Abstract
Background/objective
The aim was to evaluate the left and right ventricular functions concurrently by two-dimensional speckle tracking echocardiography (STE) in systemic sclerosis (SSc) patients without overt cardiac disease.
Methods
A total of 47 patients with SSc and 36 age- and sex-matched controls were evaluated cross-sectionally. Two-dimensional STE was used to assess the longitudinal peak systolic strains (PSS) of both ventricles including apical long-axis (APLAX), apical four-chamber (4-CH), apical two-chamber (2-CH), and global longitudinal measurements. Any association of metabolic, cardiac, and inflammatory biomarkers with PSS was investigated.
Results
The longitudinal PSS of the left ventricle [APLAX, 4-CH, 2-CH and global] were significantly lower in SSc patients than controls (− 18.2 ± 3.2 vs − 19.8 ± 2.7% p = 0.02; − 17.8 ± 3.5 vs. − 20.3 ± 3.3% p = 0.001; − 18.6 ± 3.1 vs. − 21.8 ± 3% p < 0.001; − 17.5 ± 5.7 vs. − 20.6 ± 2.7% p = 0.003, respectively). No difference was found between the groups for right ventricular strains. The longitudinal PSS-4CH correlated positively with CRP and ESR (r = 0.349, p = 0.016; r = 0.356, p = 0.014, respectively) and negatively with serum Galectin-3 (r = − 0.362, p = 0.012). Global longitudinal PSS-left ventricle (LV) correlated positively with CRP and homocysteine (r = 0.297, p = 0.043; r = 0.313, p = 0.041, respectively) and negatively with serum Galectin-3 (r = −0.314, p = 0.041). After multivariable adjustment, CRP remained the only predictor of longitudinal PSS-4CH (95% CI 0.35, 0.70, p = 0.028) and global longitudinal PSS of left ventricle (95% CI 0.004, 0.22, p = 0.043).
Conclusions
Biventricular evaluation of patients with SSc by two dimensional STE revealed reduced left ventricular longitudinal strains, despite preserved right ventricular strain, and no diastolic dysfunction. In SSc without overt cardiac disease, global cardiac assessment with 2DSTE is a promising method which seems to contribute to the detection of patients without clinical findings.
Key Points
• Two dimensional STE revealed reduced left ventricular longitudinal strains, despite preserved right ventricular strain in SSc patients without overt cardiac disease.
• CRP was the predictor of decreased longitudinal strains.
• Cardiac assessment in SSc should be made globally.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Systemic sclerosis (SSc) is a chronic connective tissue disorder characterized by three cardinal features; vasculopathy, autoimmunity, and fibrosis [1]. The general pathogenetic mechanisms in SSc, including microvascular vasospastic episodes leading to subsequent morphological vascular damage, collagen accumulation, and complex immune disturbances are thought to be involved in the pathogenesis of myocardial involvement in SSc [2].
SSc cardiac involvement typically has an insidious onset and is predictive of poor prognosis [3]. All cardiac tunicae, endocardium, myocardium, and pericardium may be involved. This may result in pericardial effusion, atrial and ventricular arrhythmias, conduction system defects, myocardial ischemia, myocardial hypertrophy, and heart failure. Fibrosis of the myocardium is the main pathological finding in postmortem studies [4].
The common consequence of myocardial impairment is considered to be left ventricular (LV) diastolic dysfunction, and less frequently systolic dysfunction, both of which may be clinically asymptomatic [5]. Impaired diastolic dysfunction is reported to be the first clinical hallmark of myocardial fibrosis [6]. Despite the belief that the systolic dysfunction usually occurs late in the disease course, some studies have reported systolic dysfunction in the absence of diastolic impairment in patients with diffuse cutaneous systemic sclerosis (dcSSc) [7].
Consequently, there is a clinical need for a sensitive and specific, non-invasive diagnostic approach for preclinical identification of myocardial manifestation in SSc patients. Conventional echocardiography is a widely available technique that has already been demonstrated to detect subclinical cardiac impairment in SSc patients with preserved left ventricular ejection fraction (LVEF) [8]. Two-dimensional speckle-tracking echocardiography (STE) is a relatively new echocardiographic technique for obtaining Doppler-independent strain and strain rate (SR) analyses, that may overcome some technical and observer derived limitations of conventional echocardiography [9].
In this study, we aimed to assess STE-derived measurements of left and right ventricular deformation concurrently, to identify early cardiac involvement in SSc. Our secondary aim was to identify a biomarker which could be associated with impaired STE results. Therefore, we investigated inflammatory and metabolic parameters routinely studied during SSc patients’ follow-up and human Galectin-3 which has been suggested to be related to the developmental process of skin and organ sclerosis in SSc [10].
Methods
Study population
Forty-seven patients who fulfilled the 2013 American College of Rheumatology/European League Against Rheumatism (ACR/EULAR) SSc classification criteria [11] and 36 gender- and age-matched, healthy subjects who were attending the outpatient clinic for general internal medicine were selectively enrolled in the study. The patients were classified into limited cutaneous (lcSSc) or dcSSc according to LeRoy’s criteria [12]. The extent of the skin involvement was evaluated by using the modified Rodnan skin score (mRSS) by a single rheumatologist [13]. The severity of disease was assessed by Medsger severity scale [14] and activity with the European Scleroderma Trials and Research Group (EUSTAR) activity index [15].
Exclusion criteria were: patients with known diabetes mellitus (i.e., patients with fasting blood glucose > 126 mg/dl or who were on treatment with oral hypoglycemic drugs or insulin,); renal involvement (creatinine > 1.3 mg/dL); respiratory disorders (asthma, chronic obstructive pulmonary disease); hypertension (i.e., patients who were on treatment with antihypertensive drugs, with systolic blood pressure > 140 mmHg, or diastolic blood pressure > 90 mmHg); overt cardiac disease (a history of angina pectoris, coronary artery disease, acute coronary syndrome or coronary revascularization, conduction disorders, valvular heart disease, pacemaker, prosthetic heart valves, stroke, left ventricular systolic dysfunction with LV ejection fractions < 55%); pulmonary arterial hypertension diagnosed by right heart catheterization; and peripheral artery disease. Patients with a pulmonary artery pressure (PAP) greater than 45 mmHg, indirectly calculated by measuring the Doppler flow of the tricuspid regurgitant jet on echocardiography, were also excluded because of the strong correlation between right heart catheterization and this estimated cutoff level [16]. Smoking, obesity, and hypertension were considered as CVD risk factors.
Clinical data concerning SSc related involvements were obtained from medical records. The following definitions were used to determine specific visceral involvements: gastrointestinal involvement (distal esophageal hypomotility or aperistalsis documented by either radiographic or manometric study, gastrointestinal symptoms defined by heartburn, dysphagia, episodes of pseudo-occlusion, anal incontinence, diarrhea, and/or fecal incontinence); pulmonary involvement (evidenced by ground-glass, honeycombing or traction bronchiectasis on thoracic high resolution computed tomography and pulmonary function test showing restrictive pulmonary disease pattern characterized by forced vital capacity (FVC) of < 70% of predicted normal and/or carbon monoxide diffusing capacity (DLCO) of < 80% of predicted normal); musculoskeletal involvement (defined by restriction of the skeletal motion due to myositis, arthritis, calcinosis, or contracture of the joints).
Metabolic parameters including waist circumference, weight, and height were measured, and body mass index (BMI) was calculated. Insulin resistance was estimated using the homeostasis model assessment of insulin resistance index (HOMA-IR) formula ((fasting insulin (μU/L) × fasting glucose (mmol/L))/22.5) [17]. Metabolic parameters including waist circumference, weight and height were measured and body mass index (BMI) was calculated. Those patients with a BMI of < 18.5 kg/m2 were considered underweight, 18.5–24.9 kg/m2 normal, 25–29.9 kg/m2 overweight, and ≥ 30 kg/m2 obese [18]. Metabolic syndrome was defined based on the National Cholesterol Education Program Adult Treatment Panel (NCEP) III criteria. Three or more of the following NCEP criteria needed to be met in order to be classified as having metabolic syndrome: waist circumference > 102 cm in men and > 88 cm in women, triglycerides ≥ 150 mg/dl, HDL < 40 mg/dl in men and < 50 mg/dl in women, high blood pressure ≥ 130/85 mmHg or use of antihypertensives, and fasting glucose ≥ 110 mg/dl [19].
The study was approved by the local ethics committee and conducted in accordance with the principles of the World Health Organization-Declaration of Helsinki. Written informed consent was obtained from all patients and controls.
Laboratory parameters
Serum samples were collected at enrollment and immediately stored at − 80 °C. A commercial ELISA kit (ThermoFisher Scientific, Massachusetts, USA) for serum human Galectin 3 (BMS279-4) with a range of detection of 0.47–30.0 ng/mL, analytical sensitivity down to 0.29 ng/mL, intra- and inter-assay coefficients of variation of 7.5%, and 5.4% was used. Blood samples were taken for C-reactive protein (CRP), erythrocyte sedimentation rate (ESR), plasma fasting glucose and insulin, homeostatic model assessment (HOMA), hemoglobin A1C (HbA1C), cholesterol, uric acid, N-terminal pro-brain natriuretic peptide (NT pro-BNP), homocysteine, antinuclear antibodies (ANA), anticentromere antibodies, and anti-topoisomerase I antibodies (Scl-70). ANA indirect immunofluorescent (IIF) testing was performed and evaluated by two experienced physicians in our laboratory.
Conventional echocardiography and tissue Doppler imaging
All subjects were imaged in the left lateral decubitus position with a commercially available system (VIVID 7, General Electric-Vingmed Ultrasound, Horten, Norway). Ejection fraction (EF) was measured with the modified biplane Simpson’s method from the apical 4-chamber (4-CH) and 2-chamber (2-CH) views [20]. Left ventricular (LV) mass was calculated according to the Devereux formula [21]. LV hypertrophy was defined as an LV mass indexed (LVMI) to body surface area (BSA) that exceeded 95 g/m2 for women and 115 g/m2 for men [22]. Concentric geometry was diagnosed if the LVMI was normal but relative wall thickness (RWT) exceeded 0.43 for both men and women [23]. Mitral inflow velocities to assess LV filling, including mitral early diastolic inflow velocity (E), atrial late filling peak velocity (A), deceleration time (DT), and E/A ratio were measured in the apical 4-CH view and the mid esophageal (ME) 4-CH view using pulsed-wave (PW) Doppler from transmitral flow [24]. Tissue Doppler imaging (TDI) of the right ventricular (RV) free wall was performed in the apical 4-CH view at end-expiration. Tissue Doppler imaging was used to measure e’, early diastolic annular velocity. The ratio E/e’ is a reliable estimate of left atrial pressure when systolic function is normal [25]. The measurements performed for the left ventricle were also recorded for the right ventricle. The tricuspid annular plane systolic excursion (TAPSE), as a parameter for RV long axis function, was measured by placing the M-mode line at the junction of the tricuspid valve annulus and the RV free wall. Conventional echocardiographic measurements were performed in accordance with the guidelines of the American Society of Echocardiography [26].
Speckle tracking echocardiography
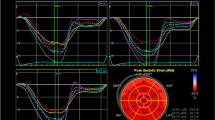
STE was measured using a commercially available speckle tracking system in an ECHOPAC (ver. 6.3, GE Vingmed, Horten, Norway) workstation. Although there are some variations in the values of data by manufacturers (for example between GE, Phillips, and TOSHIBA), it does not lead to any error for evaluation of the regional strain. In this system, the displacement of speckles of the myocardium in each spot was analyzed and tracked from frame to frame. We selected the best quality, digital, two-dimensional image cardiac cycle, and the left ventricle endocardium was traced. Regarding adequate tracking quality, the system automatically flags an acceptable or unacceptable tracking quality. We systematically accepted only segments that received an acceptable tracking quality for analysis. To optimize speckle tracking, two dimensional, gray-scale, harmonic images were obtained at a frame rate of 60–90 frames/s. Longitudinal strain was assessed with automatic functional imaging (AFI). At first, the end-systolic frame was defined in the apical long-axis view. The closure of the aortic valve was marked, and the AFI software measured the time interval between the R-wave and aortic valve closure, which was used as event timing. We manually defined three index points (two points at the base of the LV and one at the apex). The AFI algorithm automatically traced three concentric lines on the endocardial border, mid-myocardial layer, and epicardial border and followed the endocardium from this single frame throughout the cardiac cycle. The left ventricle in each apical image is divided into six segments, and the tracking quality for each segment is validated by the operator. Then, the AFI algorithm tracks the percent of wall lengthening and shortening in a set of three longitudinal, two dimensional image planes. The peak systolic longitudinal strain for each segment was displayed, based on a 17-segment model for each plane, and the results of all three planes were combined in a single bull’s-eye summary. Global longitudinal peak strain was automatically calculated as an averaged value of peak longitudinal strain in all 3-image planes [27]. We analyzed the RV strain using an apical 2-CH view, similar to the left ventricular calculations with the AFI algorithm.
Intra-observer variability
All echocardiographic studies and measurements were performed by an experienced cardiologist (T.S.) who was blinded to previously obtained data. In our laboratory, the intra-observer variability was as follows: r = 0.98 for two dimensional and M-mode echocardiographic measurements; r = 0.97 for Doppler measurements; and r = 0.98 for speckle tracking echocardiographic measurements [28].
Statistical method
Descriptive statistics for clinical and demographic characteristics of the patients were presented as frequency and percentage (%) for categorical variables and mean with standard deviation (mean ± SD) or median with interquartile range (median [Q3–Q1]) according to the distribution of the continuous variables.
Distribution of data was assessed by using Kolmogorov-Smirnov test. The categorical variables were compared between SSc and controls using Pearson chi-square test. The independent samples t test was used to analyze the variables (age, waist circumference, BMI, hemoglobin, fasting plasma glucose, total-cholesterol, HDL-cholesterol, LV mass index, LVESD, TAPSE, the strains) which were normally distributed between the groups, and Mann–Whitney U Test (Wilcoxon rank sum test) or Kruskall Wallis Test for the rest. Spearman’s rank correlation coefficients were used to calculate the bivariate relationships between categorical and continuous variables and strains in SSc. The multivariable models for the longitudinal PSS-4CH and global longitudinal PSS-LV were obtained using the enter method, in which parameters that were statistically significant in bivariate correlation analysis were included. The global longitudinal PSS-LV was not normally distributed and was therefore corrected by log-transformation.
Statistical analyses were performed using “SPSS version 20.0 software package” (IBM Inc., Chicago, IL, USA). Two-sided p values less than 0.05 were considered statistically significant (p < 0.05).
Results
Baseline characteristics of the patients with SSc
The clinical and laboratory features of SSc patients are summarized in Table 1. The mean disease duration was 8.5 ± 5.9 years, and 70.2% of the patients were in the limited disease subset. None of the patients had pulmonary hypertension (PHT) or scleroderma renal crisis. The EUSTAR activity index was 1.5 [2.76–0.71] and showed the disease was active in 15 (15.7%) of the patients with a cutoff ≥ 2.5 [14]. Twenty-eight (59.6%) of the patients had normal, 12 (25.5%) had mild, 6 (12.8%) had moderate, and 1 (2.1%) had severe disease in terms of the general domain of Medsger severity scale. For the peripheral vascular domain, 26 (55.3%) had mild, 9 (19.1%) had moderate, and 12 (25.5%) had severe disease. For the skin involvement, 37 (78.7%) had mild, 9 (19.1%) had moderate, and 1 (2.1%) had severe disease. For the joint-tendon involvement, 41 (87.2%) had normal, 4 (8.5%) had mild, and 2 (4.3%) had a moderate disease. For the gastrointestinal (GI) involvement, 42 (89.4%) had normal and 5 (10.6%) had mild disease. For the lung involvement, 11 (23.4%) had normal, 25 (53.2%) had mild, and 11 (23.4%) had moderate disease. Muscle, heart, and kidney domains of the Medsger severity scale all showed normal results. There was no relationship between the strains and both activity and severity scales.
Comparison of demographics and laboratory parameters of the study subjects
The comparison of demographics and laboratory parameters between the SSc and controls are detailed in Table 2. ESR, CRP, leukocyte and neutrophil counts, and NT pro-BNP concentration were significantly higher in patients with SSc compared with controls (18 [10–31] vs 8.5 [4–18] mm/h, p < 0.001; 0.4 [0.18–0.67] vs 0.21 [0.09–0.48] ng/mL, p = 0.012; 7510 [5990–8731] vs 6435 [5195–7360], p = 0.002; 4350 [3570–5440] vs 3390 [2903–4168], p < 0.001; 111 [74–185] vs 70 [70–127] mg/dL, p = 0.010, respectively). The fasting plasma insulin and HOMA-IR were significantly higher (6.7 [4.7–10.5] vs 4.7 [4.1–6.8], p = 0.008; 1.7 [1–2.6] vs 1.1 [0.9–1.7], p = 0.015, respectively); and total cholesterol and low-density lipoprotein cholesterol (LDL-C) were significantly lower in SSc than controls (197 ± 45 vs 284 ± 36 mg/dL, p = 0.005; 118 [84–148] vs 140 [115–180] mg/dL, p = 0.003, respectively).
Comparison of conventional echocardiography and two dimensional STE results between SSc patients and controls
No significant difference was found between the patients and controls with respect to the standard conventional echocardiography measurements (Table 3), except concentric hypertrophy which was significantly more common in patients compared with controls (17% vs 0, p < 0.001).
The results of two dimensional STE were compared between SSc and controls (Table 3). Myocardial strains for LV and RV were measured in a longitudinal direction. The longitudinal peak systolic strain (PSS) in apical long-axis view (longitudinal PSS-APLAX), longitudinal PSS in apical 4-CH view (longitudinal PSS-4CH), longitudinal PSS in apical 2-CH view (longitudinal PSS-2CH), and global longitudinal PSS of the left ventricle (global longitudinal PSS-LV) were significantly lower in SSc compared with the controls (− 18.21 ± 3.19 vs. − 19.81 ± 2.67, p = 0.018; − 17.77 ± 3.47 vs. − 20.33 ± 3.25, p = 0.001; − 18.62 ± 3.1 vs. − 21.83 ± 3.03, p < 0.001; − 17.5 ± 5.73 vs. − 20.61 ± 2.68, p = 0.003, respectively) (Fig. 1). The global right ventricular longitudinal PSS (global longitudinal PSS-RV) did not differ between the SSc patients and healthy subjects (− 17.49 ± 4.16 vs. − 18.89 ± 3.86, p = 0.121).
Comparison of conventional echocardiography and two dimensional STE results between diffuse and limited sub-types of SSc
The conventional echocardiography and two dimensional STE results showed no difference between the patients with diffuse and limited sub-types of SSc (Table 4).
Associations between two dimensional STE results and cardiometabolic risk factors, inflammatory parameters, and severity and activity indices in SSc patients
Among the patients with SSc, we tested how the strains differed according to the sex, smoking, metabolic syndrome, obesity, high HOMA, high total cholesterol, triglyceride, high waist circumference, high CRP, active disease, disease severity, and organ involvements due to SSc. The cutoff level for CRP was 0.5 mg/dl at our laboratory, and we compared the strains between the patients with CRP > 0.5 mg/dl and CRP ≤ 0.5 mg/dl. The longitudinal PSS-4CH and global longitudinal PSS-LV were significantly lower in the patients with CRP over the cutoff level compared with the ones below the cutoff (− 15.82 ± 2.94 vs − 18.87 ± 3.3, p = 0.003; − 19 [− 16.75 to − 21] vs [− 15.82 to − 19], p = 0.031, respectively. When we compared the strains between the patients with high and low waist circumference according to the National Cholesterol Education Program Adult Treatment Panel (NCEP) III criteria, we found global right ventricular strain was significantly lower in the patients with high waist circumference (− 15.5 ± 3.81 vs − 18.52 ± 4.01, p = 0.017). These results were not obtained in the controls.
The longitudinal PSS-4CH correlated positively with CRP and ESR (r = 0.349, p = 0.016; r = 0.356, p = 0.014, respectively) and negatively with serum Galectin-3 (r = − 0.362, p = 0.012). The global longitudinal PSS-LV correlated positively with CRP and homocysteine (r = 0.297, p = 0.043; r = 0.313, p = 0.041, respectively) and negatively with serum Galectin-3 (r = − 0.314, p = 0.041). After multivariable adjustment, CRP remained the only independent predictor of the longitudinal PSS-4CH (95% CI 0.35, 0.70, p = 0.028) and the global longitudinal PSS-LV (95% CI 0.004, 0.22, p = 0.043). In healthy subjects, no relationship between the strains and these parameters, except a moderate positive correlation between Galectin-3 and longitudinal PSS-APLAX (r = 0.456, p = 0.005), was demonstrated.
After defining the cutoff values of > 0.5 mg/dl for CRP and > 133 pg/ml for NT-proBNP in accordance with our routine laboratory use, we analyzed the 2DSE results in patients with SSc according to “elevated CRP”, “elevated NT pro-BNP”, “elevated CRP or NT pro-BNP”, and “elevated CRP and NT pro-BNP”. We found that apical four-chamber (4-CH) was significantly lower in patients with “elevated CRP” than the ones without (16.5 [18.25–13.75] vs − 19 [22–17], p = 0.003) and global longitudinal PSS-RV was significantly higher in patients with “elevated NT pro-BNP” than the ones without (− 19 [22–16.5] vs − 16 [19.75–7.75], p = 0.036). The longitudinal peak systolic strains (PSS) of both ventricles did not change in patients with the effects of “elevated CRP or NT pro-BNP” or “elevated CRP and NT pro-BNP”.
Two dimensional STE results showed no difference according to the activity index, severity scale results, or organ involvements. Moreover, there wasn’t a correlation between the strains and disease activity and severity indices (data not shown).
Post hoc power analysis
We appreciate for your informative comments on our work that provided new perspective. We performed the post hoc power analysis for individual 2DSTE measurements including the longitudinal peak systolic strains (PSS) of the left ventricle (APLAX, 4-CH, 2-CH, and global). Based on the results, the power of our study ranged between 0.666 and 0.997 depending on the PSS. Our sample size was enough to reject the null hypothesis with the best probability of 99.7% (α = 0.05, β = 0.003) and the worst probability of 66.6% (α = 0.05, β = 0.334).
Discussion
The present study demonstrated that SSc patients without overt cardiac disease had impaired LV and normal RV measurements by two dimensional STE compared with healthy subjects, despite the lack of any impairment evident by conventional echocardiography. As an additional result, the impaired longitudinal PSS-4CH and global longitudinal PSS-LV were independently associated with CRP.
The decreased left ventricular longitudinal strains, despite the preserved LVEF and dimensions by conventional echocardiography, were consistent with the current knowledge that two-dimensional speckle-tracking strain analysis has been proposed as a more sensitive and accurate method for the evaluation of subtle myocardial dysfunction [29,30,31] (Table 5). One noticeable result of our study was the decreased left ventricular strains in SSc, despite right global longitudinal strain being preserved. Since studies relevant to STE investigations mostly analyzed single ventricular function in SSc, our results are important in terms of providing data for evaluation of both ventricles concurrently. Similarly, designed studies that assessed both ventricles reported contradictory results. A recent study by Guerra et al. showed decreased global longitudinal left and right ventricular strains in SSc patients without a systolic impairment by conventional echocardiography [7]. Kepez et al. reported impaired left ventricular and preserved right ventricular average strains consistent with our results [32]. They demonstrated impaired strain in one of two segments of the RV, but no difference in average RV strain between the patients and controls. They suggested using the global indices like average strains, based on the data derived from the left ventricle which showed that all the regional strains were consistently decreased with the average strain. Similar to the study by Kepez et al., our study population included patients with a milder disease than most of the previously reported studies, which may be a reason for better right ventricular strains. In contrast to most of the previous reports, both the exclusion cutoff level for mean pulmonary arterial pressure on conventional echocardiography, and the severity and frequency of interstitial lung disease were less in our patients [33, 34]. Also, we hypothesize that the right ventricular endocardial fibers, which mainly influence the longitudinal function, are not thick enough to exhibit measurable subclinical cardiac impairment but the left ventricular fibers are. This might be an additional factor contributed to the apparently preserved right ventricular strains.
In our study, the conventional echocardiography results related to diastolic dysfunction were not different between SSc patients and controls. Although many studies published to date have confirmed that diastolic dysfunction in SSc was more prevalent cardiac involvement [35, 36], there is contradiction depending on the method and lack of accounting for the influence of age or the parameters which were assumed as an indicator of diastolic function [37]. Moreover, there is evidence suggesting that vasculopathy seems to precede the deposition of extracellular matrix and fibrosis [38]. This may be an explanation of why the distensibility related parameters remained unchanged and vasculopathy related parameters, which might be associated with subclinical cardiac damage, significantly decreased in our study. Although there is insufficient evidence as yet, the growing data indicating systolic impairment without a diastolic dysfunction is notable and may modify the widely accepted pathogenic mechanisms of primary cardiac involvement in SSc [7]. We believe that systolic dysfunction is underestimated in many studies due to more widespread reporting of diastolic dysfunction in previous studies.
In order to identify biomarkers of subtle cardiac impairment, we investigated the association between the inflammatory and metabolic parameters and decreased strains. We demonstrated a moderate association between the longitudinal PSS-4CH and inflammatory markers including CRP and ESR. We also found a mild association between global longitudinal PSS-LV and CRP. We found longitudinal PSS-4CH was significantly lower in SSc patients with CRP above the cutoff level than the ones below. Multivariable regression analysis revealed that CRP was the only independent predictor of longitudinal PSS-4CH and global longitudinal PSS-LV. The adverse effect of chronic inflammation on left ventricular remodeling has been previously shown [39]. In addition, there are reports which demonstrated that chronic inflammation may accelerate cardiac damage by atherosclerosis, cardiac fibrosis, apoptosis, and necrosis [40]. We suggest that CRP may be indicative for ongoing subtle damage in cardiac tissue due to atherosclerotic or disease-related mechanisms. However, this suggestion may not go beyond speculation, because our results should be confirmed by further studies with objective evidence.
Based on the accumulating data [41, 42] which has shown that during myocardial remodeling galectin-3 contributed to myocardial fibrosis and studies which have reported an association between galectin-3 and SSc [10, 43], we measured serum galectin-3 concentrations of the study subjects. Although serum galectin-3 was not different between SSc patients and controls in our study, it was associated with longitudinal PSS-4CH and global longitudinal PSS-LV in SSc, and with longitudinal PSS-APLAX in controls. In contrast to the results of previous studies that reported serum galectin-3 was related to active disease or specific organ involvements, in our study patients with SSc galectin-3 exhibited no significant results due to disease activity, severity, or organ involvements. In the study by Hromádka et al., Galectin-3 levels were higher in SSc than controls [44]. They reported a correlation between Galectin-3 and global longitudinal peak systolic strain and also an association of Galectin-3 and disease activity. However, disease activity was not defined with well-known disease activity indices in their study, and the parameters used for evaluating the disease activity were insufficient to clearly define active disease. In our study, Galectin-3 concentration did not differ between the patients with active and inactive disease. They demonstrated that cardiac MRI fibrosis parameters were significantly higher in SSc patients compared with controls and correlated with Galectin-3 concentration. In our opinion, it is more accurate to think that Galectin-3 elevation is due to increased cardiac fibrosis. This question remains until conclusive evidence is available.
As a limitation of our study, cardiac ischemia was not fully documented by coronary angiography or myocardial perfusion scintigraphy. Since all of the patients had no clinical evidence of cardiac disease and were asymptomatic in this regard, we did not perform an additional investigation. Fourteen of our patients (30%) were under immunosuppressive treatment including potentially cardiotoxic agents including cyclophosphamide, methotrexate, and azathioprine. We should not disregard the potential effect of these medications. Although there are limitations to our study, it is more comprehensive than previous reports in investigating the association between longitudinal strains and laboratory and metabolic parameters.
In summary, the patients with SSc had reduced LV longitudinal strains as measured by 2-D speckle tracking echocardiography despite no cardiac symptoms and preserved conventional echocardiography results when compared with matched controls. Our results demonstrated that 2DSTE is a good method for regular monitoring the patients with SSc in routine clinical practice, as well as an available tool for clinical even in the patients without overt cardiac disease. Additionally, we suggest that the clinicians should consider assessing cardiac functions globally rather than focusing on regional evaluation in SSc.
References
Gabrielli A, Avvedimento EV, Krieg T (2009) Scleroderma. N Engl J Med 360(19):1989–2003. https://doi.org/10.1056/NEJMra0806188
Ferri C, Giuggioli D, Sebastiani M, Colaci M, Emdin M (2005) Heart involvement and systemic sclerosis. Lupus 14(9):702–707
Tyndall AJ, Bannert B, Vonk M, Airò P, Cozzi F, Carreira PE et al (2010) Causes and risk factors for death in systemic sclerosis: a study from the EULAR scleroderma trials and research (EUSTAR) database. Ann Rheum Dis 69(10):1809–1815. https://doi.org/10.1136/ard.2009.114264
Meier FM, Frommer KW, Dinser R, Walker UA, Czirjak L, Denton CP et al (2012) Update on the profile of the EUSTAR cohort: an analysis of the EULAR scleroderma trials and research group database. Ann Rheum Dis 71:1355–1360. https://doi.org/10.1136/annrheumdis-2011-200742
Lambova S (2014) Cardiac manifestations in systemic sclerosis. World J Cardiol 6(9):993–1005. https://doi.org/10.4330/wjc.v6.i9.993
Ross L, Prior D, Proudman S, Vacca A, Baron M, Nikpour M (2018) Defining primary systemic sclerosis heart involvement: a scoping literature review. Semin Arthritis Rheum 48:874–887. https://doi.org/10.1016/j.semarthrit.2018.07.008
Guerra F, Stronati G, Fischietti C, Ferrarini A, Zuliani L, Pomponio G, Capucci A, Danieli MG, Gabrielli A (2018) Global longitudinal strain measured by speckle tracking identifies subclinical heart involvement in patients with systemic sclerosis. Eur J Prev Cardiol 25(15):1598–1606. https://doi.org/10.1177/2047487318786315
Meune C, Avouac J, Wahbi K, Cabanes L, Wipff J, Mouthon L, Guillevin L, Kahan A, Allanore Y (2008) Cardiac involvement in systemic sclerosis assessed by tissue-doppler echocardiography during routine care: a controlled study of 100 consecutive patients. Arthritis Rheum 58(6):1803–1809. https://doi.org/10.1002/art.23463
Blessberger H, Binder T (2010) Two dimensional speckle tracking echocardiography: basic principles. Heart 96(9):716–722. https://doi.org/10.1136/hrt.2007.141002
Taniguchi T, Asano Y, Akamata K, Noda S, Masui Y, Yamada D et al (2012) Serum levels of galectin-3: possible association with fibrosis, aberrant angiogenesis, and immune activation in patients with systemic sclerosis. J Rheumatol 39(3):539–544. https://doi.org/10.3899/jrheum.110755
Van den Hoogen F, Khanna D, Fransen J, Johnson SR, Baron M, Tyndall A et al (2013) Classification criteria for systemic sclerosis: an American college of rheumatology/European league against rheumatism collaborative initiative. Ann Rheum Dis 72(11):1747–1755. https://doi.org/10.1136/annrheumdis-2013-204424
LeRoy EC, Black C, Fleischmajer R, Jablonska S, Krieg T, Medsger TA Jr et al (1988) Scleroderma (systemic sclerosis): classification, subsets and pathogenesis. J Rheumatol 15(2):202–205
Clements P, Lachenbruch P, Siebold J, White B, Weiner S, Martin R, Weinstein A, Weisman M, Mayes M, Collier D (1995) Inter and intraobserver variability of total skin thickness score (modified Rodnan TSS) in systemic sclerosis. J Rheumatol 22:1281–1285
Medsger TA Jr, Bombardieri S, Czirjak L, Scorza R, Della Rossa A, Bencivelli W (2003) Assessment of disease severity and prognosis. Clin Exp Rheumatol 21(3 Suppl 29):S42–S46
Valentini G, Iudici M, Walker UA, Jaeger VK, Baron M, Carreira P (2017) The European Scleroderma Trials and Research group (EUSTAR) task force for the development of revised activity criteria for systemic sclerosis: derivation and validation of a preliminarily revised EUSTAR activity index. Ann Rheum Dis 76(1):270–276. https://doi.org/10.1136/annrheumdis-2016-209768
Hsu VM, Moreyra AE, Wilson AC, Shinnar M, Shindler DM, Wilson JE, Desai A, Seibold JR (2008) Assessment of pulmonary arterial hypertension in patients with systemic sclerosis: comparison of noninvasive tests with results of right-heart catheterization. J Rheumatol 35(3):458–465
Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC (1985) Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 28:412–419
W. H. O (1995) Physical status: the use and interpretation of anthropometry. Report of a WHO expert committee. World Health Organ Tech Rep Ser 854:1–452
Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (2001) Executive summary of the third report of the National Cholesterol Education Program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (adult treatment panel III). JAMA 285(19):2486–2497. https://doi.org/10.1001/jama.285.19.2486
Lang RM, Bierig M, Devereux RB, Flachskampf FA, Foster E, Pellikka PA, Picard MH, Roman MJ, Seward J, Shanewise JS, Solomon SD, Spencer KT, Sutton MS, Stewart WJ, Chamber Quantification Writing Group, American Society of Echocardiography's Guidelines and Standards Committee, European Association of Echocardiography (2005) Chamber quantification writing group; American Society of Echocardiography’s guidelines and standards committee; European Association of Echocardiography. Recommendations for chamber quantification: a report from the American Society of Echocardiography’s guidelines and standards committee and the chamber quantification writing group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr 18(12):1440–1463. https://doi.org/10.1016/j.echo.2005.10.005
Devereux RB, Alonso DR, Lutas EM, Gottlieb GJ, Campo E, Sachs I, Reichek N (1986) Echocardiographic assessment of left ventricular hypertrophy: comparison to necropsy findings. Am J Cardiol 57:450–458
Mancia G, Fagard R, Narkiewicz K, Redon J, Zanchetti A, Böhm M, Christiaens T, Cifkova R, de Backer G, Dominiczak A, Galderisi M, Grobbee DE, Jaarsma T, Kirchhof P, Kjeldsen SE, Laurent S, Manolis AJ, Nilsson PM, Ruilope LM, Schmieder RE, Sirnes PA, Sleight P, Viigimaa M, Waeber B, Zannad F, Redon J, Dominiczak A, Narkiewicz K, Nilsson PM, Burnier M, Viigimaa M, Ambrosioni E, Caufield M, Coca A, Olsen MH, Schmieder RE, Tsioufis C, van de Borne P, Zamorano JL, Achenbach S, Baumgartner H, Bax JJ, Bueno H, Dean V, Deaton C, Erol C, Fagard R, Ferrari R, Hasdai D, Hoes AW, Kirchhof P, Knuuti J, Kolh P, Lancellotti P, Linhart A, Nihoyannopoulos P, Piepoli MF, Ponikowski P, Sirnes PA, Tamargo JL, Tendera M, Torbicki A, Wijns W, Windecker S, Clement DL, Coca A, Gillebert TC, Tendera M, Rosei EA, Ambrosioni E, Anker SD, Bauersachs J, Hitij JB, Caulfield M, de Buyzere M, de Geest S, Derumeaux GA, Erdine S, Farsang C, Funck-Brentano C, Gerc V, Germano G, Gielen S, Haller H, Hoes AW, Jordan J, Kahan T, Komajda M, Lovic D, Mahrholdt H, Olsen MH, Ostergren J, Parati G, Perk J, Polonia J, Popescu BA, Reiner Z, Rydén L, Sirenko Y, Stanton A, Struijker-Boudier H, Tsioufis C, van de Borne P, Vlachopoulos C, Volpe M, Wood DA (2013) 2013 ESH/ESC guidelines for the management of arterial hypertension: the task force for the Management of Arterial Hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). Heart J 34(28):2159–2219. https://doi.org/10.1093/eurheartj/eht151
Ganau A, Saba PS, Roman MJ, de Simone G, Realdi G, Devereux RB (1995) Ageing induces left ventricular concentric remodelling in normotensive subjects. J Hypertens 13:1818–1822
Nagueh SF, Appleton CP, Gillebert TC, Marino PN, Oh JK, Smiseth OA, Waggoner AD, Flachskampf FA, Pellikka PA, Evangelisa A (2009) Recommendations for the evaluation of left ventricular diastolic function by echocardiography. Eur J Echocardiogr 10(2):165–193. https://doi.org/10.1093/ejechocard/jep007
Caballero L, Kou S, Dulgheru R, Gonjilashvili N, Athanassopoulos GD, Barone D, Baroni M, Cardim N, Gomez de Diego JJ, Oliva MJ, Hagendorff A, Hristova K, Lopez T, Magne J, Martinez C, de la Morena G, Popescu BA, Penicka M, Ozyigit T, Rodrigo Carbonero JD, Salustri A, van de Veire N, von Bardeleben RS, Vinereanu D, Voigt JU, Zamorano JL, Bernard A, Donal E, Lang RM, Badano LP, Lancellotti P (2015) Echocardiographic reference ranges for normal cardiac Doppler data: results from the NORRE study. Eur Heart J Cardiovasc Imaging 16(9):1031–1041. https://doi.org/10.1093/ehjci/jev083
Porter TR, Shillcutt SK, Adams MS, Desjardins G, Glas KE, Olson JJ, Troughton RW (2015) Guidelines for the use of echocardiography as a monitor for therapeutic intervention in adults: a report from the American Society of Echocardiography. J Am Soc Echocardiogr 28(1):40–56. https://doi.org/10.1016/j.echo.2014.09.009
Delgado V, Ypenburg C, van Bommel RJ, Tops LF, Mollema SA, Marsan NA, Bleeker GB, Schalij MJ, Bax JJ (2008) Assessment of left ventricular dyssynchrony by speckle tracking strain imaging comparison between longitudinal, circumferential, and radial strain in cardiac resynchronization therapy. J Am Coll Cardiol 51(20):1944–1952. https://doi.org/10.1016/j.jacc.2008.02.040
Yilmazer B, Sahin T, Cefle A (2016) Impaired myocardial deformation in psoriatic arthritis patients assessment by speckle tracking echocardiography. Acta Rheumatol Port 41(2):131–137
Spethmann S, Dreger H, Schattke S, Riemekasten G, Borges AC, Baumann G, Knebel F (2012) Two-dimensional speckle tracking of the left ventricle in patients with systemic sclerosis for early detection of myocardial involvement. Eur Heart J Cardiovasc Imaging 13(10):863–870. https://doi.org/10.1093/ehjci/jes047
Perk G, Tunick PA, Kronzon I (2007) Non-Doppler two-dimensional strain imaging by echocardiography–from technical considerations to clinical applications. J Am Soc Echocardiogr 20(3):234–243. https://doi.org/10.1016/j.echo.2006.08.023
Russo C, Jin Z, Elkind MS, Rundek T, Homma S, Sacco RL et al (2014) Prevalence and prognostic value of subclinical left ventricular systolic dysfunction by global longitudinal strain in a community-based cohort. Eur J Heart Fail 16(12):1301–1309. https://doi.org/10.1002/ejhf.154
Kepez A, Akdogan A, Sade LE, Deniz A, Kalyoncu U, Karadag O, Hayran M, Aytemir K, Ertenli I, Kiraz S, Calguneri M, Kabakcı G, Tokgozoglu L (2008) Detection of subclinical cardiac involvement in systemic sclerosis by echocardiographic strain imaging. Echocardiography 25(2):191–197. https://doi.org/10.1111/j.1540-8175.2007.00582.x
D'Andrea A, Stisi S, Bellissimo S, Vigorito F, Scotto di Uccio F, Tozzi N et al (2005) Early impairment of myocardial function in systemic sclerosis: non-invasive assessment by Doppler myocardial and strain rate imaging. Eur J Echocardiogr 6(6):407–418. https://doi.org/10.1016/j.euje.2005.01.002
Schattke S, Knebel F, Grohmann A, Dreger H, Kmezik F, Riemekasten G et al (2010) Early right ventricular systolic dysfunction in patients with systemic sclerosis without pulmonary hypertension: a Doppler tissue and speckle tracking echocardiography study. Cardiovasc Ultrasound 8:3. https://doi.org/10.1186/1476-7120-8-3
de Groote P, Gressin V, Hachulla E, Carpentier P, Guillevin L, Kahan A, Cabane J, Frances C, Lamblin N, Diot E, Patat F, Sibilia J, Petit H, Cracowski JL, Clerson P, Humbert M, for the ItinerAIR-Scleroderma Investigators (2008) Evaluation of cardiac abnormalities by Doppler echocardiography in a large nationwide multicentric cohort of patients with systemic sclerosis. Ann Rheum Dis 67(1):31–36. https://doi.org/10.1136/ard.2006.057760
Maione S, Cuomo G, Giunta A, Tanturri de Horatio L, La Montagna G, Manguso F et al (2005) Echocardiographic alterations in systemic sclerosis: a longitudinal study. Semin Arthritis Rheum 34(5):721–727. https://doi.org/10.1016/j.semarthrit.2004.11.001
Hinchcliff M, Desai CS, Varga J, Shah SJ (2012) Prevalence, prognosis, and factors associated with left ventricular diastolic dysfunction in systemic sclerosis. Clin Exp Rheumatol 30(2 Suppl 71):S30–S37
Mueller KA, Mueller II, Eppler D, Zuern CS, Seizer P, Kramer U et al (2015) Clinical and histopathological features of patients with systemic sclerosis undergoing endomyocardial biopsy. PLoS One 10(5):e0126707. https://doi.org/10.1371/journal.pone.0126707
Shang Q, Tam LS, Yip GW, Sanderson JE, Zhang Q, Li EK et al (2011) High prevalence of subclinical left ventricular dysfunction in patients with psoriatic arthritis. J Rheumatol 38(7):1363–1370. https://doi.org/10.3899/jrheum.101136
Kania G, Blyszczuk P, Eriksson U (2009) Mechanisms of cardiac fibrosis in inflammatory heart disease. Trends Cardiovasc Med 19(8):247–252. https://doi.org/10.1016/j.tcm.2010.02.005
Besler C, Lang D, Urban D, Rommel KP, von Roeder M, Fengler K et al (2017) Plasma and cardiac galectin-3 in patients with heart failure reflects both inflammation and fibrosis: implications for its use as a biomarker. Circ Heart Fail 10(3):e003804. https://doi.org/10.1161/CIRCHEARTFAILURE
Song X, Qian X, Shen M, Jiang R, Wagner MB, Ding G, Chen G, Shen B (2015) Protein kinase C promotes cardiac fibrosis and heart failure by modulating galectin-3 expression. Biochim Biophys Acta 1853(2):513–521. https://doi.org/10.1016/j.bbamcr.2014.12.001
Koca SS, Akbas F, Ozgen M, Yolbas S, Ilhan N, Gundogdu B, Isik A (2014) Serum galectin-3 level in systemic sclerosis. Clin Rheumatol 33(2):215–220. https://doi.org/10.1007/s10067-013-2346-8
Hromádka M, Seidlerová J, Suchý D, Rajdl D, Lhotský J, Ludvík J, Rokyta R, Baxa J (2017) Myocardial fibrosis detected by magnetic resonance in systemic sclerosis patients- relationship with biochemical and echocardiography parameters. Int J Cardiol 249:448–453. https://doi.org/10.1016/j.ijcard.2017.08.072
Acknowledgments
The authors are grateful to Mr. Jeremy Jones of the Academic Writing Department of Kocaeli University, Izmit, Turkey, for his assistance in editing the English language usage used and for his help and advice concerning the contents of this manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
This article does not contain any studies with animals performed by any of the authors. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. The study was approved by Kocaeli University School of Medicine Ethics Committee for non-invasive clinical trials with protocol number 178 and name “Evaluation of the ventricular dysfunction by two-dimensional speckle tracking echocardiography in SSc patients without pulmonary hypertension” in 16 June 2015 (KOU KAEK 2015/178).
Informed consent
Informed consent was obtained from all individual participants included in the study.
Disclosures
None.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Karadag, D.T., Sahin, T., Tekeoglu, S. et al. Evaluation of left and right ventricle by two-dimensional speckle tracking echocardiography in systemic sclerosis patients without overt cardiac disease. Clin Rheumatol 39, 37–48 (2020). https://doi.org/10.1007/s10067-019-04604-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10067-019-04604-3