Abstract
Drosophila cell lines are used extensively to study replication timing, yet data about DNA replication in larval and adult tissues are extremely limited. To address this gap, we traced DNA replication in polytene chromosomes from nurse cells of Drosophila melanogaster otu mutants using bromodeoxyuridine incorporation. Importantly, nurse cells are of female germline origin, unlike the classical larval salivary glands, that are somatic. In contrast to salivary gland polytene chromosomes, where replication begins simultaneously across all puffs and interbands, replication in nurse cells is first observed at several specific chromosomal regions. For instance, in the chromosome 2L, these include the regions 31B-E and 37E and proximal parts of 34B and 35B, with the rest of the decondensed chromosomal regions joining replication process a little later. We observed that replication timing of pericentric heterochromatin in nurse cells was shifted from late S phase to early and mid stages. Curiously, chromosome 4 may represent a special domain of the genome, as it replicates on its own schedule which is uncoupled from the rest of the chromosomes. Finally, we report that SUUR protein, an established marker of late replication in salivary gland polytene chromosomes, does not always colocalize with late-replicating regions in nurse cells.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
With S phase of every cell cycle genome must be replicated completely and it must do so once and only once (Sclafani and Holzen 2007). DNA replication begins at numerous genomic sites known as replication origins. Timing of replication is tightly scheduled, and some origins become activated early in the S phase, whereas others fire in the late S phase. One of the key factors setting replication timing of a region is transcription, with active regions typically replicating early in the S phase, and silent regions replicating late. Notably, this correlation is not absolute. About 30 % of active Drosophila genes replicate late, and about 30 % of inactive genes replicate early (Schübeler et al. 2002; Gilbert 2002; MacAlpine and Bell 2005; Schwaiger and Schübeler 2006; Hiratani et al. 2009). Transcriptional activity and replication timing are intimately coupled with differentiation of the cell during development. For instance, the profiles of early replication origins studied in different Drosophila cell types (embryos vs brain tissue) showed only partial overlap (Eaton et al. 2011). When embryonic and wing imaginal disc cells were compared, distinct replication timing was observed for 20 % of Drosophila genomic sequences (Schwaiger et al. 2009). Thus, each cell type has its own schedule of origin activation.
In dipterans, most of the larval tissues (and imaginal tissues to a lesser extent) use a modified version of cell cycle called endocycle. In this case, no cell division occurs, S and G phases alternate which results in formation of polyploid nuclei. Should sister chromatids remain laterally synapsed at this time, giant polytene chromosomes form (Edgar and Orr-Weaver 2001). Polytene chromosomes provide a unique opportunity to directly observe the consequences of late origin firing. Whereas normally the S phase continues until all genomic regions are replicated (including the ones replicating the latest), this is not the case in endocycling cells, where S phase may end before replication of some regions has ended. As a result, genome duplication is incomplete, and some regions of polytene chromosomes may in fact have fewer DNA strands than do neighboring regions. This gives rise to the phenomenon of DNA underreplication. Morphologically, this looks like a constriction or a break of a chromosome, and such regions are referred to as intercalary heterochromatin (Zhimulev et al. 1982).
The protein known to be associated with late-replicating and underreplicated regions in polytene chromosomes is the Suppressor of Underreplication (SUUR) (Makunin et al. 2002). The exact function of this protein remains unclear, but it is able to interfere with replication fork progression (Sher et al. 2012), probably by interacting with proliferating cell nuclear antigen (PCNA) that is a component of replication complex (Kolesnikova et al. 2013). Mapping of SUUR binding sites in Kc cell culture confirmed that its target genes tend to be late-replicating and silent in these cells (Pindyurin et al. 2007). A physical interaction between SUUR and HP1 proteins was reported (Pindyurin et al. 2008).
Genome-wide analyses of DNA replication are typically performed using cell lines, and less frequently using specific tissues as a source of material. Drosophila larval salivary gland (SG) polytene chromosomes are one such example. Data on how replication is coordinated and organized in other tissues are very scarce, and to fill in this gap, we analyzed patterns of DNA replication in polytene chromosomes from oocyte nurse cells (NCs). This cell type is of particular interest since NCs are not merely distinct from SG cells, but rather represent an entirely different tissue type; whereas SGs are composed of larval somatic cells, NCs are of adult female germline origin.
Drosophila ovaries are made of individual ovarioles, each of which consists of a chain of maturing egg chambers. Each chamber is a syncytium of 16 cells, one of them will give rise to an oocyte, with 15 remaining cells forming NCs. The entire process of egg chamber development from the first division of a progenitor cell until egg formation is subdivided into 14 stages. Primary polytene chromosomes are first detectable at stage 2; however, polyteny is lost by stage 5 and cell nuclei become polyploid (Mahowald and Kambysellis 1980). Several D. melanogaster mutations are known that result in the maintenance of or even increase in lateral conjugation between chromatids in NCs throughout their growth. This may lead to the formation of NC polytene chromosomes comparable ‘quality-wise’ to those of SGs. One of such gene mutations, ovarian tumor (otu) (King et al. 1981), is used in the present work. Phenotypically, it results in female sterility, although several allele combinations, such as otu 7/otu 11, were reported to be fertile (Mal’ceva et al. 1997). Detailed morphology analysis of NC chromosomes from otu mutants indicated that their banding pattern was very similar, yet not entirely identical to that of SG polytene chromosomes (Heino 1989; Mal’ceva et al. 1995; 1997). Notably, pericentric heterochromatin is polytenized in otu NCs significantly more than in SG polytene chromosomes and may form structures with reproducible banding pattern (Mal’ceva and Zhimulev 1993; Koryakov et al. 1996; 1999; 2003). Moreover, the degree of DNA underreplication and hence the frequency of chromosome breaks are much lower in intercalary heterochromatin regions of NC polytene chromosomes vs those of SGs (Mal’ceva et al. 1995; Koryakov et al. 2006).
Even though DNA replication has been previously studied in the context of NCs, most of these reports used wild-type background lacking polytene chromosomes (Hammond and Laird 1985; Lilly and Spradling 1996; Dej and Spradling 1999), so replication process in NC polytene chromosomes is characterized only partially. NC polytenes undergo a maximum of 12 endocycles and show the expected levels of DNA for the first six cycles. The DNA fraction failing to replicate during subsequent cycles may be as small as 10 % (Rasch et al. 1984). DNA synthesis patterns in NC polytene chromosomes are overall similar to the pattern seen in polytene nuclei of larval SGs. Moreover, comparison of late-replicating sites in NC chromosomes with those of SGs showed a remarkable similarity in the two cell types (Sinha et al. 1987). In the present work, we analyzed patterns of DNA replication in NC polytene chromosomes and compared them to the typical replication patterns known from SG polytenes.
Materials and methods
Fly stocks
Fly stock y w sn 3 otu 11/ FM3 was used in this work. Homozygous otu 11 females are sterile and have polytene chromosomes in their NCs. The flies were kept on standard fly medium at +16–18 °C. Freshly enclosed y w sn 3 otu 11/ y w sn 3 otu 11 females were collected and kept at the same temperature and fresh food for 5–7 days prior to ovary dissection. This was followed by incorporation of a synthetic thymidine analogue 5-bromo-2′-deoxyuridine (BrdU). Flies were aged because egg chambers had to develop until stages 9–10 (see below), when NCs occupy nearly half of the egg chamber.
BrdU incorporation
Females were dissected and ovaries were isolated in Ephrussi-Beadle solution (129 mM NaCl, 4.7 mM KCl, 1.9 mM CaCl2). Next, ovaries were transferred into a new vial with fresh Ephrussi-Beadle solution supplemented with 0.2 mM BrdU. Incubation lasted for 30–40 min at room temperature, and ovaries were fixed in a 3:1 mixture of ethanol and glacial acetic acid at −20 °C for at least 1 h. Fixed ovaries were transferred in a droplet of 45 % acetic acid, where they were cut into individual egg chambers. Only stage 9–10 egg chambers were used for further analysis. Egg chambers were squashed under a coverslip and snap-frozen in liquid nitrogen; coverslips were removed and the slides were stored at −20 °C in 70 % ethanol.
For immunodetection, slides were washed twice in phosphate-buffered saline (PBS, 137 mM NaCl, 2.7 mM KCl, 10 mM Na2HPO4, 1.76 mM KH2PO4, pH 7.4) for 5 min at room temperature and incubated for 10 min in 2 N HCl. Next, the slides were rinsed with 100 mM Na2B4O7 and washed thrice in PBST (PBS + 0.2 % Tween-20) for 5 min. Immunostaining with BrdU-specific antibodies (mouse anti-BrdU in 3 % BSA, Beckton Dickinson) was done overnight at +4 °C, which was followed by three PBST washes, 5 min each. Staining with secondary antibody conjugates (goat anti-mouse-FITC in 3 % BSA, Sigma-Aldrich) was performed for 2 h at room temperature, followed by PBST washes as above. To visualize DNA, the slides were briefly incubated with PBS+DAPI (1 μg/ml) solution, air-dried, and covered with Vectashield medium. Chromosomes were examined using epifluorescence optics (Olympus BX50 microscope) and photographed with CCD Olympus DP50. A total of 90 nuclei showing immunostaining signals were analyzed.
Results
General features of replication
We investigated DNA replication in D. melanogaster NC polytene chromosomes using bromodeoxyuridine (BrdU) incorporation. Most of the nuclei displayed no evidence of ongoing replication, and only a handful of nuclei could be used to analyze different stages of replication. This was expected and is in line with the earlier study by Sinha et al. (1987), where replication was reported only in 35 % of NC nuclei. Notably, we used stage 9–10 egg chambers, where polytenization and “cytological quality” of NC polytene chromosomes are the highest. At subsequent stages, NCs progressively degrade and we attribute the observed low frequency of replicating nuclei at stages 9–10 to the fact that most of the chromosomes have already completed replication by this time, so we could “catch” only the very last round of endocycle in some nuclei.
Previous studies extensively used incorporation of a radioactive DNA precursor, 3H-T, into SG polytene chromosomes of D. melanogaster. It was demonstrated that different regions of chromosomes begin and complete replication at distinct stages of the S phase. Appropriately, distinct patterns of 3H-T labeling were observed which was reflected in the names of S phase stages. Since there are no independent cytogenetic hallmarks of S phase stages, only relative terms like “early” or “late” replication are used. Regions that are the first to enter replication in SG polytene chromosomes are transcriptionally active sites of the genome, known as puffs and interbands, as well as the thinnest bands. This stage of the S phase was coined “early discontinuous labeling” stage. This is followed by the “continuous labeling” stage, when large dense bands, intercalary heterochromatin, and pericentric heterochromatin regions join replication and so the entire chromosome is covered by the label. Next, puffs, interbands, and bands complete replication, which appears as a “late discontinuous labeling” stage. Replication of intercalary and pericentric heterochromatins continues until the very end of the S phase (reviewed in Zhimulev 1999).
Previous studies of DNA replication in NCs uncovered several striking features of this process. Specifically, endocycles 1 through 5 displayed evidence of both early and late replication going on, whereas endocycles 6 to 11 completely lacked detectable late replication (Lilly and Spradling 1996; Dej and Spradling 1999). This was interpreted as reorganization of the S phase mechanism which precluded late replication. Notably, when NC polytene chromosomes from otu mutants were studied, all replication stages of the S phase, including late replication, were found (Sinha et al. 1987). Similarly, our analysis also showed replication patterns characteristic of all S phase stages; however, we found a number of features distinct from what is typically observed in SG polytene chromosomes.
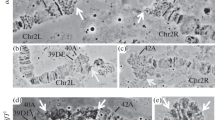
By comparing various BrdU incorporation patterns, we could establish how replication progresses through the chromosomes. We observed that only a handful of regions, rather than all interbands and puffs, would simultaneously begin replication. For instance, in the chromosome 2 L, the regions 31B-E, proximal parts of 34B and 35B, as well as 37E are the first to begin replication (Fig. 1a). Next, BrdU incorporation becomes detectable in the rest of the decondensed regions (Fig. 1b); however, by this time, replication at the region 31B-E fades away (Fig. 1c). At “continuous labeling” stage, when pronounced BrdU incorporation occurs along the entire chromosome 2L, the region 31B-E has already completed replication (Fig. 1d). We note that this is consistent with an earlier study as this pattern has been previously reported (Fig. 4e from Sinha et al. (1987)). The late stage of “discontinuous labeling” follows next (Fig. 1e, f), and the last regions to complete replication in 2L are intercalary heterochromatin regions such as 22A, 24EF, 25A, 25F, 26A, 29F, 30A, 33A, etc. (Fig. 1g).
Detailed analysis of different chromosomes at “continuous labeling” stage confirmed that this stage does not truly show “continuous” labeling, as many BrdU-negative chromosomal regions are present (Fig. 2). Importantly, whereas some regions (such as the region 31) have already replicated, intercalary heterochromatin regions have not yet started replication. Replication of these regions begins later and finishes at the very end of the S phase. One example is the region 3C of chromosome X: at the “continuous labeling” stage it is not yet replicating (Fig. 3а), and while the neighboring regions are BrdU-positive, 3C shows no signal (arrowhead on the insert in Fig. 3а). When many chromosomal regions have already completed replication, 3C is only about to begin replication, which appears as a very faint BrdU signal (Fig. 3b, c). Active replication of 3C occurs when only a few discrete sites of late replication are present on the chromosomes (Fig. 3d, e); appropriately, this region is one of the last regions to finish replication (Fig. 3f).
Distal part of the X chromosome at different replication stages, from the “continuous” stage (a) till the latest stage of the S phase (f). Left column shows chromosomal distribution of BrdU-signal, whereas the right column shows DAPI staining. Homologous regions are connected with lines. The rest of the explanations are provided in the main text. Bar - 5 μm
Regions of late replication
Next, we proceeded to analyze in detail the late replication stage. This stage was defined by the pattern when about 35 or fewer regions were found replicating in each chromosome arm. Altogether, 157 late-replicating regions were mapped, and their positions are shown in Fig. 4a–e. The key protein which is known to specifically decorate late-replicating regions in SG chromosomes and to slow down replication fork progression is SUUR (Makunin et al. 2002; Sher et al. 2012). To compare positions of late-replicating regions and regions associated with SUUR, the data published previously were used (Koryakov et al. 2006). We revised the list of SUUR-positive chromosomal sites, and added several regions showing weak yet reproducible SUUR binding, not referenced in the aforementioned work. Surprisingly, we find that whereas in SG polytene chromosomes, the overlap between SUUR binding and late replication is nearly perfect (Makunin et al. 2002), this is not entirely the case in NCs. Vast majority of late-replicating regions (150 out of 157) indeed display SUUR binding; however, seven regions (4E, 22F, 50D, 62E, 92A, 94A, and 100A) stand out as they replicate late despite the absence of detectable SUUR signal. Furthermore, there are many more SUUR-positive sites (265) than late-replicating regions (157) (Fig. 4f).
Late replication in the chromosomes X (a), 2L (b), 2R (c), 3L (d), and 3R (e). X axis shows Bridges’ map cytology coordinates, Y axis shows the number of late S phase chromosomes analyzed. The higher the bar, the later this region ceases replication. Regions where chromosome breaks have been reported in NC are colored red (Koryakov et al. 2006). Venn diagrams (f) showing overlap between late-replicating regions (LR) and SUUR-binding sites in individual chromosomes and across all chromosomes combined
Late replication in polytene chromosomes results in underreplication, which manifests as chromosome breaks. Overall, in contrast to SGs (Zhimulev et al. 1982), the frequency of chromosome breaks in NCs is remarkably low (Koryakov et al. 2006). Of 42 such regions, only 24 correspond to late replication sites (Fig. 4a–e). Intriguingly, late replication was not perfectly correlated with chromosome breaks. Many late-replicating regions, even those replicating the latest (for instance, 3C, 19A, 25E-F, 29E-30A, 33A, 67A, 74A, 95A, 97C, etc.), demonstrated no evidence of chromosome breaks whatsoever. Similar situation was previously observed for the X chromosome in SG cells. Whereas late-replicating regions show chromosome breaks in the context of female X chromosomes (i.e., they are underreplicated), this is not the case in the male X, where late replication exists in the absence of DNA underreplication (Zhimulev et al. 2003).
Comparison of late replication patterns in SGs and NCs indicated that most of the regions (137) are replicating late in both tissues; however, we consistently observed tissue-specific differences, which were not unexpected. Many chromosomal regions (among them 10A, 10B, 14B, 33D, 35C, 48A, 50A, 70D) displayed a shift in replication timing from late S phase in SGs into early or mid S phase in NCs. Conversely, 20 regions replicating late in the context of NCs, were found to start replication sooner in SGs. Of these, three regions (2B, 68C, and 85F) appeared as large puffs in SG polytene chromosomes, indicative of active transcription, whereas in NCs, no puffing was observed at these regions, and they replicated late in the S phase. In the case of the region 33D, the opposite situation is observed, i.e. this region has a puff morphology and replicates early in NCs, whereas in SGs polytene chromosomes 33D region shows no puffing and replicates late. Altered replication timing is accompanied in most cases by changes in SUUR binding. As a rule, the regions that replicate late in one cell type but early in another, tend to be SUUR-positive in the former case, and SUUR-negative in the latter, which agrees very well with developmental dynamics of SUUR (Maksimov et al. 2014). Additionally, whereas in SGs late-replicating regions are invariably formed by dense bands, we observed that several late-replicating regions in NC polytene chromosomes (such as the distal part of 2B and 62E) appear loosely compacted and lack prominent large bands.
Comparison of BrdU incorporation patterns in the region 2B shows that it encompasses both early- and late-replicating DNA sequences. Figure 3a illustrates that BrdU incorporation occurs in this region at the “continuous labeling” stage, although the signal strength appears weaker than in the neighboring sites. Next, replication becomes undetectable in this region (Fig. 3b, с) and resumes at a later stage, when most of the chromosome has already completed replication (Fig. 3d). Region 2B finishes replication at the time when replication peaks at 3C (Fig. 3e).
Of special interest is the replication timing of pericentric heterochromatin, which is the most late-replicating domain in SG polytene chromosomes (Zhimulev et al. 2003). As it turned out, pericentric heterochromatin in NCs largely replicates in early and mid S phases, and much less so in the late S phase. We observed a clear BrdU signal in the pericentric region of the chromosome 2L, 40B-F (denoted as PH in Fig. 1a), concomitantly with the signal in the region 31B-E, i.e. very early in the S phase, long before interbands and puffs would start replication. Similarly, replication of the region 41 in chromosome 2R occurs in the early S phase and coincides with replication of puffs and other decondensed chromosomal regions. Figure 5 demonstrates an image of incomplete nucleus with all the autosomes present. Pericentric heterochromatin of chromosome 2 and less so heterochromatin of chromosome 3 appear actively replicating. Additionally, BrdU incorporation is prominent in many other regions, including 31B-E, 34B, and 37E, indicating that this nucleus was imaged at an early S phase stage. In chromosomes X, 2L, and 3R, intercalary heterochromatin regions are not yet replicating at the “continuous labeling” stage, whereas pericentric heterochromatin (shown as PH in Figs. 1d and 2) is already actively incorporating the label, similarly to euchromatin. By the end of the S phase, when replication of intercalary heterochromatin occurs, pericentric heterochromatin has either completed replication or is weakly BrdU-positive. Figure 1f shows that at this stage, BrdU incorporation is very diffuse and much weaker than in the rest of the chromosomal regions. Our observations are in good agreement with the data reported by Sinha et al. (1987): Fig. 4e–g in this paper shows that pericentric heterochromatin replicates at the “continuous labeling” stage, whereas 3H-T incorporation is very weak during the late S phase.
Asynchronous replication of the chromosome 4
Our analysis demonstrates that replication of chromosome 4 appears to be uncoupled from the rest of the genome. Figure 6a illustrates the situation, when BrdU incorporation occurs in decondensed regions of large arms (early S phase), and both eu- and heterochromatic regions of chromosome 4 are BrdU-positive. As the cell transitions into the “continuous labeling” stage, we observe nuclei showing no evidence of chromosome 4 replication (Fig. 6b), as well as nuclei where chromosome 4 replicates along with the rest of the chromosomes (Fig. 6c). No replication of chromosome 4 is observed at the late stage of “discontinuous labeling” (Fig. 6d), or during the late S phase (Fig. 6e). However, another nucleus clearly demonstrates that pericentric heterochromatin of chromosome 4 replicates during late S phase (Fig. 6f). Two nuclei shown in Fig. 6g, h display no traces of replication of large chromosome arms, whereas the entire (Fig. 6g) or just a proximal part (Fig. 6h) of chromosome 4 appears BrdU-positive. Thus, we could not detect any correlation between replication timing of chromosome 4 and the rest of the genome. Replication of chromosome 4 does not appear to be generally delayed or advanced as compared to other chromosomes: it forms a stand-alone domain that seems to replicate according to its own schedule, which is not linked to the replication stages of the rest of the genomic regions.
Discussion
We explored replication patterns in NC polytene chromosomes from stage 9–10 ovaries, i.e. at the very last endocycle, shortly before NCs degenerate. We found patterns characteristic of all S phase stages, early, mid, and late. At the same time, previous analyses of NC chromosome structure, total genome DNA content and heterochromatic DNA copy number concluded that the sixth and all the subsequent endocycles lacked any late S phase. Moreover, only early BrdU incorporation patterns were observed at stage 6 (Lilly and Spradling 1996; Dej and Spradling 1999). Our analysis uses polytene, rather than polyploid cells, which affords better resolution, and may so provide an explanation why the pattern typical for late replication was not observed. We show that at least during the last endocycle, replication timing of pericentric heterochromatin is shifted toward the early S phase, and that intercalary heterochromatin regions predominantly replicate during the late S phase. Given that pericentric heterochromatin constitutes a significant portion of the genome, its late replication was likely observed in the end of S phase of the first endocycles in the above-mentioned studies. As we show here, final endocycles are characterized by late replication of mostly intercalary heterochromatin, which is too small to be detected in polyploid nuclei, unlike in polytene chromosomes. Our observations are also in line with the results of Hammond and Laird (1985), who demonstrated that during the last endocycle, the regions underreplicated at earlier stages undergo complete replication. This, as well as partial polytenization of pericentric heterochromatin (Mal’ceva and Zhimulev 1993) can be attributed to its earlier replication in NCs as compared to SG polytene chromosomes. However, we must underline that early replication results in only partial polytenization of heterochomatin, with its considerable fraction remaining underreplicated.
Previous studies have described several incorporation patterns of isotope-labeled nucleotides into replicating chromosomes, and thereby provided naming to appropriate S phase stages (reviewed in Zhimulev 1999). One of them was termed “continuous labeling” stage, for the entire bodies of chromosomes were covered with photoemulsion silver grains. In the present paper, we show that at this stage, the BrdU incorporation pattern is in fact discontinuous, and intercalary heterochromatin appears BrdU-negative. However, we believe that this is not a particular feature of NC polytene chromosomes, but is rather a consequence of distinct detection methodologies used. In the case of BrdU, we detect anti-BrdU labeling directly at the sites where BrdU has been incorporated into DNA. In contrast, detection of 3H-T incorporation in autoradiography protocols has lower resolution and relies on the formation of silver grains upon reduction with electrons emitted by the radiolabeled DNA precursor. Electrons are known to travel some distance away from 3H-T-incorporated region, which leads to nucleation of silver grains both at the replication site and around it. Thus, continuous labeling pattern observed in 3H-T incorporation experiments is partially based on the overlay between clouds of silver grains from adjacent replication sites.
Different studies showed that replication schedule of a specific genomic region may change throughout development and differentiation (Schübeler et al. 2002; Gilbert 2002; MacAlpine and Bell 2005; Schwaiger and Schübeler 2006; Hiratani et al. 2009). Our data indicate that most of the regions replicating late in NCs are also late-replicating in SGs (137 out of 157). This extensive overlap is attributable to the genetic make-up of these regions. It has been previously demonstrated that testis-specific genes tend to be clustered and form large transcriptional territories that are concomitantly depleted of ovary-specific genes (Belyakin et al. 2005; 2010). Such regions enriched in testis-specific genes constitute most of the transcriptionally silent late-replicating regions both in SGs and NCs. Despite overall similarity, we also noted tissue-specific differences that may also be explained by distinct expression profiles. Four regions that were clearly distinct between SGs and NCs encompass morphological structures characteristic of active transcription known as puffs. The regions 2B, 68C, and 85F are decorated with BrdU label in the late S phase in NCs, but form puffs and replicate early in SGs. In contrast, region 33D appears as a puff and replicates early in NCs but forms a band and replicates late in SGs. According to the modENCODE RNA-seq data (Graveley et al. 2011), the above-mentioned regions contain a number of tissue- and stage-specific genes. For instance, 2B region harbors a prominent gene BR-C, which is active in SGs (30 RPKM (reads per kilobase transcript per million) and encodes one of the key transcription factors required for ecdysone-dependent metamorphosis. In NCs, this gene is silent (2 RPKM in ovaries). Region 68C also hosts genes, whose expression is restricted to SGs (Sgs-3, Sgs-7, Sgs-8, with 60,686, 130,637, and 18,730 RPKM in SGs vs 1, 3, and 1 RPKM in ovaries, respectively). Region 33D replicates early in NCs likely due to expression of a gene aret, which is silent in SGs (0 in SG vs 114 RPKM in ovaries). Six more regions showing no evidence of puffing in NCs, but replicating early in NCs and late in SGs (10A, 10B, 14B, 35C, 50A, and 70D) harbor one to four ovary-specific genes.
We found many more SUUR-positive sites than late-replicating regions. This inconsistency may be explained by several factors. SUUR binding sites display a wide range of signal intensities corresponding to different amount of SUUR associated with this region. It is possible that seven “exceptional” regions target enough SUUR for it to cause slower and thereby later, replication, yet insufficient for immunodetection. Another plausible explanation is that the distance between replication origins may be a contributing factor. When origins happen to be positioned close to each other, replication may finish before late S phase stage even if the region is bound by SUUR. This situation is also formally compatible with the scenario described previously for SG polytene chromosomes. Namely, SUUR binding pattern was shown to be dynamic and to differ between S phase stages, with more SUUR-positive sites found in chromosomes at early rather than late stages (Kolesnikova et al. 2013). It is possible that our earlier analysis of SUUR localization in NC polytene chromosomes was somewhat biased and included predominantly early and mid S phase stages having more SUUR-positive regions (Koryakov et al. 2006). Finally, we speculate that even though SUUR is known to be intimately connected to late replication, it may nevertheless not be obligatory for late replication to occur.
Region 2B and pericentric heterochromatin were found to incorporate BrdU both in early and late S phases. Thus, these regions should not be regarded as uniformly composed structures, but rather represent a mosaic of sequences replicating at different stages of the S phase. Several lines of evidence exist which indicate that regulation of replication timing occurs on a domain-wide, rather than on a gene-by-gene basis (MacAlpine and Bell 2005). Our data suggest that control of replication timing may also occur on a much smaller, i.e., gene-level, scale. One of the facts arguing in favor of such idea is that upon integration of early replication origin into the late-replicating region, the effect is very local as the entire region does not become early-replicating (Koryakov et al. 2012). Yet another fact supporting replication independence of individual genes within one region is illustrated by the presence of late replication in the regions lacking large bands or even in the puffing regions. We suggest that in these cases, late replication is restricted to individual genes. For instance, the puffing region is present proximal to the band 3C1-2, and it could be expected to replicate early in the S phase. However, fine yet reproducible site of replication is found here at the beginning of the discontinuous labeling stage. This is likely due to the relatively late replication of a testis-specific gene CG10793. Region 62E encompasses a series of very faint bands and has complex genetic composition: it harbors dos gene active in the ovary, as well as four testis-specific genes, whose late replication we likely observed. Similarly, the puffing region 4E harbors a female germline-specific gene ovo, whose activity apparently drives puff formation. At the same time, two testis-specific genes also map to this region, and these may be causative for the late replication observed for this region.
We were somewhat puzzled to find asynchronous replication of chromosome 4, even though it is an unusual chromosome in many respects. Euchromatic part of chromosome 4 is similar to heterochromatin, as it bears extensively repeated DNA sequences, which may cause gene silencing, and is enriched with HP1 and Н3K9me2 (Riddle et al. 2008, 2012). Unlike in the rest of the chromosomes, methylation of H3K9 in chromosome 4 is SU(VAR)3-9-independent, but is rather controlled by dSETDB1 (Tzeng et al. 2007). Furthermore, chromosome 4 is the only autosome that is known to be associated with a chromosome-specific protein, POF (Larsson et al. 2001). Intriguingly, chromosome 4 was demonstrated to function ancestrally as a sex chromosome, but then it conveyed this function to the modern X in Drosophila lineage. Yet, similarly to X, D. melanogaster chromosome 4 still shows sex-biased gene expression during early embryogenesis, oogenesis, and spermatogenesis (Vicoso and Bachtrog 2013). All these peculiar features of chromosome 4 point to the possibility that it may indeed form a special domain in the nucleus.
In fact, asynchronous replication of individual chromosomes has been previously described. For example, male X chromosome in D. melanogaster SGs completes replication sooner than do autosomes or female X chromosome (Berendes 1966). This observation was later independently confirmed at a finer resolution upon genome-wide analysis of replication (Schwaiger et al. 2009). In mammals, active and inactive X chromosomes are also known to replicate at different times during the S phase (Gilbert et al. 1962; Chadwick and Willard 2003; Koren and McCarroll 2014). Asynchronous replication of different autosomes within one SG nucleus was reported for D. pseudoobscura, although it only appeared as a slightly faster replication in one autosome vs another after the “continuous labeling” stage (Chatterjee et al. 1976). Next, when individual mammalian chromosomes were observed to fail mitotic condensation, this was accompanied with significant shift in replication timing, and chromosomes that remained abnormally decondensed would begin replication after completion of replication by the rest of the chromosomes (Painter 1961; Wang et al. 1982; Thayler 2012). The said phenotype was a consequence of malignant cell transformation, as it was only observed in cancer cell lines, but not in normal fibroblasts (Wang et al. 1982). It must be emphasized that our analysis did not use wild-type NCs, as these cells lack polytene chromosomes, but rather NCs from otu mutants. This mutation results in disrupted organization of actin filaments and cytoskeleton in NCs (Rodesch et al. 1997), which may lead to aberrant cell division and abnormal formation of egg chamber. Three main classes of otu alleles are recognized. One of them is referred to as “oncogenic”, for egg chambers tend to be composed of numerous small cells instead of 16 large cells (King et al. 1986). The allele used in our work (otu 11) belongs to this class even though the proportion of “oncogenic” egg chambers is relatively low and most of NCs appear morphologically normal. Similar to human cancer cells, asynchronous replication could well be absent in wild-type NCs, but rather resulted from abnormal NC development caused by otu mutation.
Our data are compatible with the idea that a potential mechanism for asynchronous replication of individual chromosomes may operate in NCs. Yet, it remains unclear how origin firing in such chromosomes is controlled independently from the rest of the chromosomes. One option is that association of all the required proteins occurs in all chromosomes simultaneously, but the onset of replication is delayed. Alternatively, binding of replication machinery to the origins may be chromosome-specific. For instance, human chromosome 6 has a locus of non-coding RNA ASAR6; when mutant, chromosome fails to condense and its replication timing is greatly delayed (Stoffregen et al. 2011). It was speculated that all mammalian chromosomes may have similar cis-elements that control replication of each individual chromosome (Thayler 2012). Accordingly, analogous mechanisms may be in place in fruitflies, and asynchronous replication of chromosome 4 may serve as one such example.
References
Belyakin SN, Christophides GK, Alekseyenko AA, Kriventseva EV, Belyaeva ES, Nanayev RA, Makunin IV, Heidelberg Fly Array Consortium, Kafatos FC, Zhimulev IF (2005) Genomic analysis of Drosophila chromosome underreplication reveals a link between replication control and transcriptional territories. Proc Natl Acad Sci U S A 102:8269–8274
Belyakin SN, Babenko VN, Maksimov DA, Shloma VV, Kvon EZ, Belyaeva ES, Zhimulev IF (2010) Gene density profile reveals the marking of late replicated domains in the Drosophila melanogaster genome. Chromosoma 119:589–600
Berendes HD (1966) Differential replication of male and female X-chromosomes in Drosophila. Chromosoma 20:32–43
Chadwick BP, Willard HF (2003) Barring gene expression after XIST: maintaining facultative heterochromatin on the inactive X. Semin Cell Dev Biol 14:359–367
Chatterjee SN, Mondal SN, Mukherjee AS (1976) Interchromosomal asynchrony of DNA replication in polytene chromosomes of Drosophila pseudoobscura. Chromosoma 54:117–125
Dej KJ, Spradling AC (1999) The endocycle controls nurse cell polytene chromosome structure during Drosophila oogenesis. Development 126:293–303
Eaton ML, Prinz JA, MacAlpine HK, Tretyakov G, Kharchenko PV, MacAlpine DM (2011) Chromatin signatures of the Drosophila replication program. Genome Res 21:164–174
Edgar BA, Orr-Weaver TL (2001) Endoreplication cell cycles: more for less. Cell 105:297–306
Gilbert DM (2002) Replication timing and transcriptional control: beyond cause and effect. Curr Opin Cell Biol 14:377–383
Gilbert CW, Muldal S, Lajtha LG, Rowley J (1962) Time-sequence of human chromosome duplication. Nature 195:869–873
Graveley BR, May G, Brooks AN, Carlson JW, Cherbas L, Davis CA, Duff M, Eads B, Landolin J, Sandler J, Wan KH, Andrews J, Brenner SE, Cherbas P, Gingeras TR, Hoskins R, Kaufman T, Celniker SE (2011). The D. melanogaster transcriptome: modENCODE RNA-Seq data for dissected tissues. http://www.modencode.org/Celniker.shtml
Hammond MP, Laird CD (1985) Chromosome structure and DNA replication in nurse and follicle cells of Drosophila melanogaster. Chromosoma 91:267–278
Heino TI (1989) Polytene chromosomes from ovarian pseudonurse cells of the Drosophila melanogaster otu mutant. I. Photographic map of chromosome 3. Chromosoma 97:363–373
Hiratani I, Takebayashi S, Lu J, Gilbert DM (2009) Replication timing and transcriptional control: beyond cause and effect-part II. Curr Opin Genet Dev 19:142–149
King RC, Riley SF, Cassidy JD, White PE, Paik YK (1981) Giant polytene chromosomes from the ovaries of a Drosophila mutant. Science 212:441–443
King RC, Mohler D, Riley SF, Storto PD, Nicolazzo PS (1986) Complementation between alleles at the ovarian tumor locus of Drosophila melanogaster. Dev Genet 7:1–20
Kolesnikova TD, Posukh OV, Andreyeva EN, Bebyakina DS, Ivankin AV, Zhimulev IF (2013) Drosophila SUUR protein associates with PCNA and binds chromatin in a cell cycle-dependent manner. Chromosoma 122:55–66
Koren A, McCarroll SA (2014) Random replication of the inactive X chromosome. Genome Res 24:64–69
Koryakov DE, Belyaeva ES, Alekseyenko AA, Zhimulev IF (1996) Alpha and beta heterochromatin in polytene chromosome 2 of Drosophila melanogaster. Chromosoma 105:310–319
Koryakov DE, Alekseyenko AA, Zhimulev IF (1999) Dynamic organization of the b-heterochromatin in the Drosophila melanogaster polytene X chromosome. Mol Gen Genet 260:503–509
Koryakov DE, Domanitskaya EV, Belyakin SN, Zhimulev IF (2003) Abnormal tissue-dependent polytenization of a block of chromosome 3 pericentric heterochromatin in Drosophila melanogaster. J Cell Sci 116:1035–1044
Koryakov DE, Reuter G, Dimitri P, Zhimulev IF (2006) The SuUR gene influences the distribution of heterochromatic proteins HP1 and SU(VAR)3-9 on nurse cell polytene chromosomes of Drosophila melanogaster. Chromosoma 115:296–310
Koryakov DE, Pokholkova GV, Maksimov DA, Belyakin SN, Belyaeva ES, Zhimulev IF (2012) Induced transcription results in local changes in chromatin structure, replication timing, and DNA polytenization in a site of intercalary heterochromatin. Chromosoma 121:573–583
Larsson J, Chen JD, Rasheva V, Rasmuson-Lestander A, Pirrotta V (2001) Painting of fourth, a chromosome-specific protein in Drosophila. Proc Natl Acad Sci U S A 98:6273–6278
Lilly MA, Spradling AC (1996) The Drosophila endocycle is controlled by Cyclin E and lacks a checkpoint ensuring S-phase completion. Genes Dev 10:2514–2526
MacAlpine DM, Bell SP (2005) A genomic view of eukaryotic DNA replication. Chromosome Res 13:309–326
Mahowald AP, Kambysellis MP (1980) Oogenesis. In: Ashburner M, Wright TRF (eds) The genetics and biology of Drosophila. Acad Press Inc. V. 2d. pp 141–225
Maksimov DA, Koryakov DE, Belyakin SN (2014) Developmental variation of the SUUR protein binding correlates with gene regulation and specific chromatin types in D. melanogaster. Chromosoma 123:253–264
Makunin IV, Volkova EI, Belyaeva ES, Nabirochkina EN, Pirrotta V, Zhimulev IF (2002) The Drosophila suppressor of underreplication protein binds to late-replicating regions of polytene chromosomes. Genetics 160:1023–1034
Mal’ceva NI, Zhimulev IF (1993) Extent of polytene in the pericentric heterochromatin of polytene chromosomes of pseudonurse cells of otu (ovarian tumor) mutants of Drosophila melanogaster. Mol Gen Genet 240:273–276
Mal’ceva NI, Gyurkovics H, Zhimulev IF (1995) General characteristics of the polytene chromosome from ovarian pseudonurse cells of the Drosophila melanogaster otu 11 and fs(2)B mutants. Chromosome Res 3:191–200
Mal’ceva NI, Belyaeva ES, King RC, Zhimulev IF (1997) Nurse cell polytene chromosomes of Drosophila melanogaster otu mutants: morphological changes accompanying interallelic complementation and position effect variegation. Dev Genet 20:163–174
Painter RB (1961) Asynchronous replication of HeLa S3 chromosomal deoxyribonucleic acid. J Biophys Biochem Cytol 11:485–488
Pindyurin AV, Moorman C, de Wit E, Belyakin SN, Belyaeva ES, Christophides GK, Kafatos FC, van Steensel B, Zhimulev IF (2007) SUUR joins separate subsets of PcG, HP1 and B-type lamin targets in Drosophila. J Cell Sci 120:2344–2351
Pindyurin AV, Boldyreva LV, Shloma VV, Kolesnikova TD, Pokholkova GV, Andreyeva EN, Kozhevnikova EN, Ivanoschuk IG, Zarutskaya EA, Demakov SA, Gorchakov AA, Belyaeva ES, Zhimulev IF (2008) Interaction between the Drosophila heterochromatin proteins SUUR and HP1. J Cell Sci 121:1693–1703
Rasch EM, King RC, Rasch RW (1984) Cytophotometric studies on cells from the ovaries of otu mutants of Drosophila melanogaster. Histochemistry 81:105–110
Riddle NC, Leung W, Haynes KA, Granok H, Wuller J, Elgin SCR (2008) An investigation of heterochromatin domains on the fourth chromosome of Drosophila melanogaster. Genetics 178:1177–1191
Riddle NC, Jung YL, Gu T, Alekseyenko AA, Asker D, Gui H, Kharchenko PV, Minoda A, Plachetka A, Schwartz YB, Tolstorukov MY, Kuroda MI, Pirrotta V, Karpen GH, Park PJ, Elgin SCR (2012) Enrichment of HP1a on Drosophila chromosome 4 genes creates an alternate chromatin structure critical for regulation in this heterochromatic domain. PLoS Genet 8(9):e1002954
Rodesch C, Pettus J, Nagoshi RN (1997) The Drosophila ovarian tumor gene is required for the organization of actin filaments during multiple stages in oogenesis. Dev Biol 190:153–164
Schübeler D, Scalzo D, Kooperberg C, van Steensel B, Delrow J, Groudine M (2002) Genome-wide DNA replication profile for Drosophila melanogaster: a link between transcription and replication timing. Nat Genet 32:438–442
Schwaiger M, Schübeler D (2006) A question of timing: emerging links between transcription and replication. Curr Opin Genet Dev 16:177–183
Schwaiger M, Stadler MB, Bell O, Kohler H, Oakeley EJ, Schübeler D (2009) Chromatin state marks cell-type- and gender-specific replication of the Drosophila genome. Genes Dev 23:589–601
Sclafani RA, Holzen TM (2007) Cell cycle regulation of DNA replication. Annu Rev Genet 41:237–280
Sher N, Bell GW, Li S, Nordman J, Eng T, Eaton ML, MacAlpine DM, Orr-Weaver TL (2012) Developmental control of gene copy number by repression of replication initiation and fork progression. Genome Res 22:64–75
Sinha P, Mishra A, Lakhotia SC (1987) Chromosomal organization of Drosophila tumours. I. Polytene chromosome organization and DNA synthesis in ovarian pseudonurse cells in otu mutants of D. melanogaster. Chromosoma 95:108–116
Stoffregen EP, Donley N, Stauffer D, Smith L, Thayer MJ (2011) An autosomal locus that controls chromosome-wide replication timing and mono-allelic expression. Hum Mol Genet 20:2366–2378
Thayer MJ (2012) Mammalian chromosomes contain cis-acting elements that control replication timing, mitotic condensation, and stability of entire chromosomes. Bioessays 34:760–770
Tzeng TY, Lee CH, Chan LW, Shen CK (2007) Epigenetic regulation of the Drosophila chromosome 4 by the histone H3K9 methyltransferase dSETDB1. Proc Natl Acad Sci U S A 104:12691–12696
Vicoso B, Bachtrog D (2013) Reversal of an ancient sex chromosome to an autosome in Drosophila. Nature 499:332–335
Wang N, Trend B, Kaung HL, Wang T (1982) Chromosomal replication asynchrony of a human breast carcinoma cell line. I. Studied by continuous BrdU incorporation and G-banding analysis. Cancer Genet Cytogenet 7:173–180
Zhimulev IF (1999) Genetic organization of polytene chromosomes. Adv Genet 39:1–599
Zhimulev IF, Semeshin VF, Kulichkov VA, Belyaeva ES (1982) Intercalary рeterochromatin in Drosophila. I. Localization and general characteristics. Chromosoma 87:197–228
Zhimulev IF, Belyaeva ES, Makunin IV, Pirrotta V, Volkova EI, Alekseyenko AA, Andreyeva EN, Makarevich GF, Boldyreva LV, Nanayev RA, Demakova OV (2003) Influence of the SuUR gene on intercalary heterochromatin in Drosophila melanogaster polytene chromosomes. Chromosoma 111:377–398
Acknowledgments
The authors are grateful to A.A. Gorchakov, O.V. Demakova, D.A. Maksimov, and E.S. Belyaeva for their comments and help in data interpretation. The work was supported by the grant of the Russian Science Foundation (14-14-00934).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Koryakov, D.E., Zhimulev, I.F. DNA replication in nurse cell polytene chromosomes of Drosophila melanogaster otu mutants. Chromosoma 124, 95–106 (2015). https://doi.org/10.1007/s00412-014-0487-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00412-014-0487-4