrity of the optic nerve, with complete loss of fibers in the superior half of the nerve and a peripheral fiber loss in the other half [16]. The three-dimensional anatomic configuration of a NAION infarct was recently described in a patient who died 20 days after the initial diagnosis of NAION [21]. The fiber loss was in the superior part of the nerve, and the periphery of the uninvolved inferior portion of the nerve was normal in this case.
Given that histologic studies can rarely be performed in eyes with acute-onset NAION, recent ONH imaging technologies have gained the interest of many researchers, as these diagnostic modalities can easily provide us with information about the ONH anatomy at any stage of the disease. Data from scanning laser polarimetry (SLP) have revealed, for example, that RNFL thickness is reduced in cases of NAION-related ONH atrophy; unfortunately, the same technology does not seem able to detect and quantify RNFL edema in the acute stage of this optic neuropathy[1, 4, 5,18]. Optical coherence tomography (OCT), on the other hand, can measure both RNFL thickening in the acute stage and RNFL thinning after resolution of ONH edema caused by NAION [6, 20].
Quantification of RNFL changes is likely to enhance our knowledge of the pathophysiology and natural history of NAION. Since the RNFL changes occurring in NAION have never been prospectively evaluated, we aimed to longitudinally investigate them in the acute stage and during a 6-month follow-up. We also aimed to analyze whether, in the case of NAION, specific sectorial changes of the RNFL can be associated with different categories of visual field (VF) defects.
Methods
This was a prospective, observational case series study performed in a private eye clinic (Centro Salus, Bologna, Italy). All patients definitively diagnosed with NAION between November 2004 and March 2006 were enrolled. The criteria required for diagnosis of NAION included: (1) history of sudden onset of visual loss with absence of other ocular and neurologic diseases that might influence or explain the patient’s visual symptoms, (2) ONH edema at onset and spontaneous resolution of ONH edema within 2 to 3 months, and (3) absence of systemic symptoms of giant cell arteritis or a high erythrocyte sedimentation rate (>50 mm/hour). No patient presented evidence of inflammatory, demyelinating, or compressive causes of optic neuropathy. All participants gave their informed consent according to the Declaration of Helsinki. The internal review board of Centro Salus approved this investigation.
A detailed ophthalmic and medical history was obtained at the patient’s first visit to our clinic, which in all cases took place within 2 weeks of the onset of visual loss. Patients underwent a comprehensive ophthalmic evaluation, including the assessment of visual acuity and achromatic VF with Humphrey perimeter (Humphrey System, Dublin, CA, USA) utilizing a Swedish Interactive Testing Algorithm (SITA) strategy, program 30–2. For the purposes of the present study, abnormal visual fields were classified as diffuse or localized loss. For localized deficits, the principal location of VF loss was identified (i.e., inferior quadrant, inferior arcuate, inferior altitudinal, central or centrocecal scotoma) [11]. All VF test results were reliable, with less than 33% fixation losses, false-positive responses, and false-negative responses.
For the sake of simplicity, we assumed that arcuate, quadrantile, and altitudinal defects in the same hemifield represent different degrees of infarction within that hemifield [22]. Patients were then divided into three different study groups: (1) patients with VF defect confined to a single hemifield, (2) patients with diffuse VF loss, and (3) patients with central or centrocecal scotoma.
Optic nerve photography and fluorescein fundus angiography were performed in all patients at the first examination, during the acute stage of the disease.
The commercially available OCT 3 (StratusOCT, software version 4.0; Carl Zeiss Ophthalmic Systems Inc., Humphrey Division, Dublin, CA, USA) was used to measure peripapillary RNFL thickness (RNFL Thickness 3.4 acquisition protocol). In all NAION eyes, the mean value of 360° average RNFL thickness and mean temporal, superior, nasal and inferior optic disc quadrant RNFL thickness obtained from three good-quality images (signal strength ≥6) were considered for statistical purposes [20]. Patients underwent OCT measurements at the initial examination and serially after 30 days, 2, 3 and 6 months during the follow-up period. Each time, OCT was performed in parallel with an automated VF test. No patient failed any follow-up examination.
The absolute measurements of RNFL thickness in NAION eyes were compared to the corresponding values obtained from a control group. Since patients with NAION have been reported to have small optic discs [2, 3, 7, 14] and ONH size is known to influence RNFL analysis by StratusOCT [19], we used the fellow unaffected eyes of the same patients as controls.
Statistical analyses were performed using SPSS software, version 12.01 (SPSS Inc., 2004). A paired t-test was used to compare affected with unaffected eyes at each stage of follow-up. The mean RNFL values obtained from all 16 NAION eyes were compared with the control group (15 eyes). Moreover, the measurements found in the three different VF study groups were compared with the corresponding normal values. A P value less than .05 was accepted as statistically significant.
Results
Sixteen consecutive eyes of 15 patients (nine male and six female; mean age, 65.8 ± 10 years) affected with NAION were enrolled in this study. Five out of the 16 cases had already been included in a previous investigation during their acute phase of NAION [20]. In the present study, we completed the follow-up protocol and included 11 more cases. In our single case of bilateral NAION, the bilateral involvement was not simultaneous, and we included the RNFL measurements of the unaffected eye in the control group (15 eyes).
Retinal nerve fiber layer thickness at presentation (within 15 days of the onset of visual loss) and at the end of follow-up are summarized in Table 1. The initial mean RNFL thickness from 360° average in the affected eyes was 189 μm ± 56.6, statistically increased compared with the RNFL thickness of the normal fellow eyes (95.7 μm ± 9.7). The mean RNFL thickness from temporal, superior, nasal and inferior quadrants were significantly higher than the corresponding normal values. At the end of 6 months follow-up, the RNFL thickness from each optic disc quadrant was found to be significantly lower than in the control group.
At the first examination, five out of 16 NAION eyes showed a VF defect confined to the inferior hemifield (group 1), seven patients revealed a diffuse VF loss (group 2) and, finally, four eyes showed a central or centrocecal scotoma (group 3). In all patients, VF defect remained stable during the whole follow-up.
Table 2 shows the mean RNFL thickness in the three different VF study groups. At presentation, the OCT mean value from the 360° average was significantly greater in all NAION groups than in the control group. The temporal, superior, nasal and inferior quadrants were separately compared with those of the control group. Group 1 showed a significant increase in the mean RNFL thickness in the temporal (p < 0.001), superior (p < 0.001) and nasal (p = 0.005) optic disc sectors, with sparing of the inferior disc quadrant. Group 2 and group 3 revealed diffuse RNFL swelling, with involvement of all four optic disc quadrants (p < 0.001).
Figure 1 shows the OCT pattern of RNFL involvement with corresponding VF exams during the acute phase of NAION in the three study groups. In case 1 (group 1), localized RNFL swelling of the superior half of the ONH is evident. By contrast, in cases 8 and 13 (groups 2 and 3) the entire ONH is involved, with diffuse RNFL edema as detected by OCT. In all cases, a hyporeflective subretinal space, possibly representing subretinal fluid, was detected between the edematous RNFL and the retinal pigment epithelium in the peripapillary area.
Acute phases of nonarteritic anterior ischemic optic neuropathy (first examination). In case 1 (group 1) the superior half of the optic disc is involved, as shown by retinal angiography and peripapillary OCT. In cases 8 (group 2) and 13 (group 3) the entire optic disc is affected with diffuse retinal nerve fiber layer edema involving all four disc quadrants, as shown by OCT. (T: temporal, S: superior, N: nasal, I: inferior)
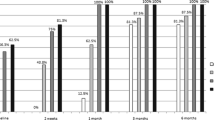
Figure 2 shows, for each study group, the mean RNFL thickness values in all four optic disc quadrants at different stages of follow-up. In group 1, the mean RNFL thickness in the temporal, superior and nasal quadrants was significantly greater at the first examination than the corresponding control group values. Gradually, ONH edema decreased, and OCT was capable of monitoring these changes. Optic nerve head atrophy started to be detected after 2/3 months, and at the end of 6 months’ follow-up (see also Table 2); optic atrophy was documented by a reduction in mean RNFL thickness in the temporal, superior and nasal quadrants, whereas the inferior disc sector did not show any significant difference when compared with control group. In group 2, after 2/3 months of follow-up, the mean RNFL thickness significantly reduced in all optic disc quadrants compared to controls (p < 0.001). In group 3, the mean RNFL thickness was significantly reduced after 2 months of follow-up, but only in the temporal quadrant (p = 0.03). After 6 months, RNFL atrophy was limited to the temporal and superior optic disc sectors (p < 0.001 and p = 0.01 respectively).
Discussion
The present study confirmed the ability of OCT to monitor RNFL changes following NAION. This imaging technology allowed us to identify different patterns of RNFL involvement specific to different classic VF defects, both in the acute and chronic stages of NAION, thus suggesting that NAION may manifest itself in a variety of ways, possibly correlated to different pathogenetic factors [12].
The eyes with NAION and VF loss confined to the inferior hemifield (group 1) revealed RNFL changes only in the superior half of the ONH, both in the acute and atrophic stages. Our findings are consistent with the previously reported three-dimensional reconstruction of an optic nerve infarct in a patient who died 20 days after the onset of NAION [21]. Tesser et al. reported that the infarct began at the most anterior extent of the ONH, extended approximately 1.5 mm posteriorly and was eccentrically located superiorly with normal uninvolved inferior portion of the nerve. It could be supposed that in patients from group 1 the acute ischemic damage of axons began and was limited to the superior part of the optic nerve, so that the resulting acute and chronic RNFL injury was confined to the superior half of the optic disc.
Patients with diffuse VF loss (group 2) showed diffuse RNFL involvement around the entire ONH, as demonstrated at each follow-up examination. It could be hypothesized that if the acute ischemic damage is large enough, it may lead to diffuse axon swelling that involves much of the optic nerve, thus resulting in larger VF abnormalities. In the chronic phases, most of the axons become atrophic, and the RNFL becomes extensively thinner in all four optic disc quadrants. These findings are consistent with a previous study that quantified postmortem changes in the remaining optic nerve fibers of eyes affected with NAION. Diffuse fiber loss around the entire ONH was found in two out of three reported cases [16].
Conventional knowledge states that the VF defect of NAION is classically altitudinal. However, a central scotoma was recently reported in approximately 30% of a large cohort of 256 patients affected with NAION (using manual kinetic perimetry) [10]. In the present study we found four out of 16 NAION eyes to have a single central VF defect (group 3) associated in the chronic phases with RNFL atrophy limited to the temporal and superior optic disc quadrants, despite the entire ONH appearing edematous at presentation. We can speculate that in these patients the ischemic infarction may be limited to the inner portion of the ONH, where the axons arising from the papillomacular bundle run [14, 15]. Acutely, such an event would lead to diffuse edema of the RNFL involving all four quadrants of the optic disc; in the atrophic stages of the disease, only the fibers of the papillomacular bundle would be affected, thus producing the observed thickness reduction in the temporal quadrant (and to a lesser extent in the superior one).
Other imaging technologies have been used to study the RNFL thickness in patients with NAION. Unfortunately, SLP does not seem able to detect RNFL thickening, so that our results can hardly be compared to those obtained with this technology in the acute stage. As observed by Menke [13] and Savini [20], the RNFL thickness measurements obtained using OCT differ considerably from those obtained using SLP. This discrepancy is probably related to the properties of SLP, which measures birefringence of the structural elements (neurofilaments and microtubules) within the RNFL and generates the RNFL thickness from such a measurement. Indeed, ONH edema is likely caused not by an increase in the number of neurofilaments and microtubules, but rather by intracytoplasmic swelling of ganglion cell axons [17].
However, if we look at the atrophic phase of NAION, our data and those obtained by SLP are quite similar. Using GDx with variable corneal compensation, in fact, Saito et al. found the average RNFL thickness in the superior quadrant to be significantly lower than the average in the inferior quadrant [18]. Moreover, changes in RNFL thickness as measured by SLP in NAION correlate with changes in VF sensitivity as demonstrated by Danesh-Meyer [5].
Our study is in moderate agreement with another investigation carried out using OCT. Evaluating 15 eyes affected with atrophic NAION and an altitudinal inferior VF defect, Deleón-Ortega et al. recently found a similar reduction in RNFL thickness in the superior sector of the ONH. In contrast with our results, however, they also detected a RNFL thickness reduction in the inferior sector of the ONH (corresponding to the relatively unaffected superior hemifield) [6]. Conversely, in our study patients with a VF defect confined to the inferior hemifield (group 1) did not show statistically significant differences in the inferior optic disc quadrant, in either the acute or the atrophic phase (this can be easily observed in Figs. 1 and 2). However, the difference between our findings and those of Deleón-Ortega et al. should be considered with caution, as the mean RNFL values of the inferior quadrant in our sample and in their own were very close to each other (111 μ vs 109 μ respectively). A thorough analysis of the data from both studies actually reveals that the difference is related to the control groups. Since patients with NAION have been reported to have small ONH, we used the fellow unaffected eyes of the same patients as control group, instead of healthy participants as used in the Deleón-Ortega study.
The main limitation of the present investigation is the relatively small sample size, but sixteen NAION eyes are sufficient to show that OCT can detect different patterns of RNFL damage in different groups of eyes affected with NAION, classified on the basis of VF defect. Our objective measurements corroborate previous histologic findings in optic nerves affected with NAION, and may provide useful information for diagnosing and monitoring patients affected with this disease.
References
Banks MC, Robe-Collignon NJ, Rizzo FJ III et al (2003) Scanning laser polarimetry of edematous and atrophic optic nerve heads. Arch Ophthalmol 121:484–490
Beck RW, Savino PJ, Repka MX et al (1984) Optic disc structure in anterior ischemic optic neuropathy. Ophthalmology 91:1334–1337
Burde RM (1993) Optic disc risk factors for non arteritic ischemic optic neuropathy. Am J Ophthalmol 116:759–764
Colen TP, Van Everdingen JAM, Lemij HG (2000) Axonal loss in a patient with anterior ischaemic optic neuropathy as measured with scanning laser polarimetry. Am J Ophthalmol 130:847–849
Danesh-Meyer HV, Carroll SC, Ku JYF et al (2006) Correlation of retinal nerve fiber layer measured by scanning laser polarimeter to visual field in ischemic optic neuropathy. Arch Ophthalmol 124:1720–1726
DeLeón-Ortega J, Carroll KE, Arthur SN et al (2007) Correlations between retinal nerve fiber layer and visual field in eyes with nonarteritic ischemic optic neuropathy. Am J Ophthalmol 143:288–294
Doro S, Lessell S (1985) Cup-disc ratio and ischemic optic neuropathy. Arch Ophthalmol 103:1143–1144
Hayreh SS (1981) Anterior ischemic optic neuropathy. V. Optic disc edema an early sign. Arch Ophthalmol 99:1030–1040
Hayreh SS, Joos KM, Podhajsky PA et al (1994) Systemic diseases associated with nonarteritic anterior ischemic optic neuropathy. Am J Ophthalmol 118:766–780
Hayreh SS (2005) Visual field abnormalities in nonarteritic anterior ischemic optic neuropathy. Their pattern and prevalence at initial examination. Arch Ophthalmol 123:1554–1562
Keltner JL, Jonson CA, Spurr JO, Beck RW (1993) Baseline visual field profile of optic neuritis. The experience of the optic neuritis treatment trial. Optic Neuritis Study Group. Arch Ophthalmol 111:231–234
Lessell S (1999) Nonarteritic anterior ischemic optic neuropathy. Enigma variation. Arch Ophthalmol 117:386–388
Menke MN, Feke GT, Trempe CL (2005) OCT measurements in patients with optic disc edema. Invest Ophthalmol Vis Sci 46:3807–3811
Minckler DS (1989) Histology of optic nerve damage in ocular hypertension and early glaucoma. Surv Ophthalmol 33(suppl):401–402
Ogden TE (1983) Nerve fiber layer of the macaque retina: retinotopic organization. Invest Ophtalmol Vis Sci 24:85–98
Quigley HA, Miller NR, Green WR (1985) The pattern of optic nerve fiber loss in anterior ischemic optic neuropathy. Ophthalmology 100:769–776
Sadun AA (1993) Optic atrophy and papilledema. In: Jakobiec F, Albert D (eds) Principles of ophthalmology. WB Saunders Co, Philadelphia, pp 2529–2538
Saito H, Tomidokoro A, Sugimoto E et al (2006) Optic disc topography and peripapillary retinal nerve fiber layer thickness in nonarteritic ischemic optic neuropathy and open-angle glaucoma. Ophthalmology 113:1340–1344
Savini G, Zanini M, Carelli V et al (2005) Correlation between retinal nerve fiber layer thickness and optic nerve head size: an optical coherence tomography study. Br J Ophthalmol 89:489–492
Savini G, Bellusci C, Carbonelli M et al (2006) Detection and quantification of retinal nerve fiber layer thickness in optic disc edema by StratusOCT. Arch Ophthalmol 124:1111–1117
Tesser RA, Niendorf ER, Levin LA (2003) The morphology of an infarct in nonarteritic anterior ischemic optic neuropathy. Ophthalmology 110:2031–2035
WuDunn D, Zimmerman K, Sadun AA et al (1997) Comparison of visual function in fellow eyes after bilateral nonarteritic anterior ischemic optic neuropathy. Ophthalmology 104:104–111
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bellusci, C., Savini, G., Carbonelli, M. et al. Retinal nerve fiber layer thickness in nonarteritic anterior ischemic optic neuropathy: OCT characterization of the acute and resolving phases. Graefes Arch Clin Exp Ophthalmol 246, 641–647 (2008). https://doi.org/10.1007/s00417-008-0767-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00417-008-0767-x