Abstract
Speckle tracking echocardiography (STE) has recently applied as imaging technique to accurately evaluate right ventricular (RV) function. STE provides a non-Doppler, angle-independent and objective quantification of RV myocardial deformation. Data regarding feasibility, accuracy and clinical applications of RV strain are rapidly gathering, especially in the setting of heart failure patients. This review describes the fundamental concepts of RV–STE and discusses its emerging clinical applications, focusing on the useful of this technique in the clinical management of patients with advanced heart failure.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Background
The physiological importance of the right ventricle (RV) has been always underestimated; the RV was considered mainly as a conduit, whereas its contractile performance was thought to be hemodynamically unimportant. In reality, the RV plays an important role in the morbidity and mortality of patients presenting with signs and symptoms of cardiopulmonary disease [1, 2].
Right ventricle function is strongly associated with clinical outcomes in many conditions, and the evaluation of RV structure and function in patients with cardiopulmonary disorders is an essential component of clinical management [3].
Recently, a number of studies have provided evidence that right ventricular ejection fraction is an independent prognostic factor in patients with moderate to severe heart failure (HF) [4]; in patients with moderate or advanced heart failure, RV dysfunction has been shown to predict a reduced exercise capacity, autonomic imbalance and short-term survival [5]. Moreover, the status of the RV is often determinant in the success of using left ventricular assist devices (LVADs), in patients with end-stage heart failure [6–9].
Although there have been significant improvements in cardiac imaging, the systematic assessment of right heart function is not uniformly carried out because several factors contribute to the challenges of RV assessment: the complex geometry of the RV, the limited definition of RV endocardial surface caused by the trabeculated myocardium, the retrosternal position of the RV that limits echocardiographic imaging windows and the marked load dependence of indices of RV function [10].
In clinical practice, echocardiography is the mainstay of evaluation of RV structure and function. Due to its widespread availability, echocardiography is used as the first-line imaging modality for assessment of RV size and RV function [10].
However, the quantitative assessment of RV size and function is often difficult, because of the complex shape that appears triangular when viewed from the side and crescent shaped when viewed in cross section [10].
Among the various echocardiographic parameters of RV systolic function proposed, RV fractional area change [11], the systolic velocity (S’) [12, 13] of the tricuspid annulus by tissue Doppler and the M-mode measurement of tricuspid annulus displacement (TAPSE) [14, 15] toward apex during ventricular systole are easy to obtain, reproducible and have been validated in patients with HF, but remain only a regional approach to the complex shape of RV [16].
Recently, the RV systolic function can be more easily studied thanks to a non-Doppler echocardiographic technology, called speckle tracking echocardiography (STE), that has been proposed as an alternative approach to define RV longitudinal function, enabling the quantification of myocardial deformation from standard bidimensional echo-images, without the problem of angle dependence [17]. Born as a technique for the study of left ventricle (LV) [18–20] and left atrial deformation [21–24], its application has been recently extended for the analysis of RV systolic function [25–28].
Herein, we present an overview about the technological aspects, the advantages, the limitations and the emerging clinical applications of STE applied for the analysis of RV function.
Methods
Two-dimensional strain imaging is an echocardiographic technique that uses standard B-mode images for speckle tracking analysis. The speckle pattern (acoustic backscatter generated by the reflected ultrasound beam) is followed frame by frame, using a statistical approach based on the detection of the best matching area. The displacement of this speckled pattern is considered to follow myocardial movement, and a change between speckles is assumed to represent myocardial deformation [17].
Although this new technique was introduced for the exclusive analysis of LV function, several studies have recently extended its applicability to other cardiac chamber, the left atrium and right ventricle.
For speckle tracking analysis of right chamber, apical four-chamber view images were obtained using conventional two-dimensional gray-scale echocardiography, storing the images at end-expiration and with a stable ECG recording. Care was taken to obtain the best visualization of RV, from base to apex, and a more reliable delineation of the endocardial border, always remaining in a four-chamber view. Three consecutive heart cycles are recorded and averaged. The frame rate is set between 60 and 80 frames per second; these settings are recommended to combine temporal resolution with adequate spatial definition and to enhance the feasibility of the frame-to-frame tracking technique [17].
Recordings are processed using an acoustic-tracking software (Echo Pac, GE, USA), allowing off-line semi-automated analysis of speckle-based strain.
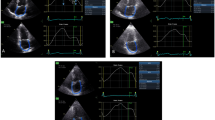
To calculate RV global strain, endocardial border is manually traced in four-chamber view, thus delineating a region of interest (ROI), composed by 6 segments. Then, after the segmental tracking quality analysis and the eventual manual adjustment of the ROI, the longitudinal strain curves are generated by the software for each RV segment. The global right ventricle longitudinal strain (global RVLS) peak was calculated by averaging values observed in all RV segments (Fig. 1).
Right ventricular free-wall strain was derived by a precise and manually delineation of the endocardial border of the only free wall, obtaining a ROI, divided in 3 segments, basal, medial and apical. The corresponding strain curves, generated by the software, allowed to calculate RV longitudinal strain for each free-wall segment, while the dashed curve, which represents the average value of the strain curves of other segments, was used to evaluate free-wall RVLS; this kind of measurement could accurately performed only on separated workstation (Echopac, GE) (Fig. 2).
The time to peak longitudinal strain (TPLS) was also measured as the average of all 6 segments (global TPLS); the temporal pattern of RV mechanical contraction was evaluated as the time needed to reach peak strain TPLS using the beginning of the QRS complex as a reference point.
In patients in whom some segments were excluded because it was not possible to achieve adequate tracking, RVLS and TPLS were calculated by averaging values measured in the remaining segments.
Recent methodological study has reported the feasibility, the reference values and the reproducibility of RVLS measured by STE in normal patients and in patients with RV dysfunction (Table 1); [25].
In patients with RV dysfunction, there is a significantly greater RV dyssynchrony that the standard echocardiographic parameters are not able to highlight for their regional nature. In a simultaneous echocardiographic-catheterization study [26], we have previously demonstrated the close negative correlation between RVLS and RV stroke work index (RVSWI), which is the hemodynamic parameter usually used to evaluate RV function in patients waiting for heart transplant; in particular, free-wall RVLS demonstrated the highest diagnostic accuracy to predict a depressed RVSWI. It may due to the fact that the free wall reflects better the function of right ventricle than global RVLS. Indeed, the interventricular septum is shared by both ventricles, and its strain pattern is also influenced by LV systolic function; so we could hypothesize that RV systolic performance appears to be closely assessed by free-wall RVLS.
Advantages and limitations
The assessment of RV strain by STE represents a relatively rapid and easy-to-perform technique to explore RV function, due to its semi-automated nature and to its off-line processing. In fact, in contrast to Doppler-derived parameters, speckle tracking has the advantage of being angle-independent, and to be less affected by reverberations, side lobes and drop out artifacts [17].
In particular, Tissue Doppler imaging measures myocardial velocities and allows quantitative assessment of RV systolic and diastolic function but can guarantee only a restricted regional evaluation to the complex RV shape and reflects merely the systolic motion of a single point of the tricuspid annulus, disregarding the contribution of the mid-apical free-wall segments. Moreover, velocity-based assessment of myocardial deformation has limitations based on alignment of the Doppler signal, the sensitivity to signal noise and the fact that any derived data are unidirectional along the signal line. This has led to the increasing replacement of these techniques with speckle tracking techniques, which tracks ultrasound reflectors within tissue that behave like tags placed on the myocardium [29].
In fact, the recent development of STE allows a precise assessment of RV deformation profile during an entire cardiac cycle, closely following RV systolic mechanic during the ejection phase; this technique is not only a reliable mean of measuring global RV function but the measurement of RVLS guarantees also the study of regional RV function.
Considering the limitations of classical indices of RV function, speckle-tracking-derived strain has overcome also TAPSE and RV-FAC, allowing the regional analysis of RV contractility. Furthermore, it identifies discrete and localized loses in contractility that are still insufficient to affect global systolic function and thus has potential diagnostic and prognostic implications. Rather than the rough estimates of global RV function provided by RV-FAC, we can now look at the right ventricle at the regional level and obtain more information concerning the pathophysiologic mechanisms leading to RV failure [25].
Although both magnetic resonance imaging and three-dimensional echocardiography have emerged as techniques that would overcome the geometric limitations of standard two-dimensional echocardiography, they are currently limited by their cost and availability [30]; thus, STE still represents a non-invasive, advantageous and user-friendly technique that can potentially enlarge our knowledge on RV function.
Nonetheless, intrinsic limitations of speckle tracking must be listened, including strict frame rate dependency, potential errors in epicardial/endocardial border tracing in subjects with suboptimal image quality and the need for an appropriate learning curve to achieve adequate experience in using analysis software. In fact, the potential difficulty of obtaining a region of interest close enough to the effective shape of right ventricle and the risk of contamination by signal components arising from structures surrounding the RV should be considered.
Moreover, RV strain was assessed only in the 4-chamber view of the 6 segments of the right ventricle, but RV longitudinal function measured in the inlet chamber accounts for about 80 % of RV function [31].
Lastly, because a dedicated software for RV strain analysis has not yet been released, the analysis is performed using a software created for the LV; for this reason, it is mandatory to be careful in the endocardial border delineation, in order to minimize the risk of artifacts.
More recently, the advent of three-dimensional (3D) speckle tracking echocardiography has the potential to overcome the limitations of 2D STE for the assessment of LV global and regional systolic function. Studies have demonstrated that it provides more accurate and convenient assessment of LV function and has been shown to be useful in the detection of subclinical LV dysfunction in patients with heart failure and hypertension [32].
In fact, respect to 2D speckle tracking, 3D STE tracks the motion of speckles within the scan volume instead of artificially dividing the myocardial displacement vector into three directions. As a result, 3D STE is free of geometric assumptions and speckles’ moving out of the scanning plane, allowing a more complete and accurate assessment of myocardial deformation in all three spatial dimensions by avoiding of out-of-plane motion, but it has been validated for the quantification of LV volumes and LV wall motion in ischemic heart disease in adult. Nonetheless, so far data are not present regarding its use in the analysis of RV function [33].
Clinical applications
The assessment of RV systolic function and pulmonary hypertension in patients with HF is of great clinical importance not only for diagnostic purposes but also for prognostication.
In this overview, we have decided to focus on this specific pathologic condition in which RV strain imaging may be helpful in clinical management of patients with only a mention to the recent application of RV strain analysis in patients affected by arrhythmogenic right ventricular cardiomyopathy.
Right ventricular deformation analysis and heart failure
Right ventricular systolic dysfunction is a strong indicator of a poor prognosis among patients with congestive HF who are refractory to medical therapy. Assessment of RV systolic function in patients with HF provides complementary information with high power to stratify prognosis [34, 35].
To the best of our knowledge, the study of Meris et al. [25] was the first one concerning patients with RV dysfunction associated with LV dysfunction, the most frequent scenario in clinical practice; they found that RV strain accurately identified reduced global RV function and that a global longitudinal strain cut-off value of −19 % can be considered a useful means of differentiating normal and impaired right ventricles.
Moreover, in this study, the magnitude of RV strain was further reduced in those patients with pulmonary hypertension; when each RV segment was considered individually, there was a clear trend toward less regional longitudinal strain in patients with RV dysfunction and pulmonary hypertension than in those without pulmonary hypertension, showing significantly greater RV dyssynchrony in the presence of pulmonary hypertension in line with the previous findings of Kalogeropoulos et al. [11].
The standard criterion to assess RV systolic function in patients waiting for a heart transplant remains the right-side heart catheterization procedure, and the hemodynamic gold standard parameter usually used to evaluate RV function is the RVSWI, which represents the effective work of RV against pulmonary pressures during cardiac cycles.
In patients with advanced systolic HF referred for cardiac transplant, it has been demonstrated a strong inverse correlation between RVSWI and RVLS, especially with free-wall RVLS [26]. Thus, the longitudinal function of RV appeared essential in determining RV output; in particular, the strongest correlation found between free-wall RVLS and RVSWI can be justified by the fact that free wall reflects better the function of right ventricle, considering that the motion of interventricular septum in this particular patient setting is mostly influenced by LV dysfunction. Recently, a prospective study has demonstrated that among patients referred for heart transplantation, free-wall RVLS is a stronger predictor of outcome than LV longitudinal strain and other conventional parameters, providing a stronger prognostic stratification [36].
Right ventricular deformation analysis and left ventricular assist device
In patients with refractory end-stage HF, the status of RV is also determinant in the success of using LVAD [37–40].
Use of implantable LVADs in the treatment for end-stage HF has recently gained broader application, not only as a bridge to heart transplantation but also as destination therapy, for patients who are not transplantation candidates [41].
One of the main and life-threatening complications inherent following the implant of LVAD is RV failure, manifested by the need for inotropic and/or nitric oxide support >14 days after LVAD implant and/or the need for right-sided mechanical circulatory support. RV failure is a major contributor of significant morbidity and mortality after LVAD placement. The complex pathophysiology of RV failure, which could potentially be related to RV myocardial dysfunction, interventricular dependence and RV afterload, has led to inconsistencies in predicting risk factors for RV dysfunction. Several strategies have evolved over the years of experience with mechanical circulatory support that have aimed to avoid as well as reduce the incidence of RV failure. It is imperative that patients who definitely need biventricular support are identified. Despite the numerous risk factors identified in many studies as well as the development of risk factor profile scores, this continues to be a challenging problem [42].
It is well recognized that where RV dysfunction is present before LVAD implant, overt postoperative RV failure may occur in the postimplant period [37].
As a consequence, better preoperative identification of risks factors for RV failure should lead to improved selection of patients.
Traditional echocardiographic parameters provide information concerning global RV function, but they miss important regional variations in contractility and are difficult to apply in this subgroup of patients with advanced heart failure; they are even more difficult to assess in the presence of ultrasound artifacts related to mechanical device [43].
The hemodynamic parameter of RVSWI is widely used in the preoperative selection of patients who would benefit from biventricular support [38]. In fact, earlier studies [44, 45] have demonstrated that a decreased RVSWI is significantly associated with severe RV failure after LVAD implantation.
Recently, by extrapolation, we were able to show in a small number of patients undergone LVAD implant the predictive role of RVLS, observing patients with advanced HF before and after LVAD implant [27]; in this study, we observed that those patients who presented a depressed RVLS value at the preoperative echocardiographic evaluation showed a worse prognosis, with development of RV failure, instead patients that presented higher values of RVLS showed progressive improvement in RV deformation.
Also, a recent study of Grant et al. [46] has demonstrated that clinical evaluation and standard preprocedural echocardiographic RV measurements seemed insufficient for predicting RV failure in patient undergoing LVAD implantation, showing that there was a significant difference in peak strain between patients with and without RV failure. Therefore, this study confirmed that reduced RVLS is associated with an increased risk of RV failure among patients undergoing LVAD implantation, showing that RVLS was incremental to the Michigan risk score as a predictor of RV failure.
Thus, even if this new echocardiographic technique requires additional validation, it has already gained wide applications; in fact, also the American Society of Echocardiography has recently proposed its use in this particular setting of patients with advanced heart failure, treated with LVAD implantation as bridge to transplantation or as destination therapy (Fig. 3); [43].
ROC curves describing the diagnostic accuracy of Michigan Score, Michigan Score + RV strain, Michigan Score + Subjective RV function evaluation in predicting RV failure after LVAD implant. Adapted from Rasalingam et al. [43]
Right ventricular deformation analysis and arrhythmogenic right ventricular cardiomyopathy
Arrhythmogenic right ventricular cardiomyopathy is a heritable disease characterized by the fibro-fatty replacement of RV myocardium leading to RV failure and arrhythmias.
The identification of RV abnormalities using echocardiography is still a major challenge because of the geometric shape of the RV and the patchy involvement of the right ventricular wall with subtle abnormalities at an early stage.
STE has broadened the utility of echocardiography in detecting and understanding the effect of contraction abnormalities of the heart, sometimes in subclinical phase of disease.
Prasaka et al. [47] have demonstrated that the value of RV strain was significantly lower than in controls, even in the subset of patients with ARVD with apparently normal right ventricles by conventional echocardiography, showing that a peak RV strain <18 % best identify patients with ARVD.
More recently, Aneq et al. [48] have demonstrated that longitudinal speckle derived strain is reduced in the right as well as in the left ventricular walls, confirming the biventricular involvement of AVRC.
In conclusion, strain analysis is superior to conventional echocardiographic parameters in identifying ARVD and may have potential clinical value in the assessment of patients with suspected ARVD in the diagnostic workup.
Conclusion
Right ventricular function carries important clinical and prognosis implications in many setting of patients. Considering the limitations of classical echocardiographic indices, this new parameter of RVLS provides more detailed information about regional and global RV mechanics and may have important clinical implications for non-invasive evaluation of RV systolic function, permitting to identify patients with subclinical RV dysfunction.
Moreover, the assessment of RV systolic function in patients with HF provides complementary information with high power to stratify prognosis and may be considered a promising non-invasive parameter to identify patients at higher risk among HF patients.
However, further prospective studies are necessary to better define its role in the intensive management of patients and the potential of this new imaging technique of STE to the analysis of RV function in patients with advanced HF and in LVAD therapy and also in the specific setting of ARVC.
References
Guglin M, Verma S (2012) Right side of heart failure. Heart Fail Rev 17(3):511–527
Rudski LG, Lai WW, Afilalo J, Hua L, Handschumacher MD, Chandrasekaran K, Solomon SD, Louie EK, Schiller NB (2010) Guidelines for the echocardiographic assessment of the right heart in adults: a report from the American Society of Echocardiography endorsed by the European Association of Echocardiography, a registered branch of the European Society of Cardiology, and the Canadian Society of Echocardiography. J Am Soc Echocardiogr 23(7):685–713
Vitarelli A, Terzano C (2010) Do we have two hearts? New insights in right ventricular function supported by myocardial imaging echocardiography. Heart Fail Rev 15(1):39–61
Ghio S, Gavazzi A, Campana C, Inserra C, Klersy C, Sebastiani R, Arbustini E, Recusani F, Tavazzi L (2001) Independent and additive prognostic value of right ventricular systolic function and pulmonary artery pressure in patients with chronic heart failure. J Am Coll Cardiol 37:183–188
Zornoff LAM, Skali H, Pfeffer MA, Sutton MSJ, Rouleau JL, Lamas GA, Plappert T, Rouleau JR, Moyé LA, Lewis SJ, Braunwald E, Solomon SD (2002) Right ventricular dysfunction and risk of heart failure and mortality after myocardial infarction. J Am Coll Cardiol 39:1450–1455
Grant AD, Smedira NG, Starling RC, Marwick TH (2012) Independent and incremental role of quantitative right ventricular evaluation for the prediction of right ventricular failure after left ventricular assist device implantation. J Am Coll Cardiol 60(6):521–528
Fitzpatrick JR, Frederick JR, Hsu VM, Kozin ED, O’Hara ML, Howell E, Dougherty D, McCormick RC, Laporte CA, Cohen JE, Southerland KW, Howard JL, Jessup ML, Morris RJ, Acker MA, Woo YJ (2008) Risk score derived from pre-operative data analysis predicts the need for biventricular mechanical circulatory support. J Heart Lung Transplant 27:1286–1292
Matthews JC, Koelling TM, Pagani FD, Aaronson KD (2008) The right ventricular failure risk score. A pre-operative tool for assessing the risk of right ventricular failure in left ventricular assist device candidates. J Am Coll Cardiol 51:2163–2172
Mancini D, Lietz K (2010) Selection of cardiac transplantation candidates in 2010. Circulation 122:173–183
Jiang L (1994) Right ventricle. In: Weyman AE (ed) Principle and practice of echocardiography. Lippincott Williams and Wilkins, Baltimore, MD, pp 901–921
Kalogeropoulos AP, Vega JD, Smith AL, Georgiopoulou VV (2011) Pulmonary hypertension and right ventricular function in advanced heart failure. Congest Heart Fail 17:189–198
Galderisi M, Severino S, Cicala S, Caso P (2002) The usefulness of pulsed tissue Doppler for the clinical assessment of right ventricular function. Ital Heart J 3(4):241–247
Meluzín J, Spinarová L, Bakala J et al (2001) Pulsed Doppler tissue imaging of the velocity of tricuspid annular systolic motion; a new, rapid, and non-invasive method of evaluating right ventricular systolic function. Eur Heart J 22(4):340–348
Miller D, Farah MG, Liner A, Fox K, Schluchter M, Hoit BD (2004) The relation between quantitative right ventricular ejection fraction and indices of tricuspid annular motion and myocardial performance. J Am Soc Echocardiogr 17(5):443–447
Puwanant S, Hamilton K, Klodell CT et al (2008) Tricuspidal annular motion as a predictor of severe right ventricular failure after left ventricular assist device implantation. J Heart Lung Transplant 27:1102–1107
Abraham J, Abraham TP (2009) The role of echocardiography in hemodynamic assessment in heart failure. Heart Fail Clin 5(2):191–208
Mondillo S, Galderisi M, Mele D et al (2011) Speckle-tracking echocardiography: a new technique for assessing myocardial function. J Ultrasound Med 30(1):71–83
Cameli M, Lisi M, Mondillo S et al (2011) Prediction of stroke volume by global left ventricular longitudinal strain in patients undergoing assessment for cardiac transplantation. J Crit Care 26(4):433
Cameli M, Ballo P, Lisi M et al (2013) Left ventricular twist in clinically stable heart transplantation recipients: a speckle tracking echocardiography study. Int J Cardiol 168(1):357–361
Cameli M, Ballo P, Righini FM, Caputo M, Lisi M, Mondillo S (2011) Physiologic determinants of left ventricular systolic torsion assessed by speckle tracking echocardiography in healthy subjects. Echocardiography 28(6):641–648
Cameli M, Caputo M, Mondillo S, Ballo P, Palmerini E, Lisi M, Marino E, Galderisi M (2009) Feasibility and reference values of left atrial longitudinal strain imaging by two-dimensional speckle tracking. Cardiovasc Ultrasound 7:6
Vianna-Pinton R, Moreno CA, Baxter CM, Lee KS, Tsang TS, Appleton CP (2009) Two-dimensional speckle-tracking echocardiography of the left atrium: feasibility and regional contraction and relaxation differences in normal subjects. J Am Soc Echocardiogr 22:299–305
Cameli M, Lisi M, Righini FM, Mondillo S (2012) Novel echocardiographic techniques to assess left atrial size, anatomy and function. Cardiovasc Ultrasound 1(10):4
Cameli M, Lisi M, Righini FM, Focardi M, Alfieri O, Mondillo S (2012) Left atrial speckle tracking analysis in patients with mitral insufficiency and history of paroxysmal atrial fibrillation. Int J Cardiovasc Imaging 28(7):1663–1670
Meris A, Faletra F, Conca C, Klersy C, Regoli F, Klimusina J, Penco M, Pasotti E, Pedrazzini GB, Moccetti T, Auricchio A (2010) Timing and magnitude of regional right ventricular function: a speckle tracking-derived strain study of normal subjects and patients with right ventricular dysfunction. J Am Soc Echocardiogr 23:823–831
Cameli M, Lisi M, Righini FM, Tsioulpas C, Bernazzali S, Maccherini M, Sani G, Ballo P, Galderisi M, Mondillo S (2012) Right ventricular longitudinal strain correlates well with right ventricular stroke work index in patients with advanced heart failure referred for heart transplantation. J Card Fail 18(3):208–215
Cameli M, Lisi M, Righini FM, Focardi M, Lunghetti S, Bernazzali S, Marchetti L, Biagioli B, Galderisi M, Maccherini M, Sani G, Mondillo S (2013) Speckle tracking echocardiography as a new technique to evaluate right ventricular function in patients with left ventricular assist device therapy. J Heart Lung Transplant 32(4):424–430
Cameli M, Bernazzali S, Lisi M et al (2012) Right ventricular longitudinal strain and right ventricular stroke work index in patients with severe heart failure: left ventricular assist device suitability for transplant candidates. Transplant Proc 44(7):2013–2015
Steeds RP (2011) Echocardiography: frontier imaging in cardiology. Br J Radiol 84:S237–S244
Jurcut R, Giusca S, La Gerche A, Vasile S, Ginghina C, Voigt JU (2010) The echocardiographic assessment of the right ventricle: what to do in 2010? Eur J Echocardiogr 11(2):81–96
Carlsson M, Ugander M, Heiberg E, Arheden H (2007) The quantitative relationship between longitudinal and radial function in left, right, and total heart pumping in humans. Am J Physiol Heart Circ Physiol 293:636–644
Li CM, Li C, Bai WJ, Zhang XL, Tang H, Qing Z, Li R (2013) Value of three-dimensional speckle-tracking in detecting left ventricular dysfunction in patients with aortic valvular diseases. J Am Soc Echocardiogr 26(11):1245–1252
Zhang L, Gao J, Mingxing X, Yin P, Liu W, Li Y, Klas B, Sun J, Balluz R, Ge S (2013) Left ventricular three-dimensional global systolic strain by real-time three-dimensional speckle-tracking in children: feasibility, reproducibility, maturational changes and normal ranges. J Am Soc Echocardiogr 26:853–859
Gavazzi A, Berzuini C, Campana C et al (1997) Value of right ventricular ejection fraction in predicting short-term prognosis of patients with severe chronic heart failure. J Heart Lung Transplant 16:774
De Groote P, Millaire A, Foucher-Hossein C et al (1998) Right ventricular ejection fraction is an independent predictor of survival in patients with moderate heart failure. J Am Coll Cardiol 32:948
Cameli M, Righini FM, Lisi M, Bennati E, Navarri R, Lunghetti S, Padeletti M, Cameli P, Tsioulpas C, Bernazzali S, Maccherini M, Sani G, Henein M, Mondillo S (2013) Comparison of right versus left ventricular strain analysis as a predictor of outcome in patients with systolic heart failure referred for heart transplantation. Am J Cardiol PMID: 24063825
Maeder M, Leet A, Andrew R, Esmore D, Kaye D (2009) Changes in right ventricular function during continuous-low left ventricular assist device support. J Heart Lung Transplant 28:360–366
Fitzpatrick JR, Frederick JR, Hsu VM, Kozin ED, O’Hara ML, Howell E et al (2008) Risk score derived from pre-operative data analysis predicts the need for biventricular mechanical circulatory support. J Heart Lung Transplant 27:1286–1292
Matthews JC, Koelling TM, Pagani FD, Aaronson KD (2008) The right ventricular failure risk score. A pre-operative tool for assessing the risk of right ventricular failure in left ventricular assist device candidates. J Am Coll Cardiol 51:2163–2172
Mancini D, Lietz K (2010) Selection of cardiac transplantation candidates in 2010. Circulation 122:173–183
Puwanant S, Hamilton K, Klodell CT, Hill JA, Schofield RS, Cleeton TS et al (2008) Tricuspidal annular motion as a predictor of severe right ventricular failure after left ventricular assist device implantation. J Heart Lung Transplant 27:1102–1107
John R, Lee S, Eckman P, Liao K (2010) Right ventricular failure—a continuing problem in patients with left ventricular assist device support. J Cardiovasc Transl Res 3(6):604–611
Rasalingam R, Johnson SN, Bilhorn KR, Huang PH, Makan M, Moazami N, Pérez JE (2011) Transthoracic echocardiographic assessment of continuous-flow left ventricular assist devices. J Am Soc Echocardiogr 24:135–148
Kavarana MN, Pessin-Minsley MS, Urtecho J, Catanese KA, Flannery M, Oz MC et al (2002) Right ventricular dysfunction and organ failure in left ventricular assist device recipients: a continuing problem. Ann Thorac Surg 73:745–750
Ochiai Y, McCarthy PM, Smedira NG, Banbury MK, Navia JL, Feng J et al. (2002) Predictors of severe right ventricular failure after implantable left ventricular assist device insertion: analysis of 245 patients. Circulation 106: I–198
Grant A, Smedira N, Starling R, Marwick T (2012) Independent and incremental role of quantitative right ventricular evaluation for the prediction of right ventricular failure after left ventricular assist device implantation. J Am Coll Cardiol 60:521–528
Prakasa KR, Wang J, Tandri H, Dalal D, Bomma C, Chojnowski R, James C, Tichnell C, Russell S, Judge D, Corretti M, Bluemke D, Calkins H, Abraham TP (2007) Utility of tissue Doppler and strain echocardiography in arrhythmogenic right ventricular dysplasia/cardiomyopathy. Am J Cardiol 100(3):507–512
Aneq MÅ, Engvall J, Brudin L, Nylander E (2012) Evaluation of right and left ventricular function using speckle tracking echocardiography in patients with arrhythmogenic right ventricular cardiomyopathy and their first degree relatives. Cardiovasc Ultrasound 10:37
Conflict of interest
The authors declare that they have no competing interests.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Cameli, M., Righini, F.M., Lisi, M. et al. Right ventricular strain as a novel approach to analyze right ventricular performance in patients with heart failure. Heart Fail Rev 19, 603–610 (2014). https://doi.org/10.1007/s10741-013-9414-7
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10741-013-9414-7