Abstract
Purpose
Sarcoidosis is a systemic inflammatory disease with unknown etiology involving several organs. Myocardial involvement, pericarditis, severe rhythm abnormalities, and heart valve disease due to papillary muscle dysfunction are some of the cardiac manifestations. Conventional echocardiographic methods remain insufficient for the determination of subclinical myocardial dysfunction in patients with sarcoidosis. In our study, we investigated the impact of sarcoidosis on bi-ventricular and atrial functions using two-dimensional (2D) speckle tracking echocardiography (STE).
Methods
Forty patients with sarcoidosis and 20 age and sex-matched controls were recruited into study. All subjects underwent a transthoracic echocardiography for the evaluation of ventricular and atrial functions with 2D STE.
Results
Left ventricular (LV) dimensions, LV ejection fraction, and right ventricular (RV) systolic velocity were similar between the two groups. Left atrial (LA) diameter was significantly higher in sarcoidosis patients than controls. Eighteen (45 %) patients in the sarcoidosis group and 1 (5 %) patient in the control group had LV diastolic dysfunction. LV global longitudinal, radial, circumferential strain, twist, untwists, and RV global longitudinal strain values were significantly lower in sarcoidosis patients compared to controls. LA and RA reservoir functions were also significantly lower in sarcoidosis patients than controls.
Conclusion
Although impaired LV diastolic function was detected using conventional parameters, only novel advanced echocardiographic modalities demonstrated impaired bi-ventricular and atrial mechanical functions in patients with sarcoidosis.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Sarcoidosis is a systemic inflammatory disease involving several organs whose etiology is unknown [1]. Cardiac manifestation is one of the important causes of morbidity and mortality due to fatal arrhythmia, as well as congestive heart failure [2]. Cardiac involvement has been demonstrated in 20–50 % of patients with sarcoidosis in autopsy series [1–3]. Early diagnosis and treatment of cardiac involvement is important because the clinical course remains asymptomatic until sudden cardiac death.
Conventional transthoracic echocardiography has been used as a method in the evaluation of cardiac sarcoidosis [4]. Cardiac functions remain normal in the early stages of the disease. Therefore, conventional echocardiographic techniques are inadequate when it comes to demonstrating subclinical forms of the disease [5]. Detection of myocardial involvement by two-dimensional (2D) speckle tracking echocardiography (STE) is a new technique for evaluating myocardial functions. Previously, it has been shown that 2D STE is a useful method for the detection of subclinical myocardial involvement in patients with sarcoidosis [6]. The aim of this study is to investigate the impact of sarcoidosis on bi-ventricular and atrial mechanics using 2D STE.
Materials and Methods
Study Population
The study population consisted of 45 consecutive patients with diagnosed sarcoidosis. The diagnosis of sarcoidosis was based on ATS/ERS/WASOG statement criteria on sarcoidosis [7]. All study population underwent a complete transthoracic echocardiography for the evaluation of cardiac functions with 2D STE. Patients were excluded from the study if they were known to have poor echogenicity, impaired left ventricular (LV) systolic function (ejection fraction (EF) < 55 %), significant valvular disease, history of coronary artery disease, malignancy, systemic arterial hypertension, storage disorders such as Fabry’s disease, and cardiac amyloidosis. Three patients had poor echogenicity and two patients had systemic arterial hypertension. After applying the exclusionary criteria, the remaining 40 patients with sarcoidosis were included in the study. The control group included 20 age and sex matched volunteers free of cardiovascular risk factors and without any cardiac and systemic disease. The investigation complies with the principles outlined in the Declaration of Helsinki. The study was approved by the local ethics committee and all participants gave written informed consent before participating.
Standard Echocardiography and 2D Speckle Tracking Echocardiography
All patients underwent a complete transthoracic echocardiographic examination with a commercially available echocardiography device (Vivid 7, GE Vingmed Ultrasound AS, Horten, Norway) by a single experienced cardiologist. Data acquisition was performed with a 3.5-MHz transducer at a depth of 16 cm in the parasternal and apical views (standard parasternal short-axis from midventricular level, apical long-axis, two-chamber, and four-chamber images). Standard M-mode, 2D, and color-coded tissue Doppler imaging (TDI) parameters were obtained during breath hold, stored in cine loop format from three consecutive beats and transferred to a workstation for further offline analysis (EchoPAC 6.1; GE Vingmed Ultrasound AS). Gain settings, filters, and pulse repetitive frequency were adjusted to optimize color saturation, and a color Doppler frame scanning rate of 100- 140 Hz was used for color TDI images and greyscale images at a frame rate of 44–82 frames/s. Conventional echocardiographic parameters were measured according to the guidelines of the American Society of Echocardiography (ASE) and LV-EF was calculated using the biplane Simpson’s method [8]. Diastolic flow velocity at the LV inflow tract was recorded with pulsed wave Doppler echocardiography as previously described [9]. In the apical four-chamber view, the pulsed wave Doppler sample volume was placed in the middle of the LV inflow tract 1 cm below the plane of the mitral annulus between the tips of the mitral leaflets, where maximal flow velocity in early (E-wave) and late diastole (A-wave) was recorded. Tissue Doppler imaging was performed in the apical views (four-chamber and long-axis) for the long-axis motion of the LV as previously described [10]. The following tissue Doppler imaging (TDI)-derived signals were obtained from the average of the basal segment of septum and lateral wall: peak systolic velocity (LVs), early diastolic velocity (LVe), and late diastolic velocity (LVa). The early diastolic mitral inflow velocity (E) and LVe velocity ratio (E/Le) were calculated. Sample volume was placed at the lateral tricuspid annulus, and tricuspid annulus TDI peak systolic velocity (RVs) was measured to assess right ventricle systolic function. LV diastolic dysfunction (DD) was defined by the presence of at least two abnormal parameters of diastolic function according to ASE guidelines [8]. Pulmonary artery systolic pressure was estimated from tricuspid regurgitation jet by continuous wave Doppler.
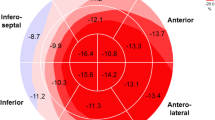
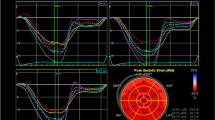
Multidirectional analysis of LV strain (in the radial, circumferential, and longitudinal directions) was performed by 2D STE as previously described [11, 12]. Apical 4-chamber, 2-chamber, and long-axis views were used for LV longitudinal strain analysis while parasternal short-axis (SAX) views at three levels (the base, the level of papillary muscles, and the apex) were used for circumferential and radial strain, twist, and untwist analysis. End-systolic regions of interest were traced on the endocardial cavity (minimum cavity area) using a point-and-click approach with special care taken to adjust the tracking of all endocardial segments. A second larger concentric circle was then automatically generated and manually adjusted near the epicardium. Software automatically analyzed frame-by-frame the movement of the speckles over the cardiac cycle. Each image was divided into six standard segments, and the corresponding strain curves were obtained. The mean strain values were calculated for each SAX view, apical long axis, apical 4- and 2-chamber views, as the sum of end-systolic strain values in six segments, divided by six. LV global longitudinal strain (GLS) was derived from the average of longitudinal strain values in the apical 4-chamber, 2-chamber, and long-axis views. The assessment of LV global radial (GRS) and circumferential strain (GCS) was performed by applying 2D speckle tracking imaging to the parasternal short-axis views of the LV. The midventricular short-axis of the LV was divided into six segments, and the values of GRS and GCS were derived from the average of the six segmental peak systolic strain values.
Two-dimensional speckle tracking analysis of parasternal SAX views at the base and the apex, revealed LV ‘rotation’ and ‘rotation rate’ curves as the angular displacement and the velocity of displacement of the LV around its central axis. Negative values indicated clockwise while positive values indicated counterclockwise rotation. LV ‘twist’ was defined as the net difference of LV peak systolic ‘rotation’ between basal (clockwise) and apical (counterclockwise) short-axis planes. Value was expressed in ‘°.’ LV ‘untwist’ was expressed as a diastolic angular motion of the LV, opposite to twist. Value was expressed in (°/s).
For right ventricular (RV) analysis, free wall endocardial border of the RV was manually tracked on the end-systolic frame and epicardial border was detected by the software. Strain curves and percentage of longitudinal deformation at basal, mid, and apical segments of RV free wall were obtained automatically. The average of RV free wall longitudinal strain values was accepted as RV global longitudinal strain (RV GLS).
For the left atrial (LA) and right atrial (RA) speckle tracking analysis, atrial-focused images in apical four-chamber view were obtained. A minimum frame rate of 40 frames per second was required for the reliable operation of this program. For two-dimensional speckle tracking strain analysis, a line was manually drawn along the LA and RA endocardial border of the apical four-chamber view after atrial contraction, when the atrium was at its minimum volume, using the point-and-click approach as previously described [11]. The software then automatically generated additional lines near the atrial epicardium and midmyocardial line, with the narrowest region of interest (ROI). The ROI then included the entire atrial myocardial wall, and a click feature increased or decreased the widths between endocardial and epicardial line for thicker or thinner walls, respectively. The software generated strain curves for each atrial segment. The value of peak early and late diastolic longitudinal strain were determined as LA or RA reservoir and conduit functions.
Statistical Analysis
Statistical analyses were performed using SPSS 16.0 statistical package for Windows. Continuous data were expressed as mean ± standard deviation while categorical data were presented as number of patients. Chi-square test was used for comparison of categorical variables while student T test or Mann–Whitney U test were used to compare parametric and nonparametric continuous variables, respectively. Normal distribution was assessed by Kolmogorov–Smirnov test. A value of p < 0.05 was considered statistically significant.
For the assessment of intraobserver variability, the analyses were repeated twice by the same observer 1 day later. For the interobserver variability assessment, a second independent observer repeated the analyses. Intraobserver variability was calculated by the average difference between the ten measurements realized. Interobserver variability was calculated as the absolute difference divided by the average of the two observations for all parameters. The intraobserver and interobserver variabilities were 5.6 and 6.2 %, respectively in our study.
Results
Age and sex distribution were similar between patients with sarcoidosis and controls (mean age 46.4 ± 10.5 years vs 41.9 ± 12.4 years, p = 0.209 and female/male 34/6 vs 17/3, p = 1, respectively). Body surface area was also similar between the two groups (1.78 ± 0.14 m2 for patient group and 1.74 ± 0.11 m2 for controls, p = 0.255). New York Heart Association (NYHA) Functional Classification of study population was as follows: 34 patients and 18 controls were NHYA Class 1, 6 patients and 2 controls were NHYA Class 2 (p = 0.591).
A comparison of the transthoracic echocardiographic parameters between the two groups is shown in Table 1. LV dimensions, EF, and RVs were similar between the two groups. LAD was higher in patients with sarcoidosis. Sarcoidosis patients also had significantly impaired LV diastolic function compared to controls. Eighteen (45 %) patients in the sarcoidosis group and one (5 %) patient in the control group exhibited impaired LV diastolic function (p = 0.002). Maximal velocity of tricuspid regurgitation was higher in patients with sarcoidosis than in controls (2.6 ± 0.3 m/s vs 2.3 ± 0.4 m/s, p = 0.039, respectively). Sarcoidosis patients with LV DD were older compared to sarcoidosis patients without LV DD (54.2 ± 6.2 years vs 40.1 ± 9.1 years, p < 0.001, respectively). Meanwhile, sex distribution was similar between the two groups (1 male in patients with LV DD, 5 males in patients without LV DD, p = 0.197). Patients with LV DD had higher LAD values than patients without LV DD (3.4 ± 0.2 cm vs 3.0 ± 0.4 cm, p = 0.002). Other conventional echocardiographic parameters were similar between the two groups.
A comparison of the 2D STE parameters between the two groups is shown in Table 2. LV GLS, GCS, GRS, twist, untwist, and RV GLS were significantly lower in patients with sarcoidosis than in controls (−21.9 ± 3.3 vs −25.7 ± 3.0, p < 0.001; −21.1 ± 3.6 vs −25.4 ± 3.1, p < 0.001; 45.0 ± 11.0 vs 49.7 ± 5.9, p = 0.015; 24.7 ± 7.3 vs 31.5 ± 8.7, p = 0.008; −126.6 ± 31.7 vs −142.9 ± 30.7, p = 0.046; and −29.2 ± 6.2 vs −35.0 ± 7.3, p = 0.004, respectively). Meanwhile, LA and RA reservoir functions were significantly lower in sarcoidosis patients than controls (31.5 ± 7.2 vs 39.1 ± 11.0, p = 0.006 and 35.2 ± 9.2 vs 42.3 ± 10.8, p = 0.019, respectively), while the LA and RA conduit functions were similar in both groups (14.1 ± 2.6 vs 14.3 ± 3.3, p = 0.962 and 16.7 ± 4.4 vs 17.1 ± 5.0, p = 0.832, respectively). We also evaluated the 2D STE parameters between sarcoidosis patients with LV DD and sarcoidosis patients without LVDD. All 2D STE parameters were similar between the two groups (Table 3).
Discussion
In this study, we demonstrated that when compared with normal subjects, 2D STE-derived LV systolic and diastolic and RV systolic function indices were significantly lower in patients with sarcoidosis. Bi-atrial reservoir function was also impaired in sarcoidosis patients. Our results suggest that 2D STE is a useful method for the early detection of LV, RV, and LA involvement in patients with sarcoidosis.
Sarcoidosis is a systemic granulomatous disease that affects several organs such as the lung, heart, skin, and eyes. Although cardiac sarcoidosis has been shown in 20–50 % of patients with sarcoidosis, it may be responsible approximately to 85 % of deaths related to sarcoidosis [13]. Therefore, early detection of cardiac involvement is very important in this patient group. Myocardial involvement, pericarditis, severe rhythm abnormalities, and heart valve disease due to papillary muscle dysfunction are major cardiac manifestations of cardiac sarcoidosis [14]. As most patients with cardiac sarcoidosis remain asymptomatic, a detailed evaluation of these patients is essential.
Psychical examination, electrocardiography, and transthoracic echocardiography are the initial methods used in outpatient clinical settings. Transthoracic echocardiography can detect cardiac involvement in up to 31 % of cardiac sarcoidosis patients [15, 16]. Previous studies have shown that pulmonary sarcoidosis is associated with LV DD [17, 18]. Aydin Kaderli et al. demonstrated that sarcoidosis patients had impaired diastolic function compared to healthy controls [19]. In another study by Fahy et al., showed that in addition to impaired echocardiographic parameters, sarcoidosis patients with LV DD had longer disease duration compared to patients without LV DD [20]. In our study, we also demonstrated that almost half of the patients with sarcoidosis had impaired LV diastolic function. These patients also had higher LA diameter measurements and impaired 2D STE–derived LA reservoir function, suggesting prolonged exposure to high LV end-diastolic pressures. Some confounding factors, including age, disease duration, infiltration of noncaseating granulomas in the myocardial interstitium, and coronary microangiopathy with consequent ischemia, might result in LV DD [20]. Sarcoidosis patients with LV DD in our group were older and had longer disease duration, which was consistent with the literature.
Two-dimensional STE is a novel technique that has been used for the detection of subclinical myocardial dysfunction in several systemic diseases [11, 12, 21]. The underlying mechanism responsible for decreased strain parameters in patients with sarcoidosis is controversial. Sarcoid myocardial infiltration usually occurs initially in the midmyocardial layer of the LV wall, suggesting early impairment of LV longitudinal strain [6]. Midmyocardial circumferential fibers are responsible for radial and circumferential shortening. Increased fibrosis in the midmyocardium and/or transmural myocardial damage may cause prominent radial and circumferential deformation. Our study also demonstrated impaired radial and circumferential strain in sarcoidosis patients. In a recent study, Aggeli et al. [6] showed significantly lower LV GLS and higher twist in patients with sarcoidosis. In our study, we demonstrated that LV GLS, twist, and untwist were lower in sarcoidosis patients compared to controls, suggesting early subclinical cardiac involvement. Kul et al. [22] showed that patients with pulmonary sarcoidosis have reduced cardiac contractility, independent of structural cardiac lesions observed through cardiac magnetic resonance imaging (CMRI), while patients with structural lesions have a more pronounced drop in strain parameters. However, these authors only used LV longitudinal and circumferential strain parameters for the evaluation of LV functions using 2D STE. Therefore, our study results provided comprehensive information about cardiac functions using 2D STE, including both ventricular and atrial functions.
Although 2D STE has been validated in the evaluation of LV function [11, 12], it is also useful in the evaluation of RV function [23, 24]. However, there are no data about RV function assessed using 2D STE in patients with sarcoidosis. In our study, we demonstrated that sarcoidosis patients had lower RV GLS, although RVs were similar between the two groups. Our results suggest that sarcoidosis not only affects LV myocardial function, but also RV myocardial function. Several mechanisms may contribute to RV myocardial dysfunction. RV free wall myocardial involvement may be one possible explanation. Patients with sarcoidosis also had higher pulmonary arterial systolic pressures responsible for RV systolic impairment. Prolonged exposure to elevated LV pressures might also impair the RV systolic function. Early detection of RV systolic deterioration in patients with cardiac sarcoidosis is important. However, conventional methods, including electrocardiography and transthoracic echocardiography, may be insufficient to distinguish cardiac sarcoidosis from other cardiac diseases. Lønborg et al. demonstrated that CMRI is a useful method for the detection of sarcoid infiltration of RV using late gadolinium enhancement [25]. Future prospective studies comparing CMRI and STE in evaluation of cardiac sarcoidosis are needed.
Previous studies have shown that atrial enlargement is closely related with LV diastolic dysfunction under several conditions [26, 27]. Atrial mechanical function can be successfully assessed using 2D STE [28]. This modality has revealed valuable information about the pathophysiological mechanisms of atrial remodeling [29, 30]. We also demonstrated impaired left and right atrial reservoir functions in patients with sarcoidosis. This might be due to direct atrial involvement or secondary to prolonged exposure to elevated LV filling pressures. Further research is needed on the consequences of atrial involvement in sarcoidosis patients.
Our research has important clinical implications. Although previous studies investigated LV function using 2D STE, we investigated both ventricles and atriums in sarcoidosis patients. Therefore, our study provided comprehensive data about cardiac functions in patients with sarcoidosis. We demonstrated that 2D STE may be used to detect subclinical cardiac dysfunction in patients with sarcoidosis, even when cardiac functions assessed by conventional transthoracic echocardiography were in the normal range in these patients. Therefore, clinicians should pay more attention in evaluating patients with sarcoidosis due to the subclinical effects of this disease on cardiac functions.
Study Limitations
A major limitation of this study is the small sample size of our study group. Our study also employed a cross-sectional design. Therefore, we did not followup the patients to obtain prognostic data. Such data might clarify the association between 2D STE-derived mechanical abnormalities and the course of sarcoid disease. The study would have been even more helpful had it been possible to include other data, such as clinical condition, arterial blood gases, chest X-rays, and pulmonary function tests. Future studies should include these parameters and evaluate the relationship with the 2D STE indices. Finally, we have no CMRI data for our patients. CMRI data might be useful in the verification of echocardiography-derived mechanical indices in patients with sarcoidosis. Further large-scale prospective studies are needed to demonstrate the prognostic value of 2D STE in sarcoidosis patients.
Conclusions
Although LV diastolic dysfunction was detected using conventional parameters, only novel advanced echocardiographic modalities demonstrated impaired bi-ventricular and atrial mechanical functions in patients with sarcoidosis. Two-dimensional speckle tracking echocardiography might be useful in the clinical evaluation of these patients.
References
Newman LS, Rose CS, Maier LA (1997) Sarcoidosis. N Engl J Med 336(17):1224–1234
Pierre-Louis B, Prasad A, Frishman WH (2009) Cardiac manifestations of sarcoidosis and therapeutic options. Cardiol Rev 17(4):153–158
Deng JC, Baughman RP, Lynch JP 3rd (2002) Cardiac involvement in sarcoidosis. Semin Respir Crit Care Med 23(6):513–527
Dubrey SW, Falk RH (2010) Diagnosis and management of cardiac sarcoidosis. Prog Cardiovasc Dis 52(4):336–346
Sharma S (2009) Cardiac imaging in myocardial sarcoidosis and other cardiomyopathies. Curr Opin Pulm Med 15(5):507–512
Aggeli C, Felekos I, Tousoulis D et al (2013) Myocardial mechanics for the early detection of cardiac sarcoidosis. Int J Cardiol 168(5):4820–4821
Hunnunghake WG, Costabel U, Ando M et al (1999) ATS/ERS/WASOG statement on sarcoidosis. Sarcoidosis Vasc Diffuse Lung Dis 16:149–173
Cheitlin MD, Armstrong WF, Aurigemma GP et al (2003) ACC/AHA/ASE 2003 guideline update for the clinical application of echocardiography: summary article. A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (ACC/AHA/ASE Committee to Update the 1997 Guidelines for the Clinical Application of Echocardiography). J Am Soc Echocardiogr 16:1091–1110
Nishimura RA, Abel MD, Hatle LK et al (1989) Assessment of diastolic function of the heart: background and current applications of Doppler echocardiography. Part II. Clinical studies. Mayo Clin Proc 64:181–204
Yu CM, Lin H, Yang H et al (2002) Progression of systolic abnormalities in patients with “isolated” diastolic heart failure and diastolic dysfunction. Circulation 105:1195–1201
Delgado V, Ypenburg C, van Bommel RJ et al (2008) Assessment of left ventricular dyssynchrony by speckle tracking strain imaging comparison between longitudinal, circumferential, and radial strain in cardiac resynchronization therapy. J Am Coll Cardiol 51:1944–1952
Donal E, Tournoux F, Leclercq C et al (2008) Assessment of longitudinal and radial ventricular dyssynchrony in ischemic and nonischemic chronic systolic heart failure: a two-dimensional echocardiographic speckle-tracking strain study. J Am Soc Echocardiogr 21:58–65
Virmani R, Bures JC, Roberts WC (1980) Cardiac sarcoidosis: a major cause of sudden death in young individuals. Chest 77:423–428
Alper AT, Güngör B, OzpamukKaradeniz F et al (2013) Malignant ventricular arrhythmia as the first manifestation of cardiac sarcoidosis. Turk Kardiyol Dern Ars 41(6):561
Lewin RF, Mor R, Spitzer S et al (1985) Echocardiographic evaluation of patients with systemic sarcoidosis. Am Heart J 110:116–122
Burstow DJ, Tajik J, Bailey KR et al (1989) Two-dimensional echocardiographic findings in systemic sarcoidosis. Am J Cardiol 63:478–482
Angomachalelis N, Hourzamanis A, Vamvalis C et al (1992) Doppler echocardiographic evaluation of left ventricular diastolic function in patients with systemic sarcoidosis. Postgrad Med J68:S52–S56
Sköld JM, Larsen FF, Rasmussen E et al (2002) Determination of cardiac involvement in sarcoidosis by magnetic resonance imaging and Doppler echocardiography. J Intern Med 252:465–471
Aydin Kaderli A, Gullulu S, Coskun F et al (2010) Impaired left ventricular systolic and diastolic functions in patients with early grade pulmonary sarcoidosis. Eur J Echocardiogr 11(10):809–813
Fahy GJ, Marwick T, McCreery CJ et al (1996) Doppler echocardiographic detection of left ventricular diastolic dysfunction in patients with pulmonary sarcoidosis. Chest 109(1):62–66
Tigen K, Sunbul M, Karaahmet T et al (2014) Left ventricular and atrial functions in hypertrophic cardiomyopathy patients with very high LVOT gradient: a speckle tracking echocardiographic study. Echocardiography 31(7):833–841
Kul S, Ozcelik HK, Uyarel H et al (2014) Diagnostic value of strain echocardiography, galectin-3, and tenascin-C levels for the identification of patients with pulmonary and cardiac sarcoidosis. Lung 192(4):533–542
Cameli M, Righini FM, Lisi M et al (2014) Right ventricular strain as a novel approach to analyze right ventricular performance in patients with heart failure. Heart Fail Rev 19(5):603–610
Kannan A, Poongkunran C, Jayaraj M et al (2014) Role of strain imaging in right heart disease: a comprehensive review. J Clin Med Res 6(5):309–313
Lønborg J, Ward M, Gill A et al (2013) Utility of cardiac magnetic resonance in assessing right-sided heart failure in sarcoidosis. BMC Med Imaging 13:2
Cuspidi C, Negri F, Sala C et al (2012) Association of left atrial enlargement with left ventricular hypertrophy and diastolic dysfunction: a tissue Doppler study in echocardiographic practice. Blood Press 21(1):24–30
Kurt M, Wang J, Torre-Amione G et al (2009) Left atrial function in diastolic heart failure. Circ Cardiovasc Imaging 2(1):10–15
Khan UA, de Simone G, Hill J et al (2013) Depressed atrial function in diastolic dysfunction: a speckle tracking imaging study. Echocardiography 30(3):309–316
Miyoshi H, Oishi Y, Mizuguchi Y et al (2014) Association of left atrial reservoir function with left atrial structural remodeling related to left ventricular dysfunction in asymptomatic patients with hypertension: evaluation by two-dimensional speckle-tracking echocardiography. Clin Exp Hypertens 22:1–11
Candan O, Ozdemir N, Aung SM et al (2014) Atrial longitudinal strain parameters predict left atrial reverse remodeling after mitral valve surgery: a speckle tracking echocardiography study. Int J Cardiovasc Imaging 30(6):1049–1056
Conflict of interest
None to declare.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tigen, K., Sunbul, M., Karaahmet, T. et al. Early Detection of Bi-ventricular and Atrial Mechanical Dysfunction Using Two-Dimensional Speckle Tracking Echocardiography in Patients with Sarcoidosis. Lung 193, 669–675 (2015). https://doi.org/10.1007/s00408-015-9748-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00408-015-9748-0