Abstract
Eukaryotic genomes are organized in large chromatin domains that maintain proper gene activity in the cell. These domains may be permissive or repressive to the transcription of underlying genes. Based on its protein makeup, chromatin in Drosophila cell culture has been recently categorized into five color-coded states. Suppressor of Under-Replication (SUUR) protein was found to be the major component present in all three repressive chromatin states named BLACK, BLUE, and GREEN and to be depleted from the active YELLOW and RED chromatin types. Here, we addressed the question of developmental dynamics of SUUR binding as a marker of repressed chromatin types. We established genomewide SUUR binding profiles in larval salivary gland, brain, and embryos using DNA adenine methyltransferase identification (DamID) technique, performed their pairwise comparisons and comparisons with the published data from Drosophila Kc cells. SUUR binding pattern was found to vary between the samples. Increase in SUUR binding predominantly correlated with local gene repression suggesting heterochromatin formation. Reduction in SUUR binding often coincided with activation of tissue-specific genes probably reflecting the transition to permissive chromatin state and increase in accessibility to specific transcription factors. SUUR binding plasticity accompanied by the regulation of the underlying genes was mainly observed in BLACK, BLUE, and RED chromatin types. Our results provide novel insight into the developmental dynamics of repressive chromatin and reveal a link to the chromatin-guided regulation of gene expression.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Different sets of proteins are recruited to characteristic chromosome regions to secure the proper gene expression. Recent successes in mapping protein–DNA interactions allowed description of the major chromatin states in Drosophila, Arabidopsis, and human chromosomes (Ernst and Kellis 2010; Filion et al. 2010; Kharchenko et al. 2011; Roudier et al. 2011). Each chromatin state is determined as a specific combination of proteins bound to discrete genome locations. In Drosophila, two independent models describing chromatin states were proposed. The five-state model was based on the DNA adenine methyltransferase identification (DamID) profiles of 53 proteins in Kc cell line (Filion et al. 2010). The nine-state model considered the distribution of 18 histone modifications over the genomes of S2 and BG3 cell lines (Kharchenko et al. 2011). Although these studies were based on different experimental data, the patterns are strikingly similar allowing drawing of parallels between the two models. In both cases, repressive and active chromatin types were clearly distinguishable.
Repressive chromatin may be subdivided into Polycomb-bound, HP1-bound, and silent domains of uncertain nature (Filion et al. 2010; Kharchenko et al. 2011). In the five-state model, these correspond to BLUE, GREEN, and BLACK chromatin types, respectively. YELLOW chromatin generally contains ubiquitously active genes, while RED chromatin type encompasses developmentally regulated genes that are active in Kc cell culture (Filion et al. 2010).
Chromatin organization is subject to developmental regulation (Hiratani et al. 2009; Kharchenko et al. 2011; Riddle et al. 2011). A recent study demonstrated plasticity of DNase I hypersensitive sites (DHS) in different chromatin domains between Drosophila embryos and Kc cells. The number of DHS gradually increased in repressive chromatin types (BLACK, GREEN, and BLUE) during the embryo development and decreased in RED chromatin, suggesting their developmental regulation (Thomas et al. 2011). Similarly, in the nine-state chromatin model, the DHS plasticity between the two cell lines occurred in the enhancerlike state 3 and correlated with the transitions to other chromatin types (Kharchenko et al. 2011). Variation of histone H3K9me2 pattern was demonstrated between cell cultures, embryos, larvae, and adult heads (Riddle et al. 2011), apparently suggesting the plasticity of the GREEN chromatin that is specifically enriched with this mark (Filion et al. 2010). Yet another study assessed Polycomb binding dynamics in Drosophila embryos and T3 imaginal disc (Kwong et al. 2008). Several dozens of differentially bound genes were detected, but strikingly, these genes had little connection to the physiology of the studied samples. Furthermore, reduction in Polycomb binding did not necessarily result in their activation (Kwong et al. 2008).
According to the five-state chromatin model (Filion et al. 2010), Suppressor of Under-Replication (SUUR) protein is one of the major components of the three repressive chromatin types. Exact function of this protein remains unclear, but it is known that it is associated with late replicating heterochromatic regions of the genome (Makunin et al. 2002) and is able to interfere with the replication fork progression (Sher et al. 2012), probably by interacting with proliferating cell nuclear antigen (PCNA) that is a component of replication complex (Kolesnikova et al. 2013). In salivary gland polytene chromosomes, this leads to local DNA under-replication at some repressed genomic sites (Zhimulev et al. 2003; Belyakin et al. 2005; Sher et al. 2012). It was recently shown that the under-replication pattern may be variable in different tissues. Moreover, in all cases analyzed so far, the under-replicated areas were SUUR dependent and disappeared in SuUR mutants, implying that SUUR distribution may differ between tissues (Nordman et al. 2011).
SUUR-bound genes in Kc cells are enriched with the repressive Polycomb mark (H3K27me3) and devoid of the histone modifications characteristic of active genes (Filion et al. 2010; Pindyurin et al. 2007). Presence of functional SUUR protein was found to be essential for H3K27 trimethylation and H3K9 di- and trimethylation at some, but not all, chromosomal regions (Sher et al. 2012; Koryakov et al. 2011). However, this effect was only limited to a subset of H3K27me3 sites, the absence of SUUR function did not lead to gene activation, and SuUR mutants were viable and fertile (Sher et al. 2012; Belyaeva et al. 1998). Mapping of SUUR binding sites in Kc cell culture confirmed that its target genes tend to be late replicating and silent in these cells (Pindyurin et al. 2007). A physical interaction between SUUR and HP1 proteins was reported (Pindyurin et al. 2008). These data suggest that SUUR is deeply connected to heterochromatic regions of the Drosophila genome. However, it remains unclear whether the correlations established in cell culture persist in other cell types.
We performed DamID chromosome profiling of SUUR protein in Drosophila embryos, larval salivary glands, and brain, where SuUR gene is known to be active. Comparison of the profiles obtained and SUUR binding profile in Kc cells (Filion et al. 2010) revealed considerable differences in SUUR binding probably reflecting the plasticity of repressive chromatin. Using the five-state chromatin model as a reference, we observed that some chromatin types are, more than others, predisposed to SUUR binding variation and to transcriptional changes in different tissues.
Materials and methods
Plasmid construction and fly crosses
The constructs for DamID (hsp70 > loxP-Stop-loxP > Dam-Myc and hsp70 > loxP-Stop-loxP > Dam-Myc-SUUR; GenBank accession numbers JN993988 and JN993989, respectively) were assembled in pUAST-attB vector. 10 aa in-frame Myc epitope served for the detection of Dam-fused proteins. Stop cassette (2,033 bp downstream Hsp70Ab stop codon (Struhl and Basler 1993) flanked by loxP recombination sites) was used to assure viability that might be compromised due to the Dam toxicity (de Wit et al. 2005). Transformation was performed using attP–attB integration system (Bischof et al. 2007) into attP18 landing site on the X chromosome (Markstein et al. 2008). Stop cassettes were deleted by crossing with the flies ubiquitously expressing CRE recombinase (Siegal and Hartl 2000). The progeny without stop cassette was viable and gave rise to homozygous strains bearing hsp70 > Dam-Myc and hsp70 > Dam-Myc-SUUR.
Western blot
Ten salivary glands of each genotype were collected by dissection in 1× PBS. Then, they were boiled for 10 min in the PAGE sample buffer and loaded on a 12 % SDS-polyacrylamide gel. The proteins were separated at 100 V for 40 min. Protein transfer was performed on Hybond-P membrane as recommended by the manufacturer (Amersham Biosciences). The membrane was blocked in 1× PBS-T (1× PBS supplemented with 0.1 % Tween 20) with 5 % dry milk for 1 h at room temperature. Next, the membrane was incubated with primary antibodies (anti-myc-FITC Ab, Invitrogen, cat. #46-0307 at 1:5,000 dilution) in the same solution overnight at 4 °C. The membrane was washed three times for 5 min in 1× PBS-T at room temperature and incubated for 1 h with secondary antibodies (Promega W402B anti-mouse IgG HRP conjugate at 1:2,500 dilution) at room temperature. After two 1× PBS-T washes, the signals were detected using Novex ECL kit (Invitrogen WP20005) on the ChemiDoc instrument (Bio-Rad).
DamID procedures and data analysis
Flies for DamID were kept at 23 °C to avoid heat shock and excessive activation of Dam constructs. Genomic DNA was isolated from 12 to 14 h embryos, dissected salivary glands, and brains of the third instar larvae of the Dam-Myc-SUUR- and Dam-Myc-expressing strains. Preparation of DamID samples was performed as described previously (Greil et al. 2006). Shortly, genomic DNA was isolated from all the experimental and reference samples and digested with DpnI restriction endonuclease that only cuts methylated GAmeTC sequences. Genomic DNA from the y,w strain was used as unmethylated control. The DNA samples were purified and annealed double-stranded adaptors were ligated. Next, the samples were digested with DpnII enzyme that cuts unmethylated GATC sequences. This step provided higher specificity of the method (Greil et al. 2006). The aliquot of this reaction was used as a template in 17 cycles of PCR with the adaptor-specific primer. Amplification was observed in Dam-expressing samples and was absent from the y,w negative control. The Dam-Myc-SUUR and Dam-Myc samples obtained were sent to the Canadian Drosophila Microarray Centre (http://www.flyarrays.com/) and hybridized with the D. melanogaster Gene Expression 4 × 72 K Arrays (NimbleGen). Two biological replicates were processed in each case.
Data were analyzed with the ArrayStar software (http://www.dnastar.com/). Each array was background corrected and quantile normalized. For each replicate, Dam-Myc-SUUR/Dam-Myc ratio was calculated for all array features. Replicates were filtered for reproducibility as was previously described (Yang et al. 2002). The filtered data from two experiments were averaged.
SUUR DamID data in Kc cells were retrieved from GEO database (GSE22069) (Filion et al. 2010). The features from this dataset that overlapped with the NimbleGen oligos were used for comparisons between the platforms. All four datasets were shifted by the genomewide median, SD scaled, and quantile normalized between the samples (Bolstad et al. 2003). Log2-transformed normalized values were used as a measure of SUUR binding.
k-means clustering was performed using the Cluster and TreeView software (Eisen et al. 1998). The data were clustered into 17 groups with 1,000 iterations. The number of groups for k-means clustering was determined as 24 + 1, where 24 = 16 is a number of possible groups that could be obtained from four datasets (for example, one group contains genes that are SUUR-depleted in all the four samples, another group comprises the genes that are depleted in one sample but enriched in the rest of the samples, and so on) and one extra group was reserved for any unexpected case. A smoothed genomewide profile was built using an averaging 5-kb sliding window with 100-bp step.
Gene expression datasets
The gene expression RNA-seq data were retrieved from modENCODE database for larval salivary glands, brain (http://www.modencode.org/), and 12–14-h embryos (Graveley et al. 2011). The data from Filion et al. (2010) were used for Kc167 cells (GEO accession GSE22069). The four datasets were quantile normalized prior to comparisons.
Gene ontology
Gene ontology (GO) analysis was performed using DAVID Bioinformatics Resources (http://david.abcc.ncifcrf.gov/tools.jsp). For Kc cells and embryos, functional annotation chart option was used to analyze the genes that bound SUUR weaker in these samples than in any of the other three samples. For salivary glands and brain, the same analysis was performed for the genes that were found SUUR-depleted specifically in these samples. All genes present on the 72 K NimbleGen arrays were used as the background gene list.
Results
To trace SUUR binding to chromosomes in flies, we used DamID approach (van Steensel and Henikoff 2000). DamID method is based on the ability of Dam (DNA adenine methyltransferase from E. coli) to methylate adenine in GATC palindrome. When fused to a chromatin protein, Dam would preferentially methylate GATC sequences around the landing sites of this protein. Methylated fragments are isolated and quantified to reveal the local enrichment of the protein under investigation. This method was previously applied to build the SUUR profile in Kc cell culture (Pindyurin et al. 2007) which was later refined to provide higher resolution (Filion et al. 2010).
To perform SUUR DamID in flies, we constructed the D. melanogaster strain expressing Dam-Myc-SUUR fusion protein under the control of the minimal hsp70 promoter of the pUAST vector (Brand and Perrimon 1993) inserted in attP18 landing site on the X chromosome (Markstein et al. 2008). The reference strain contained the control construct expressing Dam-Myc inserted in the same genomic location. The use of the minimal hsp70 promoter at room temperature is meant to avoid overexpression and assure trace amounts of Dam-Myc and Dam-Myc-SUUR proteins required for DamID (van Steensel and Henikoff 2000; Greil et al. 2006). SUUR-anchored Dam would methylate GATC sequences preferably in the vicinity of predominant SUUR binding sites, while Dam-Myc methylation is nonspecific and mainly depends on the chromatin accessibility (van Steensel and Henikoff 2000). The ratio of microarray signals from the Dam-Myc-SUUR and Dam-Myc strains was used as a measure of SUUR enrichment.
To estimate the amount of the protein produced from our constructs, we performed Western blot analysis of Dam-Myc protein in salivary gland. Dam-Myc protein was undetectable in the salivary gland at room temperature even when the membrane was overexposed, while a clear band was observed in the positive control where the Dam-Myc-expressing transgene was specifically induced in the salivary gland by the AB1 Gal4 driver (Supplementary Fig. 1). The result of this test was similar to a previously reported data on Dam expression level in cell culture; therefore, we concluded that the amounts of the Dam-Myc protein meet the requirements of the method (van Steensel and Henikoff 2000) and our constructs can be used for SUUR profiling in flies. Notably, the same experimental design has been successfully employed previously for DamID experiments in embryos and larvae (de Wit et al. 2005; Luo et al. 2011).
SUUR binding was assessed in the samples that are known to express the SuUR gene—12–14-h embryos (according to the modENCODE Temporal Expression Data (Graveley et al. 2011)), larval salivary glands, and larval brain (Kolesnikova et al. 2006). Following the standard DamID procedure (Greil et al. 2006) (“Materials and methods”), we generated SUUR binding profiles in these three samples using 72 K NimbleGen arrays (Roche). Two biological replicates were analyzed for each sample type. The results were highly reproducible between the replicates (Pearson's R > 0.92 for all samples). The outliers were filtered out as described in Yang et al. (2002) and the data were normalized and averaged for each sample. Our data were then combined with the previously published SUUR binding pattern in Kc cells obtained using tiling microarrays (Filion et al. 2010). In order to compare the datasets, we selected those array features that overlapped between the platforms. A total of 54,983 features across the genome (89 % of 72 k NimbleGen array features) passed these filters.
From previous studies, we know that native SuUR gene is expressed at different levels in different cell types (Chintapalli et al. 2007). Accordingly, the levels of endogenous SUUR protein would vary between the samples. This could result in variation of DamID signal magnitude in different samples (Supplementary Fig. 2a). Different scales of DamID signals would preclude the direct comparisons of SUUR profiles between the samples. Therefore, all four datasets were median centered, SD scaled, and quantile normalized. As a result of quantile normalization, the range of DamID signals became the same in all the four datasets rendering their statistical properties identical (Supplementary Fig. 2b) which allowed analysis of variation of SUUR profiles between the samples. The Pearson correlations calculated in all pairs of resulting datasets ranged between 0.55 and 0.76 (lower than the correlations between the biological replicates: R > 0.92 in all cases) showing that considerable developmental variation in SUUR binding exists. To illustrate this variation, we performed k-means clustering of our data into 17 groups (“Materials and methods”) and considerable part of these groups demonstrated pronounced variation of SUUR binding in one or more samples (Supplementary Fig. 2c). In practice, the number of 17 groups appeared to be somewhat excessive as some groups were quite similar, but this nevertheless illustrated the variability of SUUR binding in the studied samples.
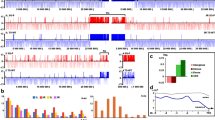
A region of 3R chromosome arm illustrating smoothed SUUR profiles (“Materials and methods”) in four samples is presented in Fig. 1. This profile demonstrates both the areas of similar SUUR binding as well as the region where SUUR profiles are distinct between the samples. Particularly, the region assigned to the RED chromatin and showing decreased SUUR binding in Kc cells (Filion et al. 2010) shows greater SUUR enrichment in other samples, especially in the brain. More examples could be found in Supplementary Fig. 3. The complete track for visualization in the UCSC Genome Browser is deposited in GEO (GSE33873).
To analyze the binding differences between the samples systematically, we averaged SUUR binding data across each gene (Supplementary Table 1). Genes showing the average Log2(Dam-Myc-SUUR/Dam-Myc) value higher than 2 and lower than −2 were considered SUUR enriched and SUUR depleted, respectively. The number of SUUR-enriched genes varied from 1,491 in Kc cells to 1,836 in salivary gland (Fig. 2a). The numbers of depleted genes varied from 2,074 in Kc cells to 2,539 in salivary gland (Fig. 2b). The overlap between the four samples comprised 220 gene targets and 611 nontarget genes (Fig. 2a). We classified these genes according to the five-state chromatin model and found that ubiquitously SUUR-bound genes belonged to the repressive chromatin types described in Kc cells and were highly enriched in BLACK chromatin (Fig. 2a, binomial test, P < 10E-12). In contrast, 95 % of genes that were SUUR depleted in all four samples were located in YELLOW chromatin in Kc cells (Fig. 2b, binomial test, P < 10E-12). The criterion for genes to be four times enriched or depleted in each of the four samples is quite stringent and the number of such genes is relatively low. However, this analysis allowed us to demonstrate that BLACK chromatin type is more persistent in SUUR binding than other repressive chromatin types. Accordingly, YELLOW chromatin demonstrated relatively stable SUUR depletion in the studied samples. Our data suggest that BLACK and YELLOW chromatin types represent the most stable chromatin types in the Drosophila genome, which is consistent with the previous report about their nature and genetic composition (Filion et al. 2010).
SUUR profiles in different biological samples. SUUR binding was measured as Log2 transformed Dam-Myc-SUUR/Dam-Myc ratio in larval brain (Br), larval salivary gland (SG), and 12–14 h embryos (Em). The data for Kc cells were taken from Filion et al. (2010) and processed as described in the text. The smoothed SUUR profile is shown with orange/blue bars over about 280 kb region of the 3R chromosome arm. The region of SUUR binding variation between the samples is shaded in gray. The span of the five color-coded chromatin types from Filion et al. (2010) is presented at the top and the bottom of the figure
SUUR protein target and nontarget genes in different samples. a Numbers of SUUR-positive and SUUR-negative genes in embryos (Em), Kc cells (Kc), salivary gland (SG), and brain (Br). The pie charts (b) demonstrate representation of genes belonging to five chromatin states among shared SUUR-positive and SUUR-negative genes and in whole genome. The genes that are SUUR-positive in all samples are enriched in BLACK chromatin, while the negative genes mostly belong to YELLOW chromatin
Next, we inspected the genes that displayed altered SUUR binding between the samples. It was previously shown that SUUR-bound chromatin in Kc cells is predominantly silent and contains developmentally regulated genes (Pindyurin et al. 2007; Filion et al. 2010). Therefore, we expected that variation of SUUR binding would reflect the changes in chromatin organization between the samples which could be accompanied by sample-specific expression changes. Two conditions were required for a gene to be considered differentially bound between two samples. First, it should demonstrate Dam-Myc-SUUR/Dam-Myc > 1 ratio in one sample, and second, it should have at least fourfold lower value in the other sample. Figure 3a demonstrates the numbers of the genes satisfying these conditions in each pair of samples. Notably, the numbers of genes showing altered SUUR binding were very close within each pair of samples. This implies that the observed differences are not due to the variable expression levels of the Dam-Myc-SUUR fusion protein between the samples because a sample with the strongly elevated Dam-Myc-SUUR protein level would systematically demonstrate higher numbers of genes bound in this sample.
Differential SUUR binding and gene activity. a Numbers of differentially bound genes in each sample pair. In each pair, light gray bars correspond to the genes that bind SUUR in the first sample stronger than in the second sample in a pair. Dark gray bars reflect the opposite situation. b Variation in SUUR binding correlates with activity of the genes in Kc–SG pair. Expression change was measured for each gene as Log2 ratio of the quantile-normalized expression data. Decrease of the SUUR binding correlated with gene activation. Significance was assessed using Mann–Whitney test. Data for all six sample pairs are presented on Supplementary Fig. 4. c Variation in SUUR binding correlates with the distribution of H3K27me3 histone mark in Kc–SG pair. Variation of the H3K27me3 was calculated for the same gene set as on the Fig. 3b. Stronger SUUR binding in the particular sample correlated with the higher level of H3K27me3 in this sample. Significance was assessed using Mann–Whitney test
To assess the expression of differentially bound genes between the samples, we used the RNA-seq modENCODE data for 12–14-h embryos (Graveley et al. 2011), larval brain, and salivary gland (www.modencode.org). The RNA-seq data on gene expression in Kc were taken from Filion et al. (2010) because they were obtained for the same cells in the same laboratory conditions as in SUUR DamID experiments. Before the comparisons, we performed quantile normalization of these datasets focusing on the genes that had numerical expression data in all four samples. Then, we measured variation of gene expression in each pair of samples as the ratio of the quantile-normalized data (Fig. 3b, Supplementary Fig. 4). We observed that gene activation in a particular tissue tends to correlate with lower SUUR binding in this tissue. This tendency was found across all six sample pairs with high confidence (Supplementary Fig. 4), suggesting that SUUR association with the developmentally regulated genes follows their chromatin state in different cell types.
It was previously shown that SUUR shares its target genes with known repressors—Pc and HP1 (Pindyurin et al. 2007). These proteins are the main determinants of BLUE and GREEN chromatin types, respectively (Filion et al. 2010), where they are associated with specific covalent histone modifications: H3K27me2 and H3K27me3 (Pc) (Schwartz and Pirrotta 2007) and H3K9me2 and H3K9me3 (HP1) (Ebert et al. 2006). It is reasonable to suggest that the genes changing SUUR binding between the samples would also manifest different levels of these histone modifications. To check this, we used published ChIP-chip data on H3K27me3 distribution in Kc cells (Filion et al. 2010) and in salivary glands (Sher et al. 2012). These data were quantile normalized and averaged for each gene. The variation in H3K27 methylation level was analyzed for the same gene set that is shown on Fig. 3b. Indeed, the genes showing lower SUUR binding in salivary gland than in Kc cells tended to have lower levels of H3K27me3. The genes showing higher SUUR binding displayed higher levels of H3K27me3 mark (Fig. 3c). This correlation between SUUR binding variation and H3K27me3 levels was even more significant than the correlation with transcription changes (Fig. 3b and c).
To obtain the functional characteristics of genes located in the regions of variable SUUR binding, we used DAVID Gene Ontology toolkit (da Huang et al. 2009). For each sample, we selected genes that bound SUUR weaker than in all other samples using the same criterion as for the Fig. 3a and performed their functional analysis. Genes that showed decreased SUUR binding in salivary gland (61 genes) manifested significant enrichment in gene categories involved in the secretory function of this organ: puparial adhesion to the substrate and molting cycle (including the genes Salivary gland secretion 3 (sgs3), sgs4, sgs 5, new glue 3 (ng3), and ng4 (Fig. 4a, Supplementary Table 2)). In larval brain sample (106 genes), the enrichment was observed in terms connected to cognition, sensory perception, and transduction. Also, the term “Immunoglobulin-like” was over-represented that includes Down syndrome cell adhesion molecule 4 (Dscam4), members of axon guidance molecules (beat family), and defective proboscis extension response (dpr) superfamily responsible for gustatory perception (Fig. 4b, Supplementary Table 3).
Gene ontology analysis of differentially SUUR-bound genes. a GO terms significantly enriched (Bonferroni-corrected P < 0.05) among the genes that had lower SUUR binding in SG compared to all other samples. b Same for Br. c GO analysis of genes that showed reduced SUUR binding in Em compared to at least one other sample. The bars represent fold enrichment for each term
In contrast to salivary gland and brain, embryonic sample consists of many cell types. Accordingly, the list of genes that specifically decreased SUUR binding in embryos was too small for GO analysis (only 32 genes). Therefore, we relaxed our criteria and allowed the genes to be SUUR depleted in at least one of other samples. GO analysis of the resulting gene set (1,222 genes) revealed enrichment in the terms connected to development and morphogenesis: heart development, mesoderm development, and specific transcription factors including HOX genes (Fig. 4c, Supplementary Table 4). In the case of Kc cell line, we also observed some significantly enriched terms (Supplementary Table 5), but it is hard to interpret their relation to the biology of the Kc cells. These observations imply that chromatin regulation reflected by SUUR dynamics in our experiments is connected to specific regulatory programs activated in different cells.
SUUR is one of the major components in the three repressive chromatin states. Therefore, we asked whether its binding variation correlated with a particular chromatin state or equally attributed to all chromatin types. In order to check this, we subdivided the genes that demonstrated SUUR binding variability (Fig. 3a) according to the five-state model (Filion et al. 2010). As this model was built for Kc cell line, we only performed pairwise comparisons of Kc to three other samples. The representation of each chromatin state was calculated for genes that displayed decreased or increased SUUR binding at least in one of the three samples. Figure 5a illustrates that among the genes that bind SUUR in embryos, salivary gland, or brain weaker than in Kc cells, the fractions of BLUE and GREEN chromatin types are increased while RED is under-represented. In turn, among the genes that bind SUUR at least in one sample stronger than in Kc cells, RED chromatin type is significantly over-represented: about 30 % genes belonging to this chromatin type in Kc cells revealed more than fourfold increase in SUUR binding at least in one of the other three samples, demonstrating that this chromatin type is highly regulated in different cells (Fig. 5a). BLACK chromatin was under-represented in this category. Taken together with the data presented on the Fig. 2b, these observations allow estimation of developmental dynamics of different chromatin types.
SUUR dynamics in different chromatin types. a The fractions of genes in five chromatin types that show differential SUUR binding in Kc cells as compared to other samples. BLUE and GREEN chromatin types tend to be SUUR-depleted, while RED chromatin accumulates SUUR in other samples. Dashed lines represent the percentages of genes expected form the total gene numbers by chance. b The correlation between the SUUR binding variation and the levels of H3K27me3 mark in five chromatin types. In all chromatin types but GREEN, increase of SUUR binding accompanied the increase of H3K27me3 level. Significance was assessed using Mann–Whitney test. c The correlation of SUUR variation and gene activity in five chromatin types. The genes in BLACK and BLUE chromatin types demonstrated a strong correlation of their expression level and SUUR binding: stronger SUUR binding coincided with repression while decrease in SUUR binding accompanied activation. In RED and YELLOW chromatin types, the significant correlation existed only for the genes increasing SUUR binding in other samples. GREEN chromatin type did not show significant correlation between SUUR binding variation and gene activity. The labeling is the same as on the Fig. 3b. Positive values correspond to gene activation while negative values reflect gene repression in comparison to Kc cells
It was possible that the observed picture resulted from the different accuracy with which the boundaries of different chromatin states were set. It seemed reasonable as the characteristic sizes of domains were very different between the five chromatin types: the smallest domains belonged to RED chromatin while the longest domains were found in BLACK type (Filion et al. 2010). If this were the case, the genes showing variable SUUR binding in different samples would tend to accumulate near the borders of chromatin domains.
To check this, we split chromatin domains into 1-kb bins starting from the border towards the center of each domain. The genes whose center was located in each bin were determined for five chromatin types. Then, for each chromatin type, a portion of genes showing the variability of SUUR binding (Fig. 5a) was calculated among the total number of genes in each bin (Supplementary Fig. 5). This analysis did not reveal any increase in SUUR binding plasticity near the borders of the domains in any chromatin type. A higher fluctuation observed in some cases when moving deeper inside the domain (in the GREEN and BLUE chromatin types in the Supplementary Fig. 5a, in the RED chromatin type on the Supplementary Fig. 5b) is due to the smaller sizes of domains and, consequently, smaller numbers of genes and is not significantly different from the distributions presented in the Fig. 5a, as estimated by the binomial test. BLACK and YELLOW chromatin types containing more genes in larger domains revealed a constant level of SUUR plasticity from border towards the center of the domain. Thus, the observed variability of SUUR binding in different chromatin types seems not to be caused by the fluctuations near the borders of domains.
Although our analysis revealed a very specific pattern of SUUR dynamics in different chromatin types (Fig. 5a), it is still possible that this picture could be at least partially attributable to some inherent features of the five-state chromatin model. For example, it was possible that protein composition of the RED chromatin type was just less developmentally stable and would demonstrate a similar variation level, whichever protein is considered.
To estimate this, we applied the analytic pipeline that we used on SUUR protein to follow the plasticity of a distinct chromatin marker between the Kc cells, where the five chromatin types were described, and a different sample, using the published data. Therefore, we chose histone H3K36me3 modification ChIP-chip dataset from Bell et al. (2010) and the modENCODE data obtained for this modification from 0 to 12-h embryos. In Kc cells, this modification was depleted in BLACK, BLUE and RED and enriched in GREEN and YELLOW chromatin types (Supplementary Fig. 6a). This pattern was highly specific and very distinct from the SUUR profile in these cells, thus H3K36me3 appeared appropriate for our test.
The same normalization steps were applied to these data as were used for SUUR DamID signals when we compared between the microarray platforms (“Materials and methods”). Namely, the data were averaged for each gene and the resulting dataset was quantile normalized to exactly reproduce the statistical characteristics of SUUR data distribution. Next, we determined the genes that changed their H3K36me3 levels between Kc cells and embryos using the same criteria as we used for SUUR to generate datasets for the Fig. 3a (see above). As a result, 383 genes decreased H3K36me3 level in embryos in comparison to Kc cells, while 488 genes revealed elevated levels of H3K36me3.
Then, we analyzed in which chromatin type these genes reside in Kc cells and observed a highly specific picture (Supplementary Fig. 6b). BLACK, RED, and YELLOW chromatin types did not show any significant variation of H3K36me3 levels. In contrast, genes from GREEN chromatin tended to lose H3K36me3, while, surprisingly, BLUE chromatin genes tended to accumulate H3K36me3 in embryos (Supplementary Fig. 6b). This picture was strikingly different from what we observed for SUUR protein (Fig. 5a), although the data were treated with the same analytic tools. This result implies that the pattern of SUUR variation in different chromatin types that we described above is highly specific and is not biased by the data processing algorithm used in our analysis.
The reduction of SUUR binding in the repressive regions of Kc cell genome could reflect formation of a more permissive chromatin state allowing their regulation in other samples. In turn, genes from the active chromatins that increased SUUR binding elsewhere could become repressed. Therefore, we checked if the observed variation in H3K27me3 level at the differentially SUUR-bound genes (Fig. 3c) is more attributable to some chromatin types than to others. Figure 5b shows that in the Kc–SG pair, the correlation between SUUR binding change and H3K27 methylation levels was observed within all chromatin types but GREEN. The lack of correlation between SUUR binding and H3K27me3 mark in GREEN chromatin type could be explained by the prevalence of HP1-SU(VAR)3-9 repressive mechanism.
Formation of permissive chromatin is not necessarily accompanied by gene activation (Schubeler et al. 2001), and even active YELLOW and RED chromatins contain a substantial fraction of silent genes (Filion et al. 2010). We asked if the observed transcriptional changes at the sites of SUUR variation (Fig. 3b and Supplementary Fig. 4) are the same in all chromatin types or are favored in some of them. To test this, we analyzed how SUUR binding variation correlates with the transcription of genes in different chromatin types. We classified the genes from the Fig. 3a according to the five-state model and used the rest of the genes as a reference (Fig. 5c). The genes from BLACK and BLUE chromatin types showed negative correlation between SUUR binding and transcription (Fig. 5c). RED and YELLOW chromatin types demonstrated this tendency only in the case of genes that increased SUUR binding in the brain, embryos, or salivary gland samples. Again, GREEN chromatin remained neutral to SUUR binding variation.
Discussion
Our data demonstrate that SUUR binding with the Drosophila chromosomes varies extensively in different cell types. Given that SUUR is one of the major determinants of repressed chromatin types (Filion et al. 2010), this confirms considerable developmental plasticity of chromosomal organization: repressed regions in one tissue may become open in other and vice versa (Hiratani et al. 2009; Kharchenko et al. 2011; Riddle et al. 2011). Indeed, we observed a pronounced correlation between SUUR binding dynamics and variation of H3K27me3 level when comparing Kc cells to salivary glands.
Nonetheless, the interplay between SUUR binding and histone H3 repressive methylation is quite intricate. From the cytological studies, we know that SUUR location only partially overlaps with the PcG sites on polytene chromosomes (Zhimulev et al. 2003) and high level of anti-SUUR immunostaining is observed in the pericentric heterochromatin (Makunin et al. 2002) that contains less H3K27 methylation and Polycomb Group proteins but is instead enriched in H3K9 methylation and HP1/Su(var)3-9 (GREEN chromatin in the five-state model (Filion et al. 2010)). Moreover, it was shown that SUUR may physically interact with HP1 (Pindyurin et al. 2008) and the genetic interaction between SuUR and Su(var)3-9 was reported (Demakova et al. 2007). Previous DamID experiments in Kc cells confirmed that PcG and HP1 often share their targets with SUUR (Pindyurin et al. 2007). This leads to the view that SUUR is a specific protein marker of silent genomic regions regardless of their repressive machinery.
It was recently shown that SuUR mutants manifest altered histone H3 methylation patterns. In particular, reduced levels of H3K9 mono- and trimethylation were observed specifically in pericentric regions of polytene chromosomes in SuUR mutants (Koryakov et al. 2011). Another study demonstrated that SuUR mutation led to the absence of the H3K27me3 mark in some specific regions of the polytene chromosomes though methylation in the other regions remained unaffected (Sher et al. 2012). These data suggest a role of SUUR in the functioning of both H3K9- and H3K27-methylated chromatins.
Existing data do not provide strong evidence allowing to clearly distinguish between the two possibilities: SUUR may play an active role in the regulation of repressive chromatin types or passively follow their developmental perturbations. Whichever scenario is correct, variation in SUUR binding clearly correlates with chromatin changes both in mutants (Koryakov et al. 2011; Sher et al. 2012) and in our developmental study.
In our work, we found that SUUR binding variation tends to correlate with gene activity. There are two lines of evidence regarding the role of SUUR in gene regulation that seem to be contradictory. First, SuUR mutation is perfectly viable (Belyaeva et al. 1998) and does not result in improper gene activation in the regions where it affects H3K27 methylation (Sher et al. 2012). Second, SuUR acts as a modifier of position-effect variegation (PEV) in various genetic assays (Belyaeva et al. 2003). The phenomenon of PEV may appear when a gene is relocated by the chromosome rearrangement into a different chromatin environment which influences its activity (for a review, see Elgin (1996), Wallrath (1998), Reuter and Spierer (1992), and Schotta et al. (2003)). In all the PEV systems tested, SUUR acted as a repressor on the gene placed into the silenced chromatin (Belyaeva et al. 2003).
Apparently, this conflict comes from the differences between the experimental systems in these two cases. It is known that chromatin does not unequivocally affect gene activity. A recent study demonstrated that variation of PcG proteins binding in different tissues did not necessarily lead to the activation of genes. Many genes associated with Pc in embryos but not in the T3 imaginal disc were related to the neural development and remained inactive in the disc even in the absence of Pc (Kwong et al. 2008). Similarly, SuUR mutation leads to reduction of H3K27me3 in some regions but does not result in activation of the underlying genes (Sher et al. 2012). Both of these examples support the idea that activation of developmentally regulated genes requires not only permissive chromatin but also the presence of specific activators.
The effect of SuUR in PEV model systems is distinct because transcriptional activators are actually present in the affected tissue, for example, all the needed activators are intact in the eye of w m4h strain demonstrating the variegation of w gene. In this case, the presence or absence of the PEV modifier would affect the chances for a variegating gene to be activated or remain repressed which is reflected, for example, as a number of pigmented facets in the Drosophila eye in the classic case of white gene variegation (Elgin 1996). In such systems, the SuUR mutation could affect these chances by decreasing the local concentration of repressive histone marks.
The effect revealed in our study is consistent with this idea. Although some genes that were clearly activated in a particular sample decreased SUUR binding, the majority of the genes did not show significant regulation upon the SUUR binding decrease. Thus, lower SUUR binding and accompanying chromatin perturbations probably allow selective activation of genes specific for this sample but are insufficient for the activation of other differentially bound genes that have different tissue specificities.
Our analysis showed that variability of SUUR binding with chromosomes is differently represented in the five chromatin types. As it turned out, BLUE and GREEN chromatins were likelier to lose SUUR, while RED chromatin tended to accumulate SUUR in other samples. Despite the fact that much like BLUE and RED, BLACK chromatin is enriched in developmentally regulated genes (Filion et al. 2010), it did not show predisposition for SUUR binding variation. This could mean that this chromatin type contains the genes that are to be activated in other Drosophila tissues that are beyond the focus of this study, for example, testis-specific genes (Belyakin et al. 2005, 2010).
The function of SUUR protein in the repressive chromatin remains elusive. Nevertheless, the recent data show that it interferes with replication fork progression at chorion gene clusters in follicular cells (Sher et al. 2012). This is in a good agreement with the phenotype in polytene chromosomes, where SuUR mutation affects the under-replicated regions allowing them to complete their replication (Belyaeva et al. 1998). Most recently, an association of SUUR with PCNA protein has been demonstrated, thereby confirming its involvement in the replication process (Kolesnikova et al. 2013). In this regard, the observed variability of SUUR binding could reflect the developmental regulation of replication timing in repressed chromatin types.
References
Bell O, Schwaiger M, Oakeley EJ, Lienert F, Beisel C, Stadler MB, Schubeler D (2010) Accessibility of the Drosophila genome discriminates PcG repression, H4K16 acetylation and replication timing. Nat Struct Mol Biol 17(7):894–900. doi:10.1038/nsmb.1825
Belyaeva ES, Zhimulev IF, Volkova EI, Alekseyenko AA, Moshkin YM, Koryakov DE (1998) Su(UR)ES: a gene suppressing DNA underreplication in intercalary and pericentric heterochromatin of Drosophila melanogaster polytene chromosomes. Proc Natl Acad Sci U S A 95(13):7532–7537
Belyaeva ES, Boldyreva LV, Volkova EI, Nanayev RA, Alekseyenko AA, Zhimulev IF (2003) Effect of the suppressor of underreplication (SuUR) gene on position-effect variegation silencing in Drosophila melanogaster. Genetics 165(3):1209–1220
Belyakin SN, Christophides GK, Alekseyenko AA, Kriventseva EV, Belyaeva ES, Nanayev RA, Makunin IV, Kafatos FC, Zhimulev IF (2005) Genomic analysis of Drosophila chromosome underreplication reveals a link between replication control and transcriptional territories. Proc Natl Acad Sci U S A 102(23):8269–8274
Belyakin SN, Babenko VN, Maksimov DA, Shloma VV, Kvon EZ, Belyaeva ES, Zhimulev IF (2010) Gene density profile reveals the marking of late replicated domains in the Drosophila melanogaster genome. Chromosoma 119(6):589–600. doi:10.1007/s00412-010-0280-y
Bischof J, Maeda RK, Hediger M, Karch F, Basler K (2007) An optimized transgenesis system for Drosophila using germ-line-specific phiC31 integrases. Proc Natl Acad Sci U S A 104(9):3312–3317
Bolstad BM, Irizarry RA, Astrand M, Speed TP (2003) A comparison of normalization methods for high density oligonucleotide array data based on variance and bias. Bioinformatics 19(2):185–193
Brand AH, Perrimon N (1993) Targeted gene expression as a means of altering cell fates and generating dominant phenotypes. Development 118(2):401–415
Chintapalli VR, Wang J, Dow JA (2007) Using FlyAtlas to identify better Drosophila melanogaster models of human disease. Nat Genet 39(6):715–720
da Huang W, Sherman BT, Lempicki RA (2009) Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat Protoc 4(1):44–57
de Wit E, Greil F, van Steensel B (2005) Genome-wide HP1 binding in Drosophila: developmental plasticity and genomic targeting signals. Genome Res 15(9):1265–1273
Demakova OV, Pokholkova GV, Kolesnikova TD, Demakov SA, Andreyeva EN, Belyaeva ES, Zhimulev IF (2007) The SU(VAR)3-9/HP1 complex differentially regulates the compaction state and degree of underreplication of X chromosome pericentric heterochromatin in Drosophila melanogaster. Genetics 175(2):609–620. doi:10.1534/genetics.106.062133
Ebert A, Lein S, Schotta G, Reuter G (2006) Histone modification and the control of heterochromatic gene silencing in Drosophila. Chromosom Res 14(4):377–392
Eisen MB, Spellman PT, Brown PO, Botstein D (1998) Cluster analysis and display of genome-wide expression patterns. Proc Natl Acad Sci U S A 95(25):14863–14868
Elgin SC (1996) Heterochromatin and gene regulation in Drosophila. Curr Opin Genet Dev 6(2):193–202
Ernst J, Kellis M (2010) Discovery and characterization of chromatin states for systematic annotation of the human genome. Nat Biotechnol 28(8):817–825
Filion GJ, van Bemmel JG, Braunschweig U, Talhout W, Kind J, Ward LD, Brugman W, de Castro IJ, Kerkhoven RM, Bussemaker HJ, van Steensel B (2010) Systematic protein location mapping reveals five principal chromatin types in Drosophila cells. Cell 143(2):212–224
Graveley BR, Brooks AN, Carlson JW, Duff MO, Landolin JM, Yang L, Artieri CG, van Baren MJ, Boley N, Booth BW, Brown JB, Cherbas L, Davis CA, Dobin A, Li R, Lin W, Malone JH, Mattiuzzo NR, Miller D, Sturgill D, Tuch BB, Zaleski C, Zhang D, Blanchette M, Dudoit S, Eads B, Green RE, Hammonds A, Jiang L, Kapranov P, Langton L, Perrimon N, Sandler JE, Wan KH, Willingham A, Zhang Y, Zou Y, Andrews J, Bickel PJ, Brenner SE, Brent MR, Cherbas P, Gingeras TR, Hoskins RA, Kaufman TC, Oliver B, Celniker SE (2011) The developmental transcriptome of Drosophila melanogaster. Nature 471(7339):473–479
Greil F, Moorman C, van Steensel B (2006) DamID: mapping of in vivo protein–genome interactions using tethered DNA adenine methyltransferase. Methods Enzymol 410:342–359
Hiratani I, Takebayashi S, Lu J, Gilbert DM (2009) Replication timing and transcriptional control: beyond cause and effect—part II. Curr Opin Genet Dev 19(2):142–149
Kharchenko PV, Alekseyenko AA, Schwartz YB, Minoda A, Riddle NC, Ernst J, Sabo PJ, Larschan E, Gorchakov AA, Gu T, Linder-Basso D, Plachetka A, Shanower G, Tolstorukov MY, Luquette LJ, Xi R, Jung YL, Park RW, Bishop EP, Canfield TK, Sandstrom R, Thurman RE, MacAlpine DM, Stamatoyannopoulos JA, Kellis M, Elgin SC, Kuroda MI, Pirrotta V, Karpen GH, Park PJ (2011) Comprehensive analysis of the chromatin landscape in Drosophila melanogaster. Nature 471(7339):480–485
Kolesnikova TD, Andreeva EN, Pindiurin AV, Anan'ko NG, Beliakin SN, Shloma VV, Iurlova AA, Makunin IV, Pokholkova GV, Volkova EI, Zarutskaia EA, Kokoza EB, Seneshin VF, Beliaeva ES, Zhimulev IF (2006) Contribution of the SuUR gene to the organization of epigenetically repressed regions of Drosophila melanogaster chromosomes. Genetika 42(8):1013–1028
Kolesnikova TD, Posukh OV, Andreyeva EN, Bebyakina DS, Ivankin AV, Zhimulev IF (2013) Drosophila SUUR protein associates with PCNA and binds chromatin in a cell cycle-dependent manner. Chromosoma 122(1–2):55–66. doi:10.1007/s00412-012-0390-9
Koryakov DE, Walther M, Ebert A, Lein S, Zhimulev IF, Reuter G (2011) The SUUR protein is involved in binding of SU(VAR)3-9 and methylation of H3K9 and H3K27 in chromosomes of Drosophila melanogaster. Chromosom Res 19(2):235–249. doi:10.1007/s10577-011-9193-8
Kwong C, Adryan B, Bell I, Meadows L, Russell S, Manak JR, White R (2008) Stability and dynamics of polycomb target sites in Drosophila development. PLoS Genet 4(9):e1000178. doi:10.1371/journal.pgen.1000178
Luo SD, Shi GW, Baker BS (2011) Direct targets of the D. melanogaster DSXF protein and the evolution of sexual development. Development 138(13):2761–2771
Makunin IV, Volkova EI, Belyaeva ES, Nabirochkina EN, Pirrotta V, Zhimulev IF (2002) The Drosophila suppressor of underreplication protein binds to late-replicating regions of polytene chromosomes. Genetics 160(3):1023–1034
Markstein M, Pitsouli C, Villalta C, Celniker SE, Perrimon N (2008) Exploiting position effects and the gypsy retrovirus insulator to engineer precisely expressed transgenes. Nat Genet 40(4):476–483. doi:10.1038/ng.101
Nordman J, Li S, Eng T, Macalpine D, Orr-Weaver TL (2011) Developmental control of the DNA replication and transcription programs. Genome Res 21(2):175–181. doi:10.1101/gr.114611.110
Pindyurin AV, Moorman C, de Wit E, Belyakin SN, Belyaeva ES, Christophides GK, Kafatos FC, van Steensel B, Zhimulev IF (2007) SUUR joins separate subsets of PcG, HP1 and B-type lamin targets in Drosophila. J Cell Sci 120(Pt 14):2344–2351
Pindyurin AV, Boldyreva LV, Shloma VV, Kolesnikova TD, Pokholkova GV, Andreyeva EN, Kozhevnikova EN, Ivanoschuk IG, Zarutskaya EA, Demakov SA, Gorchakov AA, Belyaeva ES, Zhimulev IF (2008) Interaction between the Drosophila heterochromatin proteins SUUR and HP1. J Cell Sci 121(Pt 10):1693–1703. doi:10.1242/jcs.018655
Reuter G, Spierer P (1992) Position effect variegation and chromatin proteins. Bioessays 14(9):605–612. doi:10.1002/bies.950140907
Riddle NC, Minoda A, Kharchenko PV, Alekseyenko AA, Schwartz YB, Tolstorukov MY, Gorchakov AA, Jaffe JD, Kennedy C, Linder-Basso D, Peach SE, Shanower G, Zheng H, Kuroda MI, Pirrotta V, Park PJ, Elgin SC, Karpen GH (2011) Plasticity in patterns of histone modifications and chromosomal proteins in Drosophila heterochromatin. Genome Res 21(2):147–163. doi:10.1101/gr.110098.110
Roudier F, Ahmed I, Berard C, Sarazin A, Mary-Huard T, Cortijo S, Bouyer D, Caillieux E, Duvernois-Berthet E, Al-Shikhley L, Giraut L, Despres B, Drevensek S, Barneche F, Derozier S, Brunaud V, Aubourg S, Schnittger A, Bowler C, Martin-Magniette ML, Robin S, Caboche M, Colot V (2011) Integrative epigenomic mapping defines four main chromatin states in Arabidopsis. Embo J 30(10):1928–1938
Schotta G, Ebert A, Dorn R, Reuter G (2003) Position-effect variegation and the genetic dissection of chromatin regulation in Drosophila. Semin Cell Dev Biol 14(1):67–75
Schubeler D, Groudine M, Bender MA (2001) The murine beta-globin locus control region regulates the rate of transcription but not the hyperacetylation of histones at the active genes. Proc Natl Acad Sci U S A 98(20):11432–11437
Schwartz YB, Pirrotta V (2007) Polycomb silencing mechanisms and the management of genomic programmes. Nat Rev Genet 8(1):9–22. doi:10.1038/nrg1981
Sher N, Bell GW, Li S, Nordman J, Eng T, Eaton ML, Macalpine DM, Orr-Weaver TL (2012) Developmental control of gene copy number by repression of replication initiation and fork progression. Genome Res 22(1):64–75. doi:10.1101/gr.126003.111
Siegal ML, Hartl DL (2000) Application of Cre/loxP in Drosophila. Site-specific recombination and transgene coplacement. Methods Mol Biol 136:487–495. doi:10.1385/1-59259-065-9:487
Struhl G, Basler K (1993) Organizing activity of wingless protein in Drosophila. Cell 72(4):527–540
Thomas S, Li XY, Sabo PJ, Sandstrom R, Thurman RE, Canfield TK, Giste E, Fisher W, Hammonds A, Celniker SE, Biggin MD, Stamatoyannopoulos JA (2011) Dynamic reprogramming of chromatin accessibility during Drosophila embryo development. Genome Biol 12(5):R43
van Steensel B, Henikoff S (2000) Identification of in vivo DNA targets of chromatin proteins using tethered dam methyltransferase. Nat Biotechnol 18(4):424–428
Wallrath LL (1998) Unfolding the mysteries of heterochromatin. Curr Opin Genet Dev 8(2):147–153
Yang IV, Chen E, Hasseman JP, Liang W, Frank BC, Wang S, Sharov V, Saeed AI, White J, Li J, Lee NH, Yeatman TJ, Quackenbush J (2002) Within the fold: assessing differential expression measures and reproducibility in microarray assays. Genome Biol 3(11):research0062
Zhimulev IF, Belyaeva ES, Makunin IV, Pirrotta V, Volkova EI, Alekseyenko AA, Andreyeva EN, Makarevich GF, Boldyreva LV, Nanayev RA, Demakova OV (2003) Influence of the SuUR gene on intercalary heterochromatin in Drosophila melanogaster polytene chromosomes. Chromosoma 111(6):377–398
Acknowledgments
We thank Dr. Tim Westwood and Canadian Drosophila Microarray Center who performed microarray hybridizations and Dr. Bas van Steensel for Dam-containing vectors. The datasets for this paper have been deposited at NCBI under the reference series GSE33873. The work was supported by the Russian Foundation for Basic Research grants 12-04-00160, 12-04-33080, and 12-01-31128 and by the Program of Presidium of the Russian Academy of Sciences “Molecular and Cellular Biology” (grant no. 6.3).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary Figure 1
hsp70 promoter from pUAST vector ensures a very low level of Dam-Myc expression. Western blot detection of Dam-Myc protein with the anti-Myc-tag antibodies in salivary glands of y,w strain (negative control, first lane), hsp70 > Dam-Myc strain (second lane) and in hsp70 > Dam-Myc strain induced with salivary gland-specific AB1 Gal4 driver (positive control, third lane). anti-Tubuline antibodies were used as a load control. Asterisk shows a non-specific band appearing due to over-exposure of the membrane. (JPEG 69 kb)
Supplementary Figure 2
Effect of data normalization. A – distribution of the raw DamID data in the four samples. B – the same after the normalization step. Scale – Log2(Dam-Myc-SUUR/Dam-Myc). C – K-means clustering of the normalized data in the four samples reveals a substantial variation of SUUR binding in different cell types. (JPEG 750 kb)
Supplementary Figure 3
Examples of SUUR profile variability in different biological samples. The colors and legends are the same as on the Fig. 1. (JPEG 496 kb)
Supplementary Figure 4
Correlation of SUUR binding dynamics and gene activity in six pairs of samples. The legend is the same as in Fig. 3c. (JPEG 581 kb)
Supplementary Figure 5
Variability of SUUR binding near the borders and towards the centers of domains in different chromatin types. Portions of differentially bound genes in 1 kb bins starting from the borders of the domains of different types are presented. No differences from the distributions presented in the Fig. 5a were found indicating that observed variation of SUUR binding is not a bias stemming from the domain borders. (JPEG 457 kb)
Supplementary Figure 6
A test study of H3K36me3 variability between Kc cells and 0-12h embryos. A – distribution of H3K36me3 histone mark across five chromatin types. B – variability of H3K36me3 levels in five chromatin types assessed with the same analytic tools as for SUUR. GREEN chromatin tends to loose H3K36me3 in embryos while BLUE chromatin accumulates H3K36me3. Other chromatin types do not follow this pattern. This picture is strikingly different from the observed variability of SUUR (Fig. 5a). (JPEG 227 kb)
Supplementary Table 1
(XLS 2334 kb)
Supplementary Table 2
(XLS 34 kb)
Supplementary Table 3
(XLS 42 kb)
Supplementary Table 4
(XLS 149 kb)
Supplementary Table 5
(XLS 117 kb)
Rights and permissions
About this article
Cite this article
Maksimov, D.A., Koryakov, D.E. & Belyakin, S.N. Developmental variation of the SUUR protein binding correlates with gene regulation and specific chromatin types in D. melanogaster . Chromosoma 123, 253–264 (2014). https://doi.org/10.1007/s00412-013-0445-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00412-013-0445-6